Acetylcholinesterase Inhibition and Antioxidant Activity of N-trans-Caffeoyldopamine and N-trans-Feruloyldopamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and General Tehniques
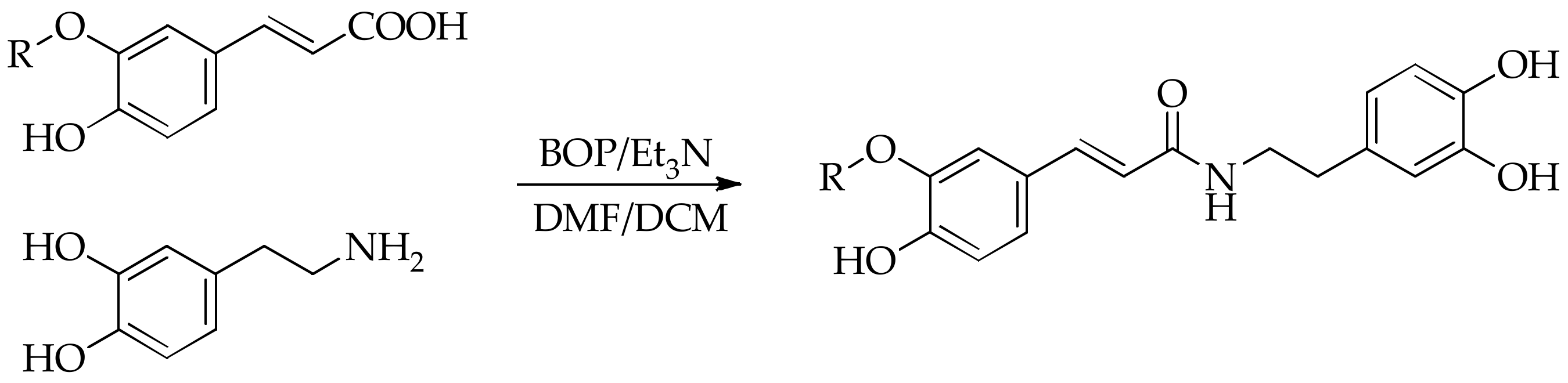
2.2. Synthesis
2.3. Acetylcholinesterase Inhibition
2.3.1. In Vitro Assay
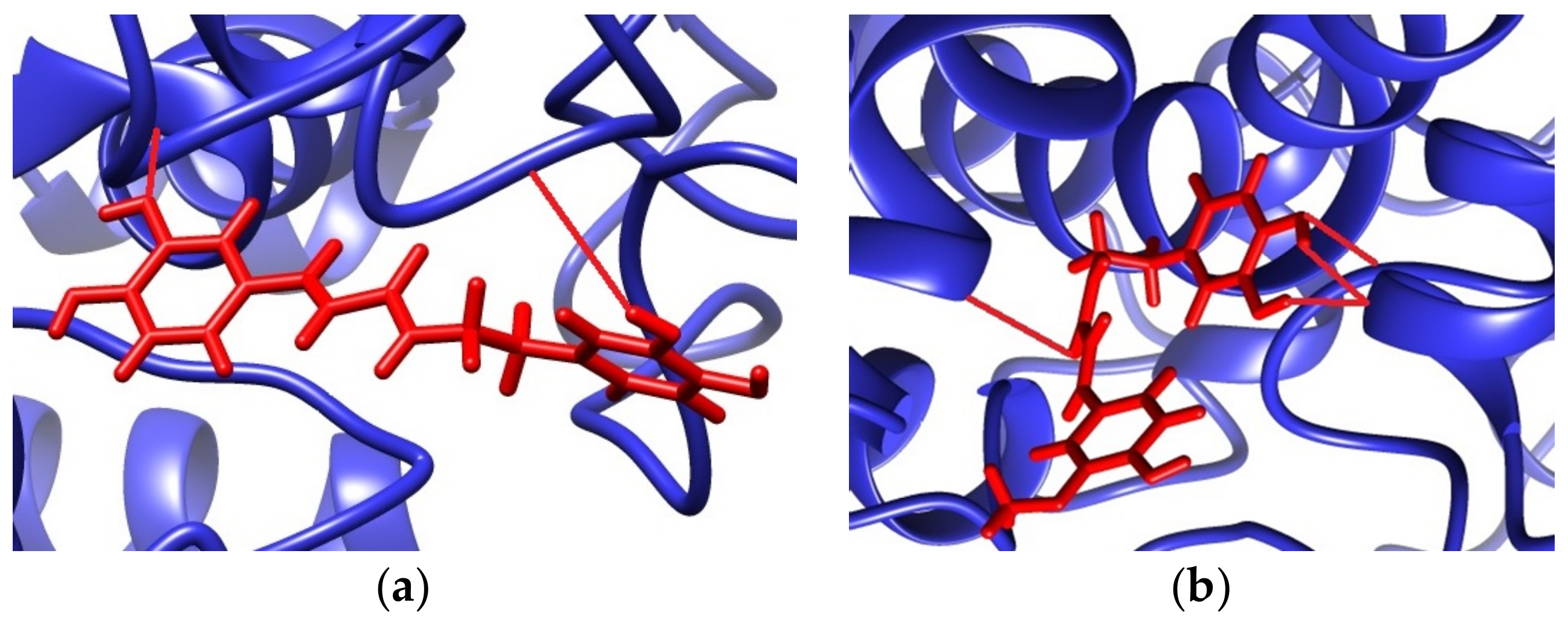
2.3.2. Molecular Docking Studies
2.4. Antioxidant Activity
2.4.1. 2,2-Diphenyl-1-Pycrylhydrazyl Free Radical Scavenging Assay
2.4.2. 2,2′-Azinobis(3-Ethylbenzothiazoline-6-Sulphonic Acid) Radical Cation Scavenging Assay
2.4.3. Ferric-Reducing Antioxidant Power Assay
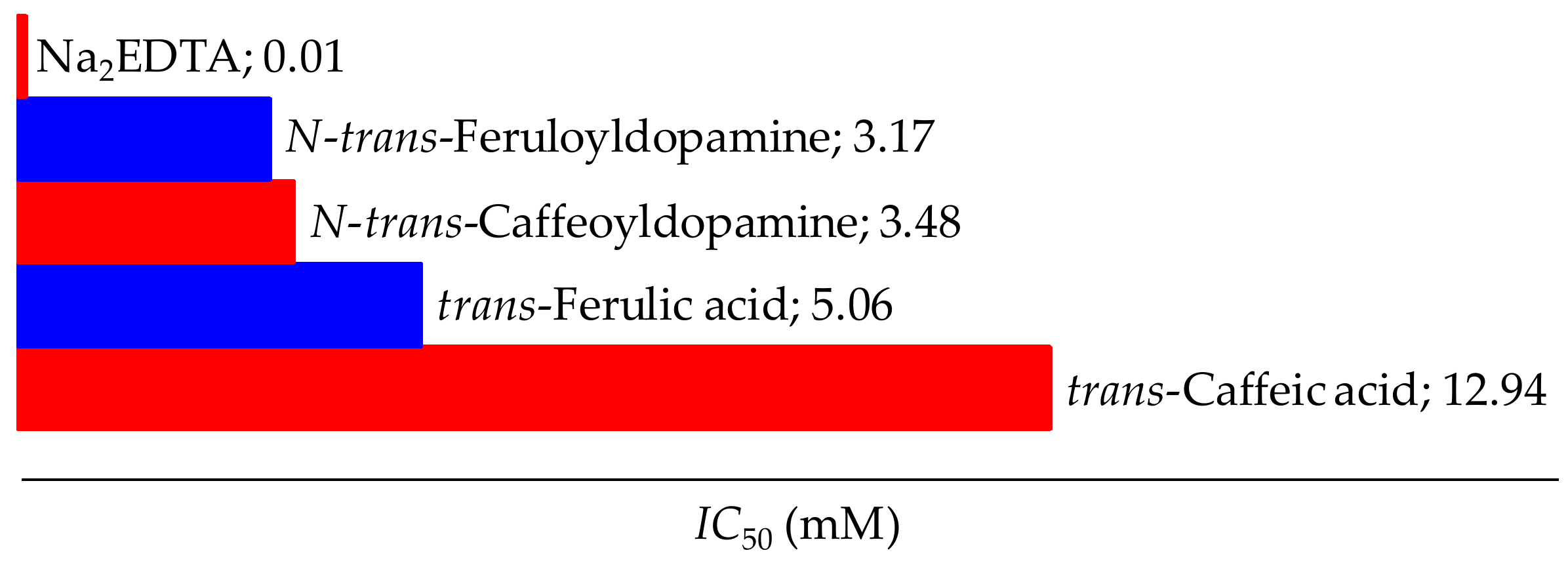
2.4.4. Fe(II)-Chelating Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Synthesis
3.1.1. N-trans-Caffeoyldopamine
3.1.2. N-trans-Feruloyldopamine
3.2. Acetylcholinesterase Inhibition
- the nature of the R substituent at the aromatic ring, which plays a key role in the inhibitory potency, and
- the presence of the dopamine moiety via the amide-coupled bond.
3.3. Antioxidant Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Y.; Xiao, H.; Zheng, J.; Liang, G. Structure-thermodynamics-antioxidant activity relationships of selected natural phenolic acids and derivatives: An experimental and theoretical evaluation. PLoS ONE 2015, 10, e0121276–20. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase activity of phenolic acids and their derivatives. Z. Naturforsch. C 2013, 68, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Maldonado, A.F.; Schieber, A.; Ganzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M.; Ferreira, I. The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Int. 2015, 76, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant activity of phenolic acids and their metabolites: Synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.H.; Leal, P.C.; Yunes, R.A.; Nunes, R.J.; Barardi, C.R.; Pinto, A.R.; Simoes, C.M.; Zanetti, C.R. Evaluation of antiviral activity of phenolic compounds and derivatives against rabies virus. Vet. Microbiol. 2006, 116, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: A structure–activity relationship study. Drug Chem. Toxicol. 2017, 40, 416–424. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic acid derivatives: In vitro and in vivo anti-inflammatory properties. Free Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Girao da Cruz, M.T.; Cordeiro, M.N.; Milhazes, N.; Borges, F.; Marques, M.P. Phenolic acid derivatives with potential anticancer properties-a structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg. Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.A.; Steffensen, S.K.; Christophersen, C.; Mortensen, A.G.; Jorgensen, L.N.; Niveyro, S.; de Troiani, R.M.; Rodriguez-Enriquez, R.J.; Barba-de la Rosa, A.P.; Fomsgaard, I.S. Synthesis and quantitation of six phenolic amides in Amaranthus spp. J. Agric. Food Chem. 2010, 58, 6306–6311. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Schoene, N. Clovamide-type phenylpropenoic acid amides, N-coumaroyldopamine and N-caffeoyldopamine, inhibit platelet-leukocyte interactions via suppressing P-selectin expression. J. Pharmacol. Exp. Ther. 2006, 317, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Shimada, C.; Uesawa, Y.; Ishihara, M.; Kagaya, H.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Takao, K.; Saito, T.; Sugita, Y.; Sakagami, H. Quantitative structure–cytotoxicity relationship of phenylpropanoid amides. Anticancer Res. 2014, 34, 3543–3548. [Google Scholar] [PubMed]
- Wu, Z.R.; Bai, Z.T.; Sun, Y.; Chen, P.; Yang, Z.G.; Zhi, D.J.; Li, Y.; Wang, X.; Du, J.J.; Yang, R.; Cui, P.; Zhang, Y.; Li, H.Y. Protective effects of the bioactive natural product N-trans-Caffeoyldopamine on hepatotoxicity induced by isoniazid and rifampicin. Bioorg. Med. Chem. Lett. 2015, 25, 5424–5426. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. N-coumaroyldopamine and N-caffeoyldopamine increase cAMP via beta 2-adrenoceptors in myelocytic U937 cells. FASEB J. 2005, 19, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Travaglia, F.; Giovannelli, L.; Coïsson, J.D.; Bordiga, M.; Pattarino, F.; Arlorio, M. Clovamide and phenolics from cocoa beans (Theobroma cacao L.) inhibit lipid peroxidation in liposomal systems. Food Res. Int. 2013, 50, 129–134. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Zhang, Y. Catalytic reaction mechanism of acetylcholinesterase determined by Born-Oppenheimer ab initio QM/MM molecular dynamics simulations. J. Phys. Chem. B 2010, 114, 8817–8825. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.; Kriz, Z.; Kuca, K.; Jun, D.; Koca, J. Acetylcholinesterases—The structural similarities and differences. J. Enzyme Inhib. Med. Chem. 2007, 22, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Retz, W.; Gsell, W.; Munch, G.; Rosler, M.; Riederer, P. Free radicals in Alzheimer’s disease. J. Neural Transm. Suppl. 1998, 54, 221–236. [Google Scholar] [PubMed]
- Konrath, E.L.; Passos Cdos, S.; Klein, L.C., Jr.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef] [PubMed]
- Pinho, B.R.; Ferreres, F.; Valentao, P.; Andrade, P.B. Nature as a source of metabolites with cholinesterase-inhibitory activity: An approach to Alzheimer’s disease treatment. J. Pharm. Pharmacol. 2013, 65, 1681–1700. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton. J. Agric. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- Cadenas, E. Mitochondrial free radical production and cell signaling. Mol. Aspects Med. 2004, 25, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Chapter 4–Purification of organic chemicals. In Purification of Laboratory Chemicals; Butterworth-Heinemann: Boston, MA, USA, 2013; pp. 103–554. ISBN 978-0-12-382161-4. [Google Scholar]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, A.; Zoete, V.; Michielin, O. Fast docking using the CHARMM force field with EADock DSS. J. Comput. Chem. 2011, 32, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Grassi, J.; Bougis, P.E.; Marchot, P. Conformational flexibility of the acetylcholinesterase tetramer suggested by x-ray crystallography. J. Biol. Chem. 1999, 274, 30370–30376. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Cai, P.; Liu, Q.H.; Yang, X.L.; Fang, S.Q.; Tang, Y.W.; Wang, C.; Wang, X.B.; Kong, L.Y. Design, synthesis, and evaluation of salicyladimine derivatives as multitarget-directed ligands against Alzheimer’s disease. Bioorg. Med. Chem. 2017, 25, 5917–5928. [Google Scholar] [CrossRef] [PubMed]
- Tomasina, F.; Carabio, C.; Celano, L.; Thomson, L. Analysis of two methods to evaluate antioxidants. Biochem. Mol. Biol. Educ. 2012, 40, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric determination of antioxidant activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanoglu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Enami, S.; Sakamoto, Y.; Colussi, A.J. Fenton chemistry at aqueous interfaces. Proc. Natl. Acad. Sci. USA 2014, 111, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Albericio, F. Peptide coupling reagents, more than a letter soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Dormoy, J.R.; Evin, G.; Selve, C. Reactifs de couplage peptidique I (1) - l′hexafluorophosphate de benzotriazolyl N-oxytrisdimethylamino phosphonium (B.O.P.). Tetrahedron Lett. 1975, 16, 1219–1222. [Google Scholar] [CrossRef]
- Chierrito, T.P.C.; Pedersoli-Mantoani, S.; Roca, C.; Requena, C.; Sebastian-Perez, V.; Castillo, W.O.; Moreira, N.C.S.; Perez, C.; Sakamoto-Hojo, E.T.; Takahashi, C.S.; et al. From dual binding site acetylcholinesterase inhibitors to allosteric modulators: A new avenue for disease-modifying drugs in Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Sheeja Malar, D.; Beema Shafreen, R.; Karutha Pandian, S.; Pandima Devi, K. Cholinesterase inhibitory, anti-amyloidogenic and neuroprotective effect of the medicinal plant Grewia tiliaefolia—An in vitro and in silico study. Pharm. Biol. 2017, 55, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.A.; Sultatos, L.G. Concentration-dependent kinetics of acetylcholinesterase inhibition by the organophosphate paraoxon. Toxicol. Sci. 2006, 90, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure-antioxidant activity relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of mction, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Guitard, R.; Nardello-Rataj, V.; Aubry, J.M. Theoretical and kinetic tools for selecting effective antioxidants: Application to the protection of omega-3 oils with natural and synthetic phenols. Int. J. Mol. Sci. 2016, 17, 1220. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Koncic, M.Z.; Barbaric, M.; Perkovic, I.; Zorc, B. Antiradical, chelating and antioxidant activities of hydroxamic acids and hydroxyureas. Molecules 2011, 16, 6232–6242. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food antioxidants: Chemical insights at the molecular level. Annu. Rev. Food. Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
| Compound | AChE Inhibition IC50 (μM) |
|---|---|
| trans-Caffeic acid | 42.81 ± 1.79 |
| trans-Ferulic acid | 20.57 ± 0.65 |
| N-trans-Caffeoyldopamine | 19.12 ± 0.83 |
| N-trans-Feruloyldopamine | 8.52 ± 0.27 |
| Galantamine | 3.89 ± 0.10 |
| Compound | DPPH• Scavenge IC50 (μM) | ABTS•+ Scavenge IC50 (μM) | FRAP AAE (μmol/mmol) |
|---|---|---|---|
| trans-Caffeic acid | 18.86 ± 0.22 | 1.19 ± 0.02 | 526.05 ± 12.87 |
| trans-Ferulic acid | 19.93 ± 0.18 | 1.62 ± 0.01 | 486.80 ± 11.75 |
| N-trans-Caffeoyldopamine | 5.95 ± 0.12 | 0.24 ± 0.00 | 822.45 ± 13.53 |
| N-trans-Feruloyldopamine | 12.29 ± 0.04 | 0.74 ± 0.00 | 661.53 ± 13.51 |
| l(+)-Ascorbic acid | 1.14 ± 0.03 | 0.11 ± 0.00 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dizdar, M.; Vidic, D.; Požgan, F.; Štefane, B.; Maksimović, M. Acetylcholinesterase Inhibition and Antioxidant Activity of N-trans-Caffeoyldopamine and N-trans-Feruloyldopamine. Sci. Pharm. 2018, 86, 11. https://doi.org/10.3390/scipharm86020011
Dizdar M, Vidic D, Požgan F, Štefane B, Maksimović M. Acetylcholinesterase Inhibition and Antioxidant Activity of N-trans-Caffeoyldopamine and N-trans-Feruloyldopamine. Scientia Pharmaceutica. 2018; 86(2):11. https://doi.org/10.3390/scipharm86020011
Chicago/Turabian StyleDizdar, Muamer, Danijela Vidic, Franc Požgan, Bogdan Štefane, and Milka Maksimović. 2018. "Acetylcholinesterase Inhibition and Antioxidant Activity of N-trans-Caffeoyldopamine and N-trans-Feruloyldopamine" Scientia Pharmaceutica 86, no. 2: 11. https://doi.org/10.3390/scipharm86020011
APA StyleDizdar, M., Vidic, D., Požgan, F., Štefane, B., & Maksimović, M. (2018). Acetylcholinesterase Inhibition and Antioxidant Activity of N-trans-Caffeoyldopamine and N-trans-Feruloyldopamine. Scientia Pharmaceutica, 86(2), 11. https://doi.org/10.3390/scipharm86020011