Qualitative and Quantitative Analysis of Different Rhodiola rosea Rhizome Extracts by UHPLC-DAD-ESI-MSn
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Reference Substances
2.2. Plant Material
2.3. Preparation of Extracts
2.4. UHPLC-DAD-ESI-MSn Instrumentation and Methods
2.5. Validation
2.5.1. Linearity
2.5.2. Precision
2.5.3. Accuracy
3. Results
3.1. UHPLC-DAD-ESI-MSn Analysis
3.1.1. Identification of Phenolic Constituents
3.1.2. Quantification of Characteristic Phenolics
3.1.3. Collection Site High Tauern
3.1.4. Collection Site Gurktal Alps
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bykov, V.A.; Zapesochnaya, G.G.; Kurkin, V.A. Traditional and biotechnological aspects of obtaining medicinal preparations from Rhodiola Rosea L. (A review). Pharm. Chem. J. 1999, 33, 29–40. [Google Scholar] [CrossRef]
- Ganzera, M.; Yayla, Y.; Khan, I.A. Analysis of the Marker Compounds of Rhodiola rosea L. (Golden Root) by Reversed Phase High Performance Liquid Chromatography. Chem. Pharm. Bull. 2001, 49, 465–467. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.; Poppleton, D.; Struchkov, A.; Ireland, R. Analysis of five bioactive compounds from naturally occurring Rhodiola rosea in eastern Canada. Can. J. Plant Sci. 2014, 94, 741–748. [Google Scholar] [CrossRef]
- Battistelli, M.; de Sanctis, R.; de Bellis, R.; Cucchiarini, L.; Dachà, M.; Gobbi, P. Rhodiola rosea as antioxidant in red blood cells:Ultrastructural and hemolytic behaviour. Eur. J. Histochem. 2005, 49, 243–254. [Google Scholar]
- De Sanctis, R.; de Bellis, R.; Scesa, C.; Mancini, U.; Cucchiarini, L.; Dachà, M. In vitro protective effect of Rhodiola rosea extract against hypochlorous acid-induced oxidative damage in human erythrocytes. BioFactors 2004, 20, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Crendal, F.; Grachev, S.; Seifulla, R.; Ziegenfuss, T. Effect of Extracts from Rhodiola rosea and Rhodiola crenulata (Crassulaceae) Roots on ATP Content in Mitochondria of Skeletal Muscles. Bull. Exp. Biol. Med. 2003, 136, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Hambardzumyan, M.; Hovhanissyan, A.; Wikman, G. The Adaptogens Rhodiola and Schizandra Modify the Response to immobilization Stress in Rabbits by Suppressing the Increase of Phosphorylated Stress-activated Protein Kinase, Nitric Oxide and Cortisol. Drug Target Insights 2007, 2, 39–54. [Google Scholar] [CrossRef]
- Mattioli, L.; Funari, C.; Perfumi, M. Effects of Rhodiola rosea L. extract on behavioural and physiological alterations induced by chronic mild stress in female rats. J. Psychopharmacol. 2009, 23, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.-A.; Hostettmann, K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009, 122, 397–401. [Google Scholar] [CrossRef]
- Pooja Bawa, A.S.; Khanum, F. Anti-inflammatory activity of Rhodiola rosea—“A second-generation adaptogen”. Phytother. Res. 2009, 23, 1099–1102. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Jang, H.-D.; Shetty, K. Evaluation of Rhodiola crenulata and Rhodiola rosea for management of type II diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 425–432. [Google Scholar] [PubMed]
- Tolonen, A.; György, Z.; Jalonen, J.; Neubauer, P.; Hohtola, A. LC/MS/MS identification of glycosides produced by biotransformation of cinnamyl alcohol in Rhodiola rosea compact callus aggregates. Biomed. Chromatogr. 2004, 18, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, J.; Gu, X.; Ding, F. Salidroside attenuates glutamate-induced apoptotic cell death in primary cultured hippocampal neurons of rats. Brain Res. 2008, 1238, 189–198. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, A.; Hou, R.; Zhang, J.; Jia, X.; Jiang, W.; Chen, J. Salidroside protects cardiomyocyte against hypoxia-induced death:A HIF-1α-activated and VEGF-mediated pathway. Eur. J. Pharmacol. 2009, 607, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Ge, Y.-K.; Zheng, X.-X.; Zhang, L. Salidroside stimulated glucose uptake in skeletal muscle cells by activating AMP-activated protein kinase. Eur. J. Pharmacol. 2008, 588, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-J.; Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Park, S.-J. Rhodiosin, an Antioxidant Flavonol Glycoside from Rhodiola rosea. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 486–492. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yamada, K.; Murata, T.; Hasegawa, T.; Takano, F.; Koga, K.; Fushiya, S.; Batkhuu, J.; Yoshizaki, F. Constituents of Rhodiola rosea Showing Inhibitory Effect on Lipase Activity in Mouse Plasma and Alimentary Canal. Planta Med. 2008, 74, 1716–1719. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S.-J.; Kim, J.H.; Kwon, H.-J.; Kim, J.H.; Park, K.H.; Rho, M.-C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef]
- Rodin, I.A.; Stavrianidi, A.N.; Braun, A.V.; Shpigun, O.A.; Popik, M.V. Simultaneous Determination of Salidroside, Rosavin, and Rosarin in Extracts from Rhodiola rosea by High Performance Liquid Chromatography with Tandem Mass Spectrometry Detection. J. Anal. Chem. 2012, 67, 1026–1030. [Google Scholar] [CrossRef]
- Ioset, K.N.; Nyberg, N.T.; van Diermen, D.; Malnoe, P.; Hostettmann, K.; Shikov, A.N.; Jaroszewski, J.W. Metabolic Profiling of Rhodiola rosea Rhizomes by ¹H NMR Spectroscopy. Phytochem. Anal. 2011, 22, 158–165. [Google Scholar] [CrossRef]
- Akgul, Y.; Ferreira, D.; Abourashed, E.A.; Khan, I.A. Lotaustralin from Rhodiola rosea roots. Fitoterapia 2004, 75, 612–614. [Google Scholar] [CrossRef]
- Tolonen, A.; Hohtola, A.; Jalonen, J. Comparison of electrospray ionization and atmospheric pressure chemical ionization techniques in the analysis of the main constituents from Rhodiola rosea extracts by liquid chromatography/mass spectrometry. J. Mass Spectrom. 2003, 38, 845–853. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.-H.; Ali, Z.; Smillie, T.J.; Filion, V.; Cuerrier, A.; Arnason, J.T.; Khan, I.A. RP-HPLC determination of phenylalkanoids and monoterpenoids in Rhodiola rosea and identification by LC-ESI-TOF. Biomed. Chromatogr. 2009, 23, 865–872. [Google Scholar] [CrossRef]
- Ali, Z.; Fronczek, F.R.; Khan, I.A. Phenylalkanoids and Monoterpene Analogues from the Roots of Rhodiola rosea. Planta Med. 2008, 74, 178–181. [Google Scholar] [CrossRef]
- Yousef, G.G.; Grace, M.H.; Cheng, D.M.; Belolipov, I.V.; Raskin, I.; Lila, M.A. Comparative phytochemical characterization of three Rhodiola species. Phytochemistry 2006, 67, 2380–2391. [Google Scholar] [CrossRef]
- Petsalo, A.; Jalonen, J.; Tolonen, A. Identification of flavonoids of Rhodiola rosea by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2006, 1112, 224–231. [Google Scholar] [CrossRef]
- Ma, G.; Li, W.; Dou, D.; Chang, X.; Bai, H.; Satou, T.; Li, J.; Sun, D.; Kang, T.; Nikaido, T.; et al. Rhodiolosides A-E, Monoterpene Glycosides from Rhodiola rosea. Chem. Pharm. Bull. 2006, 54, 1229–1233. [Google Scholar] [CrossRef]
- Rohloff, J. Volatiles from rhizomes of Rhodiola rosea L. Phytochemistry 2002, 59, 655–661. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Justino, G.C. Structural Analysis of Flavonoids and Related Compounds—A Review of Spectroscopic Applications; Phytochemicals; A Global Perspective of Their Role in Nutrition and Health; Venketeshwer, Rao; InTech: Rijeka, Croatia, 2012; Volume 1, pp. 33–56. [Google Scholar]
- Marchev, A.S.; Aneva, I.Y.; Koycheva, I.K.; Georgiev, M.I. Phytochemical variations of Rhodiola rosea L. wild-grown in Bulgaria. Phytochem. Lett. 2017, 20, 386–390. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Ladányi, M.; György, Z. Changes in the Content of the Glycosides, Aglycons and their Possible Precursors of Rhodiola rosea during the Vegetation Period. Nat. Prod. Commun. 2015, 10, 1413–1416. [Google Scholar] [CrossRef]
| Concentration (µg/mL) | Accuracy Cinnamyl Alcohol | Accuracy Salidroside | Accuracy Kaempferol-7-Neohesperoside |
|---|---|---|---|
| 75 | 98.9 | 95.1 | 109.7 |
| 300 | 104.5 | 91.2 | 93.1 |
| 600 | 98.1 | 90.5 | 95.0 |
| No | RT (min) | [M-H] (m/z) | MSn (m/z) | UV λmax (nm) | Identification | Reference |
|---|---|---|---|---|---|---|
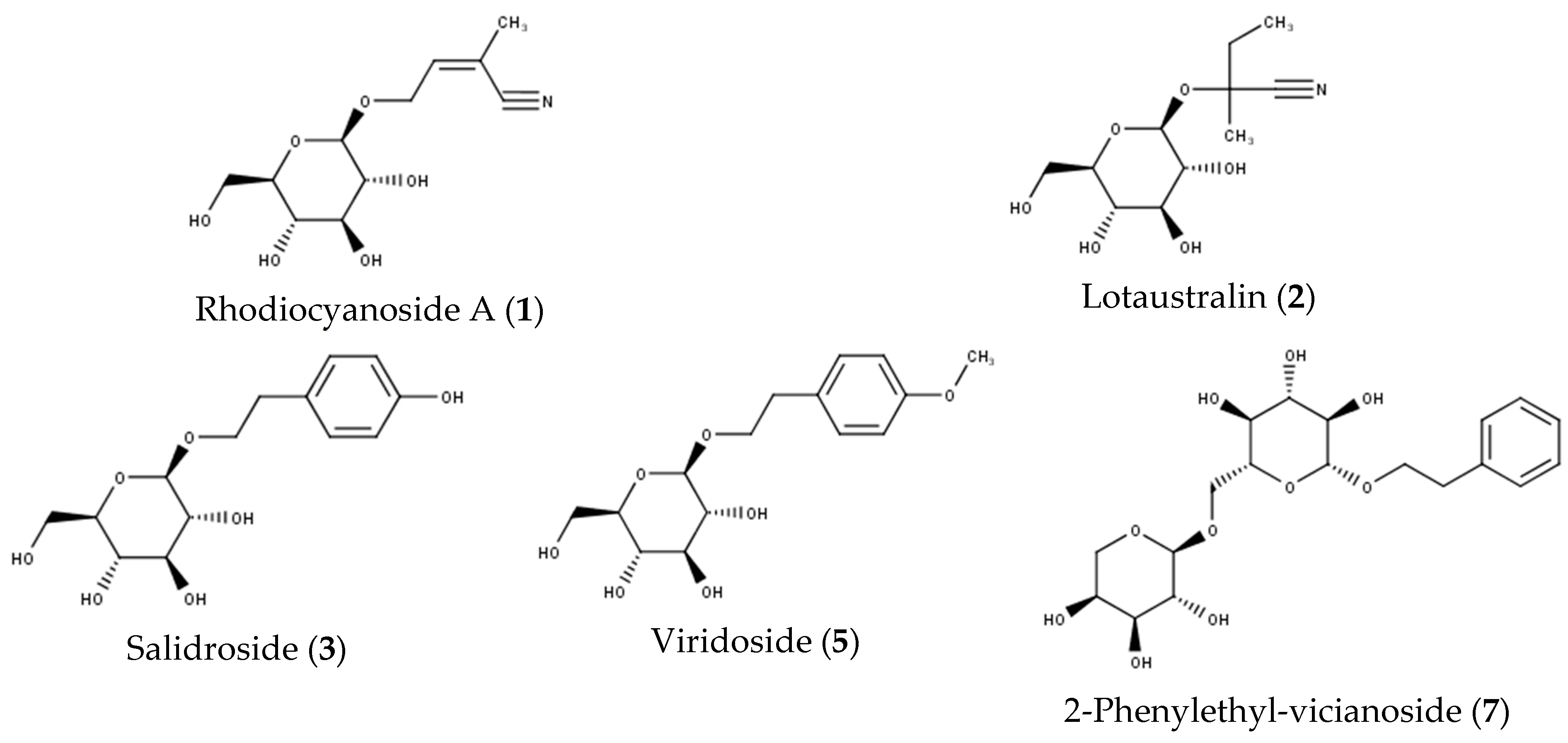
| 1 | 3.09 | 258 | MS2[304] 1: 258, 179, 161 MS3[179]: 161, 143, 131, 119, 89 | 209 | Rhodiocyanoside A | [21] |
| 2 | 4.08 | 260 | MS2[306] 1: 260, 188, 161 MS3[260]: 188, 161 | 205 | Lotaustralin | [22] |
| 3 | 4.85 | 299 | MS2[345] 1: 299 MS3[299]: 179, 161, 143, 131, 119, 113, 101, 89 | 222, 278 | Salidroside | [23,24,25] |
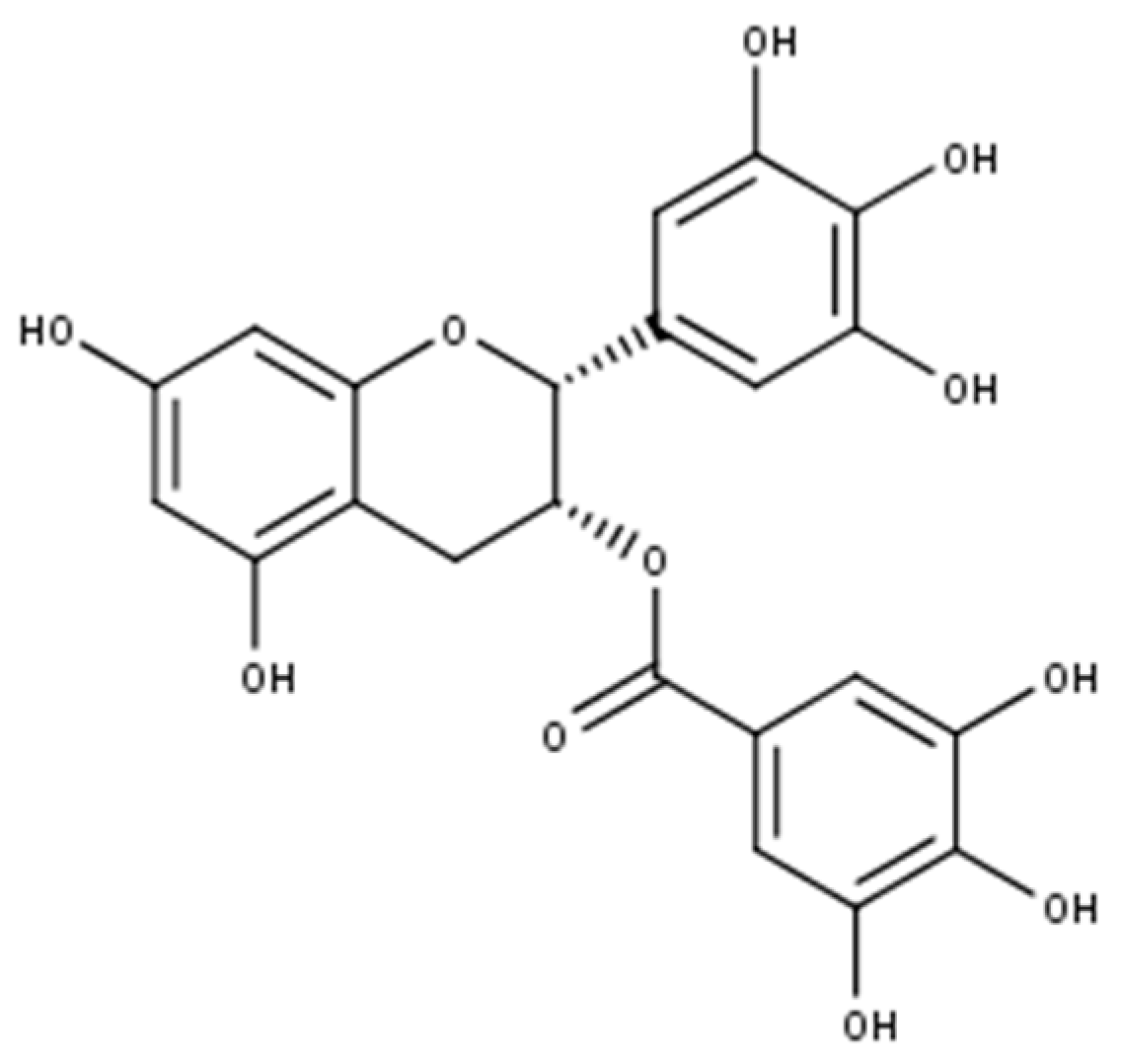
| 4 | 5.62 | 761 | MS2[761]: 635, 609, 593, 592, 575, 483, 423 MS3[423]: 405, 299, 283, 243 | 268 | Epigallocatechin-epigallocatechin gallate | [26] |
| 5 | 6.56 | 313 | MS2[359] 1: 313 MS3[313]: 151 | 226, 271 | Viridoside | [24,25] |
| 6 | 7.68 | 457 | MS2[457]: 331, 305, 169 MS3[169]: 125 | 277 | Epigallocatechin-3-O-gallate | [26] |
| 7 | 8.32 | 415 | MS2[461] 1: 415 MS3[415]: 191, 149, 131 | 2-Phenylethyl-vicianoside | [24,25] | |
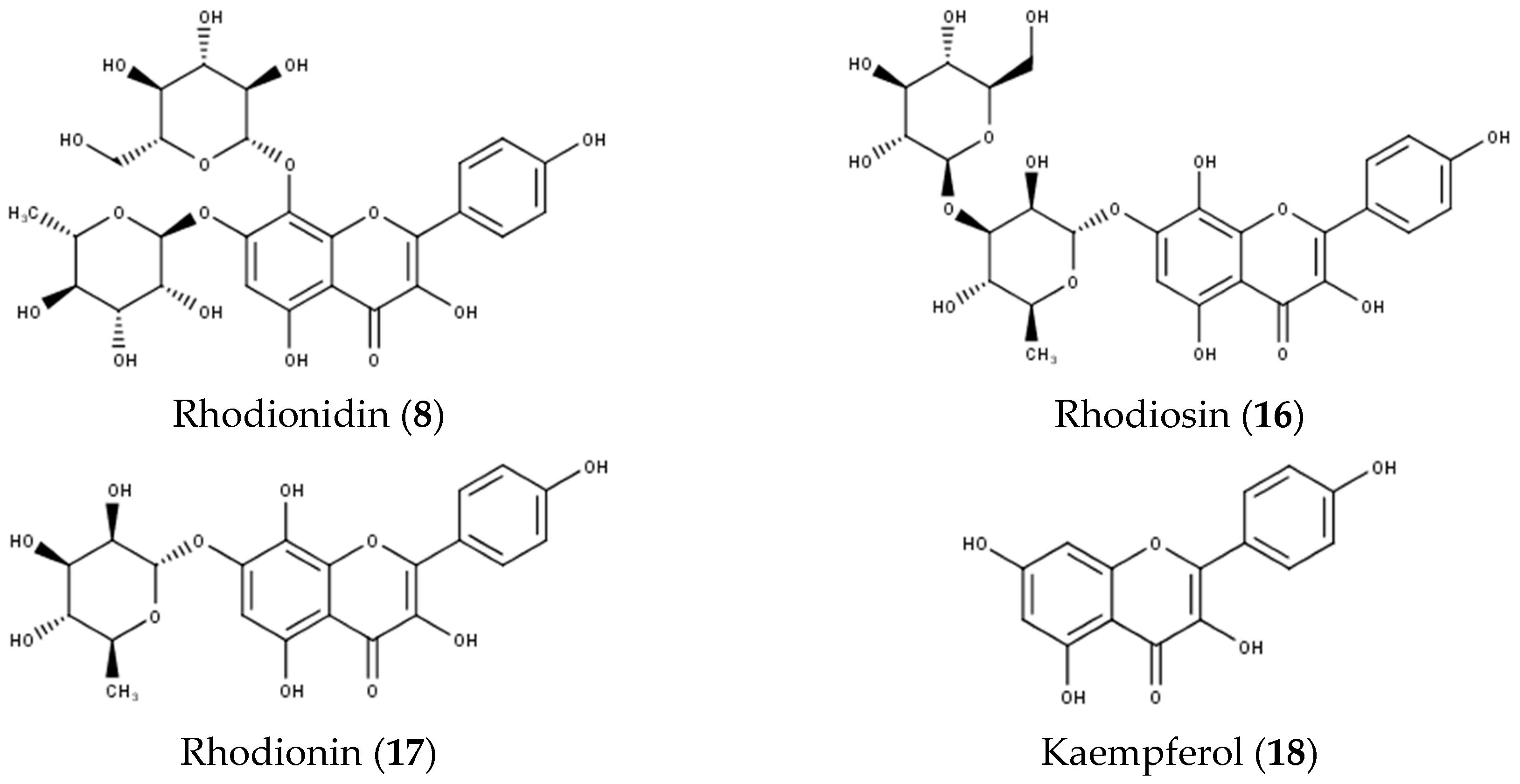
| 8 | 9.40 | 609 | MS2[609]: 463 MS3[463]: 301, 300 | 277, 329, 375(sh) | Rhodionidin | [27] |
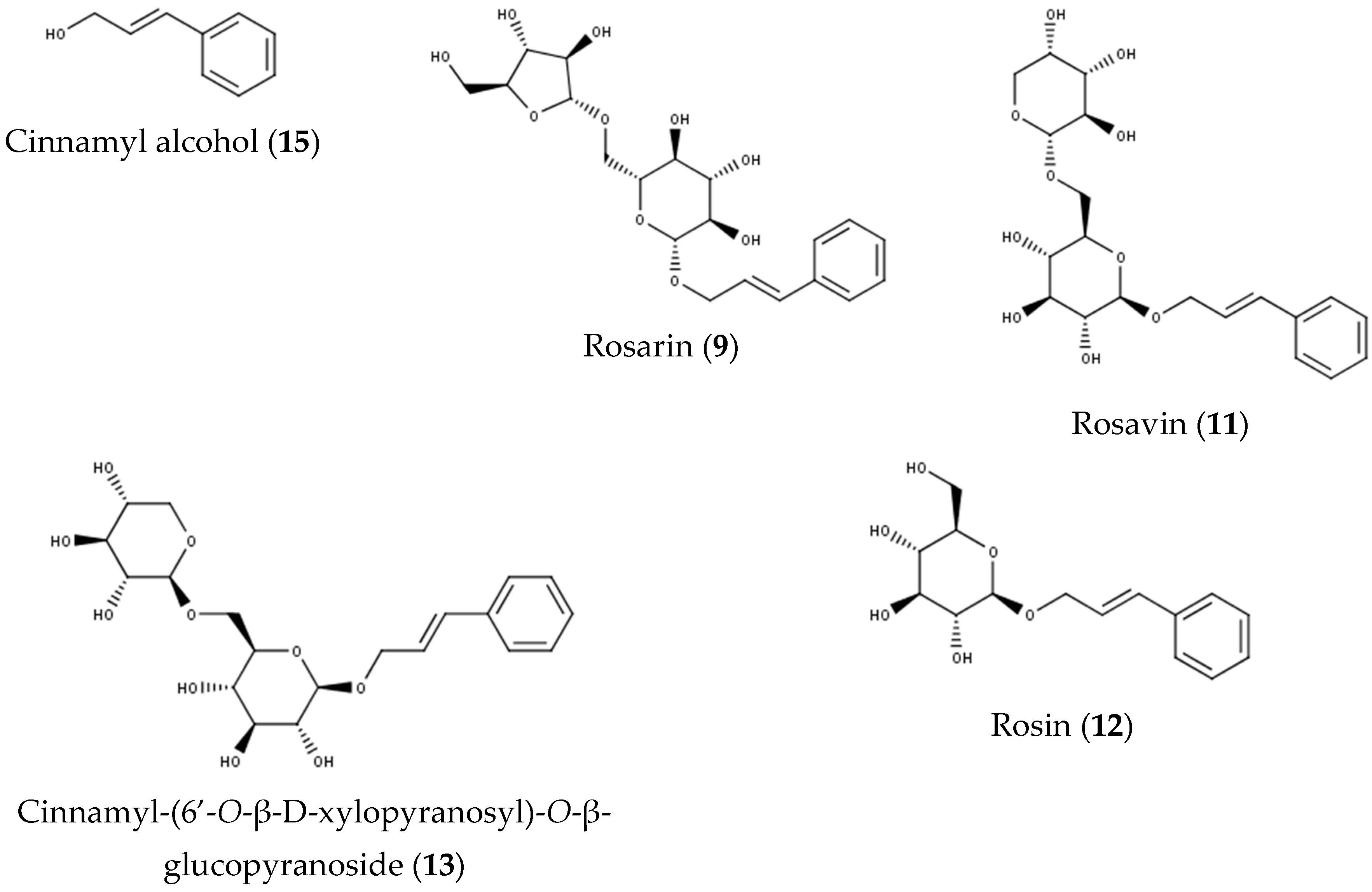
| 9 | 10.20 | 427 | MS2[473] 1: 427, 293 MS3[427]: 293, 149 | 253 | Rosarin | [23,24,25] |
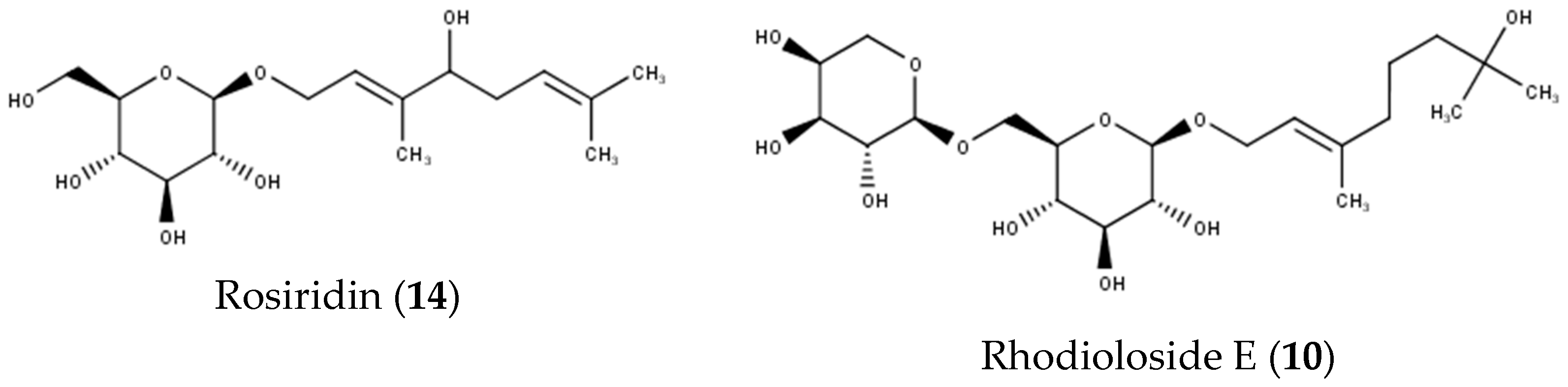
| 10 | 10.42 | 465 | MS2[511] 1: 465 MS3[465]: 333, 293, 191, 149 | Rhodioloside E | [28] | |
| 11 | 10.53 | 427 | MS2[473] 1: 427, 293 MS3[427]: 293, 149 | 253 | Rosavin | [23,25] |
| 12 | 10.70 | 295 | MS2[341] 1: 295, 179, 161 MS3[179]: 135 | 253 | Rosin | [23,24,25] |
| 13 | 10.82 | 427 | MS2[473] 1: 427, 293 MS3[427]: 293, 149 | 245 | Cinnamyl-(6’-O-β-d-xylopyranosyl)-O-β-glucopyranoside | [23,25] |
| 14 | 11.06 | 331 | MS2[377] 1: 331, 179 MS3[331]: 179, 161, 143, 113 | 192, 264(sh) | Rosiridin | [23,24,25] |
| 15 | 13.50 | - 2 | 205, 253 | Cinnamyl alcohol | [29] | |
| 16 | 14.21 | 609 | MS2[609]: 301 MS3[301]: 301, 283, 255, 229, 211, 201 | 277, 333, 385 | Rhodiosin | [17] |
| 17 | 14.56 | 447 | MS2[447]: 301 MS3[301]: 301, 283, 255, 229, 211, 201 | 277, 332, 385 | Rhodionin | [17] |
| 18 | 16.78 | 285 | MS2[285]: 285, 267, 229, 213, 185, 169, 151 MS3[151]: 107 | 267, 368 | Kaempferol | [27] |
| Cinnamyl Alcohol Derivatives | M (g/mol) | Factor |
|---|---|---|
| Cinnamyl alcohol 1 | 134.17 | - |
| Rosarin | 428 | 3.190 |
| Rosavin | 428 | 3.190 |
| Rosin | 296 | 2.206 |
| Flavonoid Derivatives | M (g/mol) | Factor |
|---|---|---|
| Kaempferol-7-neohesperoside ∙ H2O 1 | 612.53 | - |
| Rhodionidin | 610 | 0.996 |
| Rhodiosin | 610 | 0.996 |
| Rhodionin | 448.38 | 0.732 |
| Extracts High Tauern | Salidroside | Rosarin | Rosavin | Rosin | Cinnamyl Alcohol | Rhodionidin 6 | Rhodiosin 6 | Rhodionin 6 |
|---|---|---|---|---|---|---|---|---|
| A 2 ASE | 300.11 (0.75) | 439.58 (2.83) | 1565.06 (0.38) | - 5 | 31.08 (0.06) | 33.30 (1.24) | 391.39 (3.36) | 93.35 (2.71) |
| B 3 ASE | 212.70 (0.10) | 361.72 (0.51) | 1389.24 (0.82) | 34.93 (4.06) | 53.12 (0.84) | 80.98 (0.61) | 352.26 (1.26) | 81.92 (2.15) |
| C 4 ASE | 237.37 (0.13) | 251.16 (2.57) | 1010.22 (0.16) | 27.98 (2.66) | 75.17 (1.80) | 59.94 (0.80) | 460.01 (0.86) | 88.79 (2.56) |
| A 38% Ethanol | 119.94 | 71.33 | - | 8.03 | 231.47 | - | - | - |
| B 38% Ethanol | 118.64 | 57.38 | - | - | 221.16 | - | - | - |
| C 38% Ethanol | 112.22 | 48.49 | - | - | 215.76 | - | - | - |
| A 70% Ethanol | 145.94 | 94.17 | 138.17 | 16.75 | 147.11 | 2.60 | 19.81 | 5.61 |
| B 70% Ethanol | 109.93 | 77.65 | 97.25 | 11.35 | 158.07 | 4.34 | 10.76 | 2.35 |
| C 70% Ethanol | 86.51 | 81.74 | 70.22 | 18.80 | 162.45 | 4.29 | 22.23 | 5.03 |
| A 96% Ethanol | 126.79 | 93.69 | 204.74 | 28.39 | 88.76 | 3.82 | 25.90 | 5.12 |
| B 96% Ethanol | 127.89 | 77.00 | 152.84 | 30.46 | 123.32 | 7.14 | 14.55 | 2.23 |
| C 96% Ethanol | 118.85 | 79.19 | 84.89 | 25.98 | 182.23 | 7.91 | 21.92 | 3.67 |
| Extracts Gurktal Alps | Salidroside | Rosarin | Rosavin | Rosin | Cinnamyl Alcohol | Rhodionidin 6 | Rhodiosin 6 | Rhodionin 6 |
|---|---|---|---|---|---|---|---|---|
| D 2 ASE | 297.59 (1.68) | 453.54 (1.93) | 1585.28 (0.08) | - 5 | 17.25 (1.38) | 74.20 (0.27) | 619.65 (1.25) | 175.63 (4.39) |
| E 3 ASE | 402.38 (0.17) | 364.50 (2.17) | 737.94 (0.68) | 40.24 (1.27) | 159.36 (0.34) | 141.48 (0.36) | 320.38 (1.47) | 79.54 (2.71) |
| F 4 ASE | 293.97 (0.64) | 467.10 (0.50) | 1552.58 (1.33) | 41.97 (3.73) | 36.89 (1.05) | 77.50 (0.53) | 587.41 (1.58) | 131.66 (2.11) |
| D 38% Ethanol | 119.74 | 80.04 | - | 12.91 | 208.92 | - | 8.72 | 1.55 |
| E 38% Ethanol | 154.68 | 46.19 | - | 2.34 | 178.81 | - | - | - |
| F 38% Ethanol | 109.58 | 57.19 | - | - | 167.71 | - | - | - |
| D 70% Ethanol | 131.20 | 95.47 | - | 14.27 | 188.60 | - | 15.12 | 3.14 |
| E 70% Ethanol | 143.24 | 73.42 | 56.49 | 13.63 | 125.47 | 5.88 | 14.52 | 2.86 |
| F 70% Ethanol | 142.14 | 76.15 | 13.87 | - | 172.29 | - | - | - |
| D 96% Ethanol | 108.93 | 104.45 | - | 21.95 | 202.48 | 6.69 | 28.46 | 4.17 |
| E 96% Ethanol | 163.77 | 69.75 | 60.21 | 25.27 | 121.25 | 9.00 | 14.45 | 1.75 |
| F 96% Ethanol | 185.14 | 85.85 | 50.40 | 14.07 | 155.96 | - | - | - |
| Extracts High Tauern | Salidroside | Cinnamyl Alcohol Glycosides | Cinnamyl Alcohol | Flavonoid Derivatives 6 |
|---|---|---|---|---|
| A 2 ASE | 300.11 | 2004.64 | 31.08 | 518.04 |
| B 3 ASE | 212.70 | 1785.89 | 53.12 | 515.15 |
| C 4 ASE | 237.37 | 1289.36 | 75.17 | 608.74 |
| A 38% Ethanol | 119.94 | 79.36 | 231.47 | - 5 |
| B 38% Ethanol | 118.64 | 57.38 | 221.16 | - |
| C 38% Ethanol | 112.22 | 48.49 | 215.76 | - |
| A 70% Ethanol | 145.94 | 249.09 | 147.11 | 28.01 |
| B 70% Ethanol | 109.93 | 186.25 | 158.07 | 17.45 |
| C 70% Ethanol | 86.51 | 170.76 | 162.45 | 31.55 |
| A 96% Ethanol | 126.79 | 326.82 | 88.76 | 34.84 |
| B 96% Ethanol | 127.89 | 260.30 | 123.32 | 23.92 |
| C 96% Ethanol | 118.85 | 190.06 | 182.23 | 33.49 |
| Extracts Gurktal Alps | Salidroside | Cinnamyl Alcohol Glycosides | Cinnamyl Alcohol | Flavonoid Derivatives 6 |
|---|---|---|---|---|
| D 2 ASE | 297.59 | 2038.82 | 17.25 | 869.47 |
| E 3 ASE | 402.38 | 1142.68 | 159.36 | 541.40 |
| F 4 ASE | 293.97 | 2061.65 | 36.89 | 796.57 |
| D 38% Ethanol | 119.74 | 92.95 | 208.92 | 10.27 |
| E 38% Ethanol | 154.68 | 48.53 | 178.81 | - 5 |
| F 38% Ethanol | 109.58 | 57.19 | 167.71 | - |
| D 70% Ethanol | 131.20 | 109.74 | 188.60 | 18.26 |
| E 70% Ethanol | 143.24 | 143.54 | 125.47 | 23.25 |
| F 70% Ethanol | 142.14 | 90.02 | 172.29 | - |
| D 96% Ethanol | 108.93 | 126.40 | 202.48 | 39.32 |
| E 96% Ethanol | 163.77 | 155.23 | 121.25 | 25.21 |
| F 96% Ethanol | 185.14 | 150.32 | 155.96 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alperth, F.; Turek, I.; Weiss, S.; Vogt, D.; Bucar, F. Qualitative and Quantitative Analysis of Different Rhodiola rosea Rhizome Extracts by UHPLC-DAD-ESI-MSn. Sci. Pharm. 2019, 87, 8. https://doi.org/10.3390/scipharm87020008
Alperth F, Turek I, Weiss S, Vogt D, Bucar F. Qualitative and Quantitative Analysis of Different Rhodiola rosea Rhizome Extracts by UHPLC-DAD-ESI-MSn. Scientia Pharmaceutica. 2019; 87(2):8. https://doi.org/10.3390/scipharm87020008
Chicago/Turabian StyleAlperth, Fabian, Ivana Turek, Sandra Weiss, Dietmar Vogt, and Franz Bucar. 2019. "Qualitative and Quantitative Analysis of Different Rhodiola rosea Rhizome Extracts by UHPLC-DAD-ESI-MSn" Scientia Pharmaceutica 87, no. 2: 8. https://doi.org/10.3390/scipharm87020008
APA StyleAlperth, F., Turek, I., Weiss, S., Vogt, D., & Bucar, F. (2019). Qualitative and Quantitative Analysis of Different Rhodiola rosea Rhizome Extracts by UHPLC-DAD-ESI-MSn. Scientia Pharmaceutica, 87(2), 8. https://doi.org/10.3390/scipharm87020008






