Antimycobacterial Activities of N-Substituted-Glycinyl 1H-1,2,3-Triazolyl Oxazolidinones and Analytical Method Development and Validation for a Representative Compound
Abstract
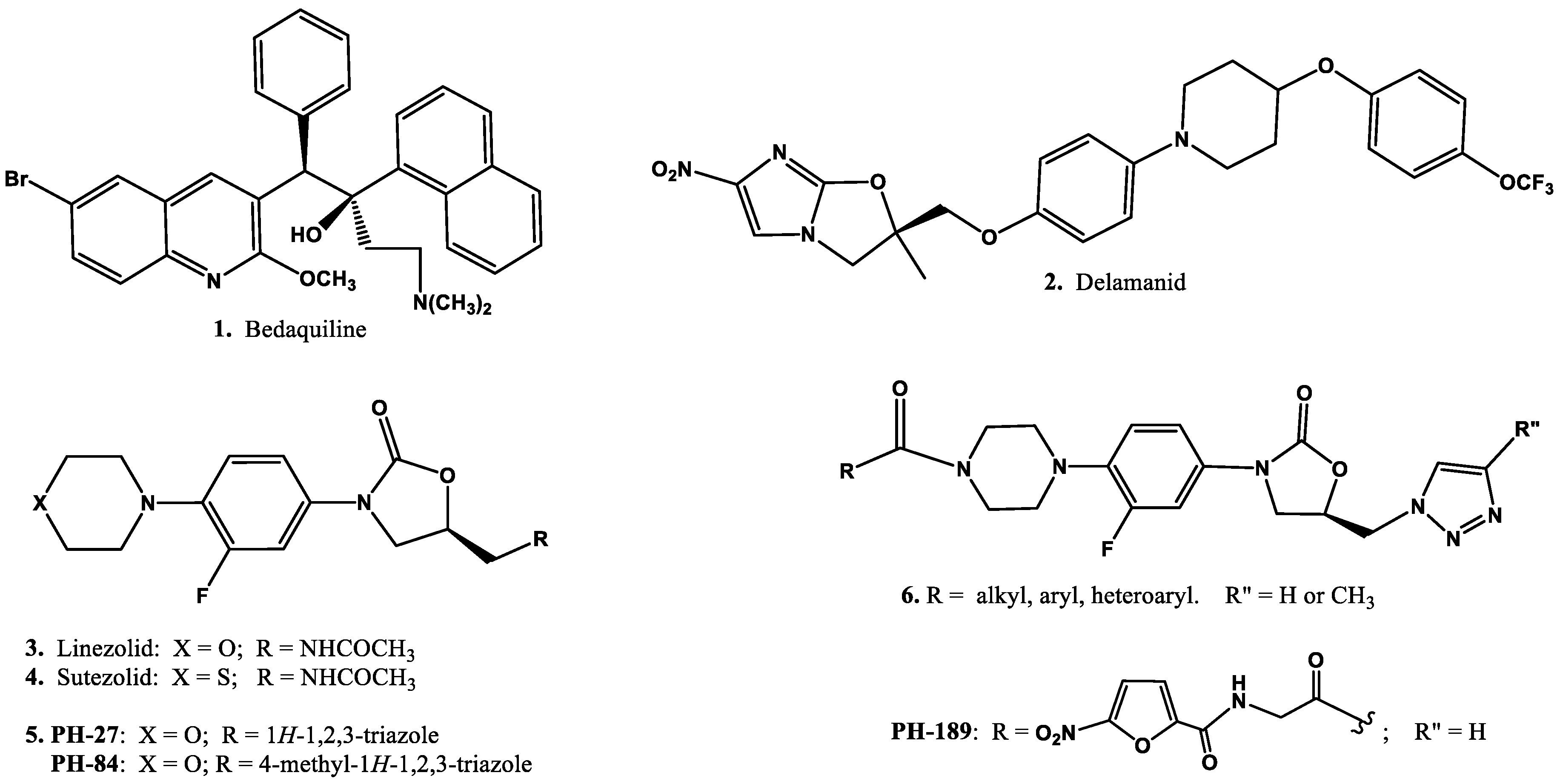
:1. Introduction
2. Chemistry, Anti-Mycobacterial Susceptibility Testing, and Liquid Chromatography–Mass Spectrometry Instrumentation
3. Results and Discussion
3.1. Antimycobacterial Activity
3.2. Analytical Methods Development and Stability Studies of PH-189
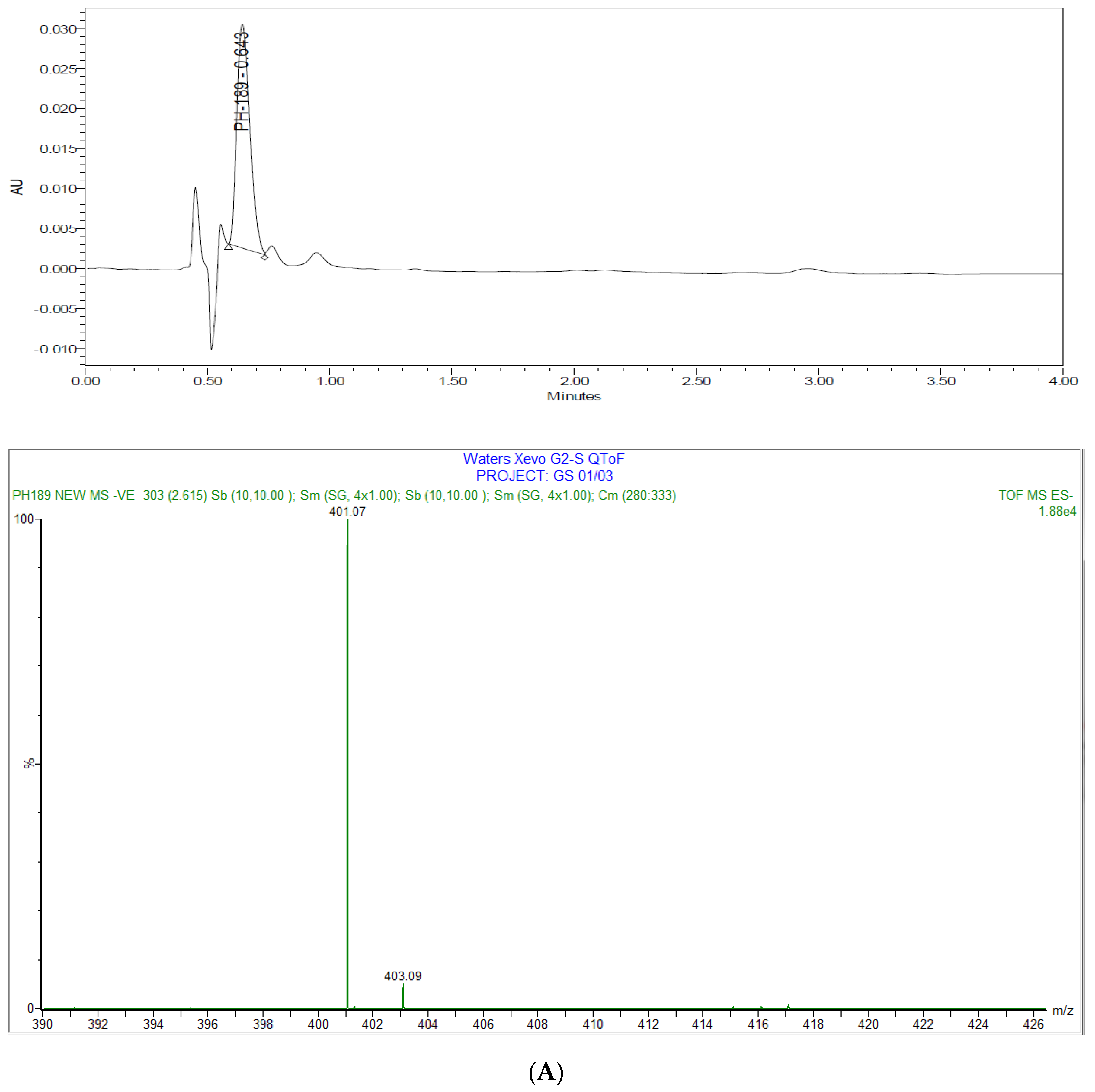
3.2.1. Method Development
3.2.2. Calibration Curve
3.2.3. Accuracy and Precision
3.2.4. Extraction Recovery
3.2.5. Limit of Quantification and Limit of Detection
3.2.6. Evaluation of PH-189 Stability in Human Plasma
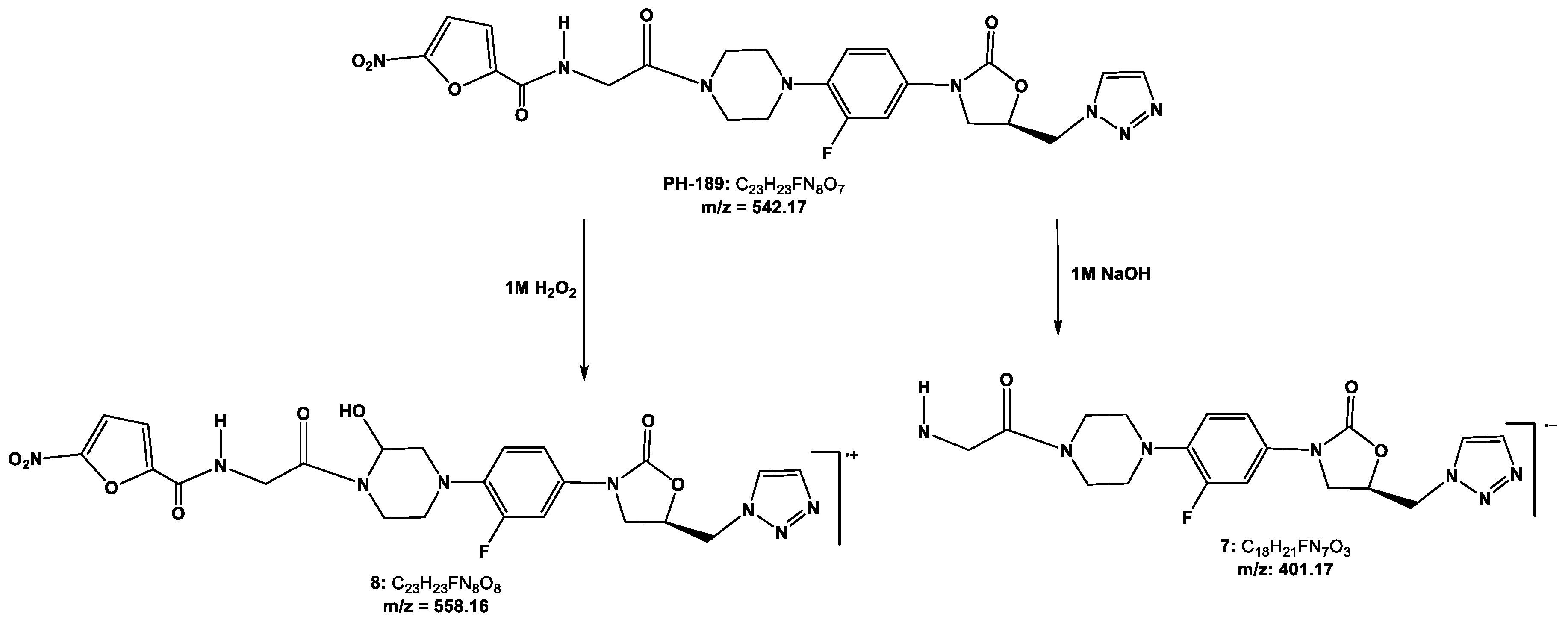
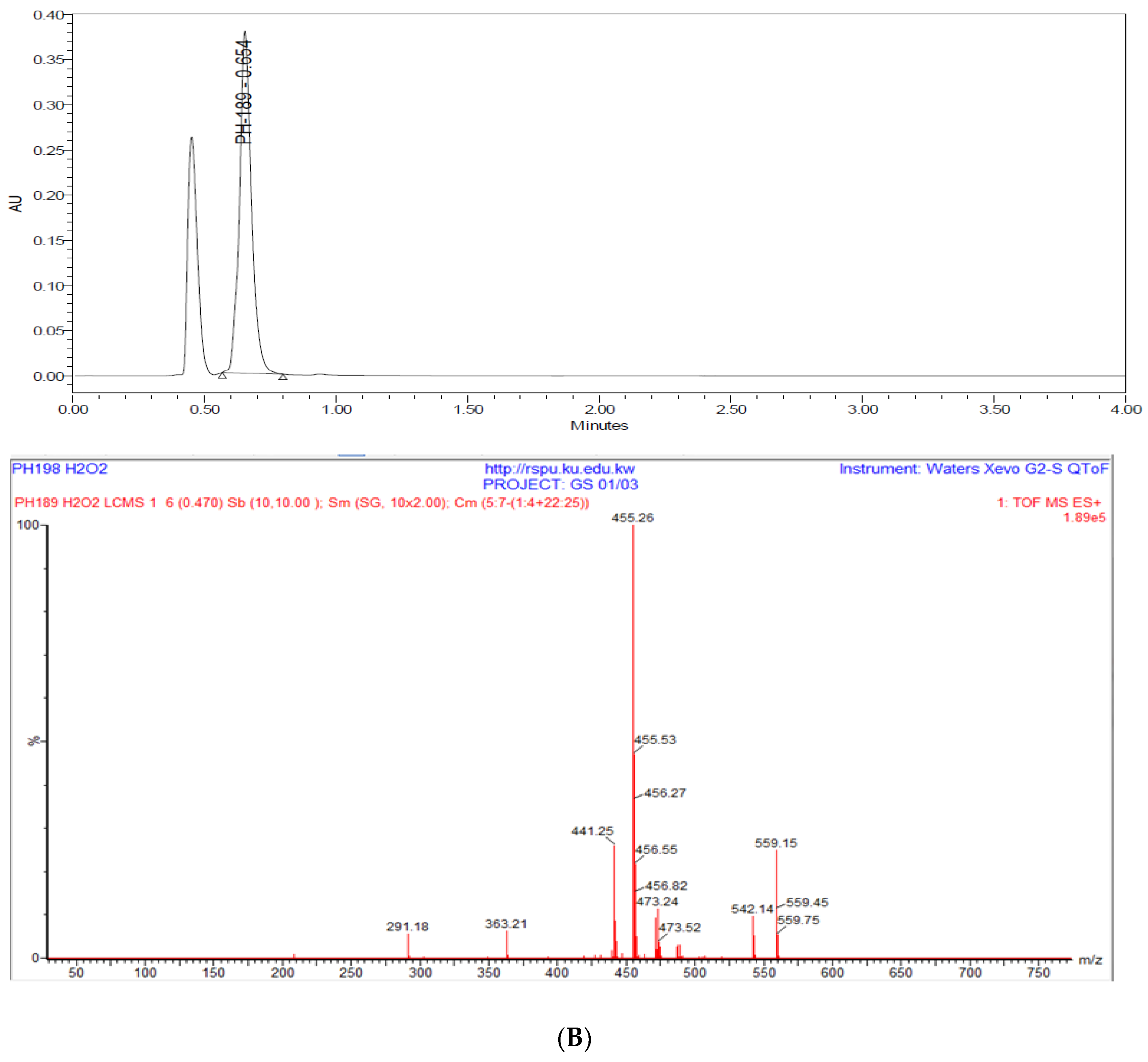
3.2.7. Degradation Studies
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Mycobacterium Tuberculosis Assay
5.3. Minimal Inhibitory Concentration (MIC)
5.4. Minimal Bactericidal Concentration
5.5. Low-Oxygen Recovery Assay
5.6. Intracellular (Macrophage) Drug Screening Assay
5.7. Method Development and Validation for Analysis of PH-189
5.7.1. Instrumentation and Chromatographic Conditions
5.7.2. Standard Solutions of PH-189 and Internal Standard
5.7.3. Human Plasma Extraction Procedure
5.8. Method Validation Criteria for PH-189
5.8.1. Calibration Curve (Linearity)
5.8.2. Accuracy and Precision
5.8.3. Sensitivity (Limit of Detection and Limit of Quantification)
5.8.4. Extraction Recovery
5.8.5. Evaluation of PH-189 Stability in Human Plasma
5.8.6. Degradation Studies
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO Global Tuberculosis Report 2016. 2016. Available online: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1 (accessed on 28 May 2017).
- Poce, G.; Cocozza, M.; Consalvi, S.; Biava, M. SAR analysis of new anti-TB drugs currently in pre-clinical and clinical development. Eur. J. Med. Chem. 2014, 86, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Brigden, G.; Hewison, C.; Varaine, F. New developments in the treatment of drug-resistant tuberculosis: Clinical utility of bedaquiline and delamanid. Infect. Drug Resist. 2015, 8, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Szumowski, J.D.; Lynch, J.B. Profile of delamanid for the treatment of multidrug-resistant tuberculosis. Drug Des. Dev. Ther. 2015, 9, 677–682. [Google Scholar]
- Butler, M.S.; Blaskovich, M.A.; Cooper, M.A. Antibiotics in the clinical pipeline at the end of 2015. J. Antibiot. 2017, 70, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.J.; Menzies, D. A Review of the Evidence for Using Bedaquiline (TMC207) to Treat Multi-Drug Resistant Tuberculosis. Infect. Dis. Ther. 2013, 2, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H. Update on linezolid: The first oxazolidinone antibiotic. Expert Opin. Pharmacother. 2005, 6, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Blaskovich, M.A.; Cooper, M.A. Antibiotics in the clinical pipeline in 2013. J. Antibiot. 2013, 66, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.C.; Hallbauer, U.M.; Seddon, J.A.; Hesseling, A.C.; Schaaf, H.S. Linezolid-containing regimens for the treatment of drug-resistant tuberculosis in South African children. Int. J. Tuberc. Lung Dis. 2012, 16, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.B.; Jiang, R.H.; Li, L.; Xiao, H.P. Linezolid in the treatment of MDR-TB: A retrospective clinical study. Int. J. Tuberc. Lung Dis. 2012, 16, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Ager, S.; Gould, K. Clinical update on linezolid in the treatment of Gram-positive bacterial infections. Infect. Drug Resist. 2012, 5, 87–102. [Google Scholar] [PubMed]
- Phillips, O.A.; Udo, E.E.; Abdel-Hamid, M.E.; Varghese, R. Synthesis and antibacterial activities of N-substituted-glycinyl 1H-1,2,3-Triazolyl oxazolidinones. Eur. J. Med. Chem. 2013, 66, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.A.; Udo, E.E.; Abdel-Hamid, M.E.; Varghese, R. Synthesis and antibacterial activity of novel 5-(4-Methyl-1H-1,2,3-triazole) methyl oxazolidinones. Eur. J. Med. Chem. 2009, 44, 3217–3227. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.A.; Udo, E.E.; Ali, A.A.; Al-Hassawi, N. Synthesis and antibacterial activity of 5-substituted oxazolidinones. Bioorg. Med. Chem. 2003, 11, 35–41. [Google Scholar] [CrossRef]
- Phillips, O.A.; Udo, E.E.; Varghese, R. Antimycobacterial Activities of Novel 5-(1H-1,2,3-Triazolyl)Methyl Oxazolidinones. Tuberc. Res. Treat. 2012, 2012, 289136. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Kim, E.J.; Kwon, J.W.; Kim, W.B.; Lee, M.G. High-performance liquid chromatographic analysis of DA-7867, a new oxazolidinone, in human plasma and urine and in rat tissue homogenates. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 794, 397–403. [Google Scholar] [CrossRef]
- Cavazos-Rocha, N.; Carmona-Alvarado, I.; Vera-Cabrera, L.; Waksman-de-Torres, N.; Salazar-Cavazos Mde, L. HPLC method for the simultaneous analysis of fluoroquinolones and oxazolidinones in plasma. J. Chromatogr. Sci. 2014, 52, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Hedaya, M.A.; Thomas, V.; Abdel-Hamid, M.E.; Kehinde, E.O.; Phillips, O.A. A validated UPLC-MS/MS method for the analysis of linezolid and a novel oxazolidinone derivative (PH027) in plasma and its application to tissue distribution study in rabbits. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1040, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.A.; Abdel-Hamid, M.E. Determination of novel antibacterial triazolylmethyl oxazolidinones concentrations in human plasma by APCI-LC-MS: Application to stability study. J. Pharm. Pharm. Sci. 2008, 11, 22s–31s. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.A.; Sharaf, L.H.; Abdel-Hamid, M.E.; Varghese, R. Assessment of the stability of novel antibacterial triazolyl oxazolidinones using a stability-indicating high-performance liquid chromatography method. Med. Princ. Pract. 2011, 20, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Warit, S.; Wan, B.; Hwang, C.H.; Pauli, G.F.; Franzblau, S.G. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2007, 51, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Susceptibility Testing of Mycobacteria, Nocaridae, and Other Aerobic Actinomycetes. Approved Standard, 2nd ed.; CLSI Document M24-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]
- Cooksey, R.C.; Crawford, J.T.; Jacobs, W.R., Jr.; Shinnick, T.M. A rapid method for screening antimicrobial agents for activities against a strain of Mycobacterium tuberculosis expressing firefly luciferase. Antimicrob. Agents Chemother. 1993, 37, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Wayne, L.G.; Hayes, L.G. An In Vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 1996, 64, 2062–2069. [Google Scholar] [PubMed]
- Barrow, E.L.; Quenelle, D.C.; Suling, W.J.; Barrow, W.W. Use of mono Mac 6 Human Moncytic Cell Line and J774 Murine Macrophage Cell Line in Parallel Antimycobacterial Drug Studies. Antimicrob. Agents Chemother. 1996, 40, 2206–2208. [Google Scholar]
- Rastogi, N.; Labrousse, V.; Goh, K.S.; De Sousa, J.P. Antimycobacterial spectrum of sparfloxacin and its activities alone and in association with other drugs against Mycobacterium avium complex growing extracellularly and intracellularly in murine and human macrophages. Antimicrob. Agents Chemother. 1991, 35, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Wu, N.; Du, G.J.; Zhao, S.Q.; Yan, M.; Gu, L.Q. Synthesis and antibacterial activities of eperezolid analogs with glycinyl substitutions. Arch. Pharm. 2009, 342, 377–385. [Google Scholar] [CrossRef] [PubMed]

| Compound Code | R′ | R″ | MIC H37Rv | % Inhibition a | MBC H37Rv | MIC INH-R b | % Inhibition | MIC RMP-R c | % Inhibition | MIC OFX-R d | % Inhibition |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PH-172 | H . CF3CO2H | H | 3.6 | 59 | N/A e | 7.2 | 68 | 3.6 | 63 | 3.6 | 62 |
| PH-151 | H . CF3CO2H | CH3 | 5.8 | 63 | N/A | 11.6 | 68 | 5.8 | 73 | 11.6 | 71 |
| PH-165 | CH3CO | H | 6.7 | 62 | N/A | 4.5 | 68 | 6.7 | 70 | 3.4 | 63 |
| PH-182 | CHCl2CO | CH3 | 1.9 | 75 | N/A | 0.95 | 67 | 0.95 | 67 | 0.47 | 68 |
| PH-169 | 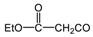 | H | 2.9 | 51 | N/A | 1.45–2.9 | 53–66 | 1.45 | 61 | 1.45 | 69 |
| PH-150 |  | CH3 | 5.8 | 79 | N/A | 2.9 | 59 | 2.9 | 66 | 2.9 | 69 |
| PH-193 | 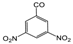 | H | 1.7 | 68 | N/A | 0.42 | 59 | 0.22 | 66 | 0.052 | 52 |
| PH-181 |  | CH3 | 3.3 | 76 | N/A | 0.85–1.64 | 51–63 | 0.82 | 60 | 0.82 | 66 |
| PH-189 | 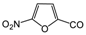 | H | 0.5 | 57 | N/A | 0.057–0.12 | 59–65 | 0.46 | 58 | 0.03 | 56 |
| PH-145 |  | CH3 | 0.5 | 58 | N/A | 0.056–0.11 | 51–64 | 0.11 | 50 | 0.056 | 65 |
| PH-195 |  | H | 3.9 | 71 | N/A | 2 | 58 | 0.98 | 75 | 0.49 | 63 |
| PH-185 |  | CH3 | 5.7–11.5 | 41–68 | N/A | 2.9 | 72 | 5.7 | 63 | 1.44 | 60 |
| RMP f | - | - | 0.06 | 66 | 1.56 | 0.06 | 58 | N/A g | N/A | 0.95 | 56 |
| INH f | - | - | N/A | N/A | N/A | N/A | N/A | 0.15 | 62 | N/A | N/A |
| Compound Code | LORA (μM) | Macrophage Log Reduction (Low Conc.) | Macrophage Log Reduction (Mid Conc.) | Macrophage Log Reduction (High Conc.) | MTT % Viability (Low Conc.) | MTT % Viability (Mid Conc.) | MTT % Viability (High Conc.) |
|---|---|---|---|---|---|---|---|
| PH-172 | >58 | 1.76 (0.36) | 5.55 (3.6) | 1.18 (36) | 87 | 72 | 71 |
| PH-151 | >46 | 1.28 (0.39) | 1.06 (3.9) | 1.34 (39) | 83 | 87 | 74 |
| PH-165 | >54 | 1.82 (1.1) | 1.30 (3.4) | 1.13 (34) | 91 | 87 | 84 |
| PH-182 | ≤0.015 | 1.72 (0.09) | 1.35 (0.95) | 1.54 (9.5) | 84 | 90 | 87 |
| PH-169 | ≤0.091 | 1.88 (0.39 | 1.34 (3.9) | 1.24 (39) | 76 | 75 | 73 |
| PH-150 | >47 | 1.85 (0.39) | 1.06 (3.9) | 0.89 (39) | 78 | 82 | 62 |
| PH-193 | ≤0.11 | 1.13 (0.17) | 1.30 (1.7) | 1.78 (17) | 100 | 100 | 74 |
| PH-181 | ≤0.10 | 1.78 (0.33) | 1.28 (3.3) | 2.09 (33) | 91 | 88 | 39 |
| PH-189 | 0.92 | 1.81 (0.18) | 1.45 (1.8) | 1.34 (18) | 98 | 88 | 86 |
| PH-145 | 0.45 | 1.41 (0.036) | 1.17 (0.36) | 1.23 (3.6) | 87 | 93 | 75 |
| PH-195 | 2 | 1.06 (0.20) | 1.35 (2.0) | 1.36 (20) | 100 | 100 | 71 |
| PH-185 | 5.7 | 1.48 (0.38) | 1.09 (3.8) | 0.89 (38) | 88 | 94 | 39 |
| RMP a | 0.95 | 1.62 (0.12) | 2.17 (1.2) | 2.43 (12) | 92 | 85 | 84 |
| Nominal/μg/mL | Mean ± s (n = 3) Observed/μg/mL | Precision a (%) | Accuracy b (%) |
|---|---|---|---|
| 1 | 1.125 ± 0.089 | 7.9 | 112.5 |
| 50 | 46.16 ± 0.665 | 1.4 | 92.32 |
| 70 | 66.28 ± 1.794 | 2.7 | 94.69 |
| 90 | 85.67 ± 2.657 | 3.1 | 95.19 |
| Nominal/μg/mL | Mean ± s (n = 3) Observed/μg/mL | Precision a (%) | Accuracy b (%) |
|---|---|---|---|
| 1 | 1.134 ± 0.091 | 8.1 | 113.4 |
| 50 | 47.50 ± 0.954 | 2.0 | 95.00 |
| 70 | 66.94 ± 2.404 | 3.6 | 95.62 |
| 90 | 84.97 ± 3.460 | 4.1 | 94.41 |
| N (Concentration Range 1–90 μg/mL) | Extracted | Non-Extracted | Recovery (%) |
|---|---|---|---|
| 1 | 0.0421 | 0.0428 | 98.36% |
| 2 | 0.0454 | 0.0462 | 98.26% |
| 3 | 0.0444 | 0.0464 | 95.69% |
| Mean | 0.0440 | 0.0451 | 97.44% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Tannak, N.F.; Phillips, O.A. Antimycobacterial Activities of N-Substituted-Glycinyl 1H-1,2,3-Triazolyl Oxazolidinones and Analytical Method Development and Validation for a Representative Compound. Sci. Pharm. 2017, 85, 34. https://doi.org/10.3390/scipharm85040034
Al-Tannak NF, Phillips OA. Antimycobacterial Activities of N-Substituted-Glycinyl 1H-1,2,3-Triazolyl Oxazolidinones and Analytical Method Development and Validation for a Representative Compound. Scientia Pharmaceutica. 2017; 85(4):34. https://doi.org/10.3390/scipharm85040034
Chicago/Turabian StyleAl-Tannak, Naser F., and Oludotun A. Phillips. 2017. "Antimycobacterial Activities of N-Substituted-Glycinyl 1H-1,2,3-Triazolyl Oxazolidinones and Analytical Method Development and Validation for a Representative Compound" Scientia Pharmaceutica 85, no. 4: 34. https://doi.org/10.3390/scipharm85040034
APA StyleAl-Tannak, N. F., & Phillips, O. A. (2017). Antimycobacterial Activities of N-Substituted-Glycinyl 1H-1,2,3-Triazolyl Oxazolidinones and Analytical Method Development and Validation for a Representative Compound. Scientia Pharmaceutica, 85(4), 34. https://doi.org/10.3390/scipharm85040034






