Evaluation of Natural Extracts in Animal Models of Pain and Inflammation for a Potential Therapy of Hemorrhoidal Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material and Extracts Preparation
2.3. Hydrogels Preparation
2.4. Animals
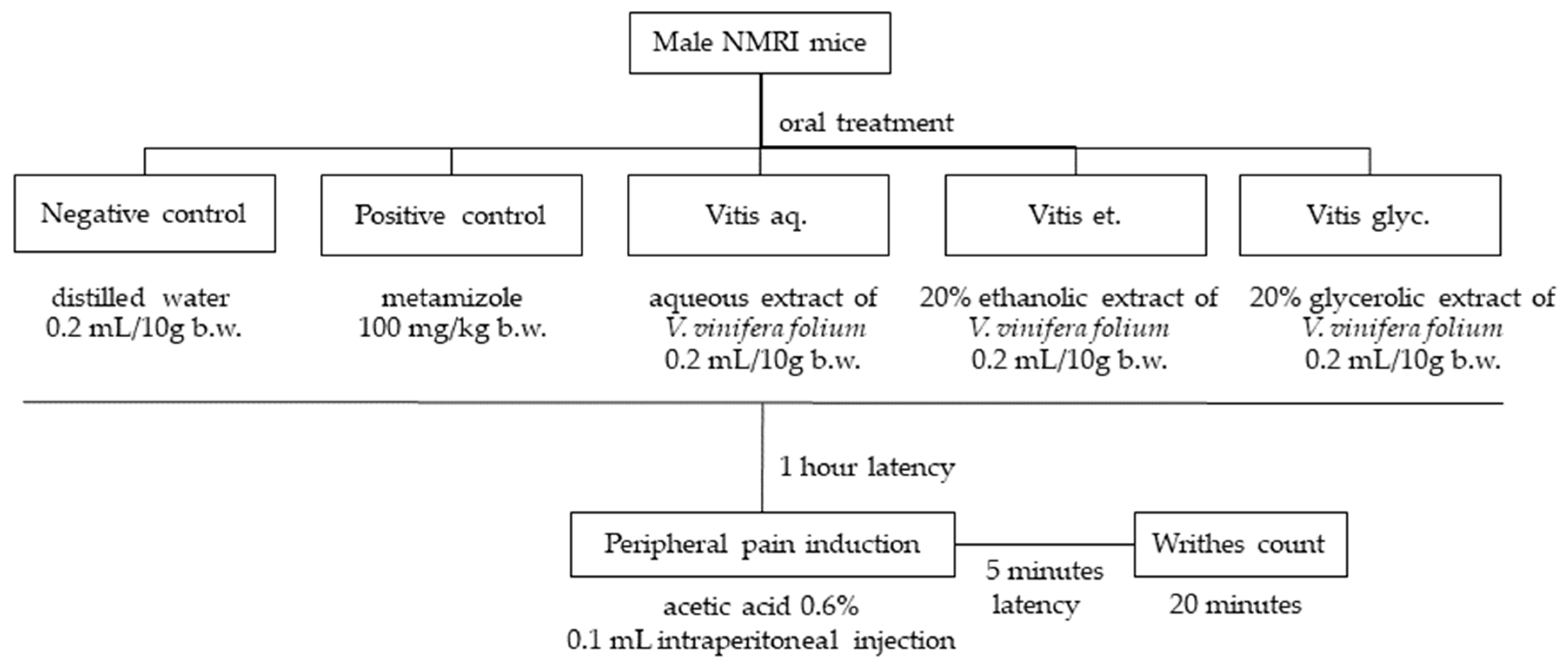
2.5. Antinociception
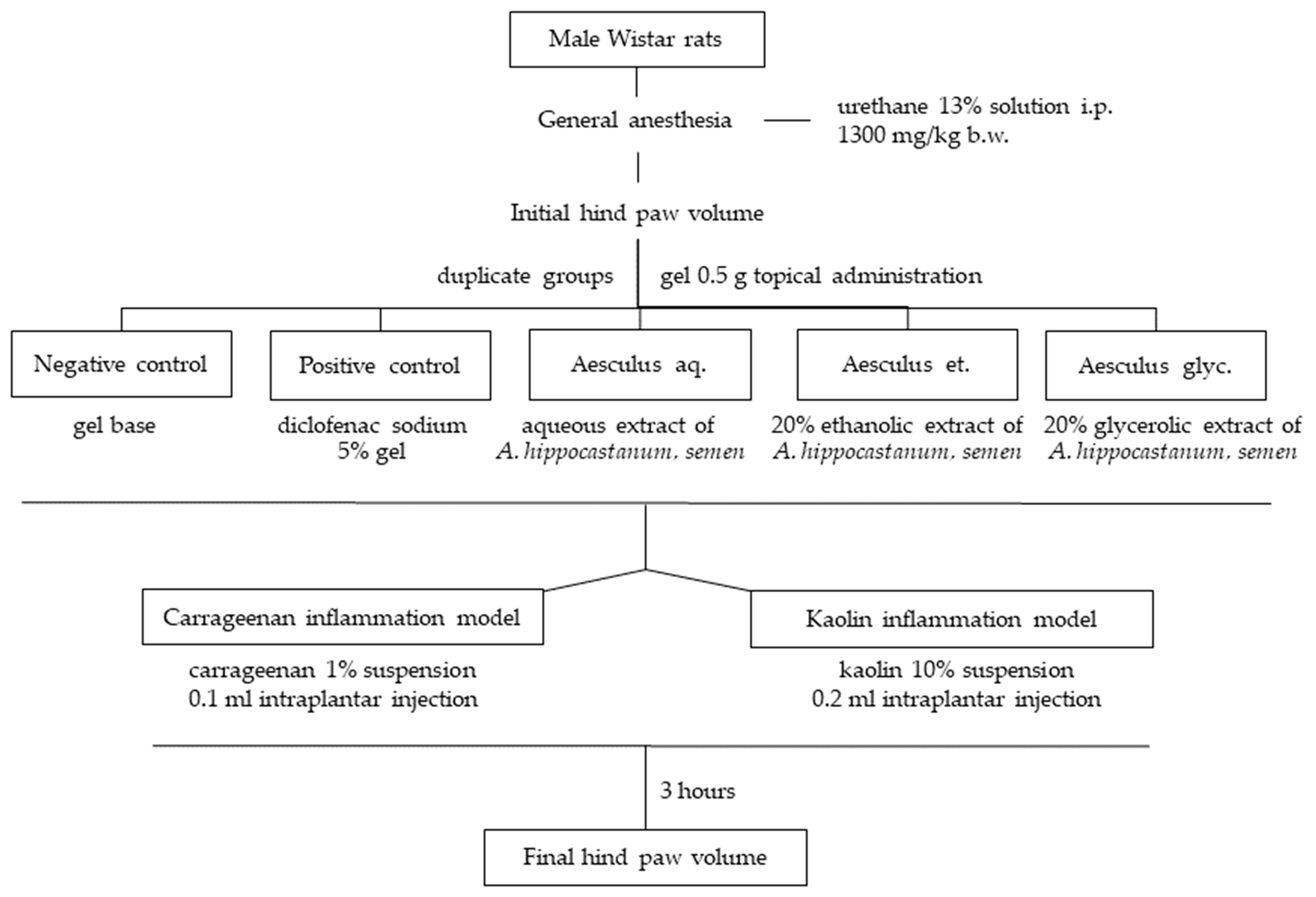
2.6. Anti-Inflammatory Effect
2.7. Statistical Analysis
3. Results
3.1. Total Polyphenolic and Flavonoid Contents
3.2. Hydrogels Preparation
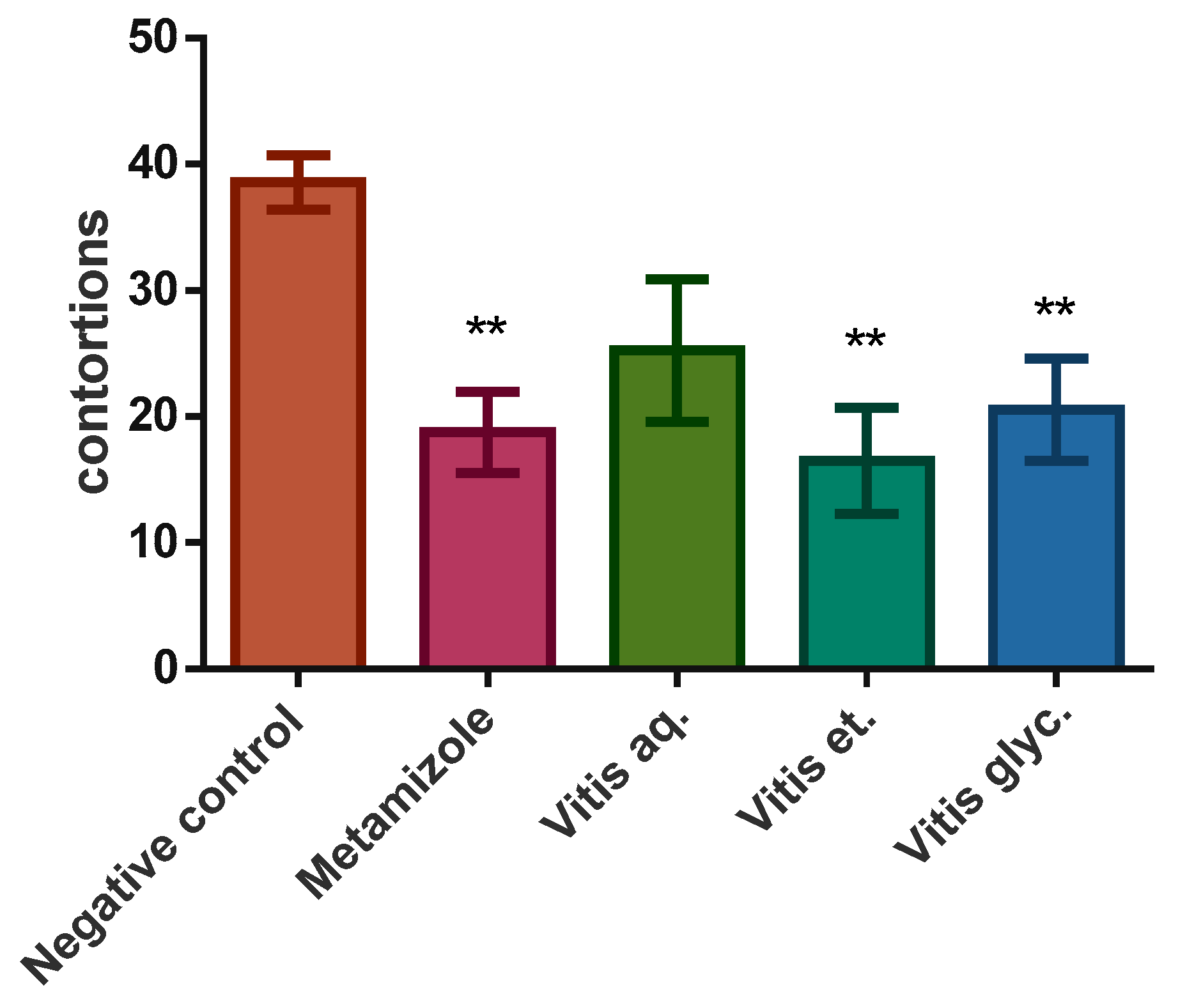
3.3. Analgesia
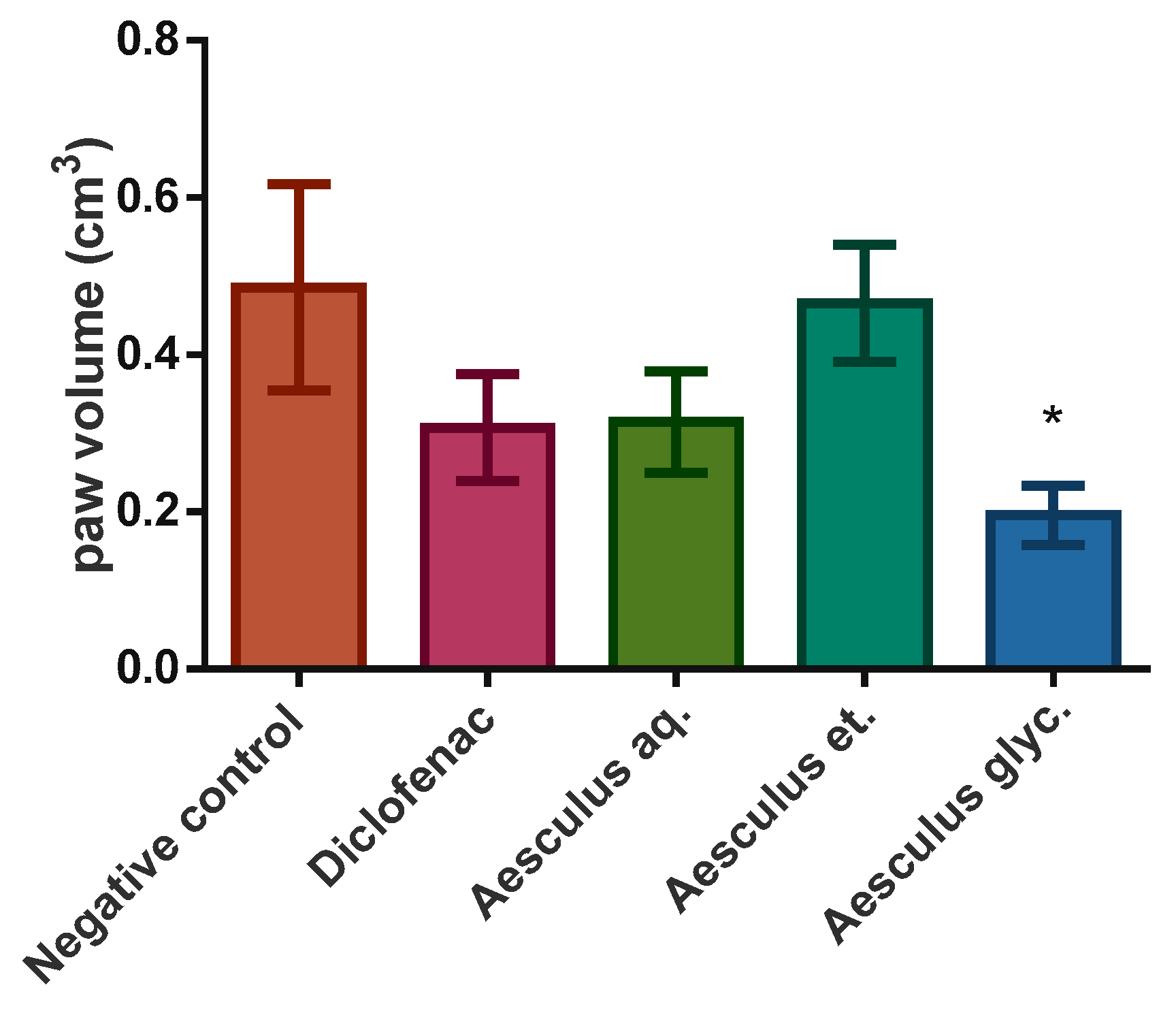
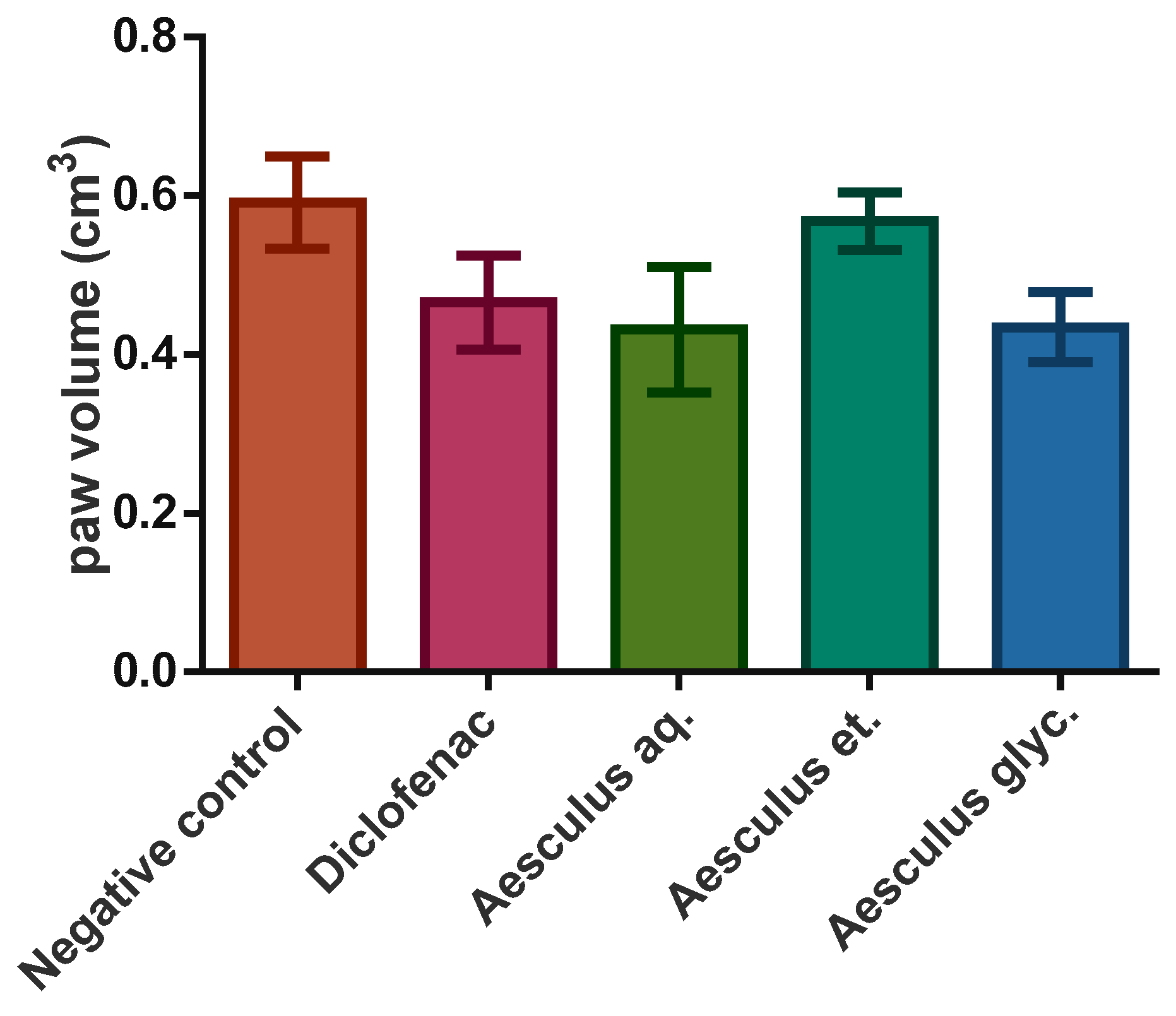
3.4. Anti-Inflamatory Effect
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Z.; Migaly, J. Review of Hemorrhoid Disease: Presentation and Management. Clin. Colon Rectal Surg. 2016, 29, 22–29. [Google Scholar] [PubMed]
- Lohsiriwat, V. Hemorrhoids: From basic pathophysiology to clinical management. World J. Gastroenterol. 2012, 18, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Mott, T.; Latimer, K.; Edwards, C. Hemorrhoids: Diagnosis and Treatment Options. Am. Fam. Physician 2018, 97, 172–179. [Google Scholar]
- Acheson, A.G.; Scholefield, J.H. Management of haemorrhoids. BMJ 2008, 336, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V. Treatment of hemorrhoids: A coloproctologist’s view. World J. Gastroenterol. 2015, 21, 9245–9252. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.R. Haemorrhoids: An update on management. Ther. Adv. Chronic Dis. 2017, 8, 141–147. [Google Scholar] [CrossRef]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P.; et al. From Plant Compounds to Botanicals and Back: A Current Snapshot. Molecules 2018, 23, 1844. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.H.S. Rediscovering the Drug Discovery with Natural Products as Therapeutic Tools. J. Nat. Sci. Biol. Med. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals—Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Hashempur, M.H.; Khademi, F.; Rahmanifard, M.; Zarshenas, M.M. An Evidence-Based Study on Medicinal Plants for Hemorrhoids in Medieval Persia. J. Evid.-Based Complement. Altern. Med. 2017, 22, 969. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, G.Ş. Horse Chestnut. Nonvitam. Nonminer. Nutr. Suppl. 2019, 295–299. [Google Scholar] [CrossRef]
- Foca, G.; Ulrici, A.; Cocchi, M.; Durante, C.; Vigni, M.L.; Marchetti, A.; Sighinolfi, S.; Tassi, L. Seeds of Horse Chestnut (Aesculus hippocastanum L.) and Their Possible Utilization for Human Consumption. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 653–661. [Google Scholar]
- Deliorman Orhan, D.; Orhan, N.; Özçelik, B.; Ergun, F. Biological activities of Vitis vinifera L. leaves. Turk. J. Biol. 2009, 33, 341–348. [Google Scholar]
- Margină, D.; Olaru, O.T.; Ilie, M.; Grădinaru, D.; GuȚu, C.; Voicu, S.; Dinischiotu, A.; Spandidos, D.A.; Tsatsakis, A.M. Assessment of the potential health benefits of certain total extracts from Vitis vinifera, Aesculus hyppocastanum and Curcuma longa. Exp. Ther. Med. 2015, 10, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Küçükkurt, I.; Ince, S.; Keleş, H.; Küpeli Akkol, E.; Avcı, G.; Yeşilada, E.; Bacak, E. Beneficial effects of Aesculus hippocastanum L. seed extract on the body’s own antioxidant defense system on subacute administration. J. Ethnopharmacol. 2010, 129, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Senatore, F.; Mścisz, A.; Mrugasiewicz, K.; Gorecki, P. Steroidal constituents and anti-inflammatory activity of the horse chestnut (Aesculus hippocastanum L.) bark. Boll. Soc. Ital. Biol. Sper. 1989, 65, 137–141. [Google Scholar]
- Wilkinson, J.A.; Brown, A.M.G. Horse Chestnut—Aesculus Hippocastanum: Potential Applications in Cosmetic Skin-care Products. Int. J. Cosmet. Sci. 1999, 21, 437–447. [Google Scholar] [CrossRef]
- MacKay, D. Hemorrhoids and varicose veins: A review of treatment options. Altern. Med. Rev. 2001, 6, 126–140. [Google Scholar]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Compounds. Phyther. Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef]
- Dehdari, S.; Hajimehdipoor, H.; Esmaeili, S.; Choopani, R.; Mortazavi, S.A. Traditional and modern aspects of hemorrhoid treatment in Iran: A review. J. Integr. Med. 2018, 16, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.; Peil, H.; Ambrosetti, L.; Petrini, O. Oedema Protective Properties of the Red Vine Leaf Extract AS 195 (Folia vitis viniferae) in the Treatment of Chronic Venous Insufficiency. Arzneimittelforschung 2011, 53, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Makuch, M.; Nska-Sroka, S. Horse chestnut—Efficacy and safety in chronic venous insufficiency: An overview. Rev. Bras. Farmacogn. 2015, 25, 533–541. [Google Scholar] [CrossRef]
- Geetha, S.; Devaraj, A. Acute oral toxicity studies of vitis vinifera (grapes) produced by microbial fertigation and foliar spray of panchagavya. J. Pharm. Sci. Innov. 2014, 3, 245–248. [Google Scholar]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Vitis vinifera (Grape)-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2014, 33, 48S–83S. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Jiang, N.; Wang, Z.; Chong, Y.; Fu, F. Anti-inflammatory effects of escin are correlated with the glucocorticoid receptor/NF-κB signaling pathway, but not the COX/PGF2α signaling pathway. Exp. Ther. Med. 2013, 6, 419–422. [Google Scholar] [CrossRef]
- Aouey, B.; Samet, A.M.; Fetoui, H.; Simmonds, M.S.J.; Bouaziz, M. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice and identification of its active constituents by LC–MS/MS analyses. Biomed. Pharmacother. 2016, 84, 1088–1098. [Google Scholar] [CrossRef]
- Radulescu, C.; Stihi, C.; Ilie, M.; Lazurcă, D.; Gruia, R.; Olaru, O.T.; Bute, O.C.; Dulama, I.D.; Stirbescu, R.M.; Teodorescu, S.; et al. Characterization of Phenolics in Lavandula angustifolia. Anal. Lett. 2017, 50, 2839–2850. [Google Scholar] [CrossRef]
- Olaru, O.T.; Venables, L.; Van De Venter, M.; Nitulescu, G.M.; Margina, D.; Spandidos, D.A.; Tsatsakis, A.M. Anticancer potential of selected Fallopia Adans species. Oncol. Lett. 2015, 10, 1323–1332. [Google Scholar] [CrossRef]
- Jantarat, C.; Sirathanarun, P.; Chuchue, T.; Konpian, A.; Sukkua, G.; Wongprasert, P. In vitro antimicrobial activity of gel containing the herbal ball extract against propionibacterium acnes. Sci. Pharm. 2018, 86, 8. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes Text with EEA relevance. Off. J. Eur. Union 2010, 28, 82–128. [Google Scholar]
- Zanfirescu, A.; Cristea, A.; Nitulescu, G.; Velescu, B.; Gradinaru, D.; Zanfirescu, A.; Cristea, A.N.; Nitulescu, G.M.; Velescu, B.S.; Gradinaru, D. Chronic Monosodium Glutamate Administration Induced Hyperalgesia in Mice. Nutrients 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Weggel, L.; Pandya, A.; Weggel, L.A.; Pandya, A.A. Acute Administration of Desformylflustrabromine Relieves Chemically Induced Pain in CD-1 Mice. Molecules 2019, 24, 944. [Google Scholar] [CrossRef] [PubMed]
- Gawade, S.P. Acetic acid induced painful endogenous infliction in writhing test on mice. J. Pharmacol. Pharmacother. 2012, 3, 348. [Google Scholar] [CrossRef] [PubMed]
- Marzoli, F.; Marianecci, C.; Rinaldi, F.; Passeri, D.; Rossi, M.; Minosi, P.; Carafa, M.; Pieretti, S.; Marzoli, F.; Marianecci, C.; et al. Long-Lasting, Antinociceptive Effects of pH-Sensitive Niosomes Loaded with Ibuprofen in Acute and Chronic Models of Pain. Pharmaceutics 2019, 11, 62. [Google Scholar] [CrossRef]
- Pashmforosh, M.; Rajabi Vardanjani, H.; Rajabi Vardanjani, H.; Pashmforosh, M.; Khodayar, M.; Pashmforosh, M.; Rajabi Vardanjani, H.; Rajabi Vardanjani, H.; Pashmforosh, M.; Khodayar, M.J. Topical Anti-Inflammatory and Analgesic Activities of Citrullus colocynthis Extract Cream in Rats. Medicina 2018, 54, 51. [Google Scholar] [CrossRef]
- Hovaneţ, M.-V.; Oprea, E.; Ancuceanu, R.V.; Duţu, L.E.; Budura, E.A.; Şeremet, O.; Ancu, I.; Moroşan, E. Wound Healing Properties of Ziziphus jujuba Mill. leaves. Rom. Biotechnol. Lett. 2016, 21, 11842–11849. [Google Scholar]
- Regina Nonato, F.; Adelita Almeida Barros, T.; Maria Lucchese, A.; Eduardo Cordeiro Oliveira, C.; Ribeiro dos Santos, R.; Botelho Pereira Soares, M.; Flora Villarreal, C. Antiinflammatory and antinociceptive activities of Blechnum occidentale L. extract. J. Ethnopharmacol. 2009, 125, 102–107. [Google Scholar] [CrossRef]
- Matsuda, H.; Li, Y.; Murakami, T.; Ninomiya, K.; Araki, N.; Yoshikawa, M.; Yamahara, J. Antiinflammatory effects of escins Ia, Ib, IIa, and IIb from horse chestnut, the seeds of Aesculus hippocastanum L. Bioorg. Med. Chem. Lett. 1997, 7, 1611–1616. [Google Scholar] [CrossRef]
- Özkan, E.E.; Mesut, B.; Polat, A.; Tan, N. In vitro skin permeation of escin in the new gel formulation of Aesculus hippocastanum (Horse Chestnut). İstanbul Üniversitesi Eczac. Fakültesi Derg. 2016, 46, 79–88. [Google Scholar]
- Nadia, Z.; Aicha, M.; Sihem, H.; Abdelmalik, B. In vivo analgesic activities and safety assessment of Vitis vinifera L. and Punica granatum L. fruits extracts. Trop. J. Pharm. Res. 2017, 16, 553. [Google Scholar] [CrossRef][Green Version]
- Koşar, M.; Küpeli, E.; Malyer, H.; Uylaşer, V.; Türkben, C.; Başer, K.H.C. Effect of Brining on Biological Activity of Leaves of Vitis vinifera L. (Cv. Sultani Çekirdeksiz) from Turkey. J. Agric. Food Chem. 2007, 55, 4596–4603. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A.; Ghorbanzadeh, B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292. [Google Scholar] [CrossRef] [PubMed]
| Sample | TFC (mg/L) | TPC (mg/L) | ||
|---|---|---|---|---|
| Mean ± SD | CI 95% | Mean ± SD | CI 95% | |
| A. hippocastanum aqueous extract | 31.01 ± 1.386 | 18.56–43.46 | 154.3 ± 0.176 | 152.7–155.8 |
| A. hippocastanum 20% ethanolic extract | 34.92 ± 8.085 | 14.84–55.01 | 160.2 ± 3.971 | 150.4–170.1 |
| A. hippocastanum 20% glycerolic extract | 53.48 ± 0.212 | 51.57–55.39 | 247.8 ± 6.991 | 239.1–256.5 |
| V. vinifera aqueous extract | 18.26 ± 1.824 | 13.73–22.79 | 266.2 ± 17.57 | 238.2–294.1 |
| V. vinifera 20% ethanolic extract | 18.47 ± 1.442 | 5.51–31.43 | 615.3 ± 34.44 | 305.8–924.8 |
| V. vinifera 20% glycerolic extract | 37.27 ± 1.174 | 26.72–47.82 | 505.3 ± 34.68 | 419.2–591.5 |
| Parameter | Treatment Groups | ||||
|---|---|---|---|---|---|
| Negative Control | Positive Control | Aesculus aq. | Aesculus et. | Aesculus glyc. | |
| Initial paw volume (Mean ± SD) | 1.73 ± 0.19 | 1.70 ± 0.06 | 1.65 ± 0.11 | 1.65 ± 0.13 | 1.81 ± 0.17 |
| Final paw volume (Mean ± SD) | 2.21 ± 0.36 | 2.01 ± 0.18 | 1.97 ± 0.15 | 2.11 ± 0.29 | 2.00 ± 0.18 |
| Paw volume increase (%) | 28.10 | 18.04 | 19.01 | 28.30 | 10.83 |
| Paired t-test (p) | * (0.0102) | ** (0.0039) | ** (0.0028) | *** (0.0008) | ** (0.0021) |
| Parameter | Treatment Groups | ||||
|---|---|---|---|---|---|
| Negative Control | Positive Control | Aesculus aq. | Aesculus et. | Aesculus glyc. | |
| Initial paw volume (Mean ± SD) | 1.69 ± 0.13 | 1.67 ± 0.18 | 1.63 ± 0.10 | 1.67 ± 0.08 | 1.75 ± 0.12 |
| Final paw volume (Mean ± SD) | 2.28 ± 0.20 | 2.14 ± 0.13 | 2.06 ± 0.22 | 2.24 ± 0.10 | 2.18 ± 0.18 |
| Paw volume increase (%) | 35.00 | 27.89 | 26.49 | 34.00 | 24.88 |
| Paired t-test (p) | *** (<0.0001) | *** (0.0002) | ** (0.0016) | *** (<0.0001) | *** (<0.0001) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, D.P.; Seremet, O.C.; Nitulescu, G.; Ivopol, M.; Sevastre, A.-S.; Negres, S.; Ivopol, G.; Nitulescu, G.M.; Olaru, O.T. Evaluation of Natural Extracts in Animal Models of Pain and Inflammation for a Potential Therapy of Hemorrhoidal Disease. Sci. Pharm. 2019, 87, 14. https://doi.org/10.3390/scipharm87020014
Mihai DP, Seremet OC, Nitulescu G, Ivopol M, Sevastre A-S, Negres S, Ivopol G, Nitulescu GM, Olaru OT. Evaluation of Natural Extracts in Animal Models of Pain and Inflammation for a Potential Therapy of Hemorrhoidal Disease. Scientia Pharmaceutica. 2019; 87(2):14. https://doi.org/10.3390/scipharm87020014
Chicago/Turabian StyleMihai, Dragos Paul, Oana Cristina Seremet, Georgiana Nitulescu, Maria Ivopol, Ani-Simona Sevastre, Simona Negres, Gabriel Ivopol, George Mihai Nitulescu, and Octavian Tudorel Olaru. 2019. "Evaluation of Natural Extracts in Animal Models of Pain and Inflammation for a Potential Therapy of Hemorrhoidal Disease" Scientia Pharmaceutica 87, no. 2: 14. https://doi.org/10.3390/scipharm87020014
APA StyleMihai, D. P., Seremet, O. C., Nitulescu, G., Ivopol, M., Sevastre, A.-S., Negres, S., Ivopol, G., Nitulescu, G. M., & Olaru, O. T. (2019). Evaluation of Natural Extracts in Animal Models of Pain and Inflammation for a Potential Therapy of Hemorrhoidal Disease. Scientia Pharmaceutica, 87(2), 14. https://doi.org/10.3390/scipharm87020014






