Abstract
In the last decade, high-performance liquid chromatography/tandem mass spectrometry (LC/MS/MS) combined with electrospray ionization (ESI) has been widely used for determining low concentrations of steroids, and derivatization has often been employed to enhance detection. In the present study, endogenous steroids were extracted using a Strata-XL polymeric reverse phase cartridge. The isolated steroids were reacted with 2-hydrazino-1-methylpyridine (HMP) at 50 °C for 30 min. A liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used in a positive mode with multiple reaction monitoring (MRM) for the quantification of testosterone (T) and its precursor, dehydroepiandrosterone (DHEA), in saliva samples collected from twenty young Saudi professional soccer players. The analytes were separated on an ACE Ultracore 2.5 Superphenylhexyl column (150 × 3.0 mm id). The extraction recovery during the pre-treatment was >89% and gave <±20% for inter- and intra-assay precision and accuracy. The limits of quantification (LOQ) were found to be 20 pg/mL for (T and DHEA) and 50 pg/mL for Epitestosterone (EPI). The results showed no significant variation in the concentration of T between pre and post training, whereas DHEA was significantly increased after short-term exercise. These results could indicate that there is no correlation between T and its precursor DHEA level following short term physical activity. EPI concentrations could not be detected with a LOQ of 50 pg/mL in the saliva samples.
1. Introduction
Testosterone (T) is a primary androgen hormone and an anabolic steroid that is secreted by the testicles of males and the ovaries of females. It stimulates the development of male characteristics by binding to androgen receptors to exert its action [1]. Dehydroepiandrosterone (DHEA) is the precursor of testosterone and other steroids and is produced by the adrenal glands [2]. Epitestosterone (EPI) is an inactive 17 alpha-epimer of testosterone [3]. A correlation between T and DHEA in biological samples has not been confirmed. Some diseases may be related to decreased levels of DHEA in serum, such as depression, osteoporosis and the metabolic syndrome [4]. However, it is not clear whether these diseases are the consequence of a decline in DHEA itself, resulting from metabolic products of DHEA, such as T [4,5].
Previous studies focused on the correlation between DHEA in saliva (Sal-DHEA) and serum samples. For example, in a study that was conducted in the Korean population, a radioimmunoassay (RIA) was used, demonstrating a positive correlation between the levels of DHEA in saliva and serum; additionally, there is an inverse variation in its level with age (n = 167 in men, n = 192 in women between 21–69 years) [6]. Another study investigated the correlation between T in saliva (Sal-T) and serum (Serum-T) samples, finding a positive correlation in men between the T level in saliva and serum (n = 104). However, there was no clear relationship of the T level in women between the saliva and serum (n = 91) [7]. Unlike serum-T, Sal-T is generally referred to as a free active steroid because of its association with the level of unbound T in serum, which is less than 2% of the circulating T [8]. By using LC-MS/MS, a Japanese group compared Sal-T and Sal-DHEA levels (n = 114), showing a low correlation between them. A variation in the activity of one or both enzymes, 3β-hydroxysteroid dehydrogenase (HSD) and 17 β -HSD, which are responsible for the conversion of DHEA to T, may control the ratio between them [9].
A recent study conducted in 2017 on a large British general population of men (n = 1675) aged between 18 and 69 years, and women (n = 2453) aged between 18 and 74 years, for the detection of Sal-T, used LC-MS/MS. The study showed an association between increased age and a reduction in the mean Sal-T level, since the annual average decline was between 1% and 1.4% in men and 1.3% and 1.5% in women [10].
There has been a debate regarding the effects of physical activity on the level of Sal-T in sports players. Previous research showed that the effects were varied among professional players. Concentrations were increased in soccer players aged 10–16 years old by activity, whereas the level was unchanged in rugby players aged 25 years [11,12]. In 2018, Australian researchers highlighted a correlation of the subsequent performance and the period of the former training load and fatigue response with the playing positions of 23 A-league football players. Regarding the impact of the training load on Sal-T, testosterone was increased for both short and long periods of training (3 to 28 days). Although the Sal-T level increased in both periods, the performance improved only for the majority of midfielders [13].
EPI is still under investigation regarding its metabolism and physiology. It is not produced from testosterone. [14]. To date, T and EPI can be detected only in urine. Athletes are suspected of androgen abuse if the ratio of T/EPI in urine is >4 [15]. Nawed et al. were for the first time able to determine T and EPI in human hair using LC-MS/MS. They reported that the correlation between the average T/EPI ratio was linear [16]. A study conducted in 2014 detected EPI in rat serum, which indicated the possibility of detecting it in human saliva [17].
Researchers have used several techniques to measure androgens in body fluids, hair and tissues, such as high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) [18,19]. In the last decade, liquid chromatography (LC) combined with mass spectrometry (MS) was introduced in various studies, resulting in a reduced analysis time and increased sensitivity in determining low concentrations of androgens [16,17,20,21,22]. Only one previous study reported the detection of T and DHEA in saliva by using LC-MS/MS, as shown in Table 1; many studies have used a radio-immunoassay.

Table 1.
Comparison of the present method with previously reported methods of detection of T and DHEA in the saliva sample.
Because some neutral steroids with low concentrations in saliva samples are particularly challenging to ionize in the ion source, chemical derivatization can be used to optimize the sensitivity for detecting analytes in saliva using ESI-MS [22,23,24]. In 2004, Higashi et al. reported that, when comparing the derivatization reagent 2-Hydrazino-1-methylpyridine derivative (HMP) with 2-fluoro-1-methylpyridine (FMP) and Girard’s reagent P (GP), the HMP reagent yielded the best results with DHEA. The detectability of the DHEA-HMP was 1600 > underivatized DHEA in ESI [22,25,26].
The current work follows the previous work by Higashi et al. [22]. The first aim of this study was to develop a sample preparation method and derivatization using HMP (Figure S1) to detect testosterone, dehydroepiandrosterone and epitestosterone in saliva samples using LC-ESI-MS/MS. The secondary target was to apply the validated method to the analysis of saliva samples collected from 20 young professional soccer players pre- and post-training on two separate days.
2. Materials and Methods
2.1. Chemicals and Materials
The following reagents were purchased from Sigma-Aldrich, UK: 2-fluoro-1-methylpyridinium-p-toluenesulfonate, hydrazine hydrate, testosterone (T), dehydroepiandrosterone (DHEA), epitestosterone (EPI), deuterated testosterone (T-d3), trifluoroacetic acid (TFA), methanol (MeOH), ethanol (EtOH). HPLC grade water, HPLC grade acetonitrile (ACN) and HPLC grade ethyl acetate were purchased from Fisher Scientific, UK. HPLC grade formic acid (98%) was obtained from BDH-Merck, UK. Individual stock solutions of T, DHEA, EPI and T-d3 were prepared in EtOH at 10 µg/mL, each followed by dilution to prepare 1 µg/mL, 10 and 1 ng/mL of working solutions in EtOH.
2.2. Preparation of 2-Hydrazino-1-Methylpyridine Derivative (HMP)
HMP was synthesized according to the work of Higashi et al. [22] as follows: hydrazine hydrate solution (80%, 132 µL) in MeCN (30 mL) was added to 2-fluoro-1-methylpyridinium-p-toluenesulfonate (FMP-TS) (300 mg) in MeCN (6 mL) at 0 °C after stirring for 10 min at 0 °C and then maintained for 20 min at room temperature under N2. The residue was dissolved for a second time in MeCN (2 mL) after this resulting mixture was concentrated. Following this, it was filtered, and the crude product was recrystallized (twice) from MeCN–ethyl acetate (5:1, v/v) to give HMP (98 mg) as colourless needles (m.p.: 133–134 °C; ESI-MS: m/z 124.3 [M]+). Finally, HMP was stored at 4 °C; this product remained stable for at least six months.
2.3. Instrumentation
An Agilent liquid chromatography–mass spectrometry (LC-MS-MS) triple, quadruple G6430A mass spectrometer equipped with an Agilent 1200 series auto-sampler, a quaternary pump SL with a degasser, and a thermostatic column compartment was used for the quantitative analysis. Positive electrospray ionization (+ESI) was used, and the mass spectrometry (MS) was operated in the multiple reaction monitoring mode (MRM). The data were recorded using Mass Hunter software version B.06.00 (Agilent technologies).
2.3.1. Liquid Chromatographic Conditions
The analytes were separated on an ACE Ultracore 2.5 Superphenylhexyl column (150 × 3.0 mm id) with a guard column, and the temperature was maintained at 30 °C. Isocratic elution was carried out with water: acetonitrile (75:25), containing 0.01% (formic acid) FA with a flow rate of 0.4 mL/min. The injection volume and run time were 10 µL and 16 min, respectively.
2.3.2. Mass Spectrometric Conditions
The androgens and testosterone-d3 (T-d3) derivatized with 2-Hydrazino-1-Methylpyridine Derivative HMP were quantified by using an Agilent liquid chromatography–mass spectrometry (LC-MS-MS) triple quadrupole G6430A mass spectrometer. The multiple reaction monitoring (MRM) conditions were examined by injecting infusion solutions directly into the electrospray ionization (ESI) source. The infusion solutions contained a concentration of 10 µg/mL of each analyte. The results are shown in Table 2. Additional mass spectrometry (MS) parameters were optimized to achieve the best sensitivity, as follows: Delta EMV, 800 V; gas temperature, 350 °C; gas flow, 9 L/min; nebulization pressure, 20 psi; sheath gas, 50 psi; auxiliary gas, 15 psi; capillary voltage, 5000 V.

Table 2.
Optimization MRM transitions of androgens and testosterone -d3 derivative with HMP.
2.4. Experimental Design
To investigate the effect of exercise on endogenous androgens in saliva using an optimised and validated method, saliva was collected from 20 young male professional soccer players (20.6 ± 1.4 years, body mass 70.2 ± 1.6 kg, height 178 ± 2 cm and Body mass index (BMI) 20.6 ± 1.4 kg/m2) using a Sarstedt Salivette polyester. The samples (pre-post) were collected over two days during the training sessions. No participant was injured, and no participant took any medication during the study. The participants attended the club one and a half hours before training starts every day. The study was approved by the Ethics Committee at King Saud University, and all players read the information sheet and signed a consent form before participating in the study.
All training sessions started at the same time each day. Two training sessions were designed for the study, beginning with a 15 min warm up. Then, the players were divided randomly to play two small side games for 30 min, followed with 4 min rest time. In addition, the coach set up a game for 40 min divided into two halves, followed with a 5 to 10 min cool down period. The intensity on the first training day and the second day was designed based on the percentage of the maximum heart rate. The heart rates were 70% and 72% of the maximum on the first and second days, respectively. The mean ambient temperature was 25 ± 3 °C, and the humidity was 18 ± 4% for the first day and 26 ± 1 °C and 16 ± 2% for the second day.
2.5. Pre-Treatment and Extraction of Samples
The stored samples were thawed and then centrifuged at 1000× g for 5 min, and 0.5 mL of supernatant was taken. The saliva samples were deprotonated with 0.5 mL of acetonitrile containing 50 pg of T-d3, vortexed for 30 s, centrifuged for 10 min and then diluted with water (1.5 mL).
For the extraction, the samples were loaded onto a Strata-XL reverse phase cartridge, which was first prepared by washing with 1 mL of methanol (MeOH) followed by 1 mL of water (H2O). After washing with 1 mL of H2O followed by 1 mL of MeOH/H2O (10:90 v/v), the retained analytes and IS were eluted under gravity with 1 mL of acetonitrile.
2.6. Derivatization of Androgens with 2-Hydrazino-1-Methylpyridine Derivative HMP
The derivatization of the standards (STDs) or extracts with deuterated testosterone (T-d3) was carried out by adding 50 µL of fresh 2-Hydrazino-1-Methylpyridine Derivative (HMP) solution (1 mg/mL) in methanol (EtOH0 containing 25 µg of trifluoroacetic acid (TFA). The sample was then incubated at 50 °C for 30 min. The mixture was then gently evaporated under the nitrogen. The residue was reconstituted using the mobile phase (200 µL) and then vortexed. Finally, 10 µL of the mixture was injected.
2.7. Method Validation
The method was validated according to the Food and Drug Administration guidelines (FDA) [34].
2.7.1. Extraction Recovery
Two sets of standards were prepared, each of which contained 50, 200 and 400 pg, corresponding to 100, 400 and 800 pg/mL of testosterone (T), dehydroepiandrosterone (DHEA)and epitestosterone (EPI), respectively. One set was made in the pooled steroid-free saliva samples (blank), and the other set was prepared in the mobile phase. The blank saliva samples (500 µL) were spiked with these three concentrations in triplicate and then extracted. The saliva samples were mixed with 100 pg of a deuterated testosterone (T-d3), referring to 200 pg/mL. The standards of the other set were directly injected after they were combined with an internal standard and then derivatized (i.e., the non-extracted samples). The peak area means of T, DHEA and EPI and the (T-d3) were obtained for the extracted and non-extracted samples in each concentration. The absolute recovery was determined for each analyte by dividing the average extracted mean by that of the non-extracted mean at the same level and then multiplying by 100 [16].
2.7.2. Matrix Effect
The matrix effect refers to any compound in the sample except the analyte of interest, which could affect the analyte response when co-eluted with it, causing an increase in ionization efficiency (i.e., enhancement) or a decrease in ionization efficiency (i.e., suppression). The consequence is an inaccurate concentration measurement. [35].
Two sets of samples were prepared in low (50 pg), medium (100 pg), and high (400 pg) concentrations of standards and deuterated testosterone (T-d3), corresponding to 100, 200 and 800 pg/mL each. Set one consisted of derivative standards that were injected six times to establish a mean peak area for each concentration. Set two involved three different pooled blank saliva samples from ten sources. Each matrix source was extracted in duplicate, and the extract was spiked in either the low, medium or high concentrations of standards and T-d3. The matrix effect was then calculated by averaging the area of each, as shown in Equation (1) [35]:
A negative value indicates signal suppression, whereas positive values suggested that enhancement occurred. The acceptable limits for enhancement or suppression are ±25% [35].
% ionisation enhancement or suppression = ((((X Area of set2 − X Area of set1)/X Area of set1) ∗ 100
2.7.3. Specificity
In the actual method, to ensure that no interference occurred between each analyte of interest by internal standard or other substances during their retention time in the saliva, three representative types of steroids, pregnenolone, dihydrotestosterone and androstenedione, were derivatized at 800 pg/mL and injected into the mass spectrometry (MS) system.
2.7.4. Linearity and Calibration Standards of the Saliva
Pooled saliva samples were prepared, and the samples were mixed for 24 h with 1 g of activated charcoal (NoritEXW, Nacalai Tesque, France) and then centrifuged at 1000× g for 20 min. The supernatant was examined to ensure that no testosterone (T), dehydroepiandrosterone (DHEA) or epitestosterone (EPI) was detected following this treatment. Aliquots of steroid-free saliva samples (blank saliva) (0.5 mL) were spiked with standards (STDs) to prepare six different concentrations of these steroids in the range of 10–400 pg, corresponding to 20–800 pg/mL of DHEA and T; and in the range of 25–600 pg, corresponding to 50–1200 pg/mL of EPI. The level of the internal standard (T-d3) was 100 pg/mL. After evaporation, the residue was dissolved in 200 µL of ethanol and then derivatized with the HMP reagent using the final optimised procedure described above. The calibration curves were determined by plotting the peak area ratio of T/T-d3, DHEA/T-d3 and EPI/T-d3 (y) against the concentrations of T, DHEA and EPI (x, pg/mL), respectively.
2.7.5. Sensitivity: Limit of Detection (LOD) and Limit of Quantification (LOQ)
Stock solutions of testosterone (T) and dehydroepiandrosterone (DHEA) were prepared at concentrations of 10–400 pg/0.5 mL, equal to 20–800 pg/mL. Meanwhile, epitestosterone (EPI) was prepared at concentrations of 25–600 pg/0.5 mL, equal to 50–1200 pg/mL. They were then spiked into the blank saliva and analyzed in triplicate following derivatization. The LOD and LOQ of T, DHEA and EPI were determined using a linear calibration curve [34]. Three calibration curves were constructed. The LOD and LOQ were calculated according to an estimate from the standard deviation of the y-intercept (Sy) and the average slope (Am), as shown in Equation 2.
LOD = 3 * Sy /Am
LOQ = 10* Sy /Am
LOQ = 10* Sy /Am
2.7.6. Accuracy and Precision
The accuracy of the results was determined by calculating the percentage recovery of five determinations at three different concentrations obtained from five sources in a linearity range of 50, 400 and 800 pg/mL. The concentrations were calculated from the corresponding regression equations.
Precision was performed for three different concentrations of pure standards of steroids (50, 400 and 800 pg/mL for testosterone (T), dehydroepiandrosterone (DHEA) and epitestosterone (EPI),, respectively) spiked into the saliva. They were analyzed in triplicate in a single day and on five following days to calculate the intra-day (i.e., repeatability) and inter-day precision (i.e., intermediate precision).
2.7.7. Stability
Two terms were investigated to study the stability of the analytes. In the short-term study, 0.5 mL of the blank saliva samples was spiked with low and high concentrations of the analytes (50 and 400 pg) and deuterated testosterone (T-d3) (50 pg), corresponding to 100 and 800 pg/mL each, as well as 100 pg/mL for the T-d3, before being stored at different temperatures (4, −20 °C and in the auto-sampler) and then analyzed after thawing and extraction. Regarding the freeze-thaw stability, two cycles in the short-term study were assessed in triplicate after 24 h and 48 h. In addition, the low and high concentrations of the analytes (100 and 800 pg/mL) and T-d3 (100 pg/mL) were stored at −20 °C for six weeks and then analyzed after more than three freeze-thaw cycles. In the long-term evaluation, 24 random samples were chosen from the overall samples, analyzed and then stored at −80 °C for six weeks, and then rerun. The first run of each concentration in the short or long-term study was considered a time-zero response. Thus, the stability was satisfactory if the response was within 15% of the time zero response.
3. Results and Discussion
3.1. Optimization of Reaction Conditions in Derivatization Steroids with HMP
Two isomeric (E &Z) peaks were formed, as a result of reacting the 2-Hydrazino-1-Methylpyridine (HMP) derivatization reagent with testosterone (T) and epitestosterone (EPI0. In contrast, dehydroepiandrosterone (DHEA-HMP) formed just one peak (possibly due to a steric hindrance from the methyl group at the 18 position in the steroid nucleus inhibiting the formation of the syn isomer).
It was found that the evaporation step was crucial since only 2% of the steroid was derivatized without evaporation, while 99% was derivatized when the solvent was evaporated with nitrogen gas. Shou et al. [36] justified this increase, reporting that when using trifluoroacetic acid (TFA )as a catalyst, it has the power to form ion pairs, which leads to the prevention and loss of the electrospray ionization (ESI) signal. To overcome this problem, nitrogen evaporation was used to dry the sample, which led to improved results.
The reaction times of 30 min and 1, 2, 4 and 24 h were used with two temperatures (room temperature and 50 °C). The results are shown in Figure S2 and indicate that the highest reaction efficiency between the androgens and HMP occurred after 30 min of incubation at both room temperature and 50 °C. However, when the androgens were heated at 50 °C, the derivatization was complete within 30 min (99.8%). Therefore, it was concluded that heating at 50 °C for 30 min yielded the best result.
3.2. Optimization of LC Conditions
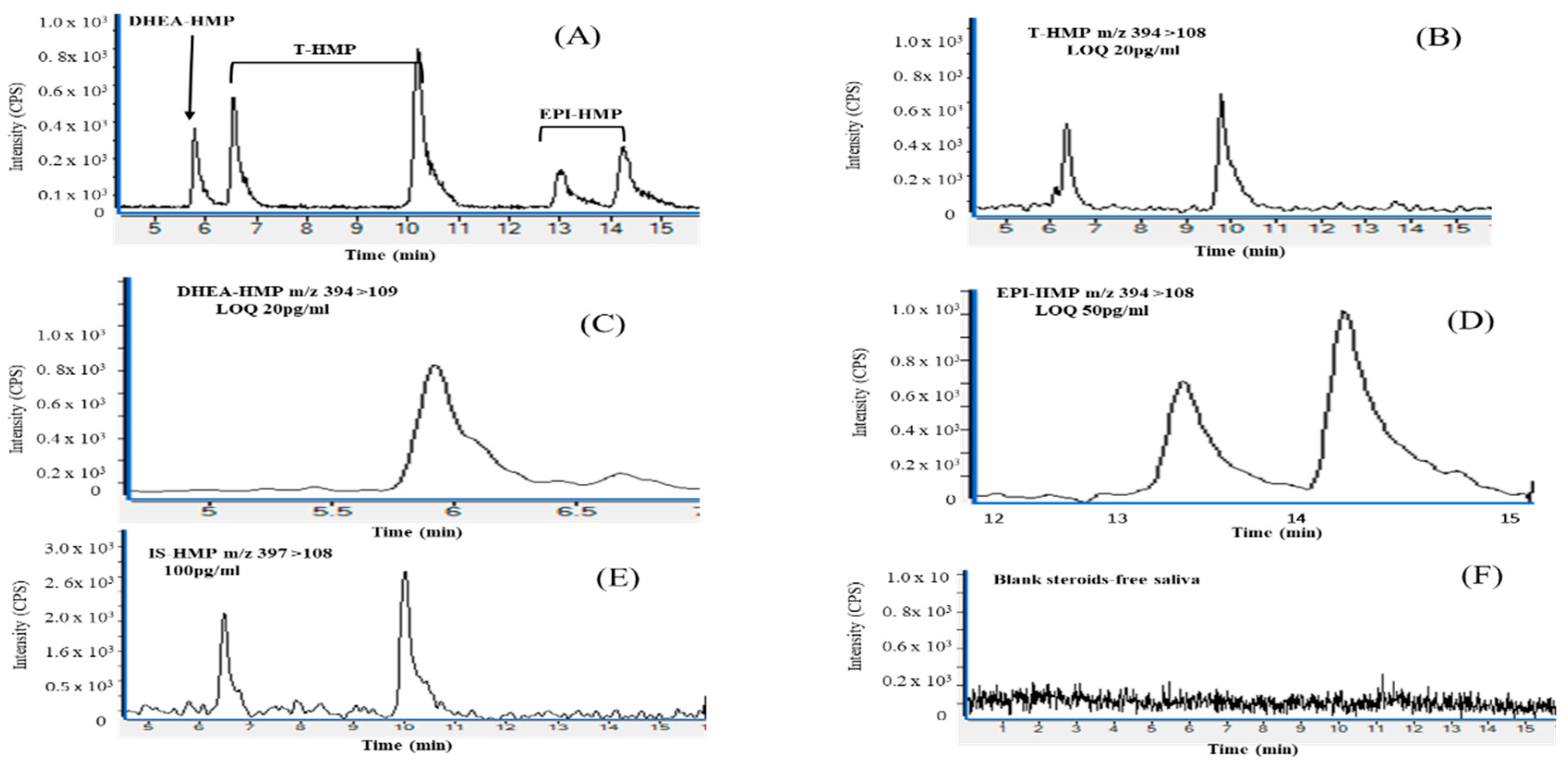
Three columns were examined to determine their efficiency in separating the steroids. This testing involved an ACE 3 C18 column (150 × 3.0 mm i.d.; Hichrom, Reading, UK), an ACE 5 C18-AR column (150 × 4.6 mm, 5 μm Hichrom) and an ACE Ultracore 2.5 Superphenylhexyl (150 × 3.0 mm id.; Hichrom). The C18 column did not provide any acceptable separation between the steroid derivatives. The C18-AR gave only a poor separation between the dehydroepiandrosterone (DHEA-HMP) peak and the first peak of testosterone (T-HMP), which led to co-elution. Because it was not possible to achieve an acceptable separation between the first peak of T-HMP and DHEA-HMP by using the C18-AR column, an ACE Ultracore 2.5 Superphenylhexyl column (150 × 3.0 mm id) was used and produced an adequate separation of the steroids. All the analyte peaks appeared within the range of 5.6 to 15 min (Figure 1), using the conditions described in Section 2.3.1; on the other hand, because of the presence of twin peaks for T-HMP and EPI-HMP (Figure S1), and since the second eluting isomers gave higher abundances, they were used for the quantification.
Figure 1.
Chromatograms of HMP derivatives of testosterone (T-HMP), dehydroepiandrosterone (DHEA-HMP), epitestosterone (EPI-HMP) and Testosterone-d3 HMP (T-d3-HMP) in healthy human saliva spiked after treatment with activated charcoal: (A) chromatographic resolution of derivative testosterone (T), dehydroepiandrosterone (DHEA) and epitestosterone (EPI), (400 pg/mL) spiked in steroid free saliva. (B) T-HMP multiple reaction monitoring (MRM) illustrating low limit of quantification (LOQ) m/z 394 > 108, (20 pg/mL); (C) DHEA-HMP limit of quantification (LOQ) m/z 394 > 109, (20 pg/mL); (D) EPI-HMP limit of quantification (LOQ) m/z 394 > 108, (50 pg/mL); (E) Testosterone-d3 HMP m/z 397> 108, (100 pg/mL); (F) Blank: saliva treated with activated charcoal, before being extracted and derivatized. The liquid chromatography-mass spectrometry (LC-MS/MS) conditions are described in Section 2.3.2. Counts per second (cps).
3.3. Optimization of MS Conditions
The parameters of the mass spectrometer (MS) operating conditions are provided in Table 3. The optimal MS parameters were achieved by the direct infusion of the derivatives of the steroids into the electrospray ionization (ESI) source in the positive mode. The infusion solution consisted of 50/50 (v/v) of aqueous and organic mobile phases at a concentration of 10 μg/mL for all the analytes. The optimal MS transitions had only one product ion with the chosen collision energy, as follows: m/z 394 > m/z 108; m/z 394 > m/z 109; m/z 394 > m/z 108; and m/z 397 > m/z 108, for testosterone (T-HMP), dehydroepiandrosterone (DHEA-HMP), epitestosterone (EPI-HMP) and deuterated testosterone (T-d3-HMP), respectively, as described in Section 2.3.2 (Table 2). The product ion is formed by the cleavage of the N-N bond of the hydrazine, as shown in Figure S1; the derivatization of the T and EPI with HMP results in hydrazones with just a fragmentation ion [M]+, whereas DHEA-HMP gave a fragmentation ion with [M + 1]+.

Table 3.
Summary of the optimum conditions for ion source parameters.
3.4. Collection of Saliva and Pre-Treatment
There are many ways to collect saliva; the most popular method is by passive drool, or spitting, as mentioned in Table 1. Saliva collection can be stimulated by chewing gum or citric acid, which could cause a reduction of the analyte concentration [37]. Also, the collection of Salina testosterone (Sal-T) and other steroids, using different devices such as Salivette® (Sarstedt) with cotton, polyester and polyethylene, were evaluated. The polyester and polyethylene Salivette have been given weak recovery for androgen. On the other hand, Salivette® cotton is not recommended [38,39]. Polyester Salivette was used for the collection of saliva samples in this project. Therefore, the evaluation of recovery from this device has been made and was compared with the direct collection by collecting a sample from a participant 5 times for both collection types following the collection protocol. Recoveries were compared and calculated by using an independent t-test. The results for the recovery of Sal-T using the Salivette device in comparison to the direct collection was 86%, and the p-value was 0.1, whereas the saliva dehydroepiandrosterone (Sal-DHEA) recovery was 83.5% and the p-value was 0.2.
3.5. Solid Phase Extraction Cartridge Selection
Two kinds of cartridges (C18-E and Strata-XL polymeric reverse phase) were examined. After suitable sorbents for the analytes were chosen, the 10-bottle optimization SPE extraction approach, which is recommended by the Agilent company, was applied [40]. The optimization was started using a procedure where 11 cartridges from each sorbent were conditioned with 1 mL methanol followed by 1 mL of water (H2O). Then, 100 µL of ethanol containing 100 pg of standards (STDs) were added to 1 mL H2O and loaded on 10 cartridges of each sorbent. Only 1 mL H2O without any STDs was loaded on the blank number 11 for each sorbent. Figure S3C shows that 10% to 20% of methanol (MeOH) and 80% to 90% of H2O could be used in the washing step because of the low quantity of steroids that was lost from both cartridges.
As shown in Figure S3A,B, 1 mL of acetonitrile () yielded the highest extraction recovery for all the steroids. Overall the Strata-XL cartridge gave a slightly better performance. Therefore, the final extraction procedure used to extract the steroids from the saliva samples was as follows: the sample was loaded onto the Strata-XL cartridge, which was prewashed with 1 mL of MeOH followed by 1 mL of H2O. After washing with 1 mL H2O followed by MeOH: H2O (10:90 v/v), the target analytes were eluted with 1 mL of ACN.
3.6. Method Validation
3.6.1. Extraction Recovery from Spiked Human Saliva
The efficiency of the extraction procedure of testosterone (T), dehydroepiandrosterone (DHEA) and epitestosterone (EPI) using solid phase extraction cartridge (SPE) was assessed by calculating the extraction recovery percentages. The extraction method was able to extract more than 93% of T, more than 86% of DHEA, and more than 96% of EPI in three different concentrations, as shown in Table 4.

Table 4.
Extraction Recovery Data on Androgens Determined in Saliva Using LC-MS/MS.
3.6.2. Matrix Effects
As shown in Figure S4, the matrix slightly enhanced the response for all steroids and the deuterated testosterone (T-d3) in the low and medium concentrations, whereas at the highest level the signal was slightly suppressed. The matrix effect was within the range of ±25%, which was acceptable.
3.6.3. Specificity
The specificity was determined using the liquid chromatography-mass spectrometry (LC-MS-MS) conditions described in Section 2.3.2. Three steroids, pregnenolone, dihydrotestosterone and androstenedione, were investigated to determine the most productive ion, and the retention time was as follows: pregnenolone (PREG) m/z 422.5 [M]+, m/z 108.0 and retention time 7.3 and 11.5; 5α dihydrotestosterone (DHT) m/z 396.2 [M]+, m/z 108.0 and retention time 7.5 and 11.8; and androstenedione (AD) m/z,497.4 [M − 1]+, m/z 108.0 and retention time 7.3 and 11.5 min, all of which had twin peaks. All these steroids were not detected by the multiple reaction monitoring (MRM)conditions used for testosterone (T), dehydroepiandrosterone (DHEA) and epitestosterone (EPI), that derivatized by 2-Hydrazino-1-Methylpyridine Derivative (HMP) when 800 pg/mL of the potentially interfering steroids were injected.
3.6.4. Linearity and Calibration of Standards Spiked into Saliva
The assay showed that the linearity was acceptable within the range of 10–400 pg corresponding respectively to 20–800 pg/mL for testosterone (T) and dehydroepiandrosterone (DHEA); and within the range of 25–600 pg corresponding respectively to 50–1200 pg/mL for epitestosterone (EPI), with correlation coefficients of ≥0.995 (Figure S5A,B). In addition, reliable resolution of the peaks and acceptable peak shapes were achieved for T, DHEA, EPI and deuterated testosterone (T-d3). The ratios obtained for DHEA and T against the (T-d3) were roughly equal; therefore, some points in the calibration curve appear as one point.
3.6.5. Sensitivity: Limit of Detection (LOD) and Limit of Quantification (LOQ)
As summarized in Table 5, LLOQ and LOD were calculated for testosterone (T) and dehydroepiandrosterone (DHEA) at concentrations of 20–800 pg/mL, and epitestosterone (EPI) at concentrations of 50–1200 pg/mL. The LOQ was 20 pg/mL for T and DHEA and 50 pg/mL for EPI. However, the LODs for T, DHEA and EPI were 4, 6 and 14 pg/mL, respectively.

Table 5.
Calibration Curve Parameters (n = 3).
3.6.6. Accuracy and Precision
The method was shown to be reproducible regarding the peak shape and retention time. The relative standard deviation (RSD%) of the intra- and inter-assay precision for testosterone (T), dehydroepiandrosterone (DHEA) and epitestosterone (EPI) was <11%. The accuracy was within the range of 93–114%. The results for the accuracy and precision of the method are shown in Table 6.

Table 6.
Intra-and Inter-Assay Precision and Accuracy Results of Androgen Determination in Saliva Using LC-MS/MS (n = 5).
3.6.7. Stability
Table 7 shows that after two cycles of freezing/ thawing at two different temperatures and on auto-sampler, there was the satisfactory stability of the analytes (testosterone (T), dehydroepiandrosterone (DHEA) and epitestosterone (EPI)) during the short term (24 and 48 h). However, there were slight decreases from zero-response in the auto-sampler. In contrast, the degradation of the analytes in low and high concentrations of 100 and 800 pg/mL, respectively, and the deuterated testosterone (T-d3) in a concentration of 100 pg/mL, was >50% when they were stored for six weeks at −20 °C after freezing/thawing for a third cycle. The stability of the derivative steroids was acceptable in 24 samples that were reanalyzed after being stored at −80 °C for six weeks. When the samples were rerun, the percentages compared with the original response were 95% and 97% for testosterone (T) and dehydroepiandrosterone (DHEA), respectively.

Table 7.
Stability of T, DHEA and EPI in the short term.
4. Method Application
Exogenous androgens in saliva samples were determined in twenty professional soccer players. All players attended the training sessions during the mid-season; the players train 90 min a day over five days a week, and they play one match per week. The samples were collected over two days before and after the training sessions.
The protocol of the injection sample in liquid chromatography-mass spectrometry (LC-MS-MS) was as follows: four samples belonging to each player, pre- and post-exercise, were injected sequentially on subsequent days. Seven pooled samples were prepared randomly from overall samples to examine technical variations. The results showed that the average of testosterone (T) and dehydroepiandrosterone (DHEA) levels in the pooled samples were 226.2 pg/mL ± 5.7% and 40.7 pg/mL ± 8.7%, respectively.
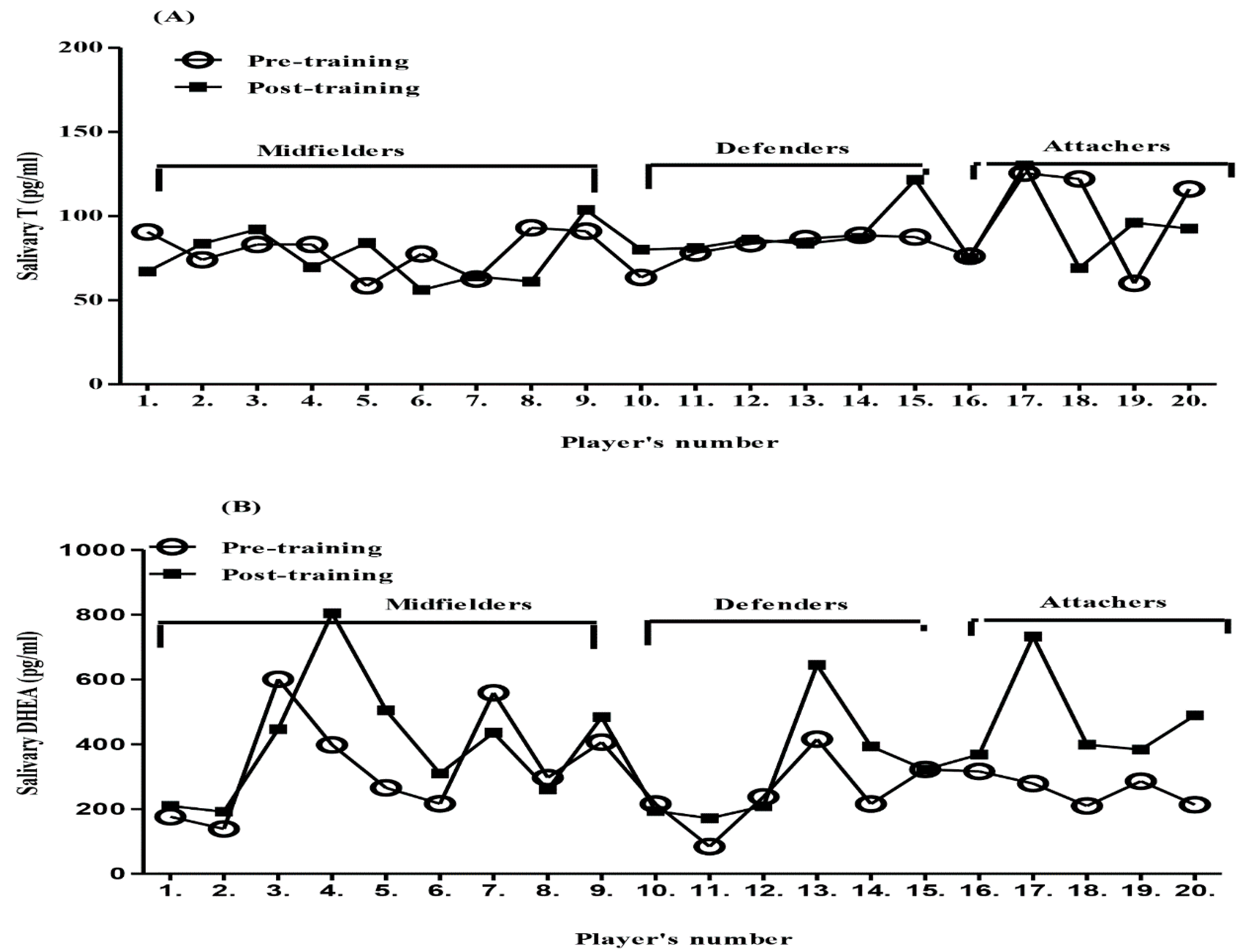
A paired t-test was used for the statistical comparison of the variables (T and DHEA) both pre- and post-exercise. The post-exercise DHEA levels increased significantly (mean = 397.45 pg/mL) by about 105 pg/mL (p-value = 0.008) compared to the pre-exercise level mean = 292.50 pg/mL. However, there was no significant change in the T levels between the post-exercise mean = 84.35 pg/mL and pre-exercise mean = 84.85 pg/mL. We compared the mean level of T in pre and post-exercise in the current study with a Japanese study [9]. They mentioned that between 20 to 30 years, for healthy men, the mean level of saliva testosterone (Sal-T) was high in the morning (rough mean= 55.0 pg/mL) while in the current study 84.35 pg/mL was obtained. The collection saliva in the current study was in the afternoon time and was also from very fit subjects. The greater T level in the current study could be due to the long term impact of physical activity, which is in agreement with other studies [13,41]. In the present study, T levels do not reveal any significant differences based on player positions (Figure 2A), and that may be because training sessions were considered as having a moderate intensity based on the percentage of the heart rate of the maximum heart beats. On the other hand, in Australian football players, they found that the T levels increased in all positions, but the performance was increased just in midfielder players, who have a higher training load than other positions [13].
Figure 2.
Pre- and post-exercise rhythms of (A) Sal-T levels and (B) Sal-DHEA levels in 20 young professional soccer players in three different positions.
ANOVA was used to compare the differences in DHEA for three positions (defender, midfielder and attacker). The results showed no specific differences in any of the positions between pre- and post-exercise. The Body mass index (BMI) and age were not compared because of small variations in both ranges. The comparison was performed using a one-way ANOVA (Figure 2B).
Previous studies have found that the highest levels of DHEA and T occurred in the early morning, which then decreased gradually during the day [9]. The current results indicated that brief, intense physical activity could significantly increase DHEA, which could lead to improved well-being and protection from many diseases [4].
To study the correlation between the level of saliva dehydroepiandrosterone (Sal-DHEA) and –T (n = 20), a Pearson correlation was calculated, and the result (Figure S6A,B) indicated that there was no correlation between them in pre or post-exercise since the Pearson correlation R2 and p-values were 0.014, 0.0002 and 0.935 for pre, and 0.407, 0.166 and 0.075 for post-training. T can be formed from DHEA via androstenedione or androst-5-ene-3β,17β-diol, and it might be that the build-up of DHEA leads to increased testosterone after a longer period of post-training. Epitestosterone was not detected in any of the collected samples.
5. Conclusions
The development of a derivatization method for exogenous androgens in a saliva sample followed by analysis by using liquid chromatography-mass spectrometry (LC-MS-MS) was achieved. The 2-Hydrazino-1-Methylpyridine Derivative (HMP) reagent was chosen for the derivatization, and the reaction conditions were optimized. The optimization of the derivatization involved several steps, including the ratio temperature and incubation time. The acceptable separation between these analytes was achieved by using an ACE Ultracore 2.5 Superphenylhexyl column.
A rapid procedure for the extraction of the steroids from the saliva samples was developed by using a polymeric reverse solid phase extraction cartridge (SPE). testosterone (T) and dehydroepiandrosterone (DHEA) were extracted from the saliva, and an LC-ESI-MS/MS method for detecting the steroids in the saliva sample was developed, and the process was validated according to FDA guidelines and had acceptable specificity, sensitivity, predictability, repeatability and accuracy.
This method was applied to the analysis of saliva samples collected from football players pre-and post-training over two days. The concentrations T and DHEA in the saliva samples were determined, whereas epitestosterone (EPI) was not detected. The levels of saliva testosterone (Sal-T) detected in the pre-and post-exercise samples showed no significant difference in Sal-T before and after training. However, the saliva dehydroepiandrosterone (Sal-DHEA) level registered a considerable difference. The correlation between the level of Sal-T and Sal-DHEA in the pre-exercise or the post-exercise samples was not high, suggesting no change in activity for the enzymes responsible for producing T from DHEA. However, the number of subjects was relatively small and it might be that a different outcome would be achieved with a larger sample size. Since the analytical method works effectively and has been shown to be stable, it would be possible to use the method to analyze samples from different cohorts of subjects undergoing different types of physical activity, e.g., ultramarathon runners [42].
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/2218-0532/87/2/11/s1, Figure S1: Structures of derivatization 2-Hydrazino-1-methylpyridine derivative (HMP) with testosterone, dehydroepiandrosterone and epitestosterone; Figure S2: Optimization of reaction conditions in derivatization steroids with HMP; Figure S3: Solid phase extraction cartridge selection.; Figure S4: Matrix Effect; Figure S5: Linearity and calibration of standards spiked into saliva; Figure S6: Correlation between the concentrations of Sal-DHEA and Sal-T (n = 20) were (A) pre-training and (B) post-training.
Author Contributions
Authors of this study contributed in the following areas: conceptualisation, M.A.A. and D.G.W.; methodology and resources, M.A.A., G.O.A., K.O.S., C.G., A.K., A.S.A., D.G.W.; formal analyses, validation and writing—original draft preparation, M.A.A.; writing—review, editing and supervision, D.G.W.
Funding
This research received no external funding.
Acknowledgments
We thank Hazel Torrance of the Department of Forensic Medicine and Science at the University of Glasgow for the use of the Agilent Tandem MS system and King Saud University. We thank the Saudi Government for a studentship for M.A.A.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mooradian, A.D.; Morley, J.E.; Korenman, S.G. Biological actions of androgens. Endocr. Rev. 1987, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.A.; Schwartz, E.B.; Booth, A.; Curran, M.; Zakaria, D. Assessing dehydroepiandrosterone in saliva: A simple radioimmunoassay for use in studies of children, adolescents and adults. Psychoneuroendocrinology 1999, 24, 567–579. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Kochakian, C.D.; Lobotsky, J. The in vitro metabolism of ∆4 -androstenedione-3,17 to testosterone, cis-testosterone, and several unidentified steroids. J. Biol. Chem. 1947, 171, 493–500. [Google Scholar] [PubMed]
- Arlt, W. Dehydroepiandrosterone and ageing. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 363–380. [Google Scholar] [CrossRef]
- Higashi, T.; Shibayama, Y.; Shimada, K. Determination of salivary dehydroepiandrosterone using liquid chromatography-tandem mass spectrometry combined with charged derivatization. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 846, 195–201. [Google Scholar] [CrossRef]
- Ahu, R.S.; Lee, Y.J.; Choi, J.Y.; Kwon, H.B.; Chun, S.I. Salivary cortisol and DHEA levels in the Korean population: Age-related differences, diurnal rhythm, and correlations with serum levels. Yonsei Med. J. 2007, 48, 379–388. [Google Scholar]
- Keevil, B.G.; MacDonald, P.; Macdowall, W.; Lee, D.M.; Wu, F.C.W.; NATSAL Team. Salivary testosterone measurement by liquid chromatography tandem mass spectrometry in adult males and females. Ann. Clin. Biochem. 2014, 51, 368–378. [Google Scholar] [CrossRef]
- Dunn, J.F.; Nisula, B.C.; Rodbard, D. Transport of Steroid Hormones: Binding of 21 Endogenous Steroids to Both Testosterone-Binding Globulin and Corticosteroid-Binding Globulin in Human Plasma. J. Clin. Endocrinol. Metab. 1981, 53, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, Y.; Higashi, T.; Shimada, K.; Odani, A.; Mizokami, A.; Konaka, H.; Koh, E.; Namiki, M. Simultaneous determination of salivary testosterone and dehydroepiandrosterone using LC-MS/MS: Method development and evaluation of applicability for diagnosis and medication for late-onset hypogonadism. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Keevil, B.G.; Clifton, S.; Tanton, C.; Macdowall, W.; Copas, A.J.; Lee, D.; Field, N.; Mitchell, K.R.; Sonnenberg, P.; Bancroft, J.; et al. Distribution of Salivary Testosterone in Men and Women in a British General Population-Based Sample: The Third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). J. Endocr. Soc. 2017, 1, 14–25. [Google Scholar]
- Di Luigi, L.; Baldari, C.; Gallotta, M.C.; Perroni, F.; Romanelli, F.; Lenzi, A.; Guidetti, L. Salivary Steroids at Rest and After a Training Load in Young Male Athletes: Relationship with Chronological Age and Pubertal Development. Int. J. Sports Med. 2006, 27, 709–717. [Google Scholar] [CrossRef]
- Beaven, C. Salivary testosterone and cortisol responses following four resistance training protocols in professional rugby players. J. Strength Cond. Res. 2008, 22, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Rowell, A.E.; Aughey, R.J.; Hopkins, W.G.; Esmaeili, A.; Lazarus, B.H.; Cormack, S.J. Effects of training and competition load on neuromuscular recovery, testosterone, cortisol, and match performance during a season of professional football. Front. Physiol. 2018, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, M.B.; Sarfaty, G.A.; Wilson, H.; Wayne Bardin, C.; Fishman, L.M. Metabolism of Testosterone and Related Steroids in Metastatic Interstitial Cell Carcinoma of the. J. Clin. Investig. 1966, 45, 1700–1709. [Google Scholar] [CrossRef]
- WADA. Prohibited List; World Anti-Doping Agency: Montreal, QC, Canada, 2017. [Google Scholar]
- Deshmukh, N.I.K.; Barker, J.; Petroczi, A.; Naughton, D.P. Detection of testosterone and epitestosterone in human hair using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 67–68, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Deshmukh, N.I.; Shah, I.; Zachar, G.; Szekely, A.D.; Petroczi, A.; Naughton, D.P. LC-MS/MS-Based Assay for Free and Deconjugated Testosterone and Epitestosterone in Rat Urine and Serum. J. Anal. Bioanal. Tech. 2014, s5. [Google Scholar] [CrossRef]
- Draper, A.J.; Madan, A.; Smith, K.; Parkinson, A. Development of a non-high pressure liquid chromatography assay to determine testosterone hydroxylase (CYP3A) activity in human liver microsomes. Drug Metab. Dispos. Biol. Fate Chem. 1998, 26, 299–304. [Google Scholar]
- Shimada, K.; Mitamura, K.; Higashi, T. Gas chromatography and high-performance liquid chromatography of natural steroids. J. Chromatogr. A 2001, 935, 141–172. [Google Scholar] [CrossRef]
- Gonzalo-Lumbreras, R.; Pimentel-Trapero, D.; Izquierdo-Hornillos, R. Development and method validation for testosterone and epitestosterone in human urine samples by liquid chromatography applications. J. Chromatogr. Sci. 2003, 41, 261–265. [Google Scholar] [CrossRef]
- Rauh, M. Steroid measurement with LC-MS/MS in pediatric endocrinology. Mol. Cell. Endocrinol. 2009, 301, 272–281. [Google Scholar] [CrossRef]
- Higashi, T.; Yamauchi, A.; Shimada, K. 2-Hydrazino-1-methylpyridine: A highly sensitive derivatization reagent for oxosteroids in liquid chromatography–electrospray ionization-mass spectrometry. J. Chromatogr. B 2005, 825, 214–222. [Google Scholar] [CrossRef]
- Büttler, R.M.; Peper, J.S.; Crone, E.A.; Lentjes, E.G.; Blankenstein, M.A.; Heijboer, A.C. Reference values for salivary testosterone in adolescent boys and girls determined using Isotope-Dilution Liquid-Chromatography Tandem Mass Spectrometry (ID-LC-MS/MS). Clin. Chim. Acta 2016, 456, 15–18. [Google Scholar] [CrossRef]
- Santa, T.; Al-Dirbashi, O.Y.; Fukushima, T. Derivatization reagents in liquid chromatography/electrospray ionization tandem mass spectrometry for biomedical analysis. Drug Discov. Ther. 2007, 1, 108–118. [Google Scholar]
- Lai, C.-C.; Tsai, C.-H.; Tsai, F.-J.; Lee, C.-C.; Lin, W.-D. Rapid monitoring assay of congenital adrenal hyperplasia with microbore high-performance liquid chromatography/electrospray ionization tandem mass spectrometry from dried blood spots. Rapid Commun. Mass Spectrom. 2001, 15, 2145–2151. [Google Scholar] [CrossRef]
- Quirke, J.M.E.; Adams, C.L.; Van Berkel, G.J. Chemical Derivatization for Electrospray Ionization Mass Spectrometry. 1. Alkyl Halides, Alcohols, Phenols, Thiols, and Amines. Anal. Chem. 1994, 66, 1302–1315. [Google Scholar] [CrossRef]
- Ostatníková, D.; Pastor, K.; Putz, Z.; Dohnányiová, M.; Mat’ašeje, A.; Hampl, R. Salivary testosterone levels in preadolescent children. BMC Pediatr. 2002, 2, 5. [Google Scholar]
- Morley, J.E.; Perry, H.M.; Patrick, P.; Dollbaum, C.M.; Kells, J.M. Validation of salivary testosterone as a screening test for male hypogonadism. Aging Male 2006, 9, 165–169. [Google Scholar] [CrossRef]
- Yasuda, M.; Honma, S.; Furuya, K.; Yoshii, T.; Kamiyama, Y.; Ide, H.; Muto, S.; Horie, S. Diagnostic significance of salivary testosterone measurement revisited: Using liquid chromatography/mass spectrometry and enzyme-linked immunosorbent assay. J. Men’s Health 2008, 5, 56–63. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Contreras, L.N.; Tumilasci, E.G.; Elbert, A.; Aguirre, E.C.; Aquilano, D.R.; Arregger, A.L. Salivary testosterone for the diagnosis of androgen deficiency in end-stage renal disease. Nephrol. Dial. Transplant. 2010, 26, 677–683. [Google Scholar] [CrossRef]
- Macdonald, P.R.; Owen, L.J.; Wu, F.C.; Macdowall, W.; Keevil, B.G. A liquid chromatography-tandem mass spectrometry method for salivary testosterone with adult male reference interval determination. Clin. Chem. 2011, 57, 774–775. [Google Scholar] [CrossRef][Green Version]
- Maya, J.; Marquez, P.; Peñailillo, L.; Contreras-Ferrat, A.; Deldicque, L.; Zbinden-Foncea, H. Salivary Biomarker Responses to Two Final Matches in Women’s Professional Football. J. Sports Sci. Med. 2016, 15, 365–371. [Google Scholar]
- Clifton, S.; Macdowall, W.; Copas, A.J.; Tanton, C.; Keevil, B.G.; Lee, D.M.; Mitchell, K.R.; Field, N.; Sonnenberg, P.; Bancroft, J.; et al. Salivary testosterone levels and health status in men and women in the British general population: Findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). J. Clin. Endocrinol. Metab. 2016, 101, jc20161669. [Google Scholar] [CrossRef]
- Food and Drug Administration. Available online: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 9 April 2019).
- Hall, T.G.; Smukste, I.; Bresciano, K.R.; Wang, Y.; McKearn, D.; Savage, R.E. Identifying and Overcoming Matrix Effects in Drug Discovery and Development. Intech Eur. 2012, 18, 389–420. [Google Scholar]
- Shou, W.; Naidong, W. Simple means to alleviate sensitivity loss by trifluoroacetic acid (TFA) mobile phases in the hydrophilic interaction chromatography–electrospray tandem mass spectrometric (HILIC–ESI/MS/MS) bioanalysis of basic compounds. J. Chromatogr. B 2005, 825, 186–192. [Google Scholar] [CrossRef]
- Choo, R.E.; Huestis, M.A. Oral fluid as a diagnostic tool. Clin. Chem. Lab. Med. 2004, 42, 1273–1287. [Google Scholar] [CrossRef]
- Gröschl, M.; Köhler, H.; Topf, H.G.; Rupprecht, T.; Rauh, M. Evaluation of saliva collection devices for the analysis of steroids, peptides and therapeutic drugs. J. Pharm. Biomed. Anal. 2008, 47, 478–486. [Google Scholar] [CrossRef]
- Gröschl, M.; Rauh, M. Influence of commercial collection devices for saliva on the reliability of salivary steroids analysis. Steroids 2006, 71, 1097–1100. [Google Scholar] [CrossRef]
- Sample Prep—SPE Method Development Tool—CHROMacademy. Available online: https://www.chromacademy.com/chromatography-SPE-Meth-Dev.html (accessed on 9 April 2019).
- Cormack, S.J.; Newton, R.U.; McGuigan, M.R.; Cormie, P. Neuromuscular and endocrine responses of elite players during an Australian rules football season. Int. J. Sports Physiol. Perform. 2008, 3, 439–453. [Google Scholar] [CrossRef]
- Howe, C.; Alshehri, A.; Muggeridge, D.; Mullen, A.; Boyd, M.; Spendiff, O.; Moir, H.; Watson, D. Untargeted Metabolomics Profiling of an 80.5 km Simulated Treadmill Ultramarathon. Metabolites 2018, 8, 14. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).