Gliadin Peptide Facilitates FITC Dextran Transport across the Non Everted Gut Sac of Rat Small Intestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Production of SOD_Cl and GliSOD_P61
2.3. In Vitro Studies Using the Non-Everted Gut Sac Method
3. Results
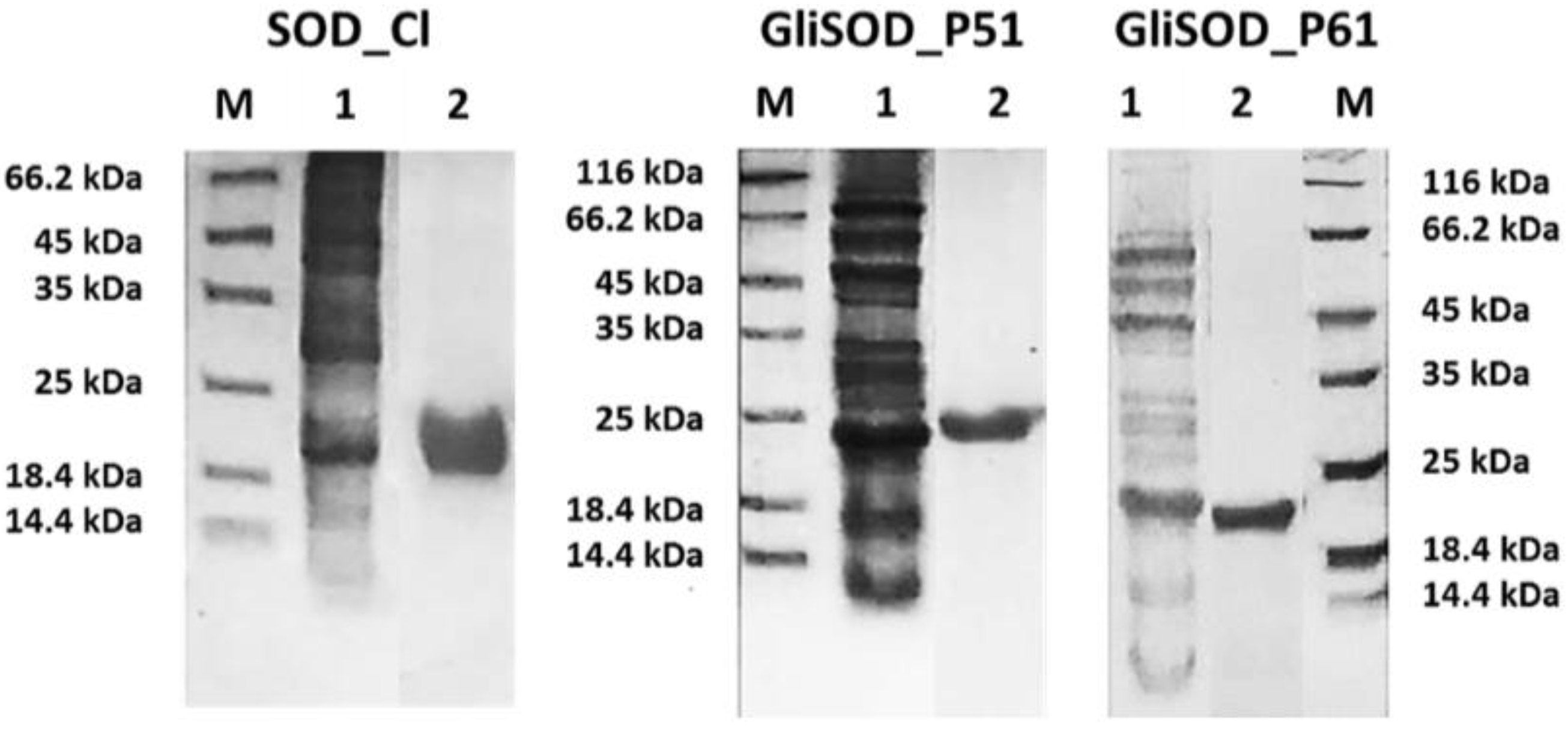
3.1. Proteins Production
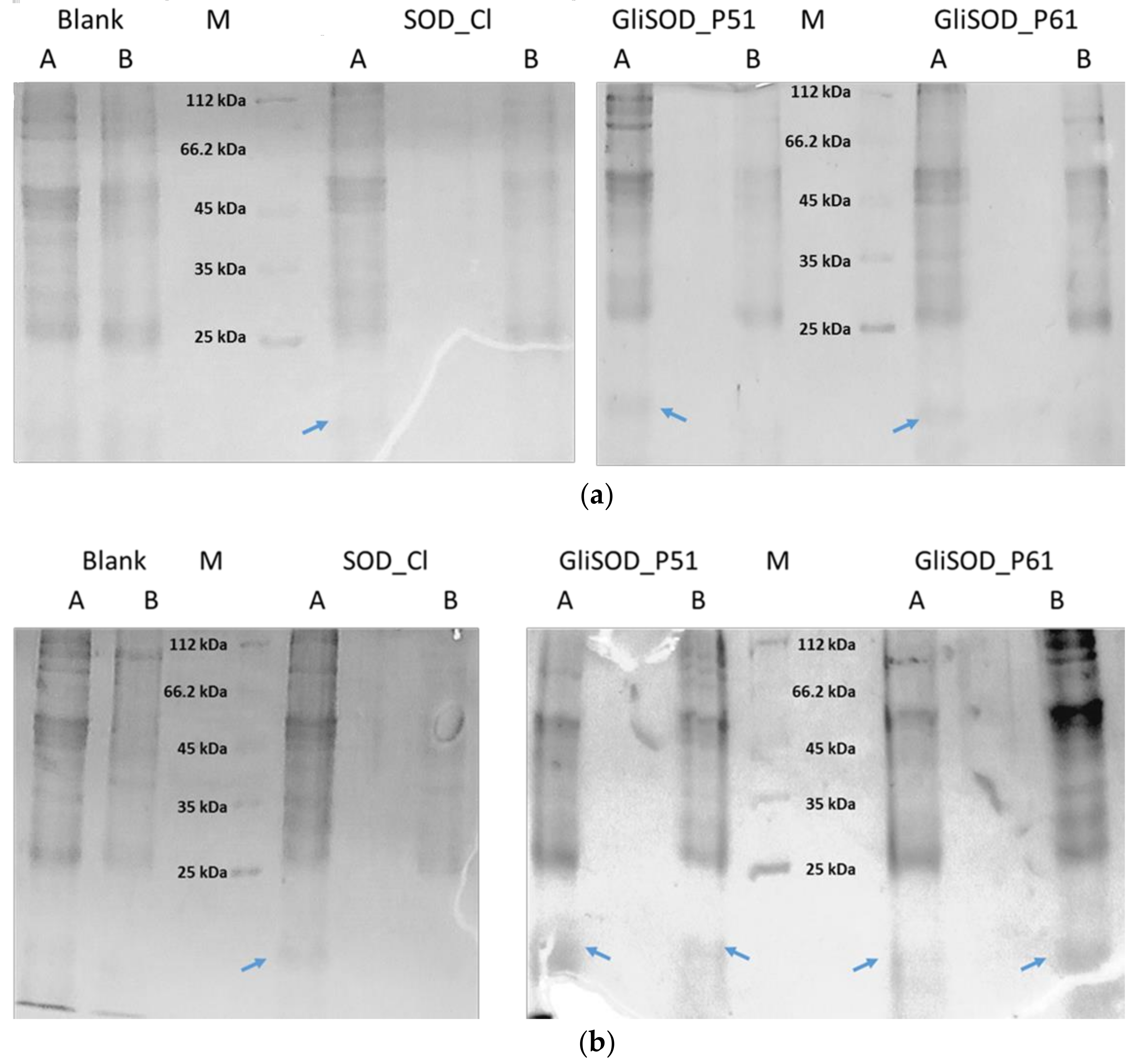
3.2. Permeability Assay
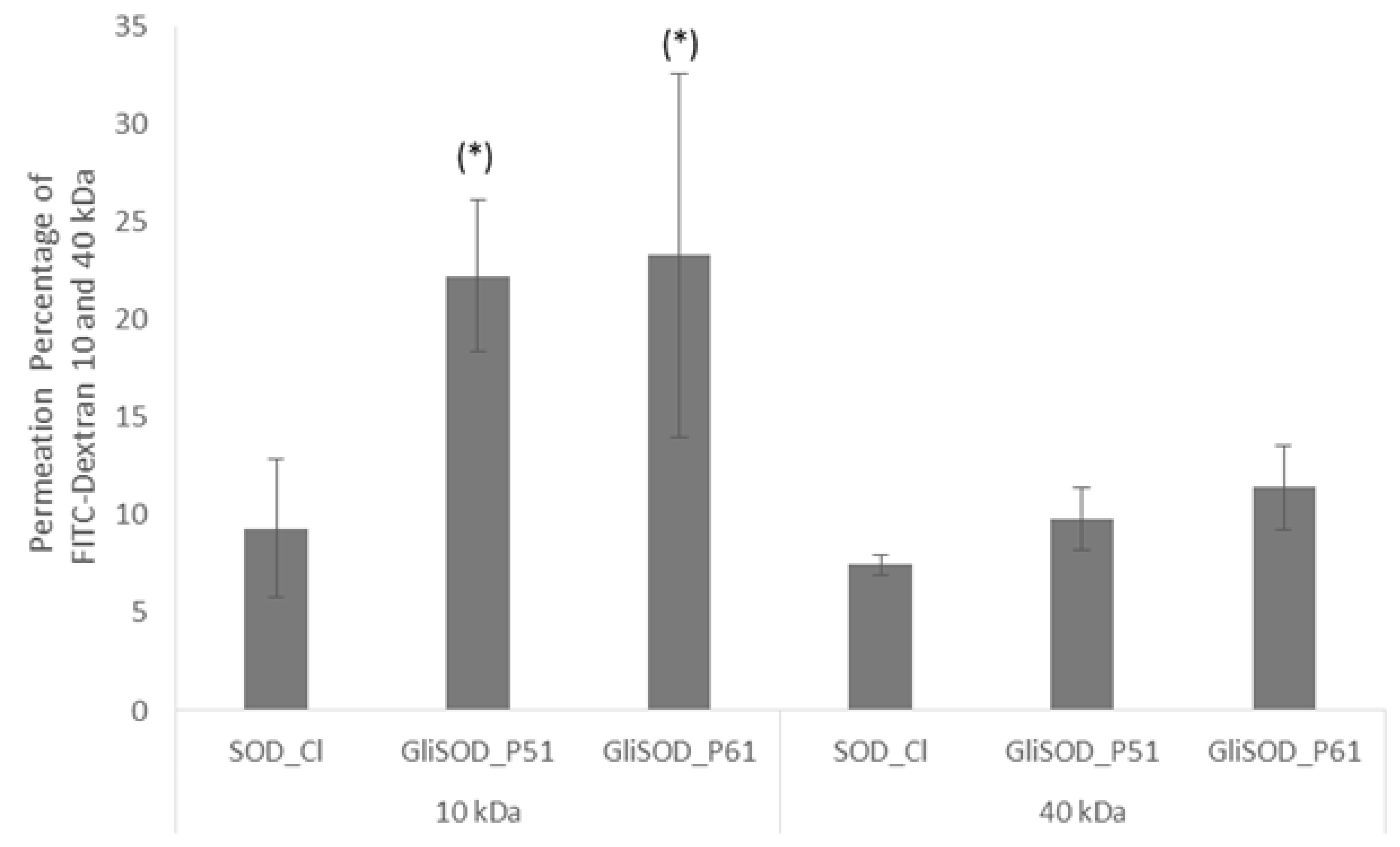
3.3. Paracellular Permeability Assay
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gopal, R.K.; Elumalai, S. Industrial Production of Superoxide Dismutase (SOD): A Mini Review. J. Probiot. Health 2017, 5. [Google Scholar] [CrossRef]
- Noor, R.; Mittal, S.; Iqbal, J. Superoxide dismutase—Applications and relevance to human diseases. Med. Sci. Monit. 2002, 8, 210–216. [Google Scholar]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A.; Dutt, S.; Kumar, A.; Kumar, S.; Ahuja, P.S. The basic and applied aspects of superoxide dismutase. J. Mol. Catal. B Enzym. 2011, 68, 129–138. [Google Scholar] [CrossRef]
- Bafana, A.; Dutt, S.; Kumar, S.; Ahuja, P.S. Superoxide dismutase: An industrial perspective. Crit. Rev. Biotechnol. 2011, 31, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Aungst, B.J. Absorption enhancers: Applications and advances. AAPS J. 2012, 14, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Choonara, B.F.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; Toit, L.C.; Pillay, V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol. Adv. J. 2014, 32, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Cho, Y.W.; Park, K. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Adv. Drug Deliv. Rev. 2013, 65, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Ellis, H.J.; Parnell, N.D.; Shidrawi, R.G.; Thomas, P.D.; O’Reilly, N.; Corazza, G.R.; Ciclitira, P.J. A non-toxic analogue of a coeliac-activating gliadin peptide: A basis for immunomodulation? Aliment. Pharmacol. Ther. 1999, 13, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Intes, L.; Bahut, M.; Nicole, P.; Couvineau, A.; Guette, C.; Calenda, A. Intestinal cell targeting of a stable recombinant Cu-Zn SOD from Cucumis melo fused to a gliadin peptide. J. Biotechnol. 2012, 159, 99–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Utami, R.A.; Aqila, A.G.; Asyarie, S.; Tjandrawinata, R.R.; Retnoningrum, D.S. In Vitro Toxicity and ZO-1 Gene Expression Analysis of GliSOD_P61 Treatment in Caco-2 Cell. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 200–206. [Google Scholar]
- Gulfam, M.; Kim, J.E.; Lee, J.M.; Ku, B.; Chung, B.H.; Chung, B.G. Anticancer drug-loaded gliadin nanoparticles induce apoptosis in breast cancer cells. Langmuir 2012, 28, 8216–8223. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.P.; Chen, S.; Yu, B.Y.; Zhu, D.N.; Cordell, G.A.; Qiu, S.X. Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur. J. Med. Chem. 2006, 41, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Everted gut sac model as a tool in pharmaceutical research: Limitations and applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pan, H.; Zhang, C.; Zhao, L.; Zhao, R.; Zhu, Y.; Pan, W. Developments in methods for measuring the intestinal absorption of nanoparticle-bound drugs. Int. J. Mol. Sci. 2016, 17, 1171. [Google Scholar] [CrossRef] [PubMed]
- Utami, R.A.; Asyarie, S.; Retnoningrum, D.S. Biochemical Characterization of Recombinant Cu–Zn SOD from Citrus limon Fused to Gliadin Peptides. J. Appl. Pharm. Sci. 2018, 8, 115–121. [Google Scholar]
- Ismaya, W.T.; Yunita, E.A.; Lai, X.; Retnoningrum, D.S.; Rachmawati, H.; Dijkstra, B.W.; Tjandrawinata, R.R. A novel immune-tolerable and permeable lectin-like protein from mushroom Agaricus bisporus. Biochem. Biophys. Res. Commun. 2016, 473, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Amasheh, M.; Dittmann, I.; Christoffel, I.; Fromm, M.; Amasheh, S. Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomaterials 2013, 34, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bruun, S.W.; Josefsen, K.; Tanassi, J.T.; Marek, A.; Pedersen, M.H.F.; Sidenius, U.; Haupt-Jorgensen, M.; Antvorskov, J.C.; Larsen, J.; Heegaard, N.H.; et al. Large Gliadin Peptides Detected in the Pancreas of NOD and Healthy Mice following Oral Administration. J. Diabetes Res. 2016, 2016, 2424306. [Google Scholar] [CrossRef] [PubMed]
- Ménard, S.; Lebreton, C.; Schumann, M.; Matysiak-Budnik, T.; Dugave, C.; Bouhnik, Y.; Malamut, G.; Cellier, C.; Allez, M.; Crenn, P.; et al. Paracellular versus Transcellular Intestinal Permeability to Gliadin Peptides in Active Celiac Disease. Am. J. Pathol. 2012, 180, 608–615. [Google Scholar]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S. Gliadin Induces an Increase in Intestinal Permeability and Zonulin Release by Binding to the Chemokine Receptor CXCR3. Gastroenterology 2008, 135, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Junginger, H.E.; Hoogstraate, J.A.; Verhoef, J.C. Recent advances in buccal drug delivery and absorption—In vitro and in vivo studies. J. Control. Release 1999, 62, 149–159. [Google Scholar] [CrossRef]
- Salama, N.N.; Eddington, N.D.; Fasano, A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Kondoh, M.; Hanada, T.; Takahashi, A.; Hamakubo, T.; Yagi, K. A claudin-4 modulator enhances the mucosal absorption of a biologically active peptide. Biochem. Pharmacol. 2010, 79, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utami, R.A.; Hakiki, A.; Asyarie, S.; Retnoningrum, D.S. Gliadin Peptide Facilitates FITC Dextran Transport across the Non Everted Gut Sac of Rat Small Intestine. Sci. Pharm. 2018, 86, 13. https://doi.org/10.3390/scipharm86020013
Utami RA, Hakiki A, Asyarie S, Retnoningrum DS. Gliadin Peptide Facilitates FITC Dextran Transport across the Non Everted Gut Sac of Rat Small Intestine. Scientia Pharmaceutica. 2018; 86(2):13. https://doi.org/10.3390/scipharm86020013
Chicago/Turabian StyleUtami, Ratna Annisa, Aunillah Hakiki, Sukmadjaja Asyarie, and Debbie Soefie Retnoningrum. 2018. "Gliadin Peptide Facilitates FITC Dextran Transport across the Non Everted Gut Sac of Rat Small Intestine" Scientia Pharmaceutica 86, no. 2: 13. https://doi.org/10.3390/scipharm86020013
APA StyleUtami, R. A., Hakiki, A., Asyarie, S., & Retnoningrum, D. S. (2018). Gliadin Peptide Facilitates FITC Dextran Transport across the Non Everted Gut Sac of Rat Small Intestine. Scientia Pharmaceutica, 86(2), 13. https://doi.org/10.3390/scipharm86020013



