N-Aryl-7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido-[3,2,1-ij]quinoline-6-carboxamides. The Synthesis and Effects on Urinary Output
Abstract
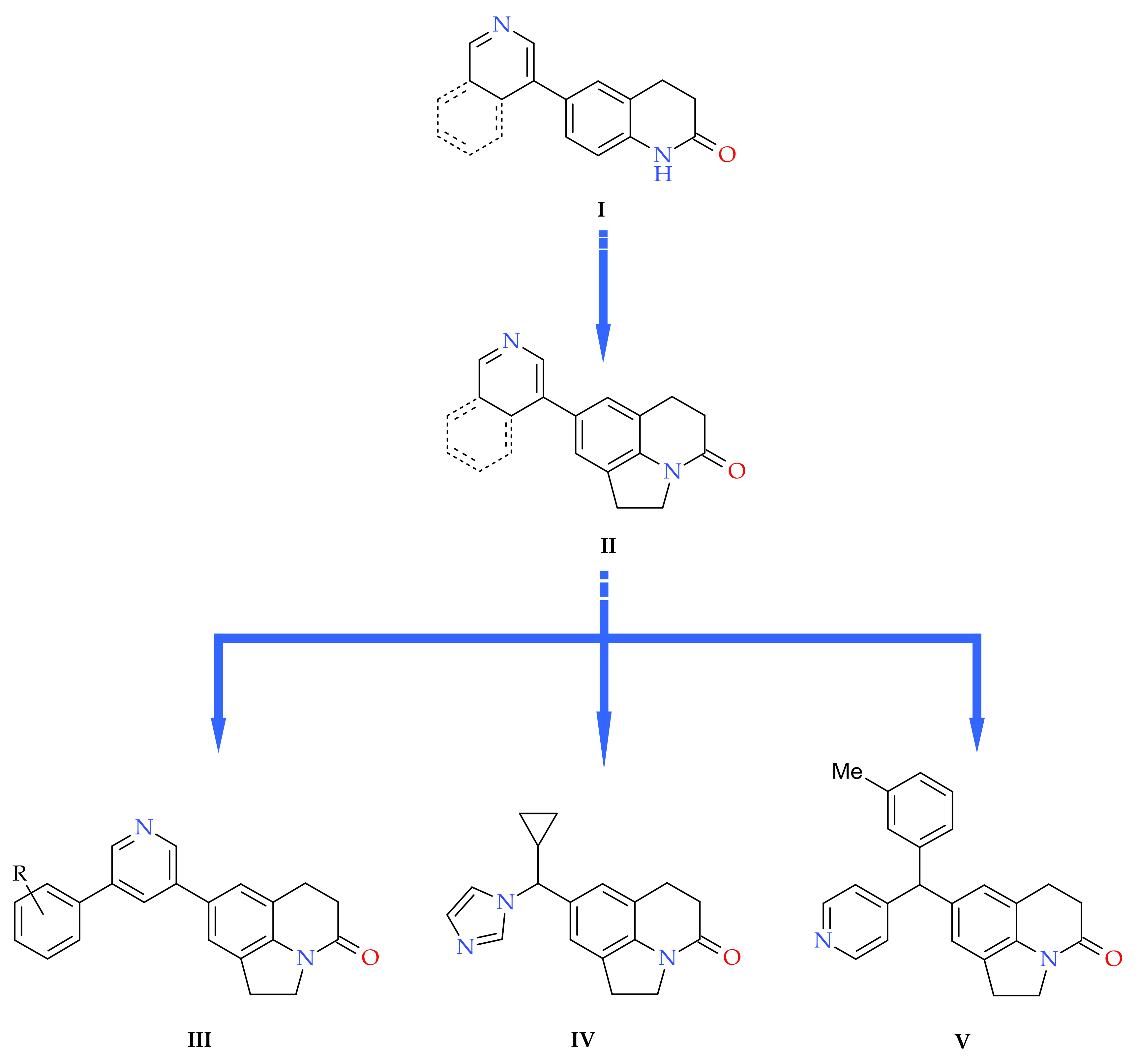
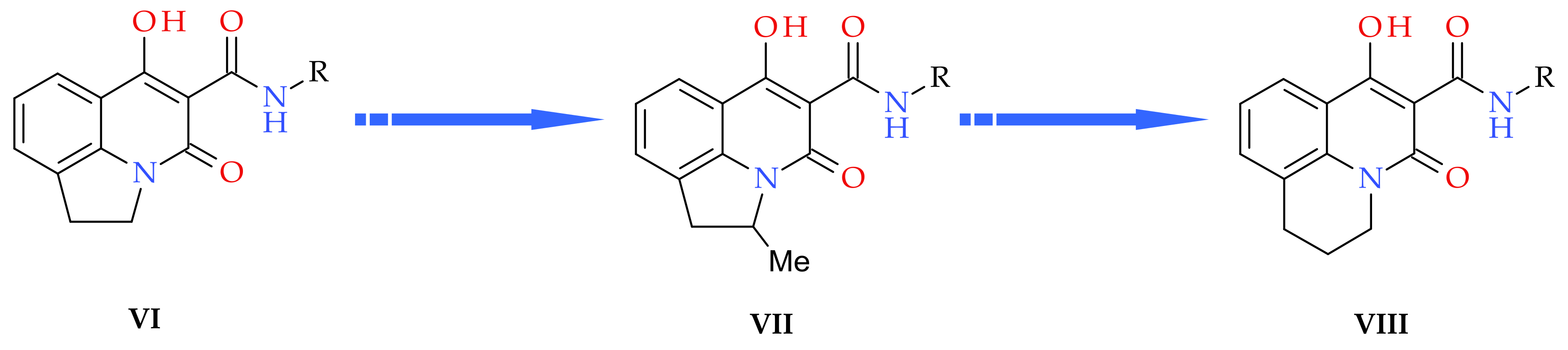
:1. Introduction
2. Materials and Methods
2.1. Chemistry
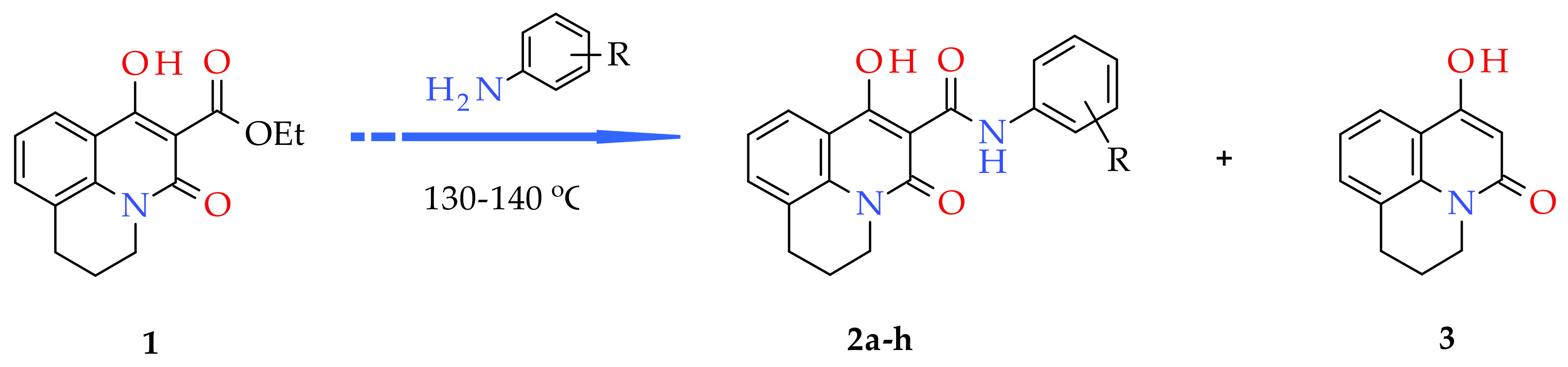
2.2. General Procedure for the Synthesis of 7-Hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-6-carboxanilides (2a–h)
2.3. Pharmacology
Diuretic Test
3. Results and Discussion
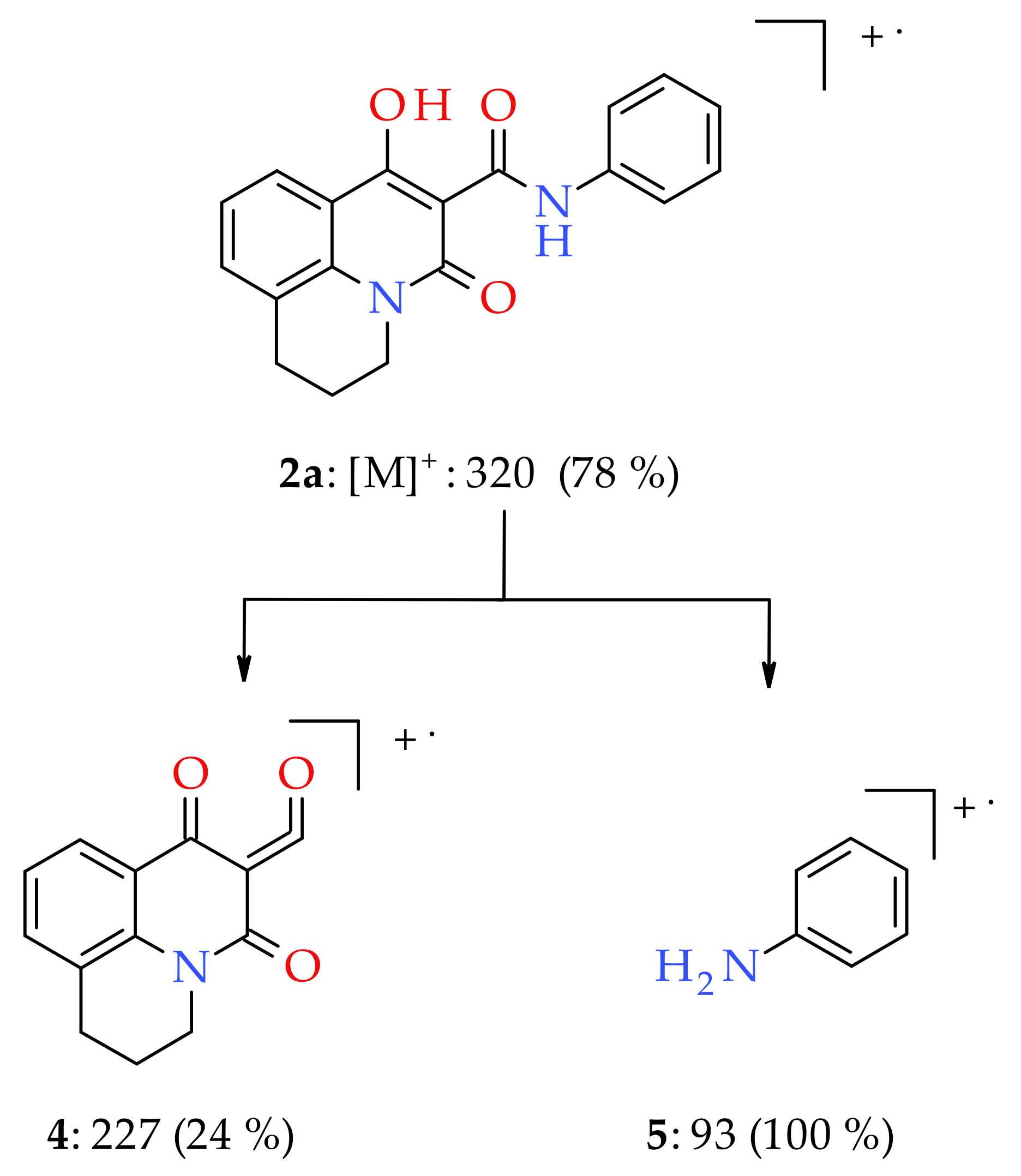
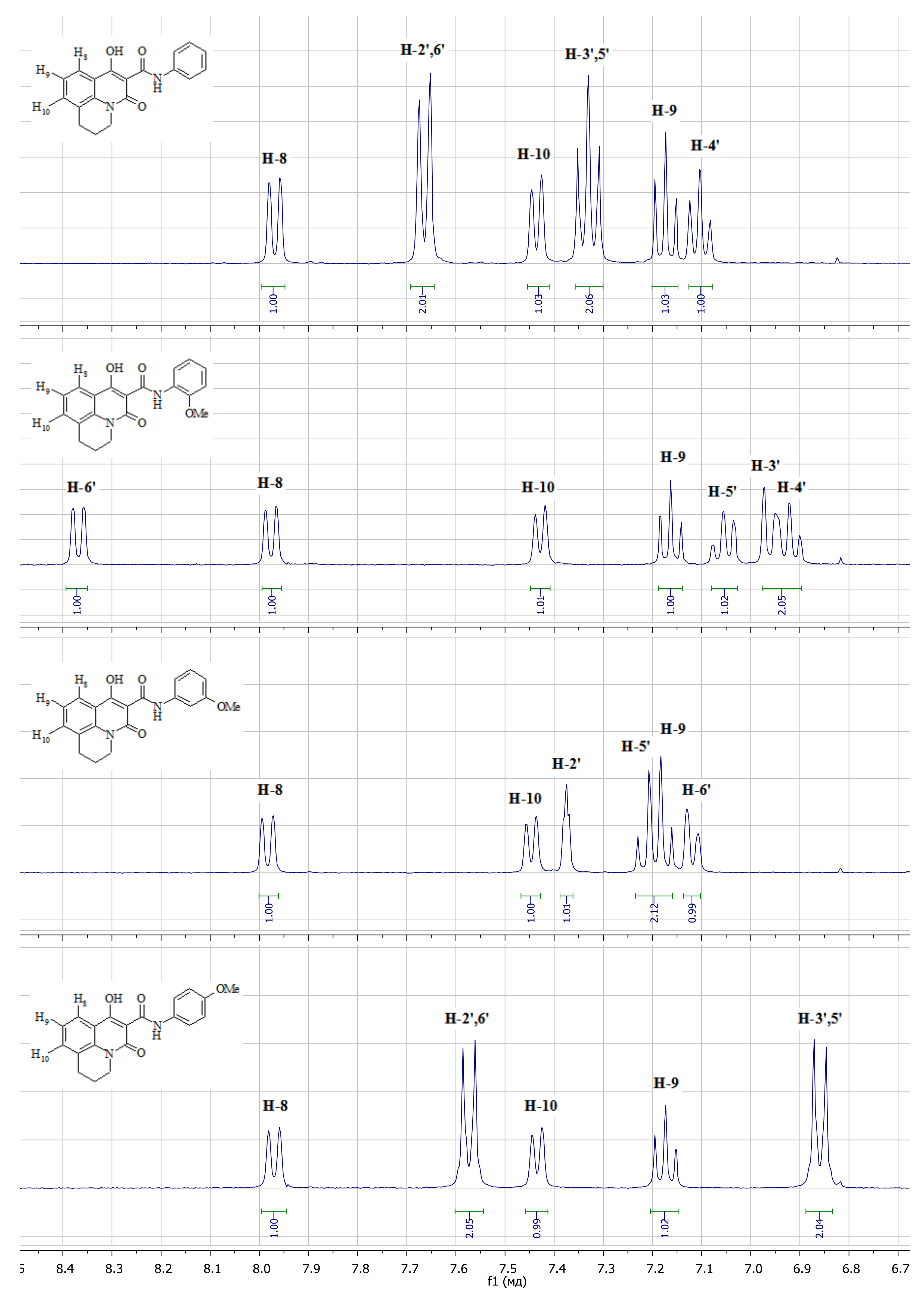
3.1. Chemistry
3.2. Evaluation of Diuretic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soutello, A.L.; Rodrigues, R.C.; Jannuzzi, F.F.; São-João, T.M.; Martinix, G.G.; Nadruz, W., Jr.; Gallani, M.C. Quality of Life on Arterial Hypertension: Validity of Known Groups of MINICHAL. Arq. Bras. Cardiol. 2015, 104, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H.; Felker, G.M. Diuretic Treatment in Heart Failure. N. Engl. J. Med. 2017, 377, 1964–1975. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Madanieh, R.; Alkan, M.; Dogar, M.U.; Kosmas, C.E.; Vittorio, T.J. A perspective on diuretic resistance in chronic congestive heart failure. Ther. Adv. Cardiovasc. Dis. 2017, 11, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M.; Valente, M.A.; Damman, K.; Hillege, H.L.; Navis, G.; Voors, A.A. Diuretic response in acute heart failure—Pathophysiology, evaluation, and therapy. Nat. Rev. Cardiol. 2015, 12, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chiong, J.R.; Cheung, R.J. Loop diuretic therapy in heart failure: The need for solid evidence on a fluid issue. Clin. Cardiol. 2010, 33, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Bereznyakova, N.L. Heterocyclic diuretics. Chem. Heterocycl. Compd. 2012, 48, 155–165. [Google Scholar] [CrossRef]
- Tamargo, M.; Tamargo, J. Future drug discovery in renin-angiotensin-aldosterone system intervention. Expert Opin. Drug Discov. 2017, 12, 827–848. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.; Goldsmith, S.R.; Gheorghiade, M. Tolvaptan for the treatment of heart failure: A review of the literature. Expert Opin. Pharmacother. 2011, 12, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.C.; Ruilope, L.M.; Segura, J. Benefits of spironolactone as the optimal treatment for drug resistant hypertension. Pathway-2 trial review. Hipertens. Riesgo Vasc. 2016, 33, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Kolkhof, P.; Bärfacker, L. 30 Years of the mineralocorticoid receptor: Mineralocorticoid receptor antagonists: 60 years of research and development. J. Endocrinol. 2017, 234, T125–T140. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Tamargo, J. Aldosterone a relevant factor in the beginning and evolution of arterial hypertension. Am. J. Hypertens. 2017, 30, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Aebi, J.D.; Hornsperger, B.; Krebs, H.J.; Kuhn, B.; Kuglstatter, A.; Alker, A.M.; Märki, H.P.; Müller, S.; Burger, D.; et al. Discovery of 4-Aryl-5,6,7,8-tetrahydroisoquinolines as potent, selective, and orally active aldosterone synthase (CYP11B2) inhibitors: In vivo evaluation in rodents and Cynomolgus monkeys. J. Med. Chem. 2015, 58, 8054–8065. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Lehmann, J.; Alzieu, T.; Lenz, M.; Carnero Corrales, M.A.; Aebi, J.D.; Märki, H.P.; Kuhn, B.; Amrein, K.; Mayweg, A.V.; et al. Synthesis of annulated pyridines as inhibitors of aldosterone synthase (CYP11B2). Org. Biomol. Chem. 2016, 14, 5922–5927. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, W.L.; Hoyt, S.B.; London, C.; McMasters, D.; Verras, A.; Struthers, M.; Cully, D.; Wisniewski, T.; Ren, N.; Bopp, C.; et al. Discovery of spirocyclic aldosterone synthase inhibitors as potential treatments for resistant hypertension. ACS Med. Chem. Lett. 2016, 8, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, S.B.; Taylor, J.; London, C.; Ali, A.; Ujjainwalla, F.; Tata, J.; Struthers, M.; Cully, D.; Wisniewski, T.; Ren, N.; et al. Discovery of indazole aldosterone synthase (CYP11B2) inhibitors as potential treatments for hypertension. Bioorg. Med. Chem. Lett. 2017, 27, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Meguro, M.; Miyauchi, S.; Kanao, Y.; Naito, S.; Suzuki, K.; Inoue, S.; Yamada, K.; Homma, T.; Chiba, K.; Nara, F.; et al. 4-Anilino-pyrimidine, novel aldosterone synthase (CYP11B2) inhibitors bearing pyrimidine structures. Bioorg. Med. Chem. Lett. 2017, 27, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Lu, F.; Qiao, L.; Chen, X.; Li, G.; Zhang, Y. Discovery of potential inhibitors of aldosterone synthase from Chinese herbs using pharmacophore modeling, molecular docking, and molecular dynamics simulation studies. Biomed. Res. Int. 2016, 2016, 4182595. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Heim, R.; Ries, C.; Schewe, K.E.; Birk, B.; Hartmann, R.W. In vivo active aldosterone synthase inhibitors with improved selectivity: Lead optimization providing a series of pyridine substituted 3,4-dihydro-1H-quinolin-2-one derivatives. J. Med. Chem. 2008, 51, 8077–8087. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Yin, L.; Ali, A.; Cooke, A.J.; Bennett, J.; Ratcliffe, P.; Lo, M.M.; Metzger, E.; Hoyt, S.; Hartmann, R.W. Novel pyridyl substituted 4,5-dihydro-[1,2,4]triazolo[4,3-a]quinolines as potent and selective aldosterone synthase inhibitors with improved in vitro metabolic stability. J. Med. Chem. 2015, 58, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Grombein, C.M.; Hu, Q.; Rau, S.; Zimmer, C.; Hartmann, R.W. Heteroatom insertion into 3,4-dihydro-1H-quinolin-2-ones leads to potent and selective inhibitors of human and rat aldosterone synthase. Eur. J. Med. Chem. 2015, 90, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Negri, M.; Heim, R.; Zimmer, C.; Hartmann, R.W. Fine-tuning the selectivity of aldosterone synthase inhibitors: Structure-activity and structure-selectivity insights from studies of heteroaryl substituted 1,2,5,6-tetrahydropyrrolo[3,2,1-ij]quinolin-4-one derivatives. J. Med. Chem. 2011, 54, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Lucas, S.; Maurer, F.; Kazmaier, U.; Hu, Q.; Hartmann, R.W. Novel imidazol-1-ylmethyl substituted 1,2,5,6-tetrahydropyrrolo[3,2,1-ij]quinolin-4-ones as potent and selective CYP11B1 inhibitors for the treatment of Cushing’s syndrome. J. Med. Chem. 2012, 55, 6629–6633. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Hu, Q.; Hartmann, R.W. Tetrahydropyrroloquinolinone type dual inhibitors of aromatase/aldosterone synthase as a novel strategy for breast cancer patients with elevated cardiovascular risks. J. Med. Chem. 2013, 56, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Bereznyakova, N.L.; Mospanova, E.V. 4-Hydroxy-2-quinolones. 121. Synthesis and biological properties of 1-hydroxy-3-oxo-5,6-dihydro-3H-pyrrolo[3,2,1-ij]quinoline-2-carboxylic acid alkylamides. Chem. Heterocycl. Compd. 2007, 43, 856–862. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Bereznyakova, N.L.; Naboka, O.I. 4-Hydroxy-2-quinolones. 138. Synthesis and study of structure—Biological activity relationships in a series of 1-hydroxy-3-oxo-5,6-dihydro-3H-pyrrolo[3,2,1-ij]quinoline-2-carboxylic acid anilides. Chem. Heterocycl. Compd. 2007, 43, 1532–1539. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Golik, N.Y.; Shemchuk, A.L.; Naboka, O.I.; Voronina, Y.V.; Turov, A.V. 4-Hydroxy-2-quinolones. 197. The search for novel diuretics amongst halo-substituted 6-hydroxy-2-methyl-4-oxo-1,2-dihydro-4H-pyrrolo[3,2,1-ij]quinoline-5-carboxylic acid anilides. Chem. Heterocycl. Compd. 2011, 47, 826–832. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Golik, N.Y.; Shemchuk, A.L.; Kravchenko, V.N. 4-Hydroxy-2-quinolones. 201. Synthesis, structure, and diuretic activity of hydroxy- and alkoxyanilides of 6-hydroxy-2-methyl-4-oxo-2,4-dihydro-1H-pyrrolo[3,2,1-ij]quinoline-5-carboxylic acid. Chem. Heterocycl. Compd. 2011, 47, 1122–1127. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Golik, N.Y.; Andreeva, K.V.; Gorokhova, O.V. 4-Hydroxy-2-quinolones. 194. 1-Hydroxy-3-oxo-6,7-dihydro-3H,5H-pyrido[3,2,1-ij]quinoline-2-carboxylic acid alkylamides. Synthesis, structure, and biological properties. Chem. Heterocycl. Compd. 2010, 46, 1459–1466. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Golik, N.Y.; Chernenok, I.N. 4-Hydroxy-2-quinolones. 233. Diuretic activity of 9-bromo-7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-6-carboxylic acid anilides. Chem. Heterocycl. Compd. 2013, 49, 1323–1330. [Google Scholar] [CrossRef]
- Kutyrev, A.; Kappe, T. Methanetricarboxylates as key reagents for the simple preparation of heteroarylcarboxamides with potential biological activity. Part 1. Reaction of methanetricarboxylates with indoline and 1,2,3,4-tetrahydroquinoline. J. Heterocycl. Chem. 1997, 34, 969–972. [Google Scholar] [CrossRef]
- Eriksoo, E.; Sandberg, E.B.; Stalhandske, L.J.T. Heterocyclic Carboxamides, Compositions Containing Such Compounds, Processes for Their Preparation and Methods of Treatment Therewith. Patent No. EP0059698(A1), 8 September 1982. [Google Scholar]
- Vogel, H.G. (Ed.) Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 459–461. [Google Scholar]
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances: Syntheses, Patents, Applications of the Most Relevant APIs, 5th ed.; Thieme: Stuttgart, Germany, 2008; p. 1800. [Google Scholar]
- Ukrainets, I.V.; Sidorenko, L.V.; Svechnikova, E.N.; Shishkin, O.V. 4-Hydroxy-2-quinolones. 130. The reactivity of ethyl 4-hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxylates. Chem. Heterocycl. Compd. 2007, 43, 1275–1279. [Google Scholar] [CrossRef]
 | ||||
|---|---|---|---|---|
| Entry | Product | R | Diuresis in 4 h | |
| mL 1 | % 2,3 | |||
| 1 | 2a | H | 7.68 ± 0.29 | +43 (−21 and −28) |
| 2 | 2b | 2-OH | 4.62 ± 0.22 | −14 (− and −12) |
| 3 | 2c | 3-OH | 8.59 ± 0.32 | +60 (− and −28) |
| 4 | 2d | 4-OH | 5.91 ± 0.30 | +10 (− and −19) |
| 5 | 2e | 2-OMe | 8.70 ± 0.32 | +62 (+44 and −58) |
| 6 | 2f | 3-OMe | 6.28 ± 0.35 | +17 (+51 and +3) |
| 7 | 2g | 4-OMe | 9.08 ± 0.37 | +69 (+126 and +68) |
| 8 | 2h | 4-OEt | 6.01 ± 0.28 | +12 (0 and +6) |
| 9 | Hydrochlorothiazide | – | 8.11 ± 0.30 | +51 |
| 10 | Control | – | 5.37 ± 0.29 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukrainets, I.V.; Sidorenko, L.V.; Golik, M.Y.; Chernenok, I.M.; Grinevich, L.A.; Davidenko, A.A. N-Aryl-7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido-[3,2,1-ij]quinoline-6-carboxamides. The Synthesis and Effects on Urinary Output. Sci. Pharm. 2018, 86, 12. https://doi.org/10.3390/scipharm86020012
Ukrainets IV, Sidorenko LV, Golik MY, Chernenok IM, Grinevich LA, Davidenko AA. N-Aryl-7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido-[3,2,1-ij]quinoline-6-carboxamides. The Synthesis and Effects on Urinary Output. Scientia Pharmaceutica. 2018; 86(2):12. https://doi.org/10.3390/scipharm86020012
Chicago/Turabian StyleUkrainets, Igor V., Lyudmila V. Sidorenko, Mykola Y. Golik, Igor M. Chernenok, Lina A. Grinevich, and Alexandra A. Davidenko. 2018. "N-Aryl-7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido-[3,2,1-ij]quinoline-6-carboxamides. The Synthesis and Effects on Urinary Output" Scientia Pharmaceutica 86, no. 2: 12. https://doi.org/10.3390/scipharm86020012
APA StyleUkrainets, I. V., Sidorenko, L. V., Golik, M. Y., Chernenok, I. M., Grinevich, L. A., & Davidenko, A. A. (2018). N-Aryl-7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido-[3,2,1-ij]quinoline-6-carboxamides. The Synthesis and Effects on Urinary Output. Scientia Pharmaceutica, 86(2), 12. https://doi.org/10.3390/scipharm86020012





