Abstract
Depression is a common disease affecting more than 300 million people worldwide. Since Lippia sidoides has shown central nervous system effects in previous works, we aimed to investigate the effect of L. sidoides essential oil and its major compound, thymol on a corticosterone-induced depression model in mice. Male mice (20–25 g) received corticosterone (20 mg/kg, subcutaneously), once a day for 22 days. From the 16th day on, mice were grouped to receive either corticosterone or L. sidoides essential oil (100 and 200 mg/kg), or thymol (25 and 50 mg/kg) or fluoxetine (35 mg/kg) by gavage. The forced swimming test, tail suspension, open field, elevated plus maze and sucrose preference tests were performed from the 19th to 22nd day. Data were analyzed by ANOVA followed by the Student-Newman-Keuls as a post hoc test and the results were considered significant when p < 0.05. It was shown that L. sidoides essential oil, thymol and fluoxetine decreased the immobility time in the tail suspension and forced swimming tests and none of these altered locomotor activity in the open field test. However, the drugs increased the amount of grooming. In the elevated plus maze, all drugs increased the number of entries and the time of permanence in the open arms. In the sucrose preference test, the L. sidoides essential oil, thymol and fluoxetine reversed anhedonia. These results suggest that the thymol and L. sidoides essential oil have an antidepressant-like effect, similar to fluoxetine. However, future studies should be encouraged to enhance understanding of the effects of essential oil and thymol for the treatment of depression.
1. Introduction
According to the World Health Organization (WHO), depression is the leading cause of mental illness and disability affecting more than 300 million people worldwide with an increase of more than 18% between 2005 and 2015 [1].
Depression is a heterogeneous multifactorial disease, with an unclear etiology and pathophysiology [2]. The main symptoms of depression are sad mood, self-deprecation, lack of concentration, energy and sleep deficits, psychosomatic disorders, anhedonia and suicidal thoughts [3,4]. The pathophysiology of depression remains poorly understood and is determined by genetic, epigenetic and physiological factors [3,4,5].
The standard form of treatment for depression generates several side effects, which often require the patient to discontinue using the drug [6]. In order to treat depression successfully, antidepressants are usually prescribed for at least four to six months. There may be a latency period of more than 3 weeks before their effects show, which requires understanding, interest and habituation to their use by patients [7]. However, it is estimated that 30–50% of depressed patients do not fully recover with currently available drug therapy [8].
Although drug treatment has proven effectiveness for depression, its effect is not always sufficient to prevent relapse in some patients [7]. As a result, drugs produced from medicinal plants can improve the antidepressants’ actions and decrease side effects in refractory patients [8]. Also, medicinal plants are easily found in nature and present a variety of chemical compounds in their extracts or essential oil which show antidepressant-like actions, such as Hypericum perforatum L. (St. John’s wort) which is widely used as herbal remedy for the treatment of mild to moderate depressive episodes [9]. Similarly, essential oils, such as the ones extracted from Lippia (Verbenaceae) species have shown pharmacological actions such as antimicrobial, antiparasitic, anesthetic, analgesic, anti-inflammatory and antitumor [10,11,12,13,14]. One of its species, Lippia sidoides Cham, known as rosemary, peppermint, rosemary-bravo, estrepa horse and rosemary-large, is popularly used to treat infections [15,16]. L. sidoides essential oil (LSEO) produces thymol monoterpene and its isomer, carvacrol; both have a powerful action in animals [17]. Carvacrol has been reported as showing anxiolytic [18] and antidepressant-like [19] effects and thymol has shown antimicrobial, larvicide, antioxidant, anti-inflammatory and analgesic activities [20,21,22,23].
Data has shown that several monoterpenes extracted from essential oils act on the central nervous system (CNS) [24]. Since carvacrol has presented anxiolytic and antidepressant-like effects in previous work, it seems relevant to investigate LSEO and thymol, the carvacrol isomer and also the major compound of LSEO, in animal models of depression and anxiety.
Studies on rodents regularly administered with corticosterone (CORT) have shown that this mimics the chronic stress-associated dysregulation of the hypothalamic-pituitary-adrenal axis [25,26]. Other groups have previously utilized this model to evaluate potential antidepressant candidates and have validated its application to study the neurobehavioral alterations as well as the pathophysiological changes associated with depression [27].
In this context, we aimed to evaluate the antidepressant efficacy of Lippia sidoides Cham. (Verbenaceae) essential oil and its major compound, thymol, against a mice model of CORT-induced depression. Our work is unprecedented, as in surveys conducted through websites, no other study using either Lippia sidoides essential oil or thymol in corticosterone-induced depression model was found.
2. Materials and Methods
2.1. Animals
Swiss mice, males, weighing 20–25 g, from INTA University Center—UNINTA animal house were used. The animals were kept in polypropylene cages with an alternating 12 h light/dark cycle, with lights on at 7 a.m., and they received specific balanced rodent chow and water ad libitum. The experimental protocols followed the ethical principles for animal experimentation adopted by the Brazilian College of Animal Experimentation (COBEA) and were evaluated by the Ethics Committee on Animal Use of UNINTA under protocol 2014.02.003-P on 16 June 2014.
2.2. Essential Oil of Lippia Sidoides Cham (Verbenaceae)—LSEO
2.2.1. Collection of Plant Material
The aerial parts (leaves and branches) of Lippia sidoides were collected in July 2014 from the Garden of Medicinal Plants, Farm of UNINTA, City of Cariré, Ceará, Brazil (3°49′51 82′′ S and 40°24′37 85′′ W). The plant was classified and a voucher specimen was deposited in the Francisco José de Abreu Matos Herbarium of the State University of Acaraú Valley (UVA) under the number: HUVA 17480.
2.2.2. Oil Extraction and Chemical Analysis
Approximately 1 kg of plant material (shoots) from the species Lippia sidoides Cham (rosemary pepper) was properly packaged and transported to INTA University Center, UNINTA experimental laboratory (NUBEM). The material was subjected to the extraction process using the hydro-distillation Cleavenger modified method for 3 h. The essential oil was dried over anhydrous sodium sulfate, maintained and kept under refrigeration until analysis of the outputs. The content of the essential oils was determined by the masses of oil in the analytical balance and expressed as a percentage weight/weight (1 g oil per 100 g of fresh weight). The extract was retained and kept in a refrigerator until analysis of the outputs. The chemical composition of the essential oil was investigated and described in previous work [28].
2.2.3. Preparation of Drugs
All drugs were administered in a volume of 0.1 mL/10 g body weight. CORT (20 mg/kg/day, subcutaneous (SC), Sigma®, São Paulo, SP, Brazil) was dissolved in a saline solution containing 0.2% dimethyl sulfoxide and 1% Tween-80 (Sigma®). Flu (35 mg/kg/day, per os—p.o., EMS®, Hortolândia, SP, Brazil) was diluted in distilled water. L. sidoides essencial oil (100 and 200 mg/kg/day, p.o.) was dissolved in 3% Tween 80 and diluted in distilled water. Thymol (25 and 50 mg/kg/day, p.o., Sigma®) was dissolved in 2% Tween 80 using distilled water as vehicle.
2.3. Experimental Protocol
For each set of experiments, thirty five animals were divided into 7 groups of 5 animals each: these included a control group (received saline water (s.c) + distilled water (p.o.)), corticosterone group (CORT) (CORT (s.c.) + distilled water (p.o.)), fluoxetine group (CORT (s.c.) + FLU 35 mg/kg (p.o.)), two thymol groups (TML) with doses of (CORT (s.c.) + TML 25 mg/kg (p.o.)) and (CORT (s.c.) + TML 50 mg/kg (p.o.)) and two Lippia sidoides essential oil (LSEO) groups at doses of (CORT (s.c.) + LSEO 100 mg/kg (p.o.)) and (CORT (s.c.) + LSEO 200 mg/kg, p.o.)). As the protocol has been performed twice, each group presented 10 animals. CORT (20 mg/kg, s.c.) was administered once daily, at 8 a.m, for 22 days to induce depressive behavior in mice. The experiments were performed from 9 a.m. to 3 p.m. On the first day of the experimental design, 30 mice (from 6 groups) received CORT (20 mg/kg/day) and 5 of them received vehicle. After 60 min, the forced swimming test (FST) was conducted for 5 min. From the 2nd day to the 14th day, mice were treated as described but were not involved in any experiments. On the 15th day, 60 min after treatment mice were again subjected to FST for 5 min.
From the 16th to 22nd days mice received respective drugs such as, vehicle, LSEO 100, LSEO 200, TML 25, TML 50 or FLU 35 after 30 min of CORT or vehicle administration. The experiments were conducted 60 min after the last treatment on the 19th, 20th, 21st and 22nd days of treatment. The sucrose preference test (SPF) was performed on the 19th, 20th, 21st days, the FST on the 20th day, the TST and OFT on the 21st day and PMT on the 22nd day. After all experiments had been finished, mice were sacrificed to obtain brain areas (Figure 1).
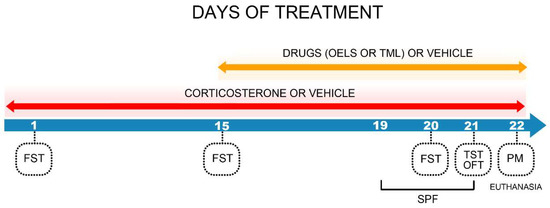
Figure 1.
Experimental design. Numbers represent the days of treatment and setting experiments. FST = forced swim test, TST = tail suspension test, OFT = open field test, PM = elevated plus maze test, SPF = sucrose preference test, OELS = Lippia sidoides essential oil, TML = thymol.
2.4. Behavioral Tests
2.4.1. Forced Swim Test
Sixty minutes after treatment, mice were individually forced to swim in an open cylinder container (diameter 15 cm; height: 40 cm) filled with 20 cm of water at 24 ± 1°C. The amount of time in which the mouse remained immobile during a 5-min period was recorded. Immobility was defined as the animal floating in the water without struggling and making only slight movements to keep its head above the water [29].
2.4.2. Open Field Test
The animals were placed on an apparatus made of acrylic (transparent black walls and floor 30 × 30 × 15 cm) and divided into 9 equal quadrants. Sixty minutes after treatment by gavage, the animals were placed, one at a time, in the center of the open field area and they were observed for 5 min. The parameters recorded were the number of crossings (spontaneous locomotor activity), the number of rearing behaviors, and the number of grooming behaviors [30].
2.4.3. Tail Suspension Test
Sixty minutes after the last treatment by gavage, each mouse was suspended by the tail on the edge of a shelf placed 58 cm above the table top. The mouse was secured in place by adhesive tape placed 1 cm from the tip of the tail. The time that the mouse remained immobile, up to 6 min was recorded [31].
2.4.4. Elevated Plus Maze Test
Sixty minutes after treatment by gavage, the animals were placed one at a time, with heads facing one of the closed arms in the center of an elevated plus maze consisting of two opposing open arms (30 × 5 cm) and two closed ones (30 × 25 × 5 cm), also opposing, crosswise. The parameters observed were the number of entries and time spent either in the open or in the closed arms [32]. For statistical analysis, the parameters considered were those related to the open arms; number of entries in the open arms (NEOA), time spent in the open arms (TSOA) and their percentage, percentage of number of entries in the open arms (PEOA) and the percentage of time spent in the open arms (PTOA).
2.4.5. Sucrose Preference Test
First, a sucrose solution was prepared at 2% to 3 L of water using 60 g of sucrose. This solution was divided into 14 bottles of 200 mL of sucrose solution at all. On the first day of the test two bottles with the solution were placed in each cage. On the second day, after 18 h, these bottles were removed, weighed and their volumes measured. After that, a bottle with sucrose solution, and another bottle with water, each containing 200 mL, were placed in the cage at the same time as the first day. On the third day, the bottles were removed and weighed to measure their respective volumes [33]. The sucrose preference was measured by the following equation:
2.5. Statistical Analysis
The statistical analysis was performed using the software Graph Pad Prism version 5.02 for Windows, Graph Pad Software, San Diego, CA, USA (Copyright© 1992–2009 Graph Pad Software Inc.). The results, which followed a parametric distribution were analyzed by one-way ANOVA followed by the Student-Newman-Keuls test as a post hoc test, and the values were represented by mean ± standard error of mean (SEM). Data were considered significant when* p < 0.05, ** p < 0.001, *** p < 0.0001.
3. Results
3.1. Forced Swim Test
Immobility Time
Animals treated with CORT showed an increase in immobility time on the 14th day (75.50 ± 18.70, n = 10) and the 20th day (105.50 ± 14.91, n = 10) as compared to treatment in the 1st day (13 ± 15.41, n = 10) (Figure 2).
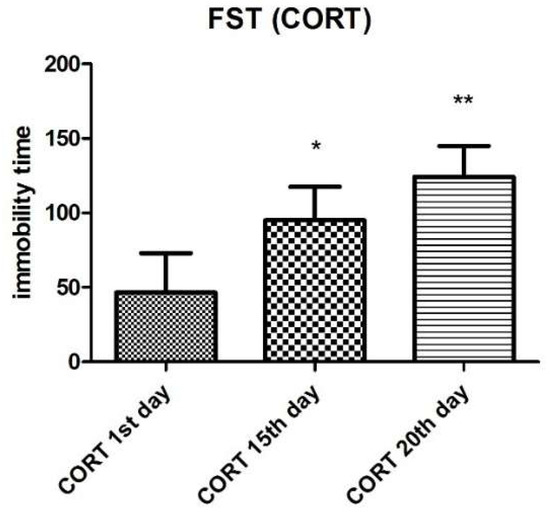
Figure 2.
CORT immobility time in the forced swimming test (FST). Values are expressed as mean ± SEM (n = 10). Statistical analysis was determined by one-way ANOVA followed by the Student-Newman-Keuls test. Significant values: * p < 0.05, ** p < 0.001 vs. CORT 1st day.
Either LSEO 100 mg/kg (52.82 ± 13.14, n = 10) or LSEO 200 mg/kg (32.64 ± 6.66, n =10) decreased the immobility time in comparison to the CORT group (135.6 ± 13.45, n =10) (Figure 3a). Similarly, TML associated with CORT, at both doses (TML 25 mg/kg = 61.70 ± 9.82, n = 10; TML 50 mg/kg = 51.78 ± 11.49, n = 10) also decreased the immobility time compared to CORT (135.6 ± 13.45, n = 10) (Figure 3b). FLU 35 mg/kg as standard, decreased the same parameter (69.82 ± 12.91, n = 10) (Figure 3a,b).
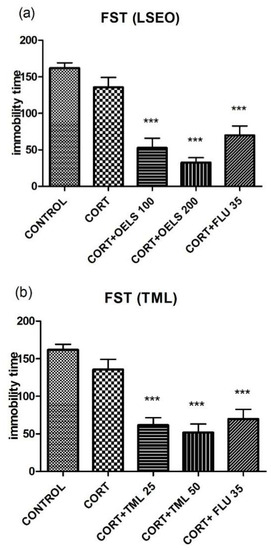
Figure 3.
Effects of repeated administration of (a) LSEO (100 and 200 mg/kg; p.o.) and (b) TML (25 and 50 mg/kg; p.o.) in animals treated with CORT (22 days, 20 mg/kg, s.c.) on the immobility time, in seconds (s), on the FST. Values are expressed as mean ± SEM (n = 10). Statistical analysis was determined by one-way ANOVA followed by the Student-Newman-Keuls test. Significant values: *** p < 0.0001 vs. CORT. LSEO Lippia sidoides essential oil; TML = thymol; CORT = corticosterone; FST = forced swimming test.
3.2. Tail Suspension Test
Immobility Time
LSEO at both doses, 100 mg/kg (40.0 ± 10.31, n = 10) and 200 mg/kg (42.73 ± 9 57, n = 10), decreased the immobility time compared to CORT (91.83 ± 4.73, n = 10) (Figure 4a). Regarding TML, the higher dose (TML 50 mg/kg = 56.50 ± 9.81, n = 10) but not the lower one (TML 25 mg/kg = 68.92 ± 12.15, n = 10) decreased this parameter as compared to CORT (91.83 ± 4.73, n = 10) (Figure 4b). FLU 35 mg/kg also decreased this parameter (43.4 ± 9.43, n = 10).
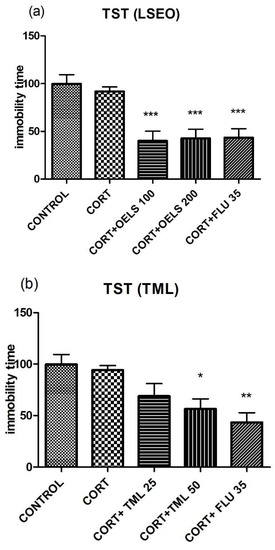
Figure 4.
Effects of repeated administration of (a) LSEO (100 and 200 mg/kg; p.o.) and (b) TML (25 and 50 mg/kg; p.o.) in animals treated with CORT (22 days, 20 mg/kg, s.c.) on the immobility time, in seconds (s), on the TST. Values are expressed as the mean ± SEM (n = 10). Statistical analysis was determined by one-way ANOVA followed by the Student-Newman-Keuls test. Significant values: * p < 0.05, ** p < 0,001, *** p < 0.0001 vs. CORT. LSEO = Lippia sidoides essential oil; TML = thymol; CORT = corticosterone; TST = tail suspension test.
3.3. Open Field
3.3.1. Number of Crossings
Both doses of LSEO, 100 mg/kg (56.13 ± 5.20, n = 10) and 200 mg/kg (49.67 ± 6.92, n = 10), were unable to alter the number of crossings compared with CORT (50 ± 4.11, n = 10) (Figure 5a). Similarly, TML either with the dose of 25 mg/kg (46.80 ± 5.53, n = 10) or with the dose of 50 mg/kg (55.64 ± 4.57, n = 10) did not alter this parameter compared with CORT (50 ± 4.11, n = 10) (Figure 5b). Also, the same pattern of behavior was shown for FLU (49.77 ± 6.16, n = 10).
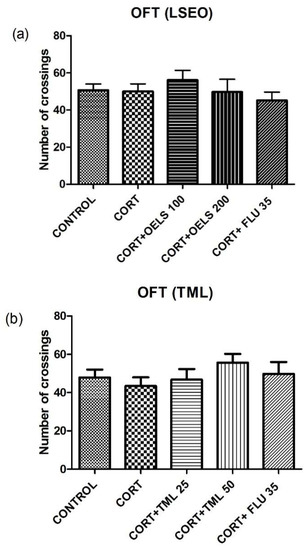
Figure 5.
Effects of repeated administration of (a) LSEO (100 and 200 mg/kg; p.o.) and (b) TML (25 and 50 mg/kg; p.o.) in animals treated with CORT (22 days, 20 mg/kg, s.c.) on the number of crossings on the OFT. Values are expressed as the mean ± SEM (n = 10). Statistical analysis was determined by one-way ANOVA followed by the Student-Newman-Keuls test. There are no significant values vs. CORT. LSEO = Lippia sidoides essential oil; TML = thymol; CORT = corticosterone; OFT = open field test.
3.3.2. Number of Rearing
Figure 6 shows that neither LSEO 100 mg/kg (12.77 ± 1.54, n = 10); LSEO 200 mg/kg (12.29 ± 2.68, n = 10), at both doses, nor TML 25 mg/kg (9.07 ± 1.73, n = 10); TML 50 mg/kg (18.60 ± 2.63, n = 10), at both doses, have changed the number of rearing as compared with CORT (12.47 ± 2.37, n = 10). Following the same pattern, FLU 35 mg/kg did not alter the parameter (9.93 ± 2.23, n = 10).
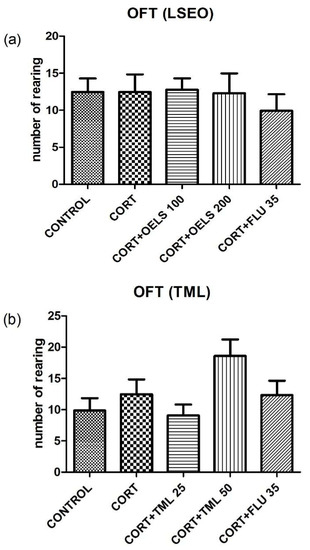
Figure 6.
Effects of repeated administration of (a) LSEO (100 and 200 mg/kg; p.o.) and (b) TML (25 and 50 mg/kg; p.o.) in animals treated with CORT (22 days, 20 mg/kg, s.c.) on the number of rearings on the OFT. Values are expressed as the mean ± SEM (n = 10). Statistical analysis was determined by one-way ANOVA followed by the Student-Newman-Keuls test. There are no significant values vs. CORT. LSEO = Lippia sidoides essential oil; TML = thymol; CORT = corticosterone; OFT = open field test.
3.3.3. Number of Grooming Behaviors
LSEO at both doses, 100 mg/kg (9.61 ± 1.92, n = 10) and 200 mg/kg (8.81 ± 1.76, n = 10), increased the amount of grooming in the open field test compared to CORT (2.08 ± 0.57, n =10) (Figure 7a). On the other hand, TML did not alter this parameter (TML 25 mg/kg = 3.21 ± 0.43, n = 10; TML 50 mg/kg = 2.92 ± 0.63, n = 10) compared to CORT (2.08 ± 0.57, n = 10) (Figure 7b). FLU 35 mg/kg, was the same as LSEO, it increased this parameter (8.45 ± 1.59, n = 10).
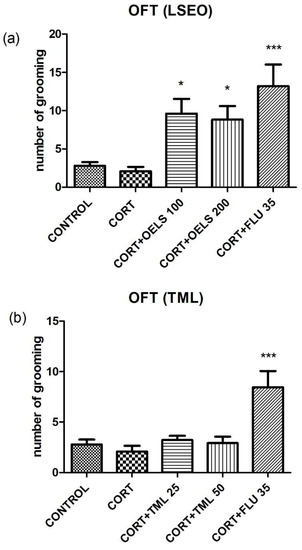
Figure 7.
Effects of repeated administration of (a) LSEO (100 and 200 mg/kg; p.o.) and (b) TML (25 and 50 mg/kg; p.o.) in animals treated with CORT (22 days, 20 mg/kg, s.c.) on the number of grooming behaviors on the OFT. Values are expressed as the mean ± SEM (n = 10). Statistical analysis was determined by one-way ANOVA followed by Student-Newman-Keuls test. Significant values: * p < 0.05, *** p < 0.0001 vs. CORT. LSEO = Lippia sidoides essential oil; TML = thymol; CORT = corticosterone; OFT = open field test.
3.4. Elevated Plus Maze Test (PM)
The parameters used to perform the statistical analysis were: number of entries in the open arms (NEOA), the percentage of entries in the open arms (PEOA), the time spent in the open arms (TSOA) and percentage of time spent in the open arms (PTBA).
3.4.1. NEOA and PEOA
LSEO at both doses, 100 mg/kg (9.33 ± 1.69, n = 10) and 200 mg/kg (9.30 ± 1.59, n = 10), increased NEOA in the plus maze test compared to CORT (2.26 ± 0.43, n = 10) (Figure 8a). Likewise, TML also increased this parameter at both doses (TML 25 mg/kg = 4.41 ± 0.84, n =10; TML 50 mg/kg = 4.66 ± 0.61, n = 10) as compared to CORT (2.26 ± 0.43, n = 10) (Figure 8b). On the other hand, FLU 35 mg/kg did not alter this parameter either in Figure 8a (4.33 ± 1.21, n = 10) or in Figure 8b (2.33 ± 0.65, n = 10).
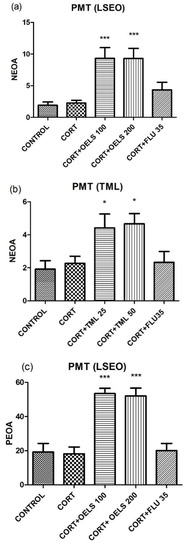
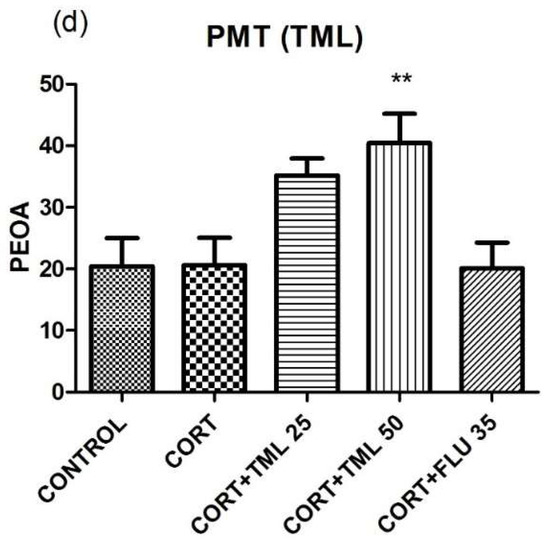
Figure 8.
Effects of repeated administration of LSEO (100 and 200 mg/kg; p.o.) in the NEOA (a) and PEOA (c) and TML (25 and 50 mg/kg; p.o.) in the NEOA (b) and PEOA (d) on animals treated with CORT (22 days, 20 mg/kg, s.c.) in the plus maze test. Values are expressed as the mean ± SEM (n = 10). Statistical analysis was determined by one-way ANOVA followed by the Student-Newman-Keuls test. Significant values: * p < 0.05, ** p < 0.001, *** p < 0.0001 vs. CORT. LSEO = Lippia sidoides essential oil; TML = thymol; CORT = corticosterone; PMT = plus maze test.
Regarding PEOA, Figure 8c shows that LSEO increased it (100 mg/kg = 53.45 ± 3.05, n = 10; 200 mg/kg = 52.08 ± 4.52, n = 10), at both doses, compared to CORT (18.14 ± 4.05, n = 10). However, TML at the dose of 50 mg/kg (40.42 ± 4.76, n = 10) but not at the dose of 25 mg/kg (35.19 ± 2.73, n = 10), increased PEOA as compared to CORT (20.59 ± 4.46, n = 10). FLU 35 mg/kg did not alter this parameter (20.10 ± 4.18, n = 10) (Figure 8d).
3.4.2. TSOA and PTOA
LSEO at both doses, 100 mg/kg (95.08 ± 14.72, n = 10) and 200 mg/kg (181.6 ± 21.57, n = 10), increased TSOA in the plus maze test compared to CORT (21.27 ± 6.48, n = 10) (Figure 9a). Likewise, TML also increased this parameter at both doses (TML 25 mg/kg = 62.70 ± 9.60, n = 10; TML 50 mg/kg = 91.10 ± 18.06, n = 10) as related to CORT (18.00 ± 5.87, n = 10) (Figure 9b). However, FLU 35 mg/kg did not alter this parameter either in Figure 9a (29.92 ± 10.83, n = 10) or in Figure 9b (30.25 ± 10.75, n = 10).
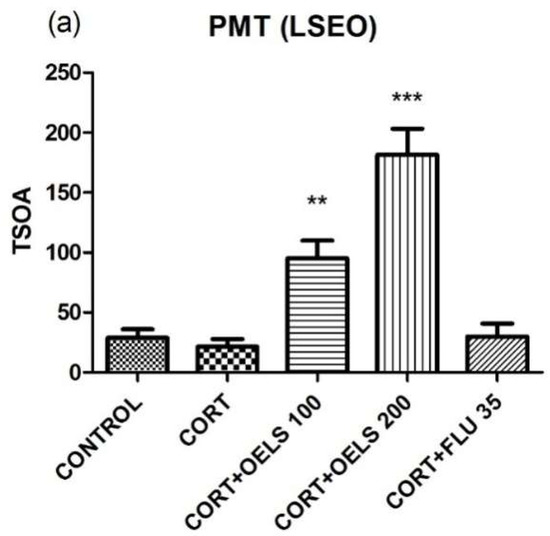
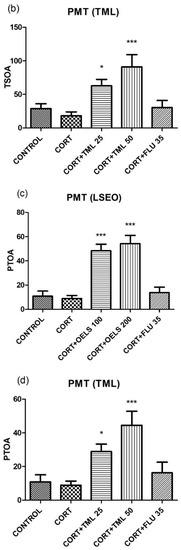
Figure 9.
Effects of repeated administration of LSEO (100 and 200 mg/kg; p.o.) on the TSOA (a) and PTOA (c) and TML (25 and 50 mg/kg; p.o.) on the TSOA (b) and PTOA (d) in animals treated with CORT (22 days, 20 mg/kg, s.c.) in the plus maze test. Values are expressed as the mean ± SEM (n = 10). Statistical analysis was determined by one-way ANOVA followed by the Student-Newman-Keuls test. Significant values: * p < 0.05, ** p < 0.001, *** p < 0.0001 vs. CORT. LSEO = Lippia sidoides essential oil; TML = thymol; CORT = corticosterone; PMT = plus maze test.
Figure 9c shows that LSEO increased PTOA (100 mg/kg = 48.34 ± 5.40, n = 10; 200 mg/kg = 54.20 ± 6.81, n = 10), at both doses, compared to CORT (8.87 ± 2.48, n = 10). Also, an increase in PTOA was observed with TML at both doses (25 mg/kg = 28.89 ± 4.40, n = 10; 50 mg/kg = 44.54 ± 8.29, n = 10) when related to CORT (8.87 ± 2.48, n = 10). FLU 35 mg/kg did not alter this parameter (20.10 ± 4.18, n = 10) (Figure 9d). FLU 35 mg/kg did not alter this parameter either in Figure 9c (13.83 ± 4.53, n = 10) or in Figure 9d (16.28 ± 6.30, n = 10).
3.5. Sucrose Preference Test
CORT (66.68 ± 0.007, n = 10) decreased the sucrose preference compared to the control as shown in Figure 10a (76.94 ± 2.56, n = 10) and Figure 10b (82.43 ± 2.67, n = 10). In addition, LSEO (100 mg/kg = 75.02 ± 1.66, n = 10; 200 mg/kg = 87.97 ± 0.98, n = 10), at both doses (Figure 10a), and TML (25 mg/kg = 89.69 ± 1.68, n = 10; 50 mg/kg = 82.01 ± 1.83, n = 10), at both doses (Figure 10b) were able to revert CORT’s effect (66.68 ± 0.007, n = 10). Likewise, FLU 35 mg/kg (80.13 ± 2.46, n = 10) also reverted CORT’s effect.
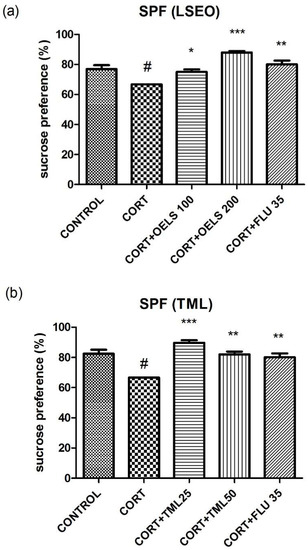
Figure 10.
Effects of repeated administration of (a) LSEO (100 and 200 mg/kg; p.o.) and (b) TML (25 and 50 mg/kg; p.o.) in animals treated with CORT (22 days, 20 mg/kg, s.c.) on the sucrose preference (%). Values are expressed as the mean ± SEM (n =10). Statistical analysis was determined by one-way ANOVA followed by the Student-Newman-Keuls test. Significant values: * p < 0.05, ** p < 0.001, *** p < 0.0001 vs. CORT; # p < 0.05 vs. control. LSEO = Lippia sidoides essential oil; TML = thymol; CORT = corticosterone; SPF = sucrose preference test.
4. Discussion
This study is the first to investigate the antidepressant-like effects of LSEO and TML in the CORT-induced depression model in mice. It has been reported that repeated administration with high doses (20 mg/kg/day), but not low, of CORT produces depressant-like behavior in rodents [34,35]. The two most widely used animal models for screening new antidepressant drugs are the forced swimming (TNF) and tail suspension (TSC) tests. Several studies using naturally occurring substances in order to investigate possible antidepressant effects [36,37,38], have shown a reduction in immobility time in the TST and FST.
In this study, CORT increased immobility time on the 14th day and 20th day of treatment in the FST, showing depressant behavior. This result suggests that CORT administered repeatedly induces depressive behavior in animals. Thus, our results are consistent with other studies [39] where the mice receiving regular CORT treatment showed significant behavioral despair, as shown by significantly increased immobility time in the FST, which validates the model used for investigating the antidepressant-like effects of LSEO and TML.
Both doses of LSEO and TML associated with CORT for 22 days were able to reverse the CORT-induced immobility time in the FST and TST, suggesting an antidepressant-like effect of the two drugs in question. This effect is similar to a study in which resveratrol, a polyphenol found in the Polygonum cuspidatum, a Japanese plant that has neuroprotective activity, when administered daily was able to decrease the immobility time in the FST and TST, reversing the depressive action of CORT [40].
In our present study it was observed that LSEO at both doses associated with CORT were able to decrease the immobility time of mice in the TST. However, only the highest dose of CORT associated with TML was able to decrease the immobility time in this test. This is similar to the study by [41] which states that repeated injections of CORT are able to increase the immobility time in the TST in mice. It has been reported in [42] that after 21 consecutive days of treatment with magnolol, which is a compound that acts on GABAA receptors of rats, and FLU (20 mg/kg) the duration of immobility in the TST decreased compared to the CORT group. These studies support our findings, allowing us to state that LSEO and TML have antidepressant actions, as well as FLU.
The TST and FST exhibit acute sensitivity and specificity for antidepressants, and also, these tests are easy to perform and provide reliability [43]. Both animal tests provide a stressful environment, where mice cannot escape and after an initial period of struggling, the animals become motionless, which mimics a depressed and desperate behavior [44]. Also, the TST has been recognized as having great importance due to its major sensitivity in detecting selective serotonin reuptake inhibitor (SSRI) type antidepressants [45]. Since both LSEO and TML effectively showed antidepressant-like behavior in the TST, as did FLU, we suggest that these drugs may have similar actions to that class of drugs. However, further studies are needed to investigate their mechanism of action.
It is known that some drugs with psychostimulant activity may have a false positive effect in the TST and FST [46]. Thus, in order to rule out the possibility that the reduction in the immobility time elicited by LSEO and TML is due to enhancement in locomotor activity, the OFT was conducted. Neither LSEO nor TML were able to change the exploratory activity of animals, as seen in the number of crossings parameter, similar to FLU. Therefore, these results confirm the antidepressant effect of LSEO and TML, observed in the FST and TST, since it excludes the possibility of these drugs having a psychostimulant effect [46]. Besides, this pattern of behavior is similar to the effects of antidepressants, such as FLU. This result is confirmed by a study of Pelargonium roseum essential oil, which contains monoterpenes, and is similar to the essential oil of Lippia sidoides Cham. The locomotor activity of the animals did not change, which also demonstrates its antidepressant effect [47].
Rearing is the animals’ ability to stand on its hind legs to explore the environment vertically and it is related to anxiety behaviors [48]. In our protocol, it was observed that neither LSEO nor TML doses were able to change this parameter. These results are similar to those found in the spontaneous locomotor activity parameter which determined horizontal exploratory activity, as was observed by the number of crossings. Both results help to confirm LSEO and TML antidepressant-like activity by eliminating the psychostimulant effect.
Grooming is a behavior that develops in a cerebrospinal flow direction and consists of several steps [49]. It is considered a sensitive measure of stress and anxiety behavior when associated with an enhancement in the OFT [49]. Grooming is related to an anxiety behavior that can be mitigated with anxiolytic drugs and thus reduce this parameter, and may be potentiated by anxiogenic agents, which tend to increase this parameter [50]. In the present study, it was observed that both doses of LSEO associated with CORT increased the number of grooming behaviors, but none of the TML doses associated with CORT were able to do this. This effect suggests LSEO, but not TML might present anxiogenic effects. In order to clarify the anxiety profile of LSEO and TML, the PMT was conducted, because it is more sensitive and specific for the detection of drugs with benzodiazepine-like anxiolytic action than the open field test.
All PMT parameters were enhanced by either LSEO or TML but not by FLU, which was used as an antidepressant standard. This effect can be explained because the PMT is specific to benzodiazepine-like drugs, not to SSRIs. TML has been described as a GABAA agonist opening Cl-channels, resembling the anesthetic agent propofol (2,6-isopropyl-phenol) [51]. These findings confirm the effects of LSEO and TML in the PMT, which is more specific for anxiolytic drugs that act by activating the GABAA receptor, such as benzodiazepines. Thus, this effect can be better analyzed by this model than the open field test [18]. Hence, we suggest that LSEO and TML present, in addition to the antidepressant effect already described, an anxiolytic effect in animals. This is similar to a study carried out by [52], using a substance of natural origin, the monoterpene 1,4-cineol, which presented a potential anxiolytic effect in the PMT test.
The sucrose preference test measures a behavior similar to anhedonia, defined as the inability to perform pleasurable activities, a common symptom in depressed patients and which may be related to depression models in rodents [53]. In the present study, it was observed that both LSEO and TML doses associated with CORT increased the animals’ sucrose preference, indicating a behavioral pattern reversal of anhedonia, in the same way that FLU did. It can be reported that animals subjected to chronic stress with CORT showed a decrease in sucrose preference when compared with control animals. Our findings are similar to a study conducted for two weeks with geniposide, a monoterpene, in which it was able to reverse sucrose preference [54] showing an anti-anhedonic effect. Hence, we suggest that LSEO and TML present the same effect.
In conclusion, our data suggest that LSEO and TML have an antidepressant-like effect, due to the decrease in immobility time in the FST and TST without altering the number of crossings and rearings, thus, discarding the psychostimulant effect. In addition, LSEO and TML reversed two very common symptoms related to depressive behavior, including anhedonia in the sucrose preference test, and anxiety in the PMT, a specific model for researching anxiolytic drugs. The main limitation of this work is that we can only assume the behavioral effects of the drugs tested but we cannot suggest how they act to provide the antidepressant-like effects. Despite those limitations, the results found provide strong evidence for their antidepressant-like effects. Therefore, many more studies can follow this one to investigate the LSEO and TML mechanisms of action; thus, contributing scientifically with new perspectives on the treatment of depression.
Author Contributions
The conceptualization was developed by C.T.V.d.M., T.S.d.M. and M.I.L. Methodology was carried out by M.S.R.P., F.R.C., N.A.C. and M.J.A. L.F.R.C. contributed with Software. Validation was conducted by C.T.V.d.M., R.M.P.S. and M.I.L. The formal analysis was performed by C.T.V.d.M. and M.S.R.P. The Investigation was conducted by T.S.d.M., M.S.R.P. and C.T.V.d.M. The Resources were provided by A.A.d.N. and F.E.A.C.J. Data curation was conducted by T.S.d.M. and M.I.L. The writing-original draft preparation was elaborated by M.S.R.P. The Writing-Review & Editing was carried out by C.T.V.d.M. T.S.d.M. and M.I.L. contributed with visualization. The supervision, project administration and funding acquisition was conducted by C.T.V.d.M.
Funding
This work was financially supported by Fundação Cearense de Apoio a Pesquisa (FUNCAP), Fortaleza, CE, 60822-130, Brazil.
Acknowledgments
FUNCAP for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (WHO). Depression: Fact Sheet No. 369. October 2017. Available online: http://www.who.int/mediacentre/factsheets/fs369/ (accessed on 17 May 2017).
- Gerhard, D.M.; Wohleb, E.S.; Duman, R.S. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov. Today 2016, 21, 454–464. [Google Scholar] [CrossRef] [PubMed]
- American Association of Suicidology. Depression and Suicide Risk. 2013. Available online: https://www.suicidology.org/portals/14/docs/resources/factsheets/2011/depressionsuicide2014.pdf (accessed on 6 June 2017).
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 16, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Martins, F. Avaliação da Eficácia do Tratamento Homeopático na Depressão: Umaanálise da Literatura. Bachelor’s Thesis, Universidade de Brasília, Ceilândia, Brasília, Brazil, 2016. [Google Scholar]
- Souza, M.S.F.; Koppittke, L. Adesãoaotratamento com Psicofármacos: Fatores de Proteção e Motivos de Não Adesão ao Tratamento Farmacológico. Rev. APS 2016, 19, 361–369. [Google Scholar]
- Afradique, M.C. Intervenção em pacientes adultos com Depressão e inadequado de Antidepressivos. Bachelor’s Thesis, Universidade Aberta do SUS, Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Heinrich, M.; Lorenz, P.; Daniels, R.; Stintzing, F.C.; Kammerer, D.R. Lipid and Phenolic Constituents from Seeds of Hypericum perforatum L. and Hypericum tetrapterumFr. and their Antioxidant Activity. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.A.; de Barros, F.M.C.; Garcia, L.d.O.; Veeck, A.P.d.L.; Heinzmann, B.M.; Loro, V.L.; Emanuelli, T.; Baldisserotto, B. Essential oil of Lippia alba: A new anesthetic for silver catfish, Rhamdia quelen. Aquaculture 2010, 306, 403–406. [Google Scholar] [CrossRef]
- Becker, A.G.; Parodi, T.V.; Heldwein, C.G.; Zeppenfeld, C.C.; Heinzmann, B.M.; Baldisserotto, B. Transportation of silver catfish, Rhamdiaquelen, in water with eugenol and the essential oil of Lippia alba. Fish Physiol. Biochem. 2012, 38, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.V.; Tavares, M.D. Espécies de Lippia (Verbenaceae), seu potencial bioativo e importância na medicina veterinária e aquicultura. Biota Amazon. 2013, 3, 109–123. [Google Scholar] [CrossRef]
- De Oliveira Hashimoto, G.S.; Neto, F.M.; Ruiz, M.L.; Acchile, M.; Chagas, E.C.; Chaves, F.C.M.; Martins, M.L. Essential oils of sidoides and Mentha piperita against monogenean parasites and their influence on the hematology of Nile tilapia. Aquaculture 2016, 450, 182–186. [Google Scholar] [CrossRef]
- Soares, B.V.; Neves, L.R.; Oliveira, M.S.B.; Chaves, F.C.M.; Reis Dias, M.K.; Chagas, E.C.; Tavares-Dias, M. Antiparasitic activity of the essential oil of Lippia alba on ectoparasites of Colossomamacropomum (tambaqui) and its physiological and histopathological effects. Aquaculture 2016, 452, 107–114. [Google Scholar] [CrossRef]
- Silva, M.I.G.; Gondim, A.P.S.; Nunes, I.F.S.; Sousa, F.C.F. Utilização de fitoterápicos nas unidades básicas de atenção à saúde da família no município de Maracanaú (CE). Rev. Bras. Farmacogn. 2006, 16, 455–462. [Google Scholar] [CrossRef]
- Veras, H.N.H.; Rodrigues, F.F.G.; Botelho, M.A.; Menezes, I.R.A.; Coutinho, H.D.M.; da Costa, J.G.M. Antimicrobial Effect of Lippia sidoides and Thymol on Enterococcus faecalisBiofilm of the Bacterium Isolated from Root Canals. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.B.; Gomes, G.A.; Santangelo, J.M.; Pontes, E.G.; Azambuja, P.; Garcia, E.S.; Carvalho, M.G. Lethal and sublethal effects of essential oil of Lippiasidoides (Verbenaceae) and monoterpenes on Chagas’ disease vector Rhodniusprolixus. Mem. Inst. Oswaldo Cruz 2017, 112, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.H.C. Investigação da ação central do Carvacrol em Modelos de Ansiedade, Depressão e Convulsão em Camundongos e Possíveis Mecanismos Farmacológicos Envolvidos. Master’s Thesis, 133f. Programa de PósGraduação em Farmacologia, Universidade Federal do Ceará, Fortaleza, Brazil, 2010. [Google Scholar]
- Melo, F.H.; Moura, B.A.; de Sousa, D.P.; de Vasconcelos, S.M.; Macedo, D.S.; Fonteles, M.M.; Viana, G.S.; de Sousa, F.C. Antidepressant-like effect of carvacrol (5-Isopropyl-2-methylphenol) in mice: Involvement of dopaminergic system. Fundam. Clin. Pharmacol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.V.; de Melo Leite, A.K.; Bertini, L.M.; de Morais, S.M.; Nunes-Pinheiro, D.C. Topical anti-inflammatory, gastroprotective and antioxidants effects of the essential oil of Lippiasidoides Cham. leaves. J. Ethnopharmacol. 2007, 111, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Larvicidal property of essential oils against Culex quinquefasciatus Say (Diptera: Culicidae). Ind. Crop. Prod. 2009, 30, 311–315. [Google Scholar] [CrossRef]
- Rivas, L.; Mcdonnell, M.J.; Burgess, C.M.; O’Brien, M.; Navarro-Villa, A.; Fanning, S.; Duffy, G. Inhibition of verocy to toxigenic Escherichia coli in model broth and rumen systems by carvacrol and thymol. Int. J. Food Microbiol. 2010, 139, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ozen, T.; Demirtas, I.; Aksit, H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011, 124, 58–64. [Google Scholar] [CrossRef]
- Silva, M.I.; Aquino, M.R.; Teixeira, P.F.; Moura, B.A.; do Amaral, J.F.; de Sousa, D.P.; Vasconcelos, S.M.; de Sousa, F.C. Central nervous system activity of acute administration of isopulegol in mice. Pharmacol. Biochem. Behav. 2007, 88, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, R.; Shen, J.; Su, H.; Xing, D.; Du, L. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008, 581, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Stanić, D.; Plećaš-Solarović, B.; Mirković, D.; Jovanović, P.; Dronjak, S.; Marković, B.; Đorđević, T.; Ignjatović, S.; Pe Šić, V. Oxytocin in corticosterone-induced chronic stress model: Focus on adrenal gland function. Psychoneuroendocrinology 2017, 80, 137–146. [Google Scholar] [CrossRef]
- Vasconcelos, A.S.; Oliveira, I.C.M.; Vidal, L.T.M.; Rodrigues, G.C.; Gutierrez, S.J.C.; Barbosa-Filho, J.M.; Vasconcelos, S.M.M.; Fonteles, M.M.F.; Gaspar, D.M.; Sousa, F.C.F. Subchronic administration of riparin III induces antidepressive-like effects and increases BDNF levels in the mouse hippocampus. Fundam. Clin. Pharmacol. 2015, 29, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Cavalcante, T.T.A.; Melo, C.T.V.; Barroso, D.L.A.; Melo, H.M.; Carvalho, M.G.; Junior, F.E.A.C. Antioxidant and antibacterial activities of essential oil of Lippia sidoides against drug-resistant Staphylococcus aureus from food. Afr. J. Biotechnol. 2018, 17, 232–238. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Archer, J. Tests for emotionality in rats and mice. A review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. Tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.G. The use of a plus maze to measure anxiety in the mouse. Psychopharmacology 1987, 25, 180–185. [Google Scholar] [CrossRef]
- Willner, P. Chronic Mild Stress (CMS) Revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005, 52, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Roy, B.; Lugli, G.; Rizavi, H.; Zhang, H.; Smalheiser, N.R. Chronic corticosterone-mediated dysregulation of microRNA network in prefrontal cortex of rats: Relevance to depression pathophysiology. Transl. Psychiatry 2015, 5, e682. [Google Scholar] [CrossRef] [PubMed]
- Marks, W.; Fournier, N.M.; Kalynchuk, L.E. Repeated expore to corticosterone increases depression-like behavior in two different versions of the forced swim test without altering nonspecific locomotor activity or muscle strength. Physiol. Behav. 2009, 98, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.P.; Melo, C.T.; De Araujo, F.L.; de Carvalho, A.M.; Silva, M.I.; Barbosa-Filho, J.M.; Macêdo, D.S.; de Barros Viana, G.S.; de Sousa, F.C. Antidepressant-like effect of riparin II from Anibariparia in mice: Evidence for the involvement of the monoaminergic system. Fundam. Clin. Pharmacol. 2011, 27, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.C.F.; Oliveira, I.C.M.; Fernandes, M.L. Advances in the Research of Essential Oils: Anxiolytic and Sedative Activity in Medicinal Essential Oils; Sousa, D.P., Ed.; Nova Biomedical: Hauppauge, NY, USA, 2012; Chapter 7; pp. 123–135. ISBN 978-1-61324-297-1. [Google Scholar]
- Amaral, J.F.; Silva, M.I.; Neto, M.R.; Neto, P.F.; Moura, B.A.; de Melo, C.T.; de Araújo, F.L.; de Sousa, D.P.; de Vasconcelos, P.F.; de Vasconcelos, S.M.; et al. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol. Pharm. Bull. 2007, 30, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Shim, I.; Lee, H.; Hahm, D.H. Angelica gigas ameliorate depression-like symptoms in rats following chronic corticosterone injection. BMC Complement. Altern. Med. 2015, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; Madhana, R.M.; Athira, K.V.; Kasala, E.R.; Bodduluru, L.N.; Pitta, S.; Mahareddy, J.R.; Lahkar, M. Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids 2015, 101, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Wang, Z.; Huang, Y.F. Chronic corticosterona exposure reduces hippocampal glycogen level and induces depression-like behavior in mice. J. Zheijian Univ. Sci. B 2015, 16, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Song, L.; Dai, G.; Xu, M.; Zhu, L.; Zhang, W.; Jing, W.; Ju, W. Antidepressant effects of magnolol in a mouse model of depression induced by chronic corticosterone injection. Steroids 2018, 135, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.S.; Lach, G.; Martins, P.R.C.; Romeiro, G.A.; De Lima, T.C.M. Evidence for the involvement of the monoaminergic system in the antidepressant-like action of two 4 amine derivatives of 10, 11dihydro5Hdibenzo cycloheptane in mice evaluated in the tail suspension test Progress in Neuro. Psychopharmacol. Biol. Psychiatry 2007, 32, 368–374. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xian, Y.F.; Ip, S.P.; Tsai, S.H.; Che, C.T. Long-term treatment with peony glycosides reverses chronic unpredictable mild stress-induced depressive-like behavior via increasing expression of neurotrophins in rat brain. Behav. Brain Res. 2008, 210, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Holmes, A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005, 4, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Abreu, T.M.; Monteiro, V.S.; Martins, A.B.S.; Teles, F.B.; Rivanor, R.L.C.; Mota, E.F.; Macedo, D.S.; Vasconcelos, S.M.M.; Junior, J.E.R.H.; Benevides, N.M.B. Involvement of the dopaminergic system in the antidepressant-like effect of the lectin isolated from the red marine alga Solieria filiformis in mice. Int. J. Biol. Macromol. 2018, 111, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M.A.; Moghaddam, A.H.; Maggi, F.; Benelli, G. Anxiolytic and antidepressant activities of Pelargonium roseum essential oil on Swiss albino mice: Possible involvement of serotonergic transmission. Phytother. Res. 2018, 32, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.C.; Oliveira, I.C.; Silva, M.I.; de Melo, C.T.; Santiago, V.R.; de Castro Chaves, R.; Fernandes, M.L.; Gutierrez, S.J.; Vasconcelos, S.M.; Macêdo, D.S.; et al. Involvement of monoaminergic system in the antidepressant-like effect of riparin I from Aniba riparia (Nees) Mez (Lauraceae) in mice. Fundam. Clin. Pharmacol. 2014, 28, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiela, M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Keeley, M.L.; Storch, E.A. Anxiety disorders in youth. J. Pediatr. Nurs. 2009, 24, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.A.; Marino, V.; Ong, J. Pharmacological actions of thymol and an analogue at GABAB autoreceptors. Clin. Exp. Pharmacol. Physiol. 2014, 41, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.B.; Feitosa, M.L.; Silva, M.I.; Noronha, E.C.; Moura, B.A.; Venâncio, E.T.; Rios, E.R.; de Sousa, D.P.; de Vasconcelos, S.M.; Fonteles, M.M.; et al. Anxiolytic-like effect of the monoterpene 1,4-cineole in mice. Pharmacol. Biochem. Behav. 2010, 96, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Y.; Li, H.Y.; Chen, J.J.; Li, R.P.; Qu, R.; Fu, Q.; Ma, S.P. Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav. Brain Res. 2015, 291, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, R.; Tang, W.J.; Meng, G.; Hu, X.Y.; Wu, T.N. Antidepressant-like effect of geniposide on chronic unpredictable mild stress-induced depressive rats by regulating the hypothalamus–pituitary–adrenal axis. Eur. Neuropsychopharmacol. 2015, 25, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).