Abstract
In order to detect new structural and biological patterns in a series of hetaryl-3-carboxylic acid derivatives, the optically pure (S)- and (R)-enantiomers of N-(1-arylethyl)-4-methyl- 2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides, their true racemates, and mechanical racemic mixtures have been synthesized in independent ways. The particular features of the 1Н- and 13С-NMR spectra of all synthesized substances, liquid chromato-mass spectrometric behavior thereof under electrospray ionization conditions, and also the results of polarimetric and X-ray diffraction studies have been discussed. Pharmacological screening on a model of carrageenan inflammation has found a clear relationship between the spatial structure of the studied objects and biological activity thereof. Enantiomers with chiral centers having (S)-configuration showed weak inhibition of pain and inflammatory reactions, while their mirror (R)-isomers exhibited very powerful analgesic and antiphlogistic properties under the same conditions, with the level of specific activity exceeding that of Lornoxicam and Diclofenac. Taking obtained data into account, a noticeable decrease in the activity of mechanical racemic mixtures, consisting of one-half of the “wrong” (S)-enantiomers, is quite natural. The true racemate of N-(1-phenylethyl)-amide proved itself in a similar way, while 4-methoxy-substituted analog thereof stood out against this background with unexpectedly high analgesic and anti-inflammatory activities. A comparative analysis of X-ray diffraction data has found that crystalline and molecular structure of racemic N-[1-(4-methoxyphenyl)ethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide is completely different from that of the original enantiomers and, moreover, very unusual for racemates. Obviously, it is the factor determining the unique character of the biological effects of the said substance.
1. Introduction
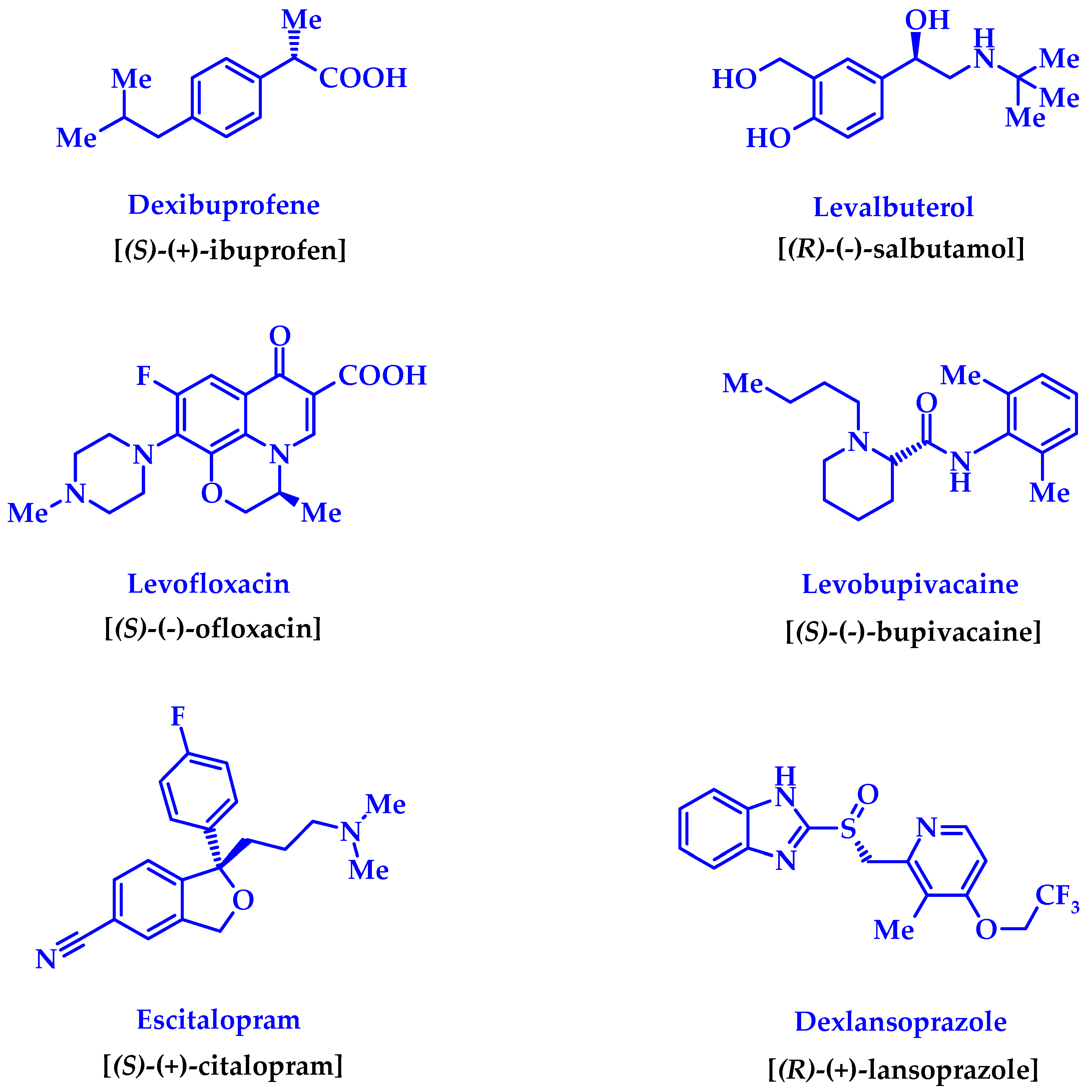
Chiral substances always attract increased attention from researchers of various specialties. The appearance of at least one asymmetric carbon atom in the molecule provides the substance with a number of unique properties, the study of which constitutes the range of scientific interests of synthetic chemists, analysts, spectroscopists, optics, crystallographers, and various other scientists. However, the practical benefits of studying chiral substances are, perhaps, to the greatest extent pharmacists and medical chemists. It is true that the awareness of the need for research in this area did not come to them immediately. It was only after racemic drugs were criticized for containing 50% of impurities [1] (although, in our opinion, this is not always true) did the pharmacy take up this problem seriously. Even a whole direction known in the literature as “chiral switches” appeared [2,3,4,5], thanks to which the “lifetime” of a number of the originally racemic drugs was prolonged in the form of their more active enantiomers [2,4] (Figure 1).
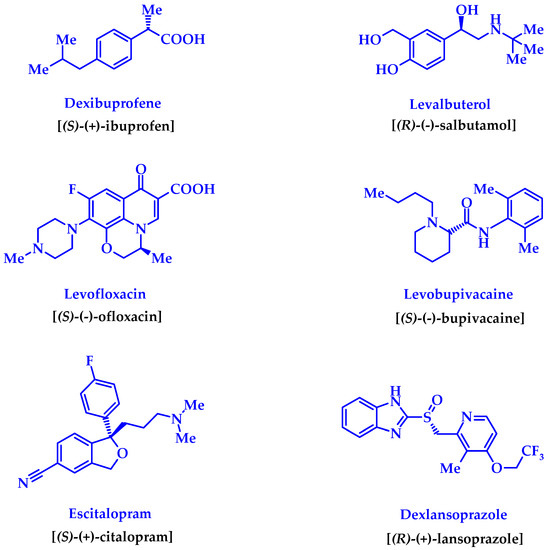
Figure 1.
Improved enantiomerically pure “copies” of the known drug-racemates created by the “chiral switches” technology [2,3,4].
However, it should be kept in mind that obtaining enantiomerically pure substances in general, and drugs in particular, as a rule, is a technologically complicated and costly practice. In addition, all the effort expended does not inevitably lead to positive results. When carrying out pharmacological tests of substances containing asymmetric carbon atoms, researchers often encounter the fact that only one of the enantiomers causes the desired biological effect, while the mirror isomer has low or no activity [6,7,8,9]. It is within the realm of possibility that enantiomers would exhibit completely different, and sometimes directly opposite, physiological properties [2], or one of the isomers would prove itself as unambiguously harmful or even dangerous [10]. Enantiomers sometimes demonstrate a completely identical clinical picture [11] that makes the “chiral switch” attempt irrelevant. One must also be prepared for the fact that the separation of the racemate may not lead to products with individual activity because enantiomers in a living organism are easily metabolized into one another [2]. It is also possible that the racemate would be much more active than each of the enantiomers [12]. Usually, such cases are interpreted as manifestations of synergism of effects, which are peculiar to each of the mirror isomers, separately [13]. However, in the case of observable pharmacological effects caused by enantiomers and racemates formed thereof, this explanation may be quite insufficient. This is exactly the situation we encountered when studying N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides, which are the object of this message.
2. Materials and Methods
2.1. Chemistry
1Н- and 13С-NMR (proton and carbon nuclear magnetic resonance) spectra were obtained on a Varian Mercury-400 (Varian Inc., Palo Alto, CA, USA) instrument (400 and 100 MHz, respectively) in hexadeuterodimethyl sulfoxide (DMSO-d6) with tetramethylsilane as the internal standard. The chemical shift values were recorded on a δ scale and the coupling constants (J) in Hertz. The following abbreviations were used in reporting spectra: s = singlet, d = doublet, t = triplet, q = quartet, quin = quintet, m = multiplet. The electrospray ionization liquid chromato-mass spectra (ESI-LC/MS) were recorded on a modular Agilent Technologies 1260 Infinity system with 6530 Accurate-Mass Q-TOF LC/MS (G6530B#200 ESI) mass-spectrometric detector (Agilent Technologies, Inc., Santa Clara, CA, USA). The chromatography conditions were: Agilent Extend-С18 column of 2.1 × 50 mm; the sorbent particle size—1.8 μm; the mobile phase flow rate—0.25 mL/min; the column temperature—30 °С; the injection volume—1.0 μL; the mobile phase composition—0.1% formic acid in methanol. The elemental analysis was performed on a Euro Vector EA-3000 (Eurovector SPA, Redavalle, Italy) microanalyzer. The melting points were determined in a capillary using an electrothermal IA9100X1 (Bibby Scientific Limited, Stone, UK) digital melting point apparatus. The specific rotations of optically active amides 3 and 4 were measured on a WWZ-2S automatic polarimeter (Zhejiang Nade Scientific instrument Co., Ltd., Yuhang, Hangzhou, Zhejiang, China). Synthesis of these compound used commercial S(–)- and R(+)-1-phenyl- and 1-(4-methoxyphenyl)- ethylamines from Fluka company (Fluka Chemie AG, Buchs, Switzerland), with optical purities of at least 99.5 and 99.0% respectively. In the synthesis of all N-(1-arylethyl)-4-methyl-2,2-dioxo-1H- 2λ6,1-benzothiazine-3-carboxamides, 3–5 described in this article the commercial N,N′-carbonyldiimidazole (CDI) and the anhydrous N,N-dimethylformamide (DMF) for peptide synthesis of Aldrich company (St. Louis, MO, USA) were used. The synthesis of the starting anhydrous 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid (1) was carried out by the method described in [14].
2.2. General Procedure for the Synthesis of N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 3, 4 and 5a
N,N′-Carbonyldiimidazole (1.78 g, 0.011 mol) was added to a solution of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid 1 (2.39 g, 0.01 mol) in anhydrous DMF (5 mL) and protected from atmospheric moisture using a CaCl2 tube. The mixture was then heated for about 2 h at 90 °C until the evolution of CO2 ceased. The reaction mixture was purged with dry argon through a thin capillary for 5 min to remove residual CO2, and then 0.01 mol of the corresponding 1-arylethylamine was added, and the mixture was kept for 5 h at the temperature of 90 °С in a tightly closed vial of thick dark glass (it is convenient to use vials of suitable volume designed for chemicals). The reaction mixture was cooled, diluted with cold water, and adjusted to pH ~3 with hydrochloric acid. The precipitate was formed, which was filtered off, washed with cold water, dried, and recrystallized from ethanol. 1-Arylethylamides 3, 4 and 5a were colorless crystals.
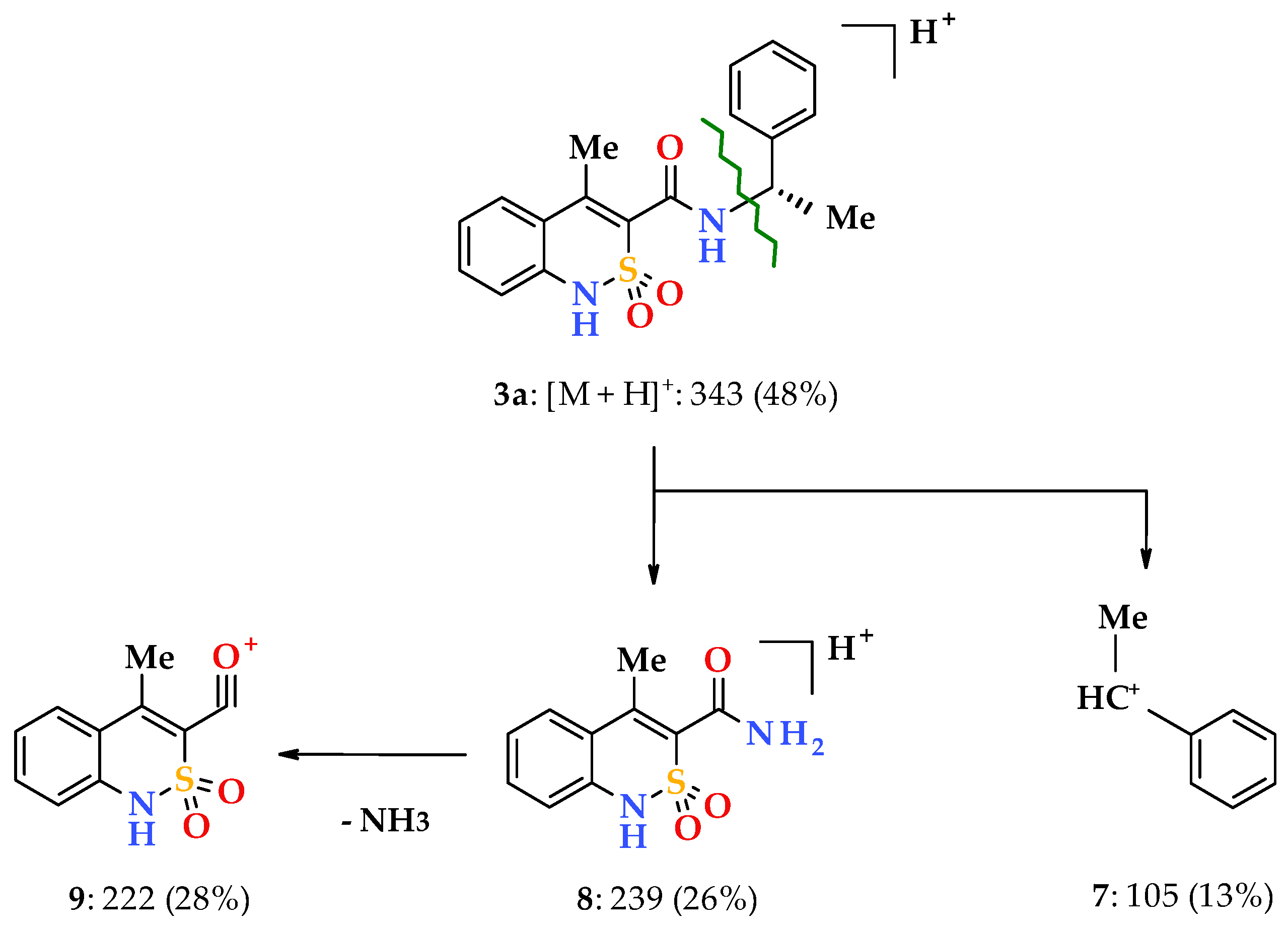
N-[(1S)-1-Phenylethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3a). The yield was: 3.21 g (94%); colorless crystals; melting point (mp) 216–218 °C; [α] = +14.5°, c = 5, dimethyl sulfoxide (DMSO); 1H-NMR (400 MHz, DMSO-d6): δ 11.70 (br. s, 1H, SO2NH), 9.15 (d, 1H, J = 7.6, CONH), 7.73 (d, 1Н, J = 7.7, Н-5), 7.50 (t, 1H, J = 7.4, H-7), 7.42 (d, 2H, J = 7.1, H-2′,6′), 7.36 (t, 2H, J = 7.2, H-3′,5′), 7.27 (t, 1H, J = 6.3, H-4′), 7.23 (t, 1H, J = 7.6, H-6), 7.15 (d, 1H, J = 7.8, H-8), 5.18-5.05 (m, 1H, NСН), 2.26 (s, 3H, 4-СН3), 1.45 (d, 3H, J = 6.7, СН-СН3). 13C-NMR (100 MHz, DMSO-d6): δ 159.5 (C=O), 144.5, 139.3, 138.1, 131.8, 131.5, 128.8 (2′,6′-С), 127.6, 127.4, 126.8 (3′,5′-С), 123.2, 121.1, 118.5 (3-C), 49.1 (СН), 22.7 (СHCH3), 17.7 (4-CH3). ESI-LC/MS (m/z, %): 343 (48) [M + H]+, 239 (26), 222 (28), 105 (13). This was analytically calculated (Anal. Calcd.) for C18H18N2O3S: C, 63.14; H, 5.30; N, 8.18; S 9.36%. We found: C, 63.17; H, 5.39; N, 8.11; S 9.25%.
N-[(1R)-1-Phenylethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (4a). The yield was: 3.07 g (90%); colorless crystals; m.p. 216–218 °C; [α] = −14.5°, c = 5, DMSO; 1H-NMR (400 MHz, DMSO-d6): δ 11.70 (br. s, 1H, SO2NH), 9.15 (d, 1H, J = 7.6, CONH), 7.73 (d, 1Н, J = 7.7, Н-5), 7.50 (t, 1H, J = 7.4, H-7), 7.42 (d, 2H, J = 7.1, H-2′,6′), 7.36 (t, 2H, J = 7.2, H-3′,5′), 7.27 (t, 1H, J = 6.3, H-4′), 7.23 (t, 1H, J = 7.6, H-6), 7.15 (d, 1H, J = 7.8, H-8), 5.18-5.05 (m, 1H, NСН), 2.26 (s, 3H, 4-СН3), 1.45 (d, 3H, J = 6.7, СН-СН3). 13C-NMR (100 MHz, DMSO-d6): δ 159.5 (C=O), 144.5, 139.3, 138.1, 131.8, 131.5, 128.8 (2′,6′-С), 127.6, 127.4, 126.8 (3′,5′-С), 123.2, 121.1, 118.5 (3-C), 49.1 (СН), 22.7 (СHCH3), 17.7 (4-CH3). ESI-LC/MS (m/z, %): 343 (44) [M + H]+, 239 (23), 222 (33), 105 (15). The Anal. Calcd. was for C18H18N2O3S: C, 63.14; H, 5.30; N, 8.18; S 9.36%. We found: C, 63.20; H, 5.37; N, 8.27; S 9.28%.
N-(1-Phenylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (5a). The yield was: 3.18 g (93%); colorless crystals; m.p. 212–214 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.71 (br. s, 1H, SO2NH), 9.15 (d, 1H, J = 7.6, CONH), 7.72 (d, 1Н, J = 7.7, Н-5), 7.50 (t, 1H, J = 7.4, H-7), 7.42 (d, 2H, J = 7.1, H-2′,6′), 7.37 (t, 2H, J = 7.2, H-3′,5′), 7.27 (t, 1H, J = 6.3, H-4′), 7.23 (t, 1H, J = 7.6, H-6), 7.16 (d, 1H, J = 7.8, H-8), 5.18-5.04 (m, 1H, NСН), 2.26 (s, 3H, 4-СН3), 1.45 (d, 3H, J = 6.7, СН-СН3). 13C-NMR (100 MHz, DMSO-d6): δ 159.5 (C=O), 144.5, 139.3, 138.1, 131.8, 131.5, 128.8 (2′,6′-С), 127.6, 127.4, 126.8 (3′,5′-С), 123.2, 121.1, 118.5 (3-C), 49.1 (СН), 22.7 (СHCH3), 17.7 (4-CH3). ESI-LC/MS (m/z, %): 343 (40) [M + H]+, 239 (22), 222 (25), 105 (10). The Anal. Calcd. was for C18H18N2O3S: C, 63.14; H, 5.30; N, 8.18; S 9.36%. We found: C, 63.22; H, 5.41; N, 8.25; S 9.30%.
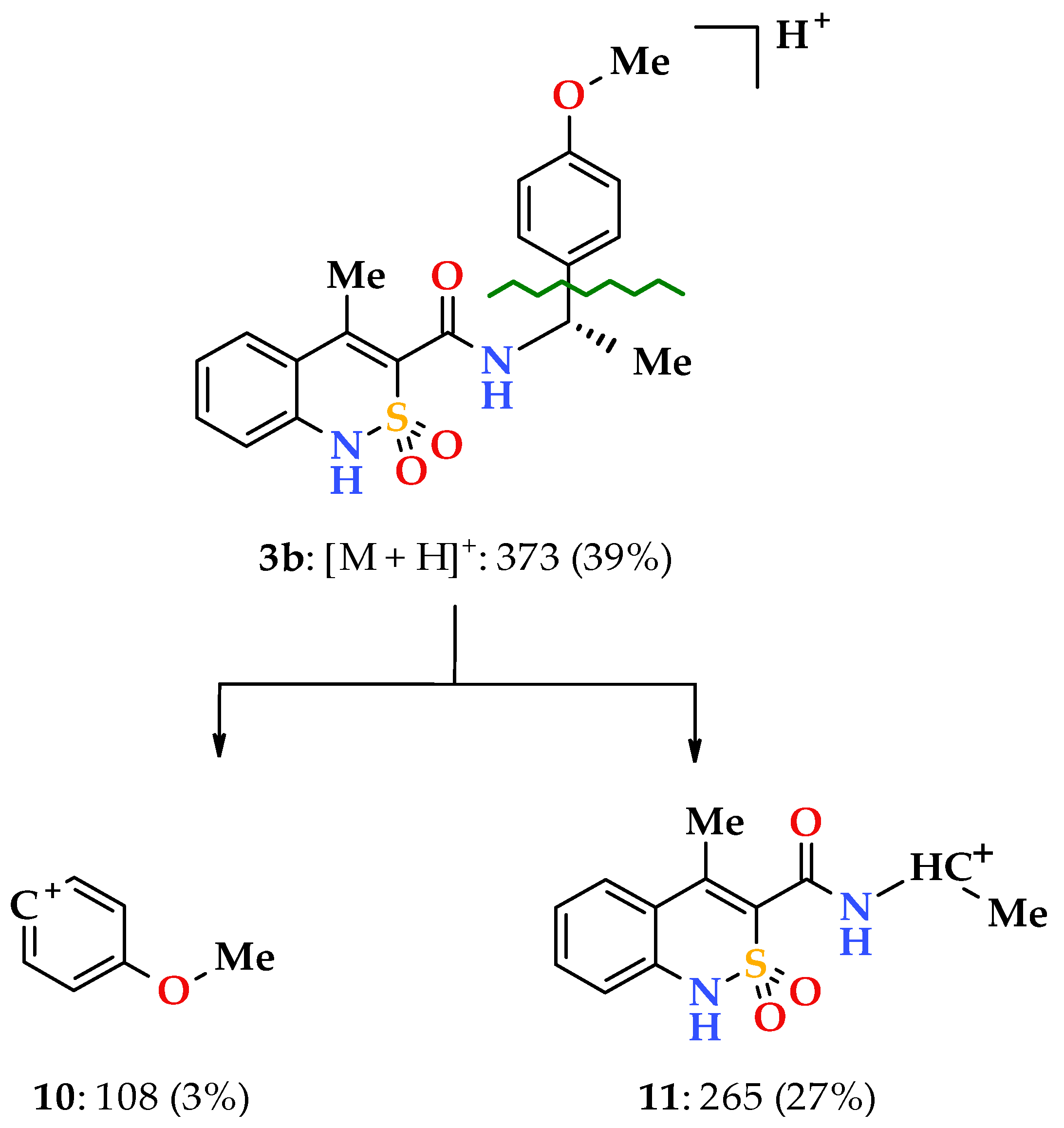
N-[(1S)-1-(4-Methoxyphenyl)ethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3b). The yield was: 3.34 g (90%); colorless crystals; m.p. 183–185 °C; [α] = +15.2°, c = 5, DMSO; 1H-NMR (400 MHz, DMSO-d6): δ 11.69 (br. s, 1H, SO2NH), 9.07 (d, 1H, J = 7.8, CONH), 7.70 (d, 1Н, J = 7.9, Н-5), 7.45 (t, 1H, J = 7.6, H-7), 7.32 (d, 2H, J = 8.1, H-2′,6′), 7.21 (t, 1H, J = 7.7, H-6), 7.12 (d, 1H, J = 8.0, H-8), 6.90 (d, 2H, J = 8.0, H-3′,5′), 5.14-5.04 (m, 1H, NСН), 3.74 (s, 3H, 4′-OMe), 2.23 (s, 3H, 4-СН3), 1.41 (d, 3H, J = 6.8, СН-СН3). 13C-NMR (100 MHz, DMSO-d6): δ 159.5 (C=O), 158.6 (4′-С-О), 139.3, 137.9, 136.3, 131.8, 131.6, 127.8 (2′,6′-С), 127.5, 123.2, 121.2, 118.5 (3-C), 114.1 (3′,5′-С), 55.5 (OCH3), 48.7 (CH), 22.7 (СHCH3), 17.6 (4-CH3). ESI-LC/MS (m/z, %): 373 (39) [M + H]+, 265 (27), 222 (14), 108 (2). The Anal. Calcd. was for C19H20N2O4S: C, 61.27; H, 5.41; N, 7.52; S 8.61. We found: C, 61.34; H, 5.49; N, 7.58; S 8.54%.
N-[(1R)-1-(4-Methoxyphenyl)ethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (4b). The yield was: 3.27 g (88%); colorless crystals; m.p. 183–185 °C; [α] = −15.2°, c = 5, DMSO; 1H-NMR (400 MHz, DMSO-d6): δ 11.68 (br. s, 1H, SO2NH), 9.07 (d, 1H, J = 7.8, CONH), 7.71 (d, 1Н, J = 7.9, Н-5), 7.45 (t, 1H, J = 7.6, H-7), 7.33 (d, 2H, J = 8.1, H-2′,6′), 7.21 (t, 1H, J = 7.7, H-6), 7.13 (d, 1H, J = 8.0, H-8), 6.90 (d, 2H, J = 8.0, H-3′,5′), 5.15-5.04 (m, 1H, NСН), 3.74 (s, 3H, 4′-OMe), 2.23 (s, 3H, 4-СН3), 1.41 (d, 3H, J = 6.8, СН-СН3). 13C-NMR (100 MHz, DMSO-d6): δ 159.5 (C=O), 158.6 (4′-С-О), 139.3, 137.9, 136.3, 131.8, 131.6, 127.8 (2′,6′-С), 127.5, 123.2, 121.2, 118.5 (3-C), 114.1 (3′,5′-С), 55.5 (OCH3), 48.7 (CH), 22.7 (СHCH3), 17.6 (4-CH3). ESI-LC/MS (m/z, %): 373 (43) [M + H]+, 265 (25), 222 (19), 108 (1). The Anal. Calcd. was for C19H20N2O4S: C, 61.27; H, 5.41; N, 7.52; S 8.61. We found: C, 61.21; H, 5.35; N, 7.45; S 8.53%.
2.3. N-[1-(4-Methoxyphenyl)ethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (5b)
1.86 g (0.005 mol) of N-[(1S)-1-(4-methoxyphenyl)ethyl]-amide 3b and 1.86 g (0.005 mol) of N-[(1R)-1-(4-methoxyphenyl)ethyl]-amide 4b were dissolved in 35 ml of boiling ethanol and filtered. The filtrate was cooled and left for 12 h at a temperature of about 10 °C. The precipitated crystals of racemate 5b were filtered off and air dried. The yield was: 3.57 g (96%); colorless crystals; m.p. 177–179 °C.
2.4. Mechanical Racemic Mixture of Optically Active N-(1-Phenylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1- benzothiazine-3-carboxamides (6a)
1.71 g (0.005 mol) of N-[(1S)-1-phenylethyl]-amide 3a and 1.71 g (0.005 mol) of N-[(1R)-1-phenylethyl]-amide 4a were thoroughly mixed until a homogeneous mass was formed. The yield was quantitative; colorless crystals; m.p. 207–209 °C.
2.5. Mechanical Racemic Mixture of Optically Active N-[1-(4-Methoxyphenyl)ethyl]-4-methyl-2,2-dioxo- 1H-2λ6,1-benzothiazine-3-carboxamides (6b)
1.86 g (0.005 mol) of N-[(1S)-1-(4-methoxyphenyl)ethyl]- amide 3b and 1.86 g (0.005 mol) of N-[(1R)-1-(4-methoxyphenyl)ethyl]-amide 4b were mixed thoroughly until a homogeneous mass was formed. The yield was quantitative; colorless crystals; m.p. 168–170 °C.
2.6. X-ray Structural Analysis of N-[(1S)-1-Phenylethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3a)
The crystals of (1S)-1-phenylethylamide 3a (C18H18N2O3S) were orthorhombic and colorless. At 20 °C: a 7.8554(5), b 8.8432(6), c 24.288(2) Å; V 1687.2(2) Å3, Z 4, space group P212121, dcalc 1.348 g/cm3, µ(MoKα) 0.210 mm−1, F(000) 720. The unit cell parameters and intensities of 10,394 reflections (4805 independent reflections, Rint = 0.077) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited, Oxford, UK) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry, Göttingen, Germany) [15]. The positions of the hydrogen atoms were found from the electron density difference maps and refined using the “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms). The hydrogen atoms of amino groups were refined within isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.119 for 4794 reflections (R1 0.060 for 2317 reflections with F > 4σ (F), S = 0.910). The final atomic coordinates, and the crystallographic data for the molecule of (1S)-1-phenylethylamide 3a have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1899013 [16].
2.7. X-ray Structural Analysis of N-(1-Phenylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (5a)
The crystals of racemic 1-phenylethylamide 5a (C18H18N2O3S) were monoclinic and colorless. At 20 °C: a 7.7803(9), b 8.8579(6), c 24.674(3) Å; β 98.37(1)°; V 1682.3(3) Å3, Z 4, space group P21/с, dcalc 1.352 g/cm3, µ(MoKα) 0.211 mm−1, F(000) 720. The unit cell parameters and intensities of 12,542 reflections (2959 independent reflections, Rint = 0.074) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 50°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [15]. The positions of the hydrogen atoms were found from the electron density difference maps and refined using the “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms). The hydrogen atoms of amino groups were refined within isotropic approximation. The hydrogen atoms of amino groups were refined within isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.257 for 2801 reflections (R1 0.090 for 2075 reflections with F > 4σ (F), S = 1.054). The final atomic coordinates and the crystallographic data for the molecule of racemic 1-phenylethylamide 5a have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1899015 [17].
2.8. X-ray Structural Analysis of N-[(1S)-1-(4-Methoxyphenyl)ethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3b)
The crystals of N-[(1S)-1-(4-methoxyphenyl)ethyl]-amide 3b (C19H20N2O4S) were monoclinic and colorless. At 20 °C: a 13.490(2), b 9.2913(9), c 15.033(2) Å; β 103.46(1)°; V 1832.4(4) Å3, Z 4, space group Р21, dcalc 1.350 g/cm3, µ(MoKα) 0.203 mm−1, F(000) 784. The unit cell parameters and intensities of 18,921 reflections (10,140 independent reflections, Rint = 0.082) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a Charge Coupled Device (CCD) detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [15]. The positions of the hydrogen atoms were found from the electron density difference map and refined using the “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms). The hydrogen atoms participating in the formation of intermolecular hydrogen bonds were refined in an isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.215 for 9948 reflections (R1 0.093 for 4991 reflections with F > 4σ (F), S 0.954). The final atomic coordinates, and the crystallographic data for the molecule of N-[(1S)-1-(4-methoxyphenyl)ethyl]-amide 3b have been deposited with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1899014 [18].
2.9. X-ray Structural Analysis of N-[(1R)-1-(4-Methoxyphenyl)ethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (4b)
The crystals of N-[(1R)-1-(4-methoxyphenyl)ethyl]-amide 4b (C19H20N2O4S) were monoclinic and colorless. At 20 °C: a 13.491(1), b 9.2746(6), c 15.050(2) Å; β 103.644(8)°; V 1829.8(3) Å3, Z 4, space group Р21, dcalc 1.352 g/cm3, µ(MoKα) 0.204 mm−1, F(000) 784. The unit cell parameters and intensities of 18,889 reflections (10,263 independent reflections, Rint = 0.077) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a Charge Coupled Device (CCD) detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [15]. The positions of the hydrogen atoms were found from the electron density difference map and refined using the “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms). The hydrogen atoms participating in the formation of intermolecular hydrogen bonds were refined in an isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.119 for 10,185 reflections (R1 0.065 for 4502 reflections with F > 4σ (F), S 0.887). The final atomic coordinates, and the crystallographic data for the molecule of N-[(1R)-1-(4-methoxyphenyl)ethyl]-amide 4b have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1899016 [19].
2.10. X-ray Structural Analysis of N-[1-(4-Methoxyphenyl)ethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (5b)
The crystals of racemic N-[1-(4-methoxyphenyl)ethyl]-amide 5b (C19H20N2O4S) were orthorhombic and colorless. At 20 °C: a 16.644(2), b 24.533(3), c 9.0188(7) Å; V 3682.5(6) Å3, Z 8, space group Pca21, dcalc 1.344 g/cm3, µ(MoKα) 0.203 mm−1, F(000) 1568. The unit cell parameters and intensities of 22,382 reflections (6429 independent reflections, Rint = 0.143) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a Charge Coupled Device (CCD) detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [15]. The positions of the hydrogen atoms were found from the electron density difference map and refined using the “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms). The hydrogen atoms participating in the formation of intermolecular hydrogen bonds were refined in an isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.091 for 6405 reflections (R1 0.063 for 3090 reflections with F > 4σ (F), S 0.865). The final atomic coordinates and the crystallographic data for the molecule of racemic N-[1-(4-methoxyphenyl)ethyl]-amide 5b have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1899017 [20].
2.11. Pharmacology
Analgesic and Anti-Inflammatory Tests
All biological experiments were carried out in full accord with the European Convention on the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes and the Ukrainian Law No. 3447-IV “On protection of animals from severe treatment” [21] (project ID 3410U14, approved 15 October 2015). The pharmacological research was carried out with the permission and under the supervision of the Commission on Bioethics (N.I. Pirogov Vinnitsa National Medical University, Vinnitsa, Ukraine).
The analgesic effect with the simultaneous assessment of the anti-inflammatory activity of all N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 3–6a,b synthesized was studied on the standard model of carrageenan edema [22,23]. The studies were conducted on white Wistar male rats weighing 200–250 g. The test substances, Lornoxicam (Wasserburger Arzneimittelwerk GmbH, Wasserburger, Germany) and Diclofenac (KRKA, Novo Mesto, Slovenia) were introduced intraperitoneally in the form of fine aqueous suspensions stabilized with Tween-80 in the screening dose of 20 mg/kg. The animals of the control group received an equivalent amount of water with Tween-80. Other details of pharmacological experiments, as well as the statistical processing of the results, were described in detail earlier [14].
3. Results and Discussion
3.1. Chemistry
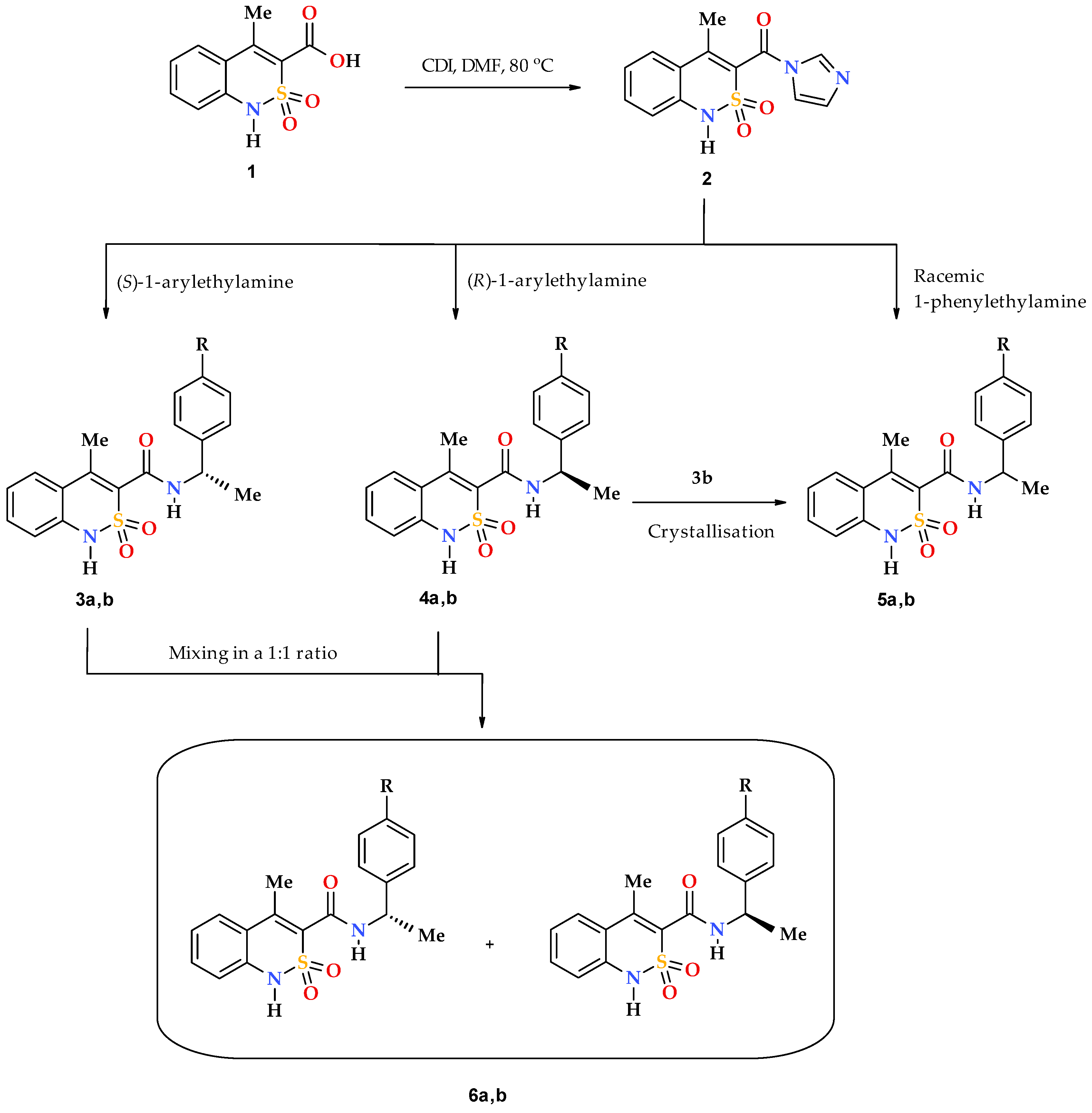
Chiral pharmaceutical substances are prepared by various methods. First of all, it is an obvious, although far from easily accomplished, separation of racemic mixtures which were originally synthesized or isolated from natural raw materials. The options for the practical implementation of this approach are constantly being improved upon and at present they allow obtaining the desired enantiomer having a high degree of optical purity, on a commercial scale [24,25,26,27]. Unfortunately, despite all the positive aspects, the separation of racemates always has one major drawback residing in the fact that it can be considered effective only if all the isolated components of the mixture find a practical use. Otherwise, at least half of the finished product will just become fruitless ballast. Therefore, more attention has been paid to the development of modern technologies of asymmetric synthesis, which would allow to immediately obtain biologically active substances with the known-good configuration of chiral centers [28,29,30,31]. The assembling of compounds of complex structure based on commercially available enantiomerically pure building blocks of natural or synthetic origin (chiral pool synthesis) has gained particular popularity [32]. In this case, the chiral fragment, as a rule, is introduced into the molecule at the final stage of the synthetic scheme, which allows for minimizing the risk of possible racemization. This strategy was implemented in the synthesis of the target objects in the present study: the previously formed bicyclic 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid (1) was transformed into imidazolide 2, which was further reacted with optical high-purity S(–)- and R(+)-1-phenyl- and 1-(4-methoxyphenyl)-ethylamines forming corresponding chiral N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 3–4 (Scheme 1). By the same principle, except using racemic 1-phenylethylamine, racemate 5а (R = H) was synthesized, which is of interest for structural and biological comparative studies. Due to lack of racemic 1-(4-methoxyphenyl)ethylamine, 4-methoxy-substituted analog thereof, 5b (R = OMe), was obtained by crystallization of a mixture of enantiomers 3b and 4b in a 1:1 ratio from ethanol. As will be shown below, the samples obtained in this way were true single crystal racemates, i.e., each crystal contained both enantiomers in a 1:1 ratio. This is their fundamental difference from mechanical racemic mixtures 6a,b, obtained by simple mixing of equimolar amounts of the corresponding optically pure enantiomers 3 and 4, also in a 1:1 ratio, but without subsequent crystallization.
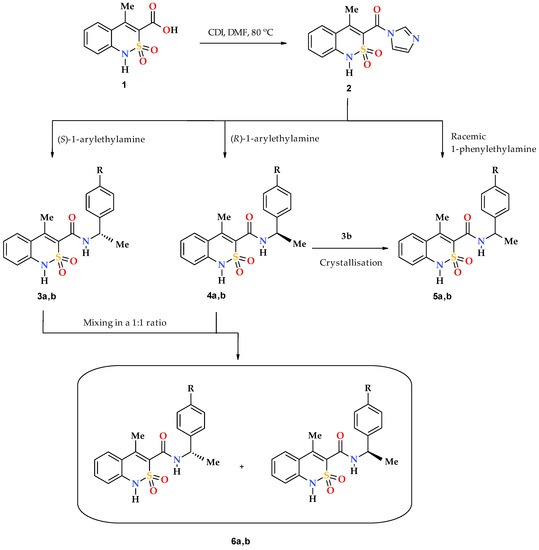
Scheme 1.
Synthesis of chiral N-(arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine- 3-carboxamides 3 and 4, their true racemates 5 and mechanical racemic mixtures 6. 3--6: а R = H; b R = OMe.
All obtained N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides are colorless crystalline substances, which at room temperature are moderately soluble in DMSO and DMF, slightly soluble in alcohols, and insoluble in water. Taking into account the structure of the studied compounds (the presence of benzothiazine bicycle, aryl ethylamide fragments, several methyl groups in different chemical environments, optical activity, etc.), we have used a standard set of analytical methods to confirm their structure: elemental analysis, 1Н- and 13С-NMR spectroscopy, electrospray ionization liquid chromato-mass spectrometry, and polarimetry. However, the most important and interesting features of the spatial structure of this group of substances in the crystalline phase have been detected using X-ray diffraction.
As expected, 1H and 13C NMR spectra of all samples in each of the triads: (S)-enantiomer – (R)-enantiomer – racemate (for example, 3a–4a–5a) were the same. All the signals of protons and most of the signals of the carbon atoms composing these spectra were easily interpreted by the characteristic chemical shifts, intensity, and multiplicity (see Section 2.2). In other words, NMR spectroscopy allows reliable confirmation of the chemical structure of the studied substances, and even their purity to some extent (on the absence of “irrelevant” peaks in the experimental spectra).
Similar information is provided by electrospray ionization liquid chromato-mass spectrometry, which, in addition to chemical identity, makes it possible to establish an analytically important molecular weight of a substance. Additional information on the structure of the sample is provided by the analysis of the initial molecule fragments formed upon registration of the mass spectrum. For example, it has been observed that, under the influence of electrospray ionization, N-(1-phenylethyl)-amides 3a, 4a, and 5a behave in the same manner and firstly form protonated molecular ions [M + H]+ (Scheme 2). Primary fragmentation of said substances occurs similarly to N-benzyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides as described in our earlier works [33], i.e., with the benzyl bond –CONH–CH(Me)Ph breaking (β-decay [34]). This produces phenethyl cation 7 and the unstable protonated cation of 4-methyl-2,2-dioxo-1H-2λ6,1- benzothiazine-3-carboxamide 8, which rapidly loses NH3 molecule and becomes more stable, and therefore typical for all derivatives of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid acylium-cation 9 with m/z 222 (Scheme 2). However, the mass spectrometric behavior of the phenethylamides 3a, 4a and 5a were different from that of the benzylamides mentioned above. In particular, one of the directions of primary destruction of protonated molecular ions in their spectra implying the breaking of the terminal amide bond –CO–NHCH(Me)Ph, which was usual for 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides, was not observable.
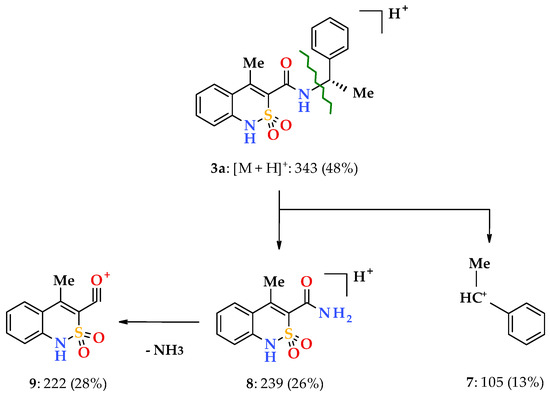
Scheme 2.
The primary fragmentation of the N-[(1S)-1-phenylethyl]-amide 3a protonated molecular ion.
When going to 4-methoxy-substituted analogs (enantiomers 3,4b and racemate 5b), a part of the regularities mentioned above remains. At the same time, the 4-methoxy group of the phenethyl fragment implies its peculiarity on the mass spectrometric behavior of the studied compounds. We have found that it promotes the appearance of a new and very unusual path of primary fragmentation of protonated molecular ions for the studied class of compounds. During its implementation, the bond between the aryl substituent and the ethylcarbamide fragment breaks first (α-decay [34]), resulting in the peaks of 4-methoxyphenyl 10 and benzothiazine-3- carbonylaminoethyl 11 cations with m/z 108 and 265, respectively (Scheme 3).
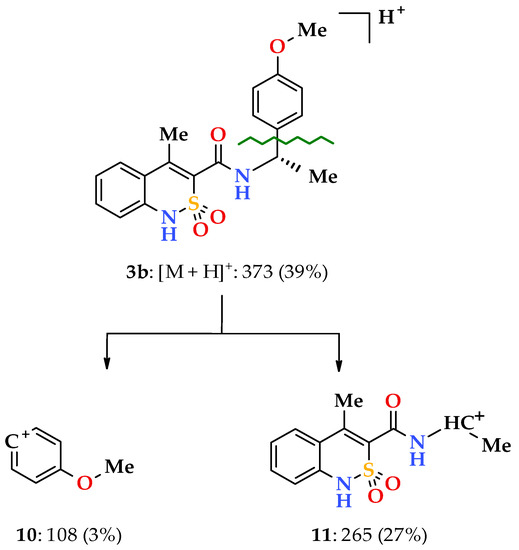
Scheme 3.
The primary fragmentation of the N-[(1S)-1-(4-methoxyphenyl)ethyl]-amide 3b protonated molecular ion.
Finally, another important analytical characteristic of the studied N-(1-arylethyl)- 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 3 and 4, which requires a mandatory definition, are their specific rotation values. Our polarimetric studies confirmed that N-(1-arylethyl)-amides 3 and 4 are true optically active substances. Moreover, the fact that enantiomer pairs 3a–4a and 3b–4b, obtained in independent ways using different asymmetric reagents had the same specific rotation values (see Section 2.2), which were different only in sign, was a fairly reliable hallmark of optical high-purity [35]. Meanwhile, the issue of the true configuration of the obtained chiral amides 3a,b and 4a,b may arise from the fact that they rotated the plane of polarization in the opposite direction than the original 1-arylethylamines. However, this fact, though curious, is not principal, since the configuration of the substance and the direction of the polarization plane rotation are completely different characteristics unrelated to each other. It is well known that specific rotation values and their sign (+ or −) can vary greatly in the same substance depending on concentration, nature of solvent, temperature, etc. [35,36]. In addition, as will be shown below, neither racemization nor the conversion of configuration occurred in the synthesis of N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 3 and 4, i.e., asymmetric carbon atoms in the finished products retained the configuration of the original chiral building blocks.
3.2. Evaluation of the Analgesic and Anti-Inflammatory Activity
A comparative analysis of the data obtained from the pharmacological tests of N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides (Table 1 and Table 2) revealed a close relationship between their spatial structure and biological activity. Therefore, enantiomers 3a,b with chiral centers having (S)-configuration showed extremely weak inhibition of the pain reaction, whereas their mirror isomers having (R)-configuration 4a,b were very powerful analgesics, superior to lornoxicam and diclofenac, under the same conditions. Such a significant difference in the activity of optical antipodes indicates that their interaction with pain receptors includes at least three sites. With regards to the anti-inflammatory properties of the enantiomeric pairs 3a–4a and 3b–4b, the similar structural and biological patterns have been detected, although in a slightly less pronounced form. Stereospecificity of action was demonstrated here only by 4-methoxy derivatives 3b and 4b; meanwhile their unsubstituted analogs 3a and 4a exhibit an anti-exudative effect of similar strength regardless of the configuration of the chiral center (Table 2). However, the appearance of a direct relationship between the spatial structure of N-R-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine- 3-carboxamides and analgesic activity thereof in the absence of any anti-inflammatory activity has been noted in our earlier works [33].

Table 1.
The analgesic activity of N-(1-arylethyl)-amides 3–6, and reference drugs.

Table 2.
The anti-inflammatory activity of N-(1-arylethyl)-amides 3–6, and reference drugs.
The significant difference between the biological properties of enantiomers of N-(1-arylethyl)- 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 3 and 4 have been, of course, unknown without experimental testing, but it was quite predictable. Meanwhile, the pharmacological study of their true racemates 5 and racemic mixtures 6 presented some very interesting surprises. At the same time, nothing unusual was observed in the behavior of racemic N-(1-phenylethyl)-amide 5a; as one would expect, it showed extremely weak analgesic activity because it consists of one half of the “wrong” (S)-enantiomer 3a. It was not surprising that the racemic mixture 6a showed approximately the same result (Table 1). For the same reason, a weak analgesic effect of the racemic mixture 6b was quite predictable. Moreover, only racemic N-[1-(4-methoxyphenyl)ethyl]-amide 5b did not fit into this almost perfect picture and clearly stood out against the background of other samples due to surprisingly high activity. We tried to find an explanation for this phenomenon in a detailed study of the spatial structure of both the racemate 5b itself and analogs thereof presented in this work.
3.3. The Molecular and Crystal Structure Study
The basis for studying the molecular and crystalline structure of the synthesized N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides was the desire to solve several problems at one stroke, both analytical, structural, and biological. Although all the methods described above confirm the structure of the studied substances, they do it only indirectly. Additionally, the character of this work required accurate information on the conformation of chiral centers in optically active amides 3 and 4, as also on the actual structure of racemates 5a,b. Otherwise, all the revealed patterns of the “structure-activity” relationships, as well as the final conclusions, would be inaccurate. The only “absolute” method that allows unambiguous solving such problems is X-ray diffraction. Here it must be remembered that the basis for determining the absolute configuration of one or another chiral center of the molecule from X-ray diffraction data is the phenomenon of X-ray scattering [37]. Noticeably, this effect appears only on atoms of the third and subsequent periods. Therefore, a direct determination of the absolute configuration of substances that do not contain atoms “heavier” than oxygen is usually impossible (see, for example, the case of 2-carbonyl analogs of amides 3 and 4 [38]). For this purpose, the molecule must be subjected to the appropriate modification: salt formation, halogenation, etc. [39].
The appearance of the fairly “heavy” sulfur atom in the molecules of N-(1-arylethyl)-4-methyl- 2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 3–4 allows solving all the issues of the absolute conformation of chiral centers without any additional chemical transformations. Therefore, in particular, it was found that N-[(1S)-1-phenylethyl]-amide 3a crystallizes in the chiral space group P212121, which indicates the presence of only one enantiomer in the crystal. The (S)-configuration of the chiral center at the С(10) atom is unambiguously determined from experimental data using the Flack parameter (−0.06(12)).
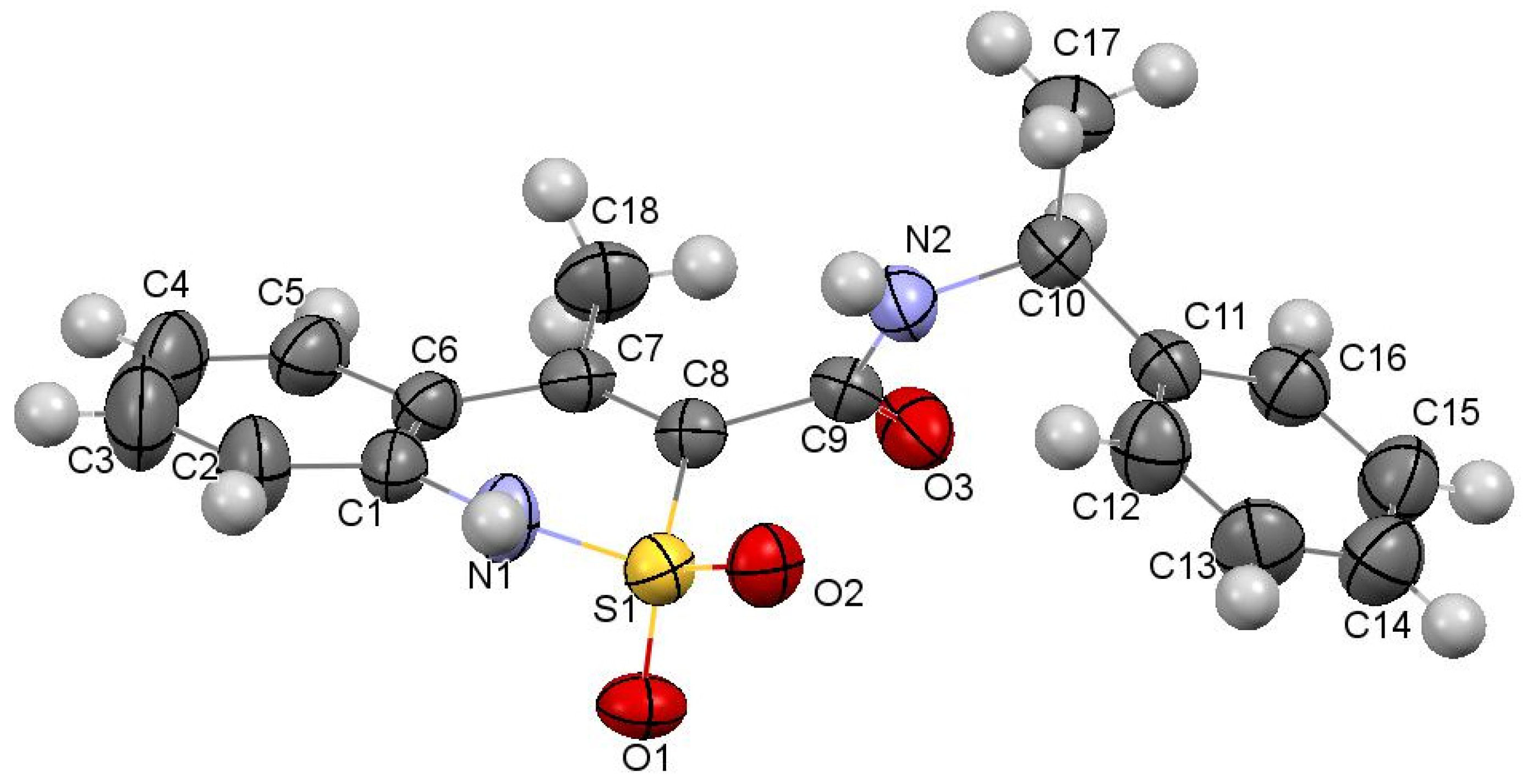
The dihydrothiazine heterocycle in this compound (Figure 2) has conformation, which is intermediate between the twist-boat and chair conformations (folding parameters [40]: S = 1.71, Θ = 88.7°, Ψ = 8.9°). The deviations of the S(1) and С(8) atoms from the mean plane of the remaining atoms of this cycle were −0.67 Å and −0.09 Å, respectively. The cyclic nitrogen atom has a planar configuration, wherein the sum of the valence angles centered on it equals to 360°. Steric repulsion has been found between the atoms of the methyl substituent at С(7) and the aromatic cycle of С(1)…С(6), as evidenced by the shortened intramolecular contacts Н(5)…С(18) 2.55 Å, H(18a)…C(5) 2.83 Å, H(18b)…C(5) 2.80 Å with the van der Waals radii sum 2.87 Å [41]. The carbamide group in the substituent at the С(8) atom is noticeably rotated relative to the endocyclic double С(7)−С(8) bond (torsion angle С(7)−С(8)−С(9)−О(3) −71.9(4)°). The methyl group at the С(10) atom is situated in the position intermediate between ас- and ар- relatively the C(9)–N(2) bond (torsion angle C(9)–N(2)–C(10)–C(17) 157.6(3)°), and the phenyl substituent at the same atom is in the –sc- conformation relatively the C(9)–N(2) bond and is rotated relatively the bond N(2)–C(10) (torsion angles C(9)–N(2)–C(10)–C(11) −78.1(4)°, N(2)–C(10)–C(11)–C(12) −47.1(4)°).
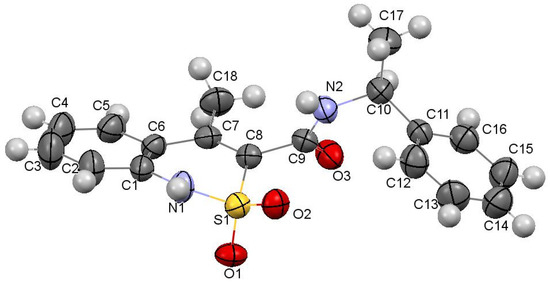
Figure 2.
The molecular structure of N-[(1S)-1-phenylethyl]-amide 3a with atoms represented by thermal vibration ellipsoids of 50% probability.
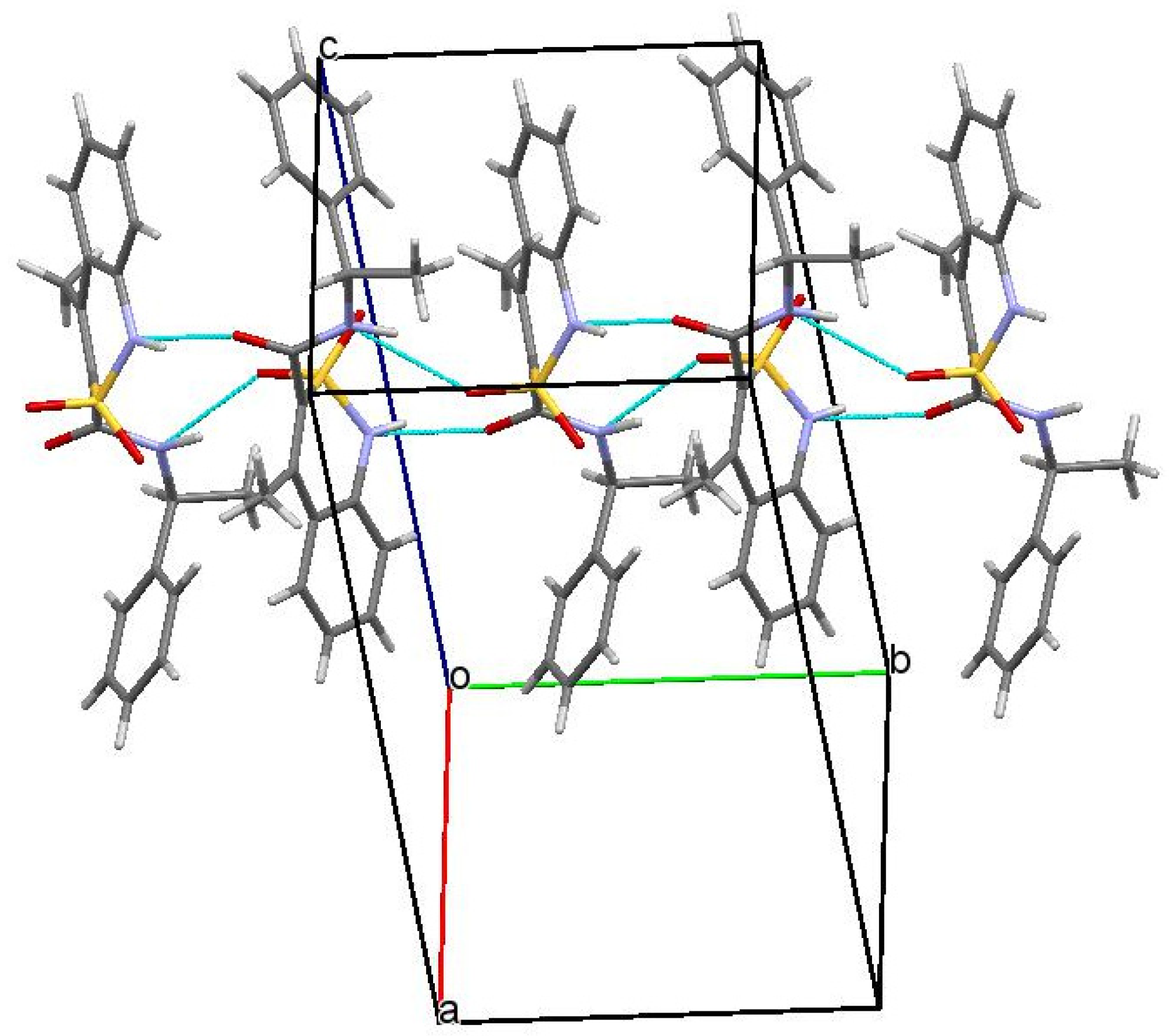
In a crystal, the molecules of N-[(1S)-1-phenylethyl]-amide 3a are linked via intermolecular hydrogen bonds N(1)–H…O(3′) (1 − x, 0.5 + y, 1.5 − z) H…O 1.99 Å, N–H…O 161°; N(2)–H…O(1′)(1 − x, 0.5 + y, 1.5 − z) H…O 2.06 Å, O–H…O 154° forming chains along the crystallographic direction [010] (Figure 3). The formation of hydrogen bonds leads to the elongation of the C(9)–O(3) bond to 1.234(4) Å (average value [42] 1.210 Å) and to the non-equivalence of S(1)–O(1) and S(1)–O(2) bonds (1.441(2) Å and 1.430(2) Å, respectively).
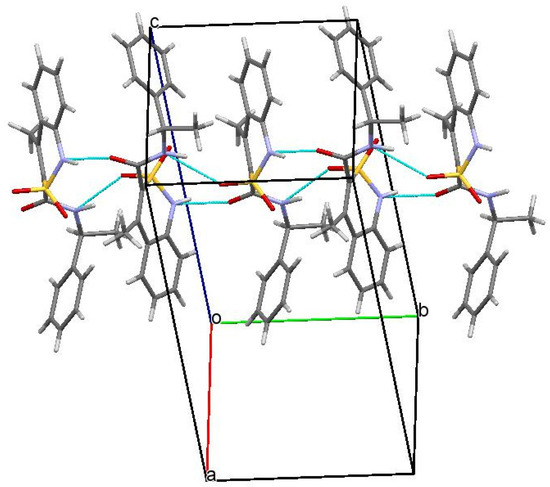
Figure 3.
Packing of N-[(1S)-1-phenylethyl]-amide 3a in the crystal phase. Projection along [0 1 0] crystallographic direction.
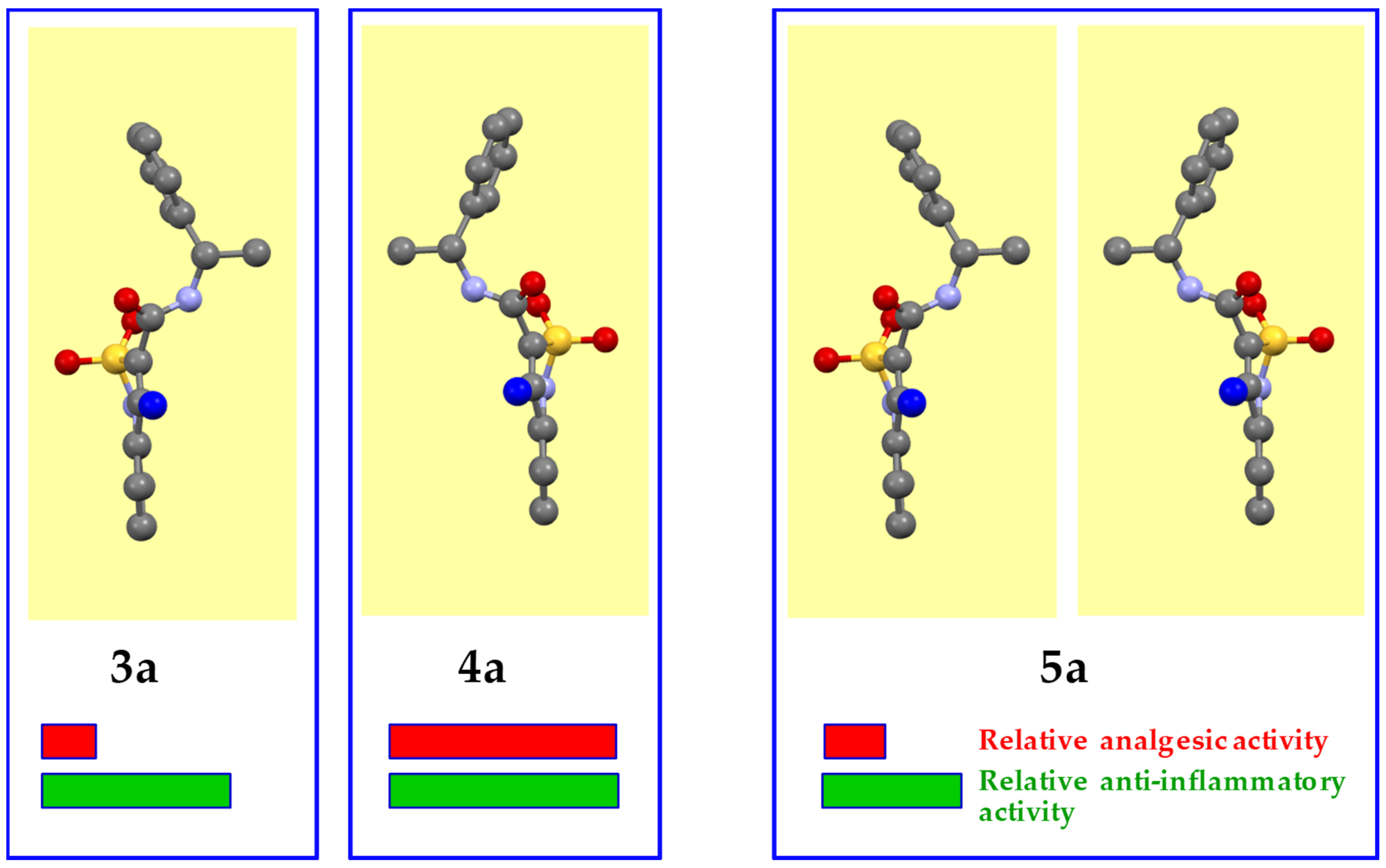
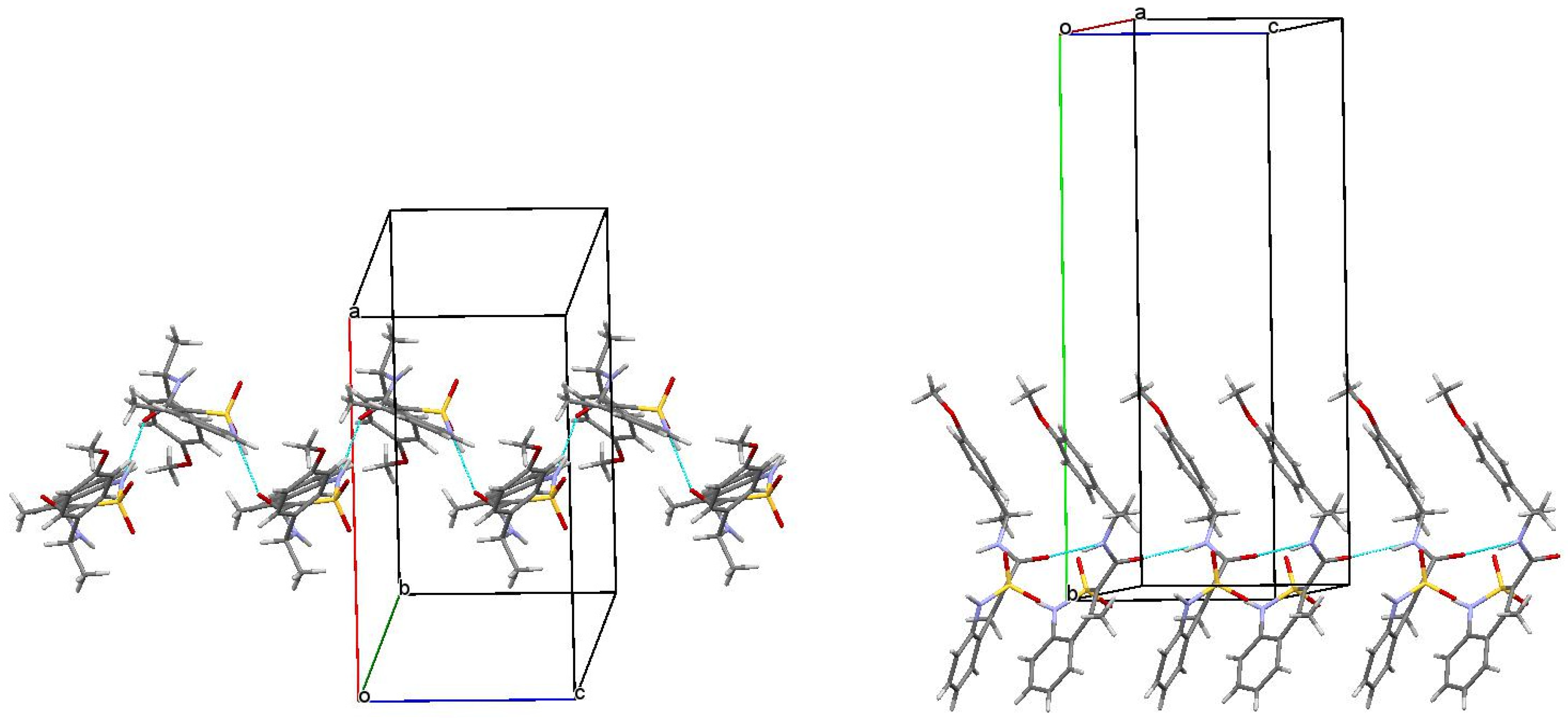
Based on X-ray diffraction data, N-(1-phenylethyl)-amide 5a is a true racemate consisting of two mirror isomers 3а and 4a in a 1:1 ratio (Figure 4). In this case, the conformations of one of the enantiomers thereof and above-described N-[(1S)-1-phenylethyl]-amide 3a were almost the same. The nature of the packing of molecules in a crystal was also not changed. Unfortunately, it was not possible to study the structure of the second enantiomer, N-[(1R)-1-phenylethyl]-amide 4a on an individual basis. However, its structure is identical to that of one of the components of racemate 5а. Therefore, in the case of the triad 3a–4a–5a we have a classic example with two optically pure enantiomers and racemate formed thereof, respectively. Hence, the results shown by enantiomers 3а and 4a, as well as the biological effects (especially analgesic) of the true racemate 5а and the mechanical racemic mixture 6а very similar in strength are quite expectable (see Table 1 and Table 2).
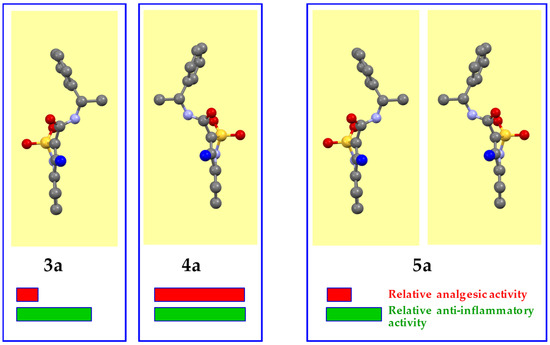
Figure 4.
Crystal conformers of N-[(1S)-1-phenylethyl]-amide 3a and racemate 5a (according to the data of X-ray diffraction analysis), and N-[(1R)-1-phenylethyl]-amide 4a (presumably). The benzothiazine fragment is located vertically and is turned to the viewer by the C-atom of the 4-methyl group (colored blue).
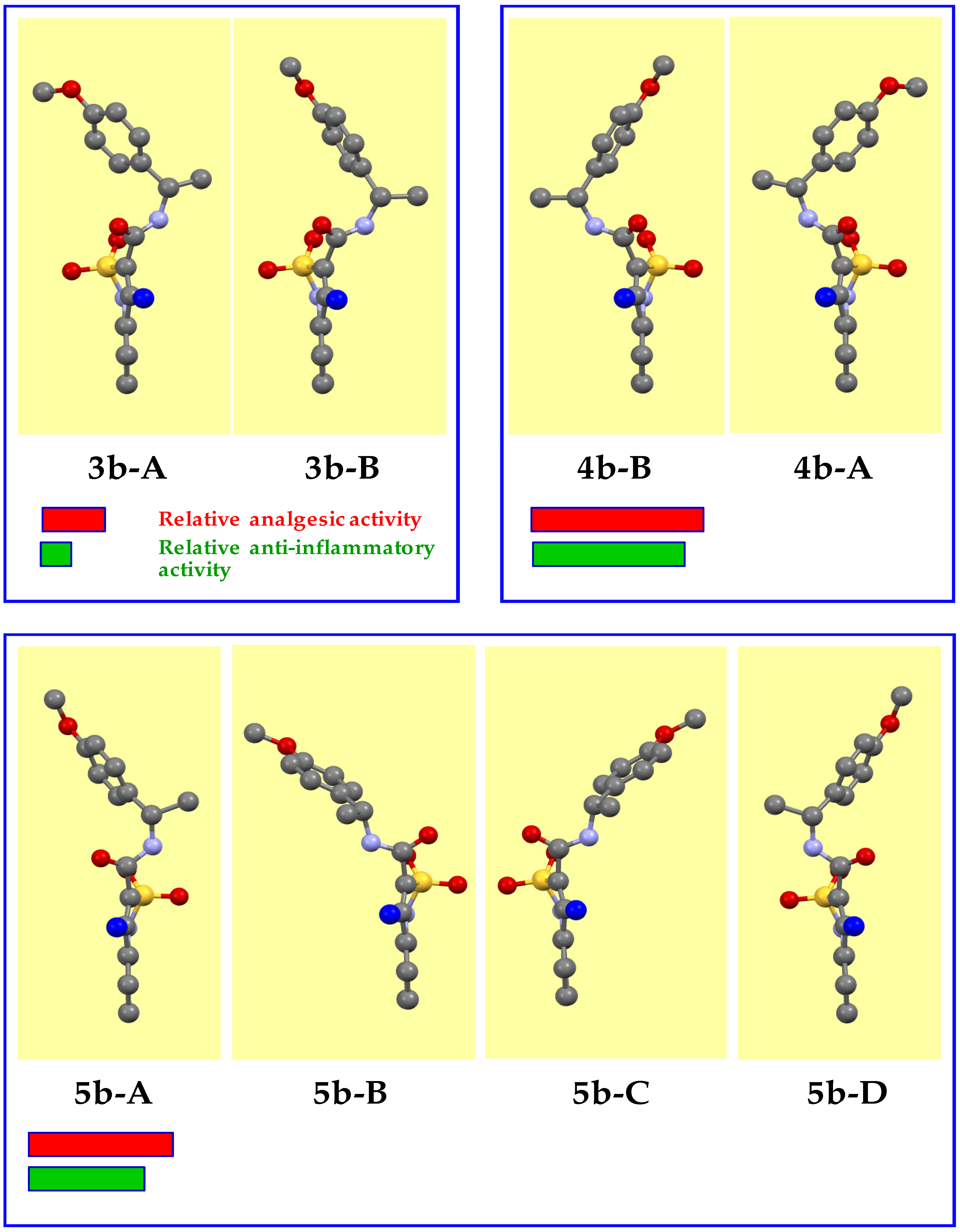
When studying the structural features of the triad 3b–4b–5b, a slightly different picture was noted. Optically pure enantiomers 3b and 4b do not raise questions. As expected, they crystallize in the non-centrosymmetric space group Р21. The difference from the unsubstituted analogs 3a and 4a described above, perhaps, is only in the fact that in the independent part of the unit cell of each of these products, two molecules (А and В) were founded, which differ from each other in both the orientation of the methoxy group and the angle of rotation of the aryl substituent around the NHCH(Me)–C bond (Figure 5). However, these differences are quite insignificant, for example, the torsion angles N(2)–C(10)–C(11)–C(12) determining the nature of rotation in molecules А and В are 150.7(6)° and 134.0(5)°, respectively. The structural similarity of enantiomers 3a/4a and 3b/4b, respectively, attracts attention. The spatial arrangement of the methyl substituent in the methoxy group, as well as the appearance of this methoxy group in the molecule, are not principal for the manifestation of analgesic and anti-inflammatory action. As a result, we have a high total activity of the 4b-A/4b-B pair having the (R)-configuration of the chiral centers and low activity of their optical antipodes 3b-A/3b-B.
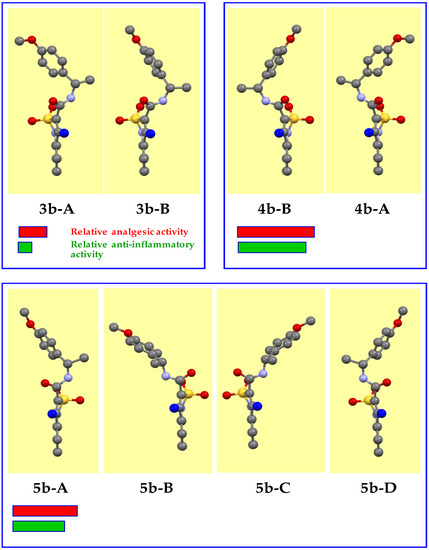
Figure 5.
Crystal conformers of N-[(1S)-1-(4-methoxyphenyl)ethyl]-amide 3b, its mirror isomer 4b, and racemate 5b (experimental data). The benzothiazine fragment is located vertically and is turned to the viewer by the C-atom of the 4-methyl group (colored blue).
The crystal structures of the mirror isomers 3b and 4b have the same unit cell parameters (see Section 2.8 and Section 2.9), which is usual for classical enantiomers. The appearance of the “heavy” sulfur atom in the studied molecules and the resulting violation of the Fridel law allows unambiguous determining the true configuration of their chiral centers using the calculated Flack parameter [37]. Therefore, in the amides 3b-A/3b-B, С(10) atoms have (S)-configuration (the Flack parameter is 0.05(10)), whereas in the amides 4b-A/4b-B they have (R)-configuration (the Flack parameter is 0.05(11)).
In crystals, molecules А and В composing both enantiomers 3b and 4b form chains of both А–А–А–А and В–В–В–В types (Figure 6) along the crystallographic direction [010]. These chains are simultaneously interconnected by two intermolecular hydrogen bonds N(1)–H…O(1′) and N(2)–H…O(3′) (symmetry operations 1 – x, 0.5 + y, 1 − z in chain А–А and −x, −0.5 + y, –z in chain В–В).
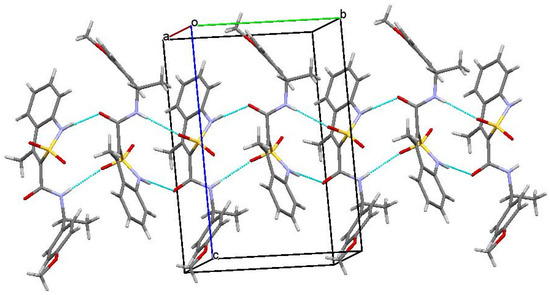
Figure 6.
The main fragment of the package in enantiomers 3b and 4b.
Similar to the triad of derivatives 3a−4a−5a with the unsubstituted aromatic core, it was logical to assume that the racemate 5b is the sum of the conformers composing the optically active amides 3b and 4b. However, in reality, everything was completely different. First of all, the non-centrosymmetric space group of racemate 5b (Рса21) was surprising. In theory, this should indicate the appearance of only one enantiomer in a crystal. However, it was found that two molecules (A and B) are present in the independent part of the unit cell, which molecules have different configurations of their chiral centers: (S)-configuration for А and (R)-configuration for В, which was confirmed by the calculated value of the Flack parameter 0.03(12).
Additionally, unlike amide 5а, the methoxy-substituted analog 5b could not be considered a usual racemate, since constituent molecules thereof, 5b-А and 5b-В (Figure 5), did not mirror reflections of each other and were not linked by a center of symmetry. In addition, a symmetry plane appeared in the amide 5b crystal. Consequently, each of the molecules 5b-А and 5b-В had a pair of 5b-C and 5b-D symmetric to them in the crystal. In other words, the structure of amide 5b contained four conformers of the studied compound (Figure 5), which could be divided into two pairs, connected by a symmetry transformation relatively the plane. The unique character of amide 5b as a racemate also lies in the fact that structure of all the conformers 5b-А, 5b-В, 5b-C, and 5b-D composing said racemate was significantly different from that of the original enantiomers 3b and 4b. For this reason, it was not possible to determine, which of these conformers provided an unusually high level of analgesic and anti-inflammatory activities of amide 5b compared to the mechanical racemic mixture 6b, which mixture consisted by one half of the enantiomer 3b with obviously low activity.
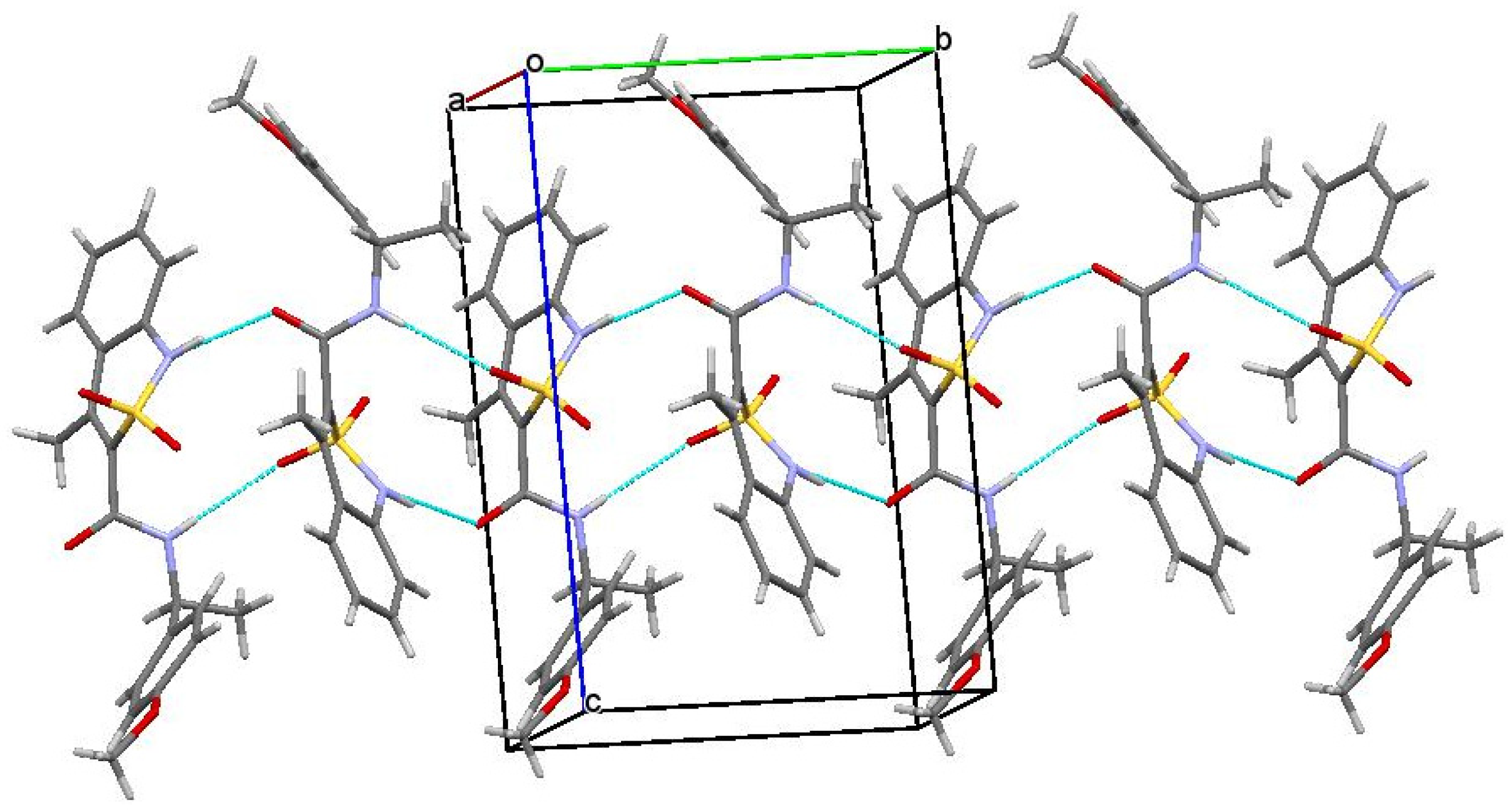
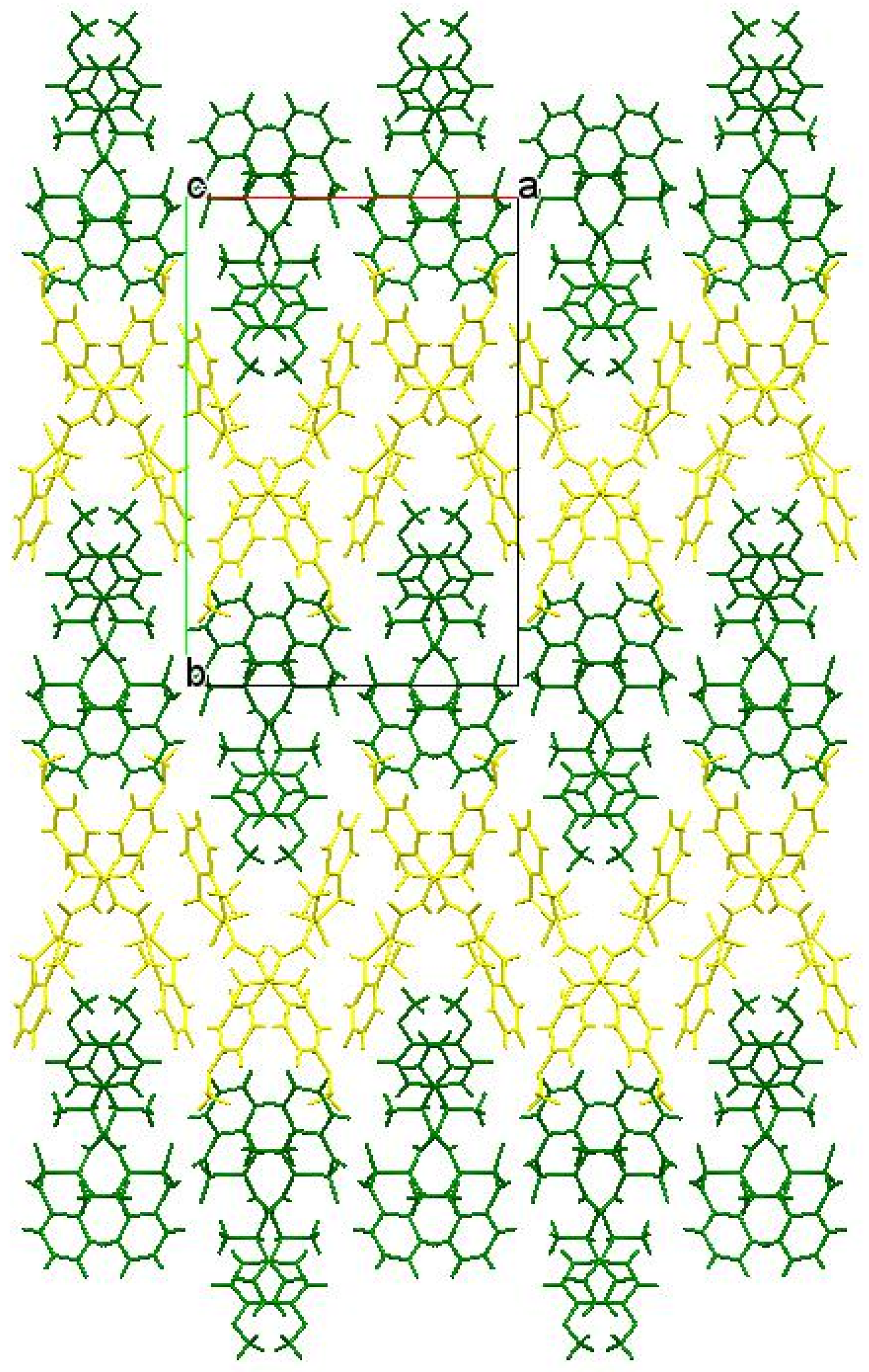
Another factor that could additionally contribute to the biological properties of amide 5b was its crystalline package. As in the case of enantiomers 3b and 4b, molecules А and В also form chains of both А–А–А–А and В–В–В–В types in the crystal of racemate 5b. However, these chains are different. More specifically, in the zigzag А–А chains, molecules were linked only by one intermolecular hydrogen bond N(1)–H…O(1), while the neighboring chains were not connected at all (Figure 7). The chains of molecules В were formed via the intermolecular hydrogen bonds N(2)–H…O(1) and were linked by the hydrogen bond N(1)–H…O(3). In the crystal, the chains А–А and В–В formed alternating layers (Figure 8).
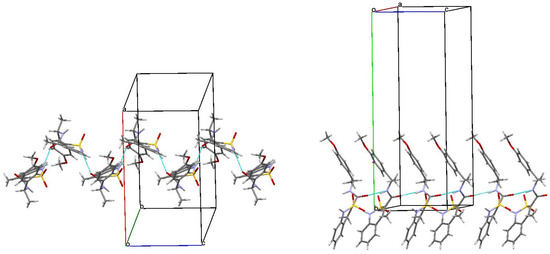
Figure 7.
The main fragment of the package of molecules А (left) and B (right) in the structure of racemate 5b. Projection along [0 0 1] crystallographic direction.
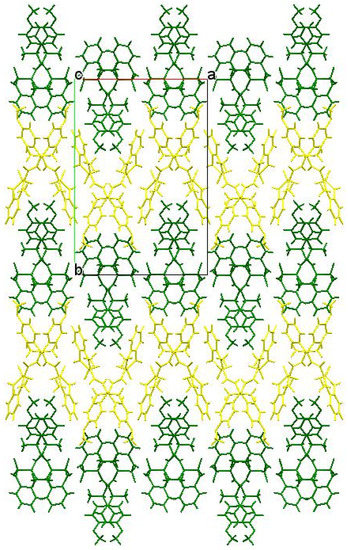
Figure 8.
Packing the chains in layers in a racemate 5b crystal. Molecules А form a layer highlighted in yellow, molecules В form green.
Therefore, our study clearly showed that the anti-inflammatory, and especially analgesic properties of N-(1-arylethyl)-4-methyl-2,2- dioxo-1H-2λ6,1-benzothiazine-3-carboxamides are largely determined by molecular conformations thereof initially fixed in crystals, and also by the nature of the molecular package in the crystalline phase.
4. Conclusions
This article is devoted to obtaining new optically pure enantiomers of N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides, their true racemates, and mechanical racemic mixtures, and also to a detailed study of the spatial structure and biological properties thereof. Elemental analysis, 1Н and 13С NMR spectroscopy, electrospray ionization liquid chromato-mass spectrometry, polarimetry, and X-ray diffraction have been used to confirm the structure of the synthesized compounds. Based on the data obtained from the pharmacological tests, chiral N-(1-arylethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides have shown apparent stereospecificity of biological properties. Therefore, against the background of low active (S)-enantiomers, mirror isomers thereof having (R)-configuration of chiral centers have proven to be more powerful analgesics and antiphlogistics than lornoxicam and diclofenac. At the same time, an unusually high level of activity has been noted in racemic N-[1-(4-methoxyphenyl)ethyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide. It was shown that the unexpected pharmacological effects of this compound had been caused by a unique crystalline and molecular structure, which is quite unusual for racemates.
Author Contributions
The synthesis of the compounds presented in this work and analysis of their characteristics were performed by I.V.U. and G.M.H. The liquid chromato-mass-spectrometric and X-ray structural studies were performed by I.A.D., G.S., and S.V.S., A.A.B., respectively. The pharmacological studies were conducted by N.I.V. and O.V.M. The manuscript was written by I.V.U., G.M.H., and O.V.M.
Funding
This research received no external funding.
Acknowledgments
We are grateful to Candidate of Chemistry Evgene S. Gladkov (SSI “Institute for Single Crystal” National Academy of Sciences of Ukraine, Kharkiv, Ukraine) for his help in registration of NMR spectra of the compounds synthesized.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ariëns, E.J. Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. Eur. J. Clin. Pharmacol. 1984, 26, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Kubinyi, H. In search of the new compounds-leaders for creation of drugs. Russ. Chem. J. 2006, 50, 5–17. Available online: http://www.chem.msu.su/rus/journals/jvho/2006-2/5.pdf (accessed on 26 May 2006).
- Agranat, I.; Caner, H.; Caldwell, J. Putting chirality to work: The strategy of chiral switches. Nat. Rev. Drug Discov. 2002, 1, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.T. Chiral switches. Lancet 2000, 355, 1085–1087. [Google Scholar] [CrossRef]
- Thacker, H.P. S-amlodipine—The 2007 clinical review. J. Indian Med. Assoc. 2007, 105, 180–182. [Google Scholar] [PubMed]
- Hardikar, M.S. Chiral non-steroidal anti-inflammatory drugs—A review. Indian Med. Assoc. 2008, 106, 615–618. [Google Scholar]
- Gordon, A.L.; Lopatko, O.V.; Somogyi, A.A.; Foster, D.J.; White, J.M. (R)- and (S)-methadone and buprenorphine concentration ratios in maternal and umbilical cord plasma following chronic maintenance dosing in pregnancy. Br. J. Clin. Pharmacol. 2010, 70, 895–902. [Google Scholar] [CrossRef]
- Matera, M.G.; Calzetta, L.; Rogliani, P.; Bardaro, F.; Page, C.P.; Cazzola, M. Evaluation of the effects of the R- and S-enantiomers of salbutamol on equine isolated bronchi. Pulm. Pharmacol. Ther. 2011, 24, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yurovskaya, М.А.; Kurkin, А.V. Some aspects of the relationship between chirality and biological activity. In Proceedings of the Synthesis and Complexation Achievements, Materials of the all-Russian Scientific Conference with International Participation, Dedicated to the International Year of Chemistry, Moscow, Russia, 18–22 April 2011; p. 19. [Google Scholar]
- Kaneko, T. Troglitazone (CS-045): A new antidiabetic agent. Horm. Metab. Res. 1997, 29, 203–213. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; Nóbrega, F.F.; Santos, C.C.; de Almeida, R.N. Anticonvulsant activity of the linalool enantiomers and racemate: Investigation of chiral influence. Nat. Prod. Commun. 2010, 5, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Friderichs, E.; Reimann, W.; Shank, R.P.; Codd, E.E.; Vaught, J.L.; Jacoby, H.I.; Selve, N. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J. Pharmacol. Exp. Ther. 1993, 267, 331–340. [Google Scholar] [PubMed]
- Ukrainets, I.V.; Hamza, G.M.; Burian, A.A.; Shishkina, S.V.; Voloshchuk, N.I.; Malchenko, O.V. 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid. Peculiarities of preparation, structure, and biological properties. Sci. Pharm. 2018, 86, 9. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1899013. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 23 February 2019).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1899015. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 23 February 2019).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1899014. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 23 February 2019).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1899016. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 23 February 2019).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1899017. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 23 February 2019).
- Ukrainian Law No. 3447-IV. On Protection of Animals from Severe Treatment. Available online: http://zakon2.rada.gov.ua/laws/show/3447-15 (accessed on 4 August 2017).
- Vogel, H.G. (Ed.) Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 1103–1106. [Google Scholar]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef]
- Hsu, L.C.; Kim, H.; Yang, X.; Ross, D. Large scale chiral chromatography for the separation of an enantiomer to accelerate drug development. Chirality 2011, 23, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Schuur, B.; Verkuijl, B.J.; Minnaard, A.J.; de Vries, J.G.; Heeres, H.J.; Feringa, B.L. Chiral separation by enantioselective liquid-liquid extraction. Org. Biomol. Chem. 2011, 9, 36–51. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Gan, J.; Kistemaker, J.C.; Pizzolato, S.F.; Chang, M.C.; Feringa, B.L. Enantiopure functional molecular motors obtained by a switchable chiral-resolution process. Chemistry 2016, 22, 7054–7058. [Google Scholar] [CrossRef]
- Kannappan, V.; Mannemala, S.S. Simultaneous enantioseparation and purity determination of chiral switches of amlodipine and atenolol by liquid chromatography. J. Pharm. Biomed. Anal. 2016, 120, 221–227. [Google Scholar] [CrossRef]
- Kato, N.; Mita, T.; Kanai, M.; Therrien, B.; Kawano, M.; Yamaguchi, K.; Danjo, H.; Sei, Y.; Sato, A.; Furusho, S.; et al. Assembly state of catalytic modules as chiral switches in asymmetric Strecker amino acid synthesis. J. Am. Chem. Soc. 2006, 128, 6768–6769. [Google Scholar] [CrossRef]
- Fuwa, H.; Suzuki, T.; Kubo, H.; Yamori, T.; Sasaki, M. Total synthesis and biological assessment of (−)-exiguolide and analogues. Chemistry 2011, 17, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Shibata, M.; Kaneko, K.; Okino, T.; Matsuda, F. Asymmetric total synthesis of danicalipin a and evaluation of biological activity. Org. Lett. 2011, 13, 904–907. [Google Scholar] [CrossRef]
- Rossi, S.; Porta, R.; Brenna, D.; Puglisi, A.; Benaglia, M. Stereoselective catalytic synthesis of active pharmaceutical ingredients in homemade 3D-printed mesoreactors. Angew. Chem. Int. Ed. 2017, 56, 4290–4294. [Google Scholar] [CrossRef] [PubMed]
- Núñez, M.C.; García-Rubiño, M.E.; Conejo-García, A.; Cruz-López, O.; Kimatrai, M.; Gallo, M.A.; Espinosa, A.; Campos, J.M. Homochiral drugs: A demanding tendency of the pharmaceutical industry. Curr. Med. Chem. 2009, 16, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Hamza, G.M.; Burian, A.A.; Voloshchuk, N.I.; Malchenko, O.V.; Shishkina, S.V.; Grinevich, L.N.; Grynenko, V.V.; Sim, G. Molecular conformations and biological activity of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides. Sci. Pharm. 2018, 86, 50. [Google Scholar] [CrossRef]
- Terentev, P.B.; Stankiavichyus, A.P. Mass Spectrometry Bioactive Nitrogenous Bases; Mokslas: Vilnius, Lithuania, 1987; pp. 60–64. [Google Scholar]
- Potapov, V.M. Stereochemistry; Khimia: Moscow, Russia, 1988; pp. 282–284. [Google Scholar]
- Sykes, P. A Guidebook to Mechanism in Organic Chemistry, 6th ed.; Pearson: London, UK, 2003; pp. 340–358. [Google Scholar]
- Glusker, J.P.; Domenicano, A. Introduction to X-ray crystallography. In Accurate Molecular Structures. Their Determination and Importance; Domenicano, A., Hargittai, I., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 158–210. [Google Scholar]
- Ukrainets, I.V.; Taran, S.G.; Likhanova, N.V.; Rybakov, V.B.; Gorokhova, O.V.; Nidal Amin Jaradat. 4-Hydroxy-2-quinolones. 38. Synthesis, structure, and anticonvulsant activity of optically active 1-phenylethylamides of 1-R-4-hydroxy-2-oxo-3-quinolinecarboxylic acids. Chem. Heterocycl. Compd. 2000, 36, 49–56. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Taran, S.G.; Likhanova, N.V.; Nidal Amin Jaradat; Shishkin, O.V. 4-Hydroxy-2-quinolones. 39. Structure of 6-bromo-4-hydroxy-1-isoamyl-2-oxo-3-quinolinecarboxylic acid. Chem. Heterocycl. Compd. 2000, 36, 57–61. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles. The case of six-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]
- Zefirov, Y.V. Reduced intermolecular contacts and specific interactions in molecular crystals. Crystallogr. Rep. 1997, 42, 865–886. [Google Scholar]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical interatomic distances in organic compounds and organometallic compounds and coordination complexes of the d- and f-block metals. In Structure Correlation; Burgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994; Volume 2, pp. 741–926. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).