All chemicals were obtained from Sigma-Aldrich & Alfa Aesar and were used without further purification. Solvents were obtained from commercial suppliers and were used without further purification. Melting points were determined on a Mettler FP1, melting point apparatus (Mettler Toledo, OH, USA) and are uncorrected. FT-IR spectra were recorded on the Nicolet Avatar 380 spectrometer. The NMR spectra were measured in DMSO-d6 or Acetone-d6 at RT using a Bruker DRX-500 spectrometer (Billerica, MA, USA). δ is given in ppm relative to tetramethylsilane as an internal reference. Silica gel column chromatography was carried out using silica gel 230–400 mesh, 60 Å (Sigma-Aldrich, St. Louis, MO, USA). Mass spectrometric analysis (HPLC-ESI-MS) was performed on a TSQ quantum (Thermo Electron Corporation, Waltham, Massachusetts) instrument equipped with an ESI source and a triple quadrupole mass detector (Thermo Finnigan, San Jose, CA, USA). The MS detection was carried out at a spray voltage of 4.2 kV, a nitrogen sheath gas pressure of 4.0 × 105 Pa, an auxiliary gas pressure of 1.0 × 105 Pa, a capillary temperature of 400 °C, and capillary voltage of 35 V. The progress of the reaction was monitored by Thin Layer Chromatography (TLC) using fluorescent pre-coated silica gel plates and detection of the components was made by short ultraviolet light at λ = 254 nm, and methylene chloride–methanol was used as an eluting system, with different ratios for each reaction.
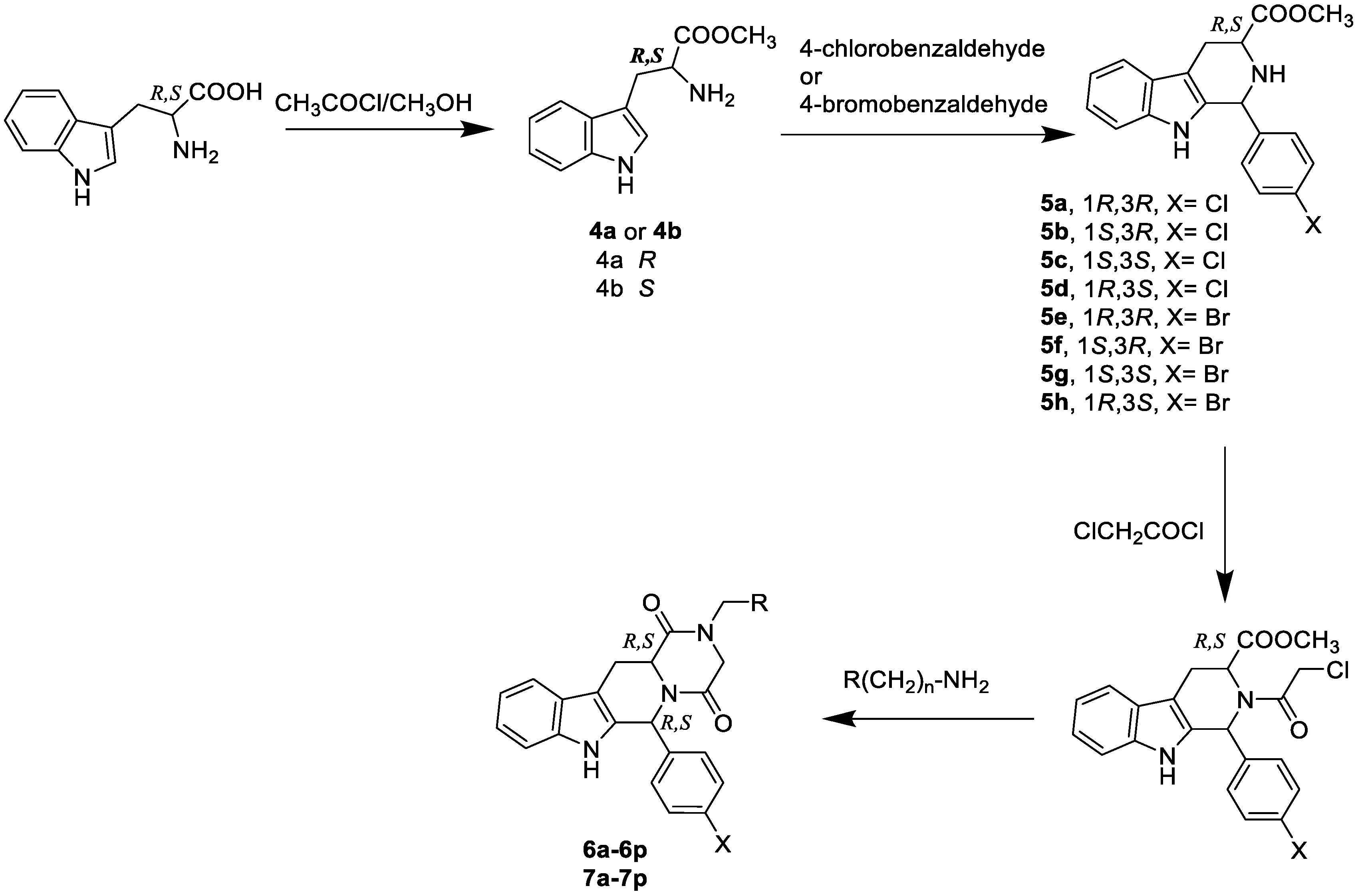
2.1.4. General Procedure for the Preparation of Compounds 6a–6p, 7a–7p
A solution of the appropriate chloroethanone derivative (1.2 mmol) in methanol (25 mL) was heated to reflux with the appropriate amine, namely ethanol amine, ethylenediamine, butanolamine, & 1,4-diaminobutane (2.4 mmol) under nitrogen atmosphere for 16 h, the reaction mixture was cooled to room temperature and then evaporated to dryness under reduced pressure. The residue was dissolved in ethyl acetate, and the organic layer was washed with water, dried over anhydrous MgSO4, filtered, and concentrated till dryness. The crude product was purified using column chromatography eluting with the CH2Cl2 & CH3OH solvent system or with the CH2Cl2, CH3OH, and triethylamine solvent system in the appropriate ratio.
(6R,12aR)6-(4-Chloro-phenyl)-2-(2-hydroxy-ethyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6a). Orange solid. Yield: 10%. mp 230–233 °C. TLC, Rf: 0.39 (CH2Cl2/CH3OH, 97:3). IR: 3056.91 (OH), 2920.61 (NH), 1658.54, 1650.29 (CO). 1H-NMR (Acetone-d6) δ: 3.02 (ddd, J = 15.5, 11.9, 1.2 Hz, 1H, CHaCHb), 3.39 (ddd, J = 14.2, 6.3, 3.7 Hz, 1H, NCHaHbCH2), 3.54 (dd, J = 15.5, 4.3 Hz, 1H, CHaCHb ), 3.71 (ddd, J = 14.1, 6.3, 3.9 Hz, 1H, NCHaHbCH2), 3.87–3.82 (m, 2H, CH2OH), 4.12 (d, J = 17.7 Hz, 1H, CHaHbC(O)N), 4.29 (dd, J = 21.3, 10.6 Hz, 2H, CHaHbC(O)N, CHC(O)), 6.99 (s, 1H, CHPh), 7.19–7.14 (m, 3H, Ar-H), 7.25–7.21 (m, 2H, Ar-H), 7.27 (d, J = 12.9 Hz, 1H, Ar-H), 7.33 (d, J = 8.1 Hz, 1H, Ar-H), 7.52 (d, J = 7.8 Hz, 1H,Ar-H), 10.22 (s, 1H, NH). ESI-MS: m/z 411 (M+ + 2), m/z 409 (M+).
(6R,12aR)-2-(2-Amino-ethyl)-6-(4-chloro-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6b). Yellow solid. Yield: 13%. mp 231–234 °C. TLC, Rf: 0.54 (CH2Cl2/CH3OH/TEA, 95:4:1). IR: 3733.97, 3662.21 (NH2), 2968 (NH), 1663.54, 1654.29 (CO). 1H-NMR (Acetone-d6) δ: 2.97 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaCHb), 3.37 (dd, J = 15.3, 4.1 Hz, 1H, CHaCHb), 3.45–3.41 (m, 1H, NCHaHbCH2), 3.59–3.51 (m, 2H, CH2NH2), 3.66–3.61 (m, 1H, NCHaHbCH2), 4.16 (dd, J = 11.8, 4.3 Hz, 1H, CHC(O)), 4.23 (dd, J = 17.8, 0.5 Hz, 1H, CHaHbC(O)N), 4.42 (dd, J = 17.8, 1.0 Hz, 1H, CHaHbC(O)N), 7.04 (s, 1H, CHPh), 7.08 (ddd, J = 8.1, 7.1, 1.0 Hz, 1H, Ar-H), 7.15 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar-H), 7.32–7.30 (m, 1H, Ar-H), 7.30–7.28 (m, 1H, Ar-H), 7.38 (d, J = 1.8 Hz, 2H, Ar-H), 7.4 (d, J = 1.3 Hz, 1H, Ar-H), 7.57 (d, J = 7.7 Hz, 1H, Ar-H), 10.22 (s, 1H, NH). ESI-MS: m/z 410 (M+ + 2), m/z 408 (M+).
(6R,12aR)-6-(4-Chloro-phenyl)-2-(4-hydroxy-butyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6c). Orange solid, yield: 48%. mp 228–230 °C. TLC, Rf: 0.31 (CH2Cl2/CH3OH, 97:3). IR: 3100.62 (OH), 2920 (NH), 1660.54, 1650 (CO). 1H-NMR (Acetone-d6) δ: 1.55–1.50 (m, 2H, CH2 CH2), 1.71–1.65 (m, 2H, CH2CH2), 3.13 (ddd, J = 16.0, 11.6, 1.3 Hz, 1H, CHaCHb), 3.46–3.40 (m, 1H, NCHaHbCH2), 3.55–3.51 (m, 1H, NCHaHbCH2), 3.58 (dd, J = 11.1, 6.3 Hz, 2H, CH2OH), 3.65 (dd, J = 16.2, 4.9 Hz, 1H, CHaCHb), 3.93 (d, J = 17.1 Hz, 1H, CHaHbC(O)N), 4.24 (dd, J = 17.1, 1.3 Hz, 1H, CHaHbC(O)N), 4.46 (dd, J = 11.5, 5.7 Hz, 1H, CHC(O)), 6.32 (s, 1H, CHPh), 7.07–7.03 (m, 1H, Ar-H), 7.11–7.07 (m, 1H, Ar-H), 7.26–7.24 (m, 1H, Ar-H), 7.28–7.26 (m, 1H, Ar-H), 7.32–7.29 (m, 1H, Ar-H), 7.38–7.37 (m, 1H, Ar-H),7.40–7.39 (m, 1H, Ar-H), 7.61–7.58 (m, 1H, Ar-H), 10.20 (s, 1H, NH). ESI-MS: m/z 440 (M+ + 2), m/z 438 (M+).
(6R,12R)-2-(4-Amino-butyl)-6-(4-chloro-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6d). Yellow solid. Yield: 5%. mp 228–232 °C. TLC, Rf: 0.35 (CH2Cl2/CH3OH/TEA, 93:5:2). IR: 3666.41, 3542.31 (NH2), 2930.4 (NH), 1661.4, 1652 (CO). 1H-NMR (DMSO-d6): δ 1.37–1.27 (m, 2H, CH2CH2), 1.57–1.47 (m, 2H, CH2CH2), 2.58–2.52 (m, 2H, CH2NH2), 2.99 (dd, J = 14.8, 11.2 Hz, 1H, CHaHb), 3.28 (dd, J = 15.1, 4.5 Hz, 1H, CHaHb), 3.52–3.40 (m, 2H, NCHaHbCH2), 4.08–3.88 (m, 2H, CHC(O), CHaHbC(O)N), 4.29 (d, J = 17.7 Hz, 1H, CHaHbC(O)N), 6.90 (s, 1H, CHPh), 7.06–7.00 (m, 1H, Ar-H), 7.15–7.08 (m, 1H, Ar-H), 7.35–7.22 (m, 4H, Ar-H), 7.44 (d, J = 8.4 Hz, 1H, Ar-H), 7.57–7.51 (m, 1H, Ar-H), 11.07 (s, 1H, NH). ESI-MS: m/z 439 (M+ + 2), m/z 437 (M+).
(6S,12aR)-6-(4-Chloro-phenyl)-2-(2-hydroxy-ethyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6e). Orange solid. Yield: 26%. mp 202–206 °C. TLC, Rf: 0.39, (CH2Cl2/CH3OH, 97:3). IR: 3056.91 (OH), 2920.61 (NH), 1658.54, 1650.29 (CO). 1H-NMR (DMSO-d6): δ 3.01 (dd, J = 15.8, 11.1 Hz, 1H, CHaHb), 3.42–3.33 (m, 3H, NCHaHbCH2, CHaHb), 3.56 (dd, J = 7.6, 3.7 Hz, 2H, CH2OH), 4.06 (dd, J = 11.7, 4.3 Hz, 1H, CHC(O)), 4.14 (d, J = 17.7 Hz, 1H, CHaHbC(O)N), 4.34 (d, J = 17.7 Hz, 1H, CHaHbC(O)N), 6.91 (s, 1H, CHPh), 7.03 (t, J = 7.4 Hz, 1H, Ar-H), 7.12 (t, J = 7.3 Hz, 1H, Ar-H), 7.24 (d, J = 8.5 Hz, 2H, Ar-H), 7.26–7.24 (m, 1H, Ar-H), 7.33 (d, J = 8.0 Hz, 1H, Ar-H), 7.47–7.42 (m, 2H, Ar-H), 7.53 (d, J = 7.4 Hz, 1H, Ar-H), 11.05 (s, 1H, NH). ESI-MS: m/z 411 (M+ + 2), m/z 409 (M+).
(6S,12aR)-2-(2-Amino-ethyl)-6-(4-chloro-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6f). Orange solid. Yield: 10%. mp 200–204 °C. TLC, Rf: 0.54, (CH2Cl2/CH3OH/TEA, 95:4:1). IR: 3733.97, 3662.21 (NH2), 2968 (NH), 1660.64, 1652.21 (CO). 1H-NMR (DMSO-d6): δ 2.75 (t, J = 7.9 Hz, 2H, CH2NH2), 3.04 (dd, J = 15.2, 11.3 Hz, 1H,CHaHb), 3.49–3.39 (m, 2H, NCHaHbCH2), 4.10 (d, J = 17.3 Hz, 2H, CHaHbC(O)N, CHC(O)), 4.34 (d, J = 18.0 Hz, 1H, CHaHbC(O)N), 6.91 (s, 1H, CHPh), 7.04 (t, J = 7.3 Hz, 1H, Ar-H), 7.12 (t, J = 7.6 Hz, 1H, Ar-H), 7.25 (d, J = 8.1 Hz, 2H, Ar-H), 7.34 (d, J = 7.9 Hz, 1H, Ar-H), 7.44 (d, J = 8.4 Hz, 2H, Ar-H), 7.53 (d, J = 7.5 Hz, 1H, Ar-H), 11.06 (s, 1H, NH). ESI-MS: m/z 410 (M+ + 2), m/z 408 (M+).
(6S,12aR)-6-(4-Chloro-phenyl)-2-(4-hydroxy-butyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6g). Orange solid. Yield: 64%. mp 200–203 °C. TLC, Rf: 0.31, (CH2Cl2/CH3OH, 97:3). IR: 3100.62 (OH), 2920 (NH), 1660.54, 1650 (CO). 1H-NMR (DMSO-d6): δ 1.43–1.33 (m, 2H, CH2CH2), 1.59–1.48 (m, 2H, CH2CH2), 2.99 (dd, J = 15.5, 11.6 Hz, 1H,CHaHb), 3.19–3.12 (m, 1H, NCHaHbCH2), 3.40 (dd, J = 11.7, 6.1 Hz, 2H, CH2OH), 3.54–3.44 (m, 1H, NCHaHbCH2), 4.07–3.96 (m, 2H, CHaHbC(O)N, CHC(O)), 4.30 (d, J = 17.6 Hz, 1H, CHaHbC(O)N), 6.90 (s, 1H, CHPh), 7.03 (t, J = 7.4 Hz, 1H, Ar-H), 7.12 (t, J = 7.5 Hz, 1H, Ar-H), 7.23 (d, J = 8.4 Hz, 2H, Ar-H), 7.33 (d, J = 7.9 Hz, 1H, Ar-H), 7.44 (d, J = 8.4 Hz, 2H, Ar-H), 7.53 (d, J = 7.7 Hz, 1H, Ar-H), 11.08 (s, 1H, NH). ESI-MS: m/z 440 (M+ + 2), m/z 438 (M+).
(6S,12R)-2-(4-Amino-butyl)-6-(4-chloro-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6h). Yellow solid. Yield: 29%. mp 200–202 °C. TLC, Rf: 0.35, (CH2Cl2/CH3OH/TEA, 93:5:2). IR: 3666.41, 3542.31 (NH2), 2930.4 (NH), 1662.3, 1654 (CO). 1H-NMR (Acetone-d6): δ 1.61–1.55 (m, 2H, CH2CH2), 1.69–1.63 (m, 2H,CH2CH2), 2.99 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaHb), 3.20 (t, J = 6.7 Hz, 2H, CH2NH2), 3.37 (dd, J = 15.4, 4.2 Hz, 1H, CHaHb), 3.60–3.57 (m, 1H, NCHaHbCH2), 3.63–3.61 (m, 1H, NCHaHbCH2), 4.03 (d, J = 17.6 Hz, 1H, CHaHbC(O)N), 4.17 (dd, J = 11.8, 4.1 Hz, 1H, CHC(O)), 4.30 (dd, J = 17.6, 1.1 Hz, 1H, CHaHbC(O)N), 7.04 (s, 1H, CHPh), 7.08 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar-H), 7.15 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar-H), 7.30–7.28 (m, 2H, Ar-H), 7.38–7.36 (m, 2H, Ar-H), 7.40–7.38 (m, 1H, Ar-H), 7.58–7.55 (m, 1H, Ar-H), 10.26 (s, 1H, NH). ESI-MS: m/z 439 (M+ + 2), m/z 437 (M+).
(6S,12aS)-6-(4-Chloro-phenyl)-2-(2-hydroxy-ethyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6i). Yellow solid. Yield: 40%. mp 231–233 °C, TLC, Rf: 0.39, (CH2Cl2/CH3OH, 97:3). IR: 3056.91 (OH), 2920.61 (NH), 1658.54, 1650.29 (CO). 1H-NMR (Acetone-d6): δ 3.01 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaHb), 3.38 (dd, J = 15.4, 4.3 Hz, 1H, CHaHb), 3.47 (ddd, J = 13.8, 6.0, 4.9 Hz, 1H, NCHaHbCH2), 3.63–3.57 (m, 1H, NCHaHbCH2), 3.75 (ddd, J = 10.4, 5.4, 2.7 Hz, 2H, CH2OH), 4.24–4.17 (m, 2H, CHC(O), CHaHbC(O)N), 4.40 (dd, J = 17.7, 1.1 Hz, 1H, CHaHbC(O)N), 7.04 (s, 1H, CHPh), 7.08 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar-H), 7.15 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar-H), 7.30–7.28 (m, 1H, Ar-H), 7.32–7.31 (m, 1H, Ar-H), 7.38–7.36 (m, 2H, Ar-H), 7.39 (d, J = 1.6 Hz, 1H, Ar-H), 7.57 (d, J = 7.9 Hz, 1H, Ar-H), 10.15 (s, 1H, NH). ). 13C-NMR (Acetone-d6): δ 166, 163.3 (NCO), 139.28, 137.8, 134.54, 131.21, 130.85, 129.52, 127.39, 122.96, 120.22, 112.21, 109.56, 60.39 (NC(O)CHaHb), 53.55 (C6), 51.83 (C12), 51.6 (NCHaHbCH2), 49.48 (CH2OH), 27.84 (CCHaHb). ESI-MS: m/z 411 (M+ + 2), m/z 409 (M+).
(6S,12aS)-2-(2-Amino-ethyl)-6-(4-chloro-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6j). Yellow solid. Yield: 30%. mp 231–233 °C, TLC, Rf: 0.54, (CH2Cl2/CH3OH/TEA, 95:4:1). IR: 3733.97, 3662.21 (NH2), 2968 (NH), 1657.4, 1650 (CO). 1H-NMR (Acetone-d6): δ 2.97 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaHb), 3.38 (dd, J = 15.4, 4.1 Hz, 1H, CHaHb), 3.47–3.40 (m, 2H, CH2NH2), 3.56–3.51 (m, 1H, NCHaHbCH2), 3.66–3.61 (m, 1H, NCHaHbCH2), 4.16 (dd, J = 11.9, 4.2 Hz, 1H, CHC(O)), 4.23 (d, J = 17.4 Hz, 1H, CHaHbC(O)N), 4.41 (dd, J = 17.8, 1.1 Hz, 1H, CHaHbC(O)N), 7.04 (s, 1H, CHPh), 7.08 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar-H), 7.15 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar-H), 7.29–7.28 (m, 1H, Ar-H), 7.31–7.30 (m, 1H, Ar-H), 7.39–7.36 (m, 2H, Ar-H), 7.40–7.39 (m, 1H, Ar-H), 7.58–7.55 (m, 1H, Ar-H), 10.23 (s, 1H, NH).13C-NMR (Acetone-d6): δ 165.62, 163.44 (NCO), 139.3, 137.8, 134.52, 131.16, 130.88, 129.52, 127.39, 122.94, 120.19, 119.05, 112.17, 109.55, 53.57 (C6), 51.79 (C12), 49.76 (NC(O)CHaHb), 47.99 (NCHaHbCH2), 47.01 (CH2NH2), 27.78 (CCHaHb). ESI-MS: m/z 410 (M+ + 2), m/z 408 (M+).
(6S,12aS)-6-(4-Chloro-phenyl)-2-(4-hydroxy-butyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6k). Yellow solid. Yield: 31%. mp: 227–229 °C. TLC, Rf: 0.31, (CH2Cl2/CH3OH, 97:3). IR: 3100.62 (OH), 2920 (NH), 1660.54, 1650 (CO). 1H-NMR (Acetone-d6): δ 1.56–1.51 (m, 2H, CH2CH2), 1.71–1.64 (m, 2H, CH2CH2), 3.13 (ddd, J = 16.0, 11.6, 1.3 Hz, 1H, CHaHb), 3.46–3.40 (m, 1H, NCHaHbCH2), 3.52–3.49 (m, 1H, NCHaHbCH2), 3.58 (dd, J = 11.7, 6.6 Hz, 2H, CH2OH), 3.65 (dd, J = 15.8, 4.5 Hz, 1H, CHaHb), 3.93 (d, J = 17.1 Hz, 1H, CHaHbC(O)N), 4.24 (dd, J = 17.1, 1.4 Hz, 1H, CHaHbC(O)N), 4.47 (ddd, J = 11.6, 4.7, 1.1 Hz, 1H, CHC(O)), 6.32 (s, 1H, CHPh), 7.07–7.03 (m, 1H, Ar-H), 7.10–7.07 (m, 1H. Ar-H), 7.26–7.25 (m, 1H, Ar-H), 7.28–7.26 (m, 1H, Ar-H), 7.30 (ddd, J = 8.0, 1.3, 0.8 Hz, 1H, Ar-H), 7.38–7.37 (m, 1H, Ar-H), 7.40–7.39 (m, 1H, Ar-H), 7.60 (ddd, J = 7.6, 1.0, 0.4 Hz, 1H, Ar-H), 10.21 (s, 1H, NH). 13C-NMR (Acetone-d6): δ 168.17, 167.37 (NCO), 143, 137.87, 134.32, 133.06, 129.55, 129.15, 127.25, 122.58, 120.17, 119.10, 112.16, 106.87, 62.07 (C6), 56.78 (C12), 56.54 (NC(O)CHaHb), 50.74 (NCHaHbCH2), 46.34 (CH2OH), 30.7 (CH2CH2), 24.315 (CCHaHb), 24.2 (CH2CH2). ESI-MS: m/z 440 (M+ + 2), m/z 438 (M+).
(6S,12S)-2-(4-Amino-butyl)-6-(4-chloro-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6l). Yellow solid. Yield: 27%. mp: 229–231 °C. TLC, Rf: 0.35, (CH2Cl2/CH3OH/TEA, 93:5:2). IR: 3666.41, 3542.31 (NH2), 2930.4 (NH), 1660.7, 1653.5 (CO). 1H-NMR (Acetone-d6): δ 1.62–1.55 (m, 2H, CH2CH2), 1.67 (ddd, J = 11.5, 6.9, 4.0 Hz, 2H, CH2CH2), 3.11 (ddd, J = 15.9, 11.6, 1.1 Hz, 1H, CHaHb), 3.22 (t, J = 6.7 Hz, 2H, CH2NH2), 3.44 (dt, J = 13.7, 7.0 Hz, 1H, NCHaHbCH2), 3.62–3.58 (m, 1H, NCHaHbCH2), 3.66 (dd, J = 11.6, 5.8 Hz, 1H, CHaHb), 3.92 (d, J = 17.0 Hz, 1H, CHaHbC(O)N), 4.24 (dd, J = 17.0, 1.3 Hz, 1H, CHaHbC(O)N), 4.44 (dd, J = 11.5, 5.6 Hz, 1H, CHC(O)), 6.35 (s, 1H, CHPh), 7.05–7.02 (m, 1H, Ar-H), 7.10–7.06 (m, 1H, Ar-H), 7.23 (t, J = 2.3 Hz, 1H, Ar-H), 7.26–7.24 (m, 1H, Ar-H), 7.34–7.31 (m, 1H, Ar-H), 7.43–7.39 (m, 2H, Ar-H), 7.60–7.57 (m, 1H, Ar-H), 10.51 (s, 1H, NH). 13C-NMR (Acetone-d6): δ 168.14, 167.4 (NCO), 143.06, 137.72, 134.28, 132.98, 129.52, 129.11, 127.2, 122.49, 120.08, 119.05, 112.17, 106.69, 56.77 (C6), 56.41 (NC(O)CHaHb), 51.23 (C12), 50.74 (NCHaHbCH2), 46.23 (CH2NH2), 28.63 (CH2CH2), 25.66 (CCHaHb), 24.17 (CH2CH2). ESI-MS: m/z 439 (M+ + 2), m/z 437 (M+).
(6R,12aS)-6-(4-Chloro-phenyl)-2-(2-hydroxy-ethyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6m). White solid. Yield: 20%. mp: 202–206 °C. TLC, Rf: 0.39, (CH2Cl2/CH3OH, 97:3). IR: 3056.91 (OH), 2920.61 (NH), 1658.54, 1650.29 (CO). 1H-NMR (DMSO-d6): δ 2.99 (ddd, J = 15.6, 11.9, 1.5 Hz, 1H, CHaCHb), 3.49 (ddd, J = 14.3, 6.1, 3.8 Hz, 1H, NCHaHbCH2), 3.58 (dd, J = 15.5, 4.2 Hz, 1H, CHaHb), 3.73 (ddd, J = 14.2, 6.2, 4.0 Hz, 1H, NCHaHbCH2), 3.89 (dd, J = 9.5, 5.8 Hz, 2H, CH2OH), 4.16 (d, J = 17.5 Hz, 1H, CHaHbC(O)N), 4.35–4.30 (m, 2H,CHC(O), CHaHbC(O)N), 7.05 (s, 1H, CHPh), 7.21–7.17 (m, 1H, Ar-H), 7.24 (t, J = 1.9 Hz, 1H, Ar-H), 7.26 (d, J = 2.2 Hz, 2H, Ar-H), 7.32–7.30 (m, 2H, Ar-H), 7.34 (d, J = 8.1 Hz, 1H, Ar-H), 7.55 (d, J = 7.4 Hz, 1H, Ar-H). ESI-MS: m/z 411(M+ + 2), m/z 409 (M+).
(6R,12aS)-2-(2-Amino-ethyl)-6-(4-chloro-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6n). White solid. Yield: 48%. mp: 200–204 °C. TLC, Rf: 0.54, (CH2Cl2/CH3OH/TEA, 95:4:1). IR: 3733.97, 3662.21 (NH2), 2968 (NH), 1656.7, 1648.11 (CO). 1H-NMR (Acetone-d6): δ 2.96 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaCHb), 3.37 (dd, J = 15.4, 4.1 Hz, 1H, CHaCHb), 3.46–3.39 (m, 2H, CH2NH2), 3.54–3.49 (m, 1H, NCHaHbCH2), 3.65–3.60 (m, 1H, NCHaHbCH2), 4.16 (dd, J = 11.8, 4.2 Hz, 1H, CHC(O), 4.22 (d, J = 17.8 Hz, 1H, CHaHbC(O)N), 4.40 (dd, J = 17.8, 1.0 Hz, 1H, CHaHbC(O)N), 7.03 (s, 1H, CHPh), 7.07 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar-H), 7.14 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar-H), 7.30–7.26 (m, 2H, Ar-H), 7.38–7.36 (m, 2H, Ar-H), 7.39 (dd, J = 2.9, 2.1 Hz, 1H, Ar-H), 7.57–7.54 (m, 1H, Ar-H), 10.27 (s, 1H, NH). ESI-MS: m/z 410 (M+ + 2), m/z 408 (M+).
(6R,12aS)-6-(4-Chloro-phenyl)-2-(4-hydroxy-butyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6o). Orange solid. Yield: 44%. mp: 199–203 °C. TLC, Rf: 0.31, (CH2Cl2/CH3OH, 97:3). IR: 3100.62 (OH), 2920(NH), 1660.54, 1650 (CO). 1H-NMR (DMSO-d6): δ 1.44–1.35 (m, 2H, CH2CH2), 1.55 (dt, J = 7.4, 6.2 Hz, 2H, CH2CH2), 2.99 (m 1H, CHaHb), 3.43–3.37 (m, 2H, CH2OH), 3.48 (dd, J = 13.8, 7.7 Hz, 1H, NCHaHbCH2), 4.02 (d, J = 17.7 Hz, 2H, CHC(O), CHaHbC(O)N), 4.29 (d, J = 17.4 Hz, 1H, CHaHbC(O)N), 6.91 (s, 1H, CHPh), 7.04 (t, J = 7.5 Hz, 1H, Ar-H), 7.13 (t, J = 7.4 Hz, 1H, Ar-H), 7.25 (d, J = 8.3 Hz, 2H, Ar-H), 7.34 (d, J = 8.1 Hz, 1H, Ar-H), 7.44 (d, J = 8.5 Hz, 2H, Ar-H), 7.53 (d, J = 7.5 Hz, 1H, Ar-H), 11.12 (s, 1H, NH). ESI-MS: m/z 440 (M+ + 2), m/z 438 (M+).
(6R,12S)-2-(4-Amino-butyl)-6-(4-chloro-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (6p). White solid. Yield: 50%. mp: 199–201 °C. TLC, Rf: 0.35, (CH2Cl2/CH3OH/TEA, 93:5:2). IR: 3666.41, 3542.31 (NH2), 2930.4 (NH), 1663.5, 1652.6 (CO). 1H-NMR (Acetone-d6): δ 1.34 (t, J = 7.3 Hz, 2H, CH2CH2), 1.47 (t, J = 7.3 Hz, 2H, CH2CH2), 3.11–3.05 (m, 1H, CHaHb), 3.37 (dd, J = 15.4, 4.3 Hz, 1H, CHaHb), 3.53–3.51 (m, 1H, NCHaHbCH2), 3.59 (t, J = 2.8 Hz, 2H, CH2NH2), 3.62–3.60 (m, 1H, NCHaHbCH2), 4.06 (d, J = 17.6 Hz, 1H, CHaHbC(O)N), 4.17 (dd, J = 11.8, 4.2 Hz, 1H, CHC(O), 4.38 (d, J = 17.4 Hz, 1H,CHaHbC(O)N), 7.05 (s, 1H, CHPh), 7.08 (dd, J = 11.0, 4.0 Hz, 1H, Ar-H), 7.14 (dd, J = 11.1, 4.1 Hz, 1H, Ar-H), 7.30 (t, J = 2.1 Hz, 1H, Ar-H), 7.32 (d, J = 2.2 Hz, 1H, Ar-H), 7.39–7.36 (m, 2H, Ar-H), 7.41–7.39 (m, 1H, Ar-H), 7.57 (d, J = 7.9 Hz, 1H, Ar-H), 10.31 (s, 1H, NH). ESI-MS: m/z 439 (M+ + 2), m/z 437 (M+).
(6R,12R)-6-(4-Bromo-phenyl)-2-(2-hydroxy-ethyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7a). Orange solid. Yield: 36.7%. mp: 233–235 °C. TLC, Rf: 0.38, (CH2Cl2/CH3OH, 97:3). IR: 3056.91 (OH), 2920.61 (NH), 1656.8, 1650.29 (CO). 1H-NMR (DMSO-d6): δ 3.00 (dd, J = 15.3, 11.1 Hz, 1H, CHaHb), 3.41–3.36 (m, 1H, NCHaHbCH2), 3.60–3.51 (m, 2H, CH2OH), 3.50 (dd, J = 13.9, 7.0 Hz, 1H, NCHaHbNC(O)), 4.18–4.00 (m, 2H, CHaHbC(O)N, CHC(O)), 4.33 (d, J = 18.1 Hz, 1H, CHaHbC(O)N), 6.89 (s, 1H, CHPh), 7.07–6.99 (m, 1H, Ar-H), 7.14–7.08 (m, 1H, Ar-H), 7.18 (d, J = 8.7 Hz, 2H, Ar-H), 7.33 (d, J = 7.8 Hz, 1H, Ar-H), 7.53 (d, J = 7.9 Hz, 2H, Ar-H), 7.58 (d, J = 7.8 Hz, 1H, Ar-H), 11.03 (s, 1H, NH). ESI-MS: m/z 456 (M+ + 2), m/z 454 (M+).
(6R,12R)-2-(2-Amino-ethyl)-6-(4-bromo-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7b). Yellow solid. Yield: 12.2%. mp: 232–236 °C. TLC, Rf: 0.49, (CH2Cl2/CH3OH/TEA, 95:4:1). IR: 3733.97, 3662.21 (NH2), 2968 (NH), 1659.2, 1651.4 (CO). 1H-NMR (Acetone-d6): δ 2.96 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaHb), 3.37 (dd, J = 15.4, 4.2 Hz, 1H, CHaHb), 3.47–3.40 (m, 2H, CH2NH2), 3.55–3.50 (m, 1H, NCHaHbCH2), 3.66–3.61 (m, 1H, NCHaHbCH2), 4.16 (dd, J = 11.8, 4.2 Hz, 1H, CHC(O)), 4.23 (d, J = 17.8 Hz, 1H, CHaHbC(O)N), 4.41 (dd, J = 17.8, 1.1 Hz, 1H, CHaHbC(O)N), 7.03 (s, 1H, CHPh), 7.08 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar-H), 7.16–7.13 (m, 1H, Ar-H), 7.25–7.22 (m, 2H, Ar-H), 7.40–7.38 (m, 1H, Ar-H), 7.55–7.52 (m, 2H, Ar-H), 7.56 (d, J = 7.8 Hz, 1H, Ar-H). 13C-NMR (Acetone-d6): 165.2, 163.45 (NCO), 139.76, 137.73, 132.52, 131.48, 130.69, 127.34, 122.92, 120.18, 119, 112, 109, 53.59 (C6), 51.79 (C12), 49.76 (NC(O)CHaHb), 47.99 (NCHaHb), 46.92 (CH2NH2), 27.79 (CCHaHb).ESI-MS: m/z 455 (M+ + 2), m/z 453 (M+).
(6R,12R)-6-(4-Bromo-phenyl)-2-(4-hydroxy-butyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7c). Orange solid. Yield: 55.6%. mp: 234–237 °C. TLC, Rf: 0.32, (CH2Cl2/CH3OH, 97:3). IR: 3100.62 (OH), 2920 (NH), 1680, 1665.4 (CO). 1H-NMR (Acetone-d6): δ 1.56–1.49 (m, 2H, CH2CH2), 1.71–1.64 (m, 2H, CH2CH2), 3.13 (ddd, J = 16.0, 11.6, 1.3 Hz, 1H, CHaHb), 3.46–3.40 (m, 1H, NCHaHbCH2), 3.61–3.56 (m, 3H, NCHaHbCH2, CH2OH), 3.65 (dd, J = 16.1, 4.8 Hz, 1H, CHaHb), 3.93 (d, J = 17.1 Hz, 1H, CHaHbC(O)N), 4.24 (dd, J = 17.1, 1.4 Hz, 1H, CHaHbC(O)N), 4.46 (ddd, J = 11.5, 4.6, 1.0 Hz, 1H, CHC(O)), 6.30 (s, 1H, CHPh), 7.07–7.03 (m, 1H, Ar-H), 7.10–7.07 (m, 1H, Ar-H), 7.31–7.29 (m, 1H, Ar-H), 7.35–7.32 (m, 2H, Ar-H), 7.43–7.40 (m, 2H, Ar-H), 7.60 (d, J = 7.6 Hz, 1H, Ar-H), 10.20 (s, 1H, NH). 13C-NMR (Acetone-d6): 168.16, 167.34 (NCO), 143.5, 137.88, 134.25, 132.14, 129.91, 127.25, 122.59, 121.15, 120.17, 119.11, 112.15, 106.89, 62.07 (C6), 56.774 (C12), 56.6 (NC(O)CHaHb), 50.73 (NCHaHb), 46.34 (CH2OH), 30.70 (CH2CH2), 24.3 (CCHaHb), 24.2 (CH2CH2).ESI-MS: m/z 484 (M+ + 2), m/z 482 (M+).
(6R,12R)-2-(4-Amino-butyl)-6-(4-bromo-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7d). Yellow solid. Yield: 38%. mp: 235–238 °C. TLC, Rf: 0.37, (CH2Cl2/CH3OH/TEA, 93:5:2). IR: 3666.41, 3542.31 (NH2), 2930.4 (NH), 1669.2, 1660.1 (CO). 1H-NMR (Acetone-d6): δ 1.57 (ddt, J = 9.3, 7.1, 4.5 Hz, 2H, CH2CH2), 1.70–1.63 (m, 2H, CH2CH2), 2.98 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaHb), 3.19 (t, J = 6.7 Hz, 2H, CH2NH2), 3.30 (dt, J = 11.6, 7.0 Hz, 1H, NCHaHbCH2), 3.37 (dd, J = 15.4, 4.2 Hz, 1H, CHaHb), 3.60–3.55 (m, 1H, NCHaHbCH2), 4.04 (d, J = 17.6 Hz, 1H, CHaHbC(O)N), 4.17 (dd, J = 11.9, 4.2 Hz, 1H, CHC(O)), 4.30 (dd, J = 17.6, 1.0 Hz, 1H, CHaHbC(O)N), 7.03(s, 1H, CHPh), 7.09–7.06 (m, 1H, Ar-H), 7.16–7.13 (m, 1H, Ar-H), 7.25–7.22 (m, 2H, Ar-H), 7.40–7.38 (m, 1H, Ar-H), 7.54–7.51 (m, 2H, Ar-H), 7.56 (d, J = 7.8 Hz, 1H, Ar-H), 10.34 (s, 1H, NH). 13C-NMR (Acetone-d6): 165.53, 163.4 (NCO), 139.74, 137.66, 132.51, 131.48, 130.69, 127.34, 122.91, 122.7, 120.17, 119, 112, 109, 53.64 (C6), 51.89 (C12), 51.32 (NC(O)CHaHb), 49.89 (NCHaHb), 46.14 (CH2NH2), 28.73 (CH2CH2), 27.85 (CCHaHb), 25.12 (CH2CH2). ESI-MS: m/z 483 (M+ + 2), m/z 481(M+).
(6S,12R)-6-(4-Bromo-phenyl)-2-(2-hydroxy-ethyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7e). Yellow solid. Yield: 55%. mp: 206–208 °C. TLC, Rf: 0.38, (CH2Cl2/CH3OH, 97:3). IR: 3056.91 (OH), 2920.61(NH), 1656.8, 1650.29 (CO). 1H-NMR (DMSO-d6): δ 3.00 (dd, J = 15.3, 11.8 Hz, 1H, CHaHb), 3.29 (dd, J = 15.7, 4.3 Hz, 1H, CHaHb), 3.57 (dd, J = 10.6, 4.9 Hz, 2H, CH2OH), 4.05 (dd, J = 11.6, 4.2 Hz, 1H, CHC(O)), 4.14 (d, J = 17.7 Hz, 1H, CHaHbC(O)N), 4.33 (d, J = 17.8 Hz, 1H, CHaHbC(O)N), 6.89 (s, 1H, CHPh), 7.03 (t, J = 7.4 Hz, 1H, Ar-H), 7.12 (t, J = 7.6 Hz, 1H, Ar-H), 7.18 (d, J = 8.3 Hz, 2H, Ar-H), 7.35–7.30 (m, 1H, Ar-H), 7.53 (d, J = 7.7 Hz, 1H, Ar-H), 7.61–7.56 (m, 2H, Ar-H), 11.05 (s, 1H, NH). ESI-MS: m/z 456 (M+ + 2), m/z 454 (M+).
(6S,12R)-2-(2-Amino-ethyl)-6-(4-bromo-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7f). Yellow solid. Yield: 41.7%. mp: 206–209 °C. TLC, Rf: 0.49, (CH2Cl2/CH3OH/TEA, 95:4:1). IR: 3733.97, 3662.21 (NH2), 2968 (NH), 1657.4, 1650.1 (CO). 1H-NMR (Acetone-d6): δ 2.97 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaHb), 3.37 (dd, J = 15.3, 4.3 Hz, 1H, CHaHb), 3.43 (dd, J = 14.6, 8.4 Hz, 2H, CH2NH2), 3.57–3.51 (m, 1H, NCHaHbCH2), 3.67–3.61 (m, 1H, NCHaHbCH2), 4.17 (dd, J = 11.9, 4.2 Hz, 1H, CHC(O)), 4.23 (d, J = 17.8 Hz, 1H, CHaHbC(O)N), 4.41 (dd, J = 17.8, 1.0 Hz, 1H, CHaHbC(O)N), 7.02 (s, 1H, CHPh), 7.08 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar-H), 7.15 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar-H), 7.26–7.22 (m, 2H, Ar-H), 7.40–7.37 (m, 1H, Ar-H), 7.55–7.53 (m, 2H, Ar-H), 7.57 (dd, J = 7.8, 1.0 Hz, 1H, Ar-H). ESI-MS: m/z 455 (M+ + 2), m/z 453 (M+).
(6S,12R)-6-(4-Bromo-phenyl)-2-(4-hydroxy-butyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7g). White solid. Yield: 43%. mp: 207–209 °C. TLC, Rf: 0.32, (CH2Cl2/CH3OH, 97:3). IR: 3100.62 (OH), 2920(NH), 1670.8, 1658.29 (CO). 1H-NMR (DMSO-d6): δ 1.45–1.33 (m, 2H, CH2CH2), 1.60–1.48 (m, 2H, CH2CH2), 2.99 (dd, J = 15.5, 12.5 Hz, 1H, CHaHb), 3.22–3.11 (m, 1H, NCHaHbCH2), 3.40 (t, J = 6.0 Hz, 2H, CH2OH), 3.55–3.44 (m, 1H, NCHaHbCH2), 4.06–3.97 (m, 2H, CHaHbC(O)N, CHC(O)), 4.30 (d, J = 17.6 Hz, 1H, CHaHbC(O)N), 6.88 (s, 1H, CHPh), 7.03 (t, J = 7.4 Hz, 1H, Ar-H), 7.19–7.09 (m, 3H, Ar-H), 7.33 (d, J = 8.1 Hz, 1H, Ar-H), 7.60–7.51 (m, 3H, Ar-H), 11.06 (s, 1H, NH). ESI-MS: m/z 484 (M+ + 2), m/z 482 (M+).
(6S,12R)-2-(4-Amino-butyl)-6-(4-bromo-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7h). White solid. Yield: 41%. mp: 210–212 °C. TLC, Rf: 0.37, (CH2Cl2/CH3OH/TEA, 93:5:2). IR: 3666.41, 3542.31 (NH2), 2930.4 (NH), 1675.2, 1665.8 (CO). 1H-NMR (Acetone-d6): δ 1.60–1.54 (m, 2H, CH2CH2), 1.70–1.63 (m, 2H, CH2CH2), 2.98 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaHb), 3.20 (t, J = 6.7 Hz, 2H, CH2NH2), 3.30 (dt, J = 13.7, 7.0 Hz, 1H, NCHaHbCH2), 3.37 (dd, J = 15.4, 4.3 Hz, 1H, CHaHb), 3.62–3.57 (m, 1H, NCHaHbCH2), 4.04 (d, J = 17.6 Hz, 1H, CHaHbC(O)N), 4.16 (dd, J = 11.8, 4.2 Hz, 1H, CHC(O)), 4.30 (dd, J = 17.6, 1.0 Hz, 1H, CHaHbC(O)N), 7.04 (s, 1H, CHPh), 7.07 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar-H), 7.16–7.12 (m, 1H, Ar-H), 7.25–7.22 (m, 2H, Ar-H), 7.40 (dt, J = 8.1, 0.9 Hz, 1H, Ar-H), 7.53–7.51 (m, 2H, Ar-H), 7.57–7.54 (m, 1H, Ar-H), 10.5 (s,1H, NH). 13C-NMR (Acetone-d6): 165.55, 163.4 (NCO), 139.77, 137.68, 132.49, 131.46, 130.73, 127.33, 122.87, 122.64, 120.13, 119.02, 112.17, 109.39, 53.65 (C6), 51.89 (C12), 49.89 (NC(O)CHaHb), 46.84 (NCHaHb), 46.13 (CH2NH2), 28.72 (CH2CH2), 27.86 (CCHaHb), 25.12 (CH2CH2).ESI-MS: m/z 483(M+ + 2), m/z 481 (M+).
(6S,12S)-6-(4-Bromo-phenyl)-2-(2-hydroxy-ethyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7i). Orange solid. Yield: 6%. mp: 233–236 °C. TLC, Rf: 0.38, (CH2Cl2/CH3OH, 97:3). IR: 3056.91 (OH), 2920.61 (NH), 1656.8, 1650.29 (CO). 1H-NMR (Acetone-d6): δ 3.13 (ddd, J = 16.0, 11.6, 1.3 Hz, 1H, CHaHb), 3.49–3.45 (m, 1H, NCHaHbCH2), 3.62–3.59 (m, 1H, NCHaHbCH2), 3.65 (dd, J = 15.5, 4.3 Hz, 1H, CHaHb), 3.78–3.74 (m, 2H, CH2OH), 4.08 (d, J = 17.2 Hz, 1H, CHaHbC(O)N), 4.34 (dd, J = 17.2, 1.4 Hz, 1H, CHaHbC(O)N), 4.47 (dd, J = 11.5, 5.7 Hz, 1H, CHC(O)), 6.30 (s, 1H, CHPh), 7.07–7.03 (m, 1H, Ar-H), 7.11–7.07 (m, 1H, Ar-H), 7.31–7.29 (m, 1H, Ar-H), 7.35–7.32 (m, 2H, Ar-H), 7.42–7.39 (m, 2H, Ar-H), 7.59 (dd, J = 4.5, 3.9 Hz, 1H, Ar-H), 10.18 (s,1H, NH). ESI-MS: m/z 456 (M+ + 2), m/z 454 (M+).
(6S,12S)-2-(2-Amino-ethyl)-6-(4-bromo-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7j). White solid. Yield: 20%. mp: 232–236 °C. TLC, Rf: 0.49, (CH2Cl2/CH3OH/TEA, 95:4:1). IR: 3733.97, 3662.21 (NH2), 2968 (NH), 1660.3, 1653.2 (CO). 1H-NMR (Acetone-d6): δ 2.95 (ddd, J = 15.4, 11.9, 1.5 Hz, 1H, CHaHb). 3.36 (dd, J = 15.4, 4.2 Hz, 1H, CHaHb), 3.45–3.39 (m, 2H, CH2NH2), 3.55–3.49 (m, 1H, NCHaHbCH2), 3.64–3.59 (m, 1H, NCHaHbCH2), 4.15 (dd, J = 11.8, 4.2 Hz, 1H, CHC(O)), 4.22 (d, J = 18.2 Hz, 1H, CHaHbC(O)N), 4.40 (dd, J = 17.8, 1.0 Hz, 1H, CHaHbC(O)N), 7.01 (s, 1H, CHPh), 7.07 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar-H), 7.14 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar-H), 7.24–7.21 (m, 2H, Ar-H), 7.37 (dt, J = 8.1, 0.9 Hz, 1H, Ar-H), 7.54–7.51 (m, 2H, Ar-H), 7.57–7.54 (m, 1H, Ar-H), 10.2 (s,1H, NH). ESI-MS: m/z 455 (M+ + 2), m/z 453 (M+).
(6S,12S)-6-(4-Bromo-phenyl)-2-(4-hydroxy-butyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7k). Brown solid. Yield: 51%. mp: 234–236 °C. TLC, Rf: 0.32, (CH2Cl2/CH3OH, 97:3). IR: 3100.62 (OH), 2920 (NH), 1670.8, 1658.29 (CO). 1H-NMR (Acetone-d6): δ 1.56–1.49 (m, 2H, CH2CH2), 1.71–1.64 (m, 2H, CH2CH2), 3.13 (ddd, J = 16.0, 11.6, 1.2 Hz, 1H, CHaHb), 3.46–3.39 (m, 1H, NCHaHbCH2), 3.60–3.54 (m, 3H, NCHaHbCH2 , CH2OH), 3.65 (dd, J = 16.1, 4.9 Hz, 1H, CHaHb), 3.93 (d, J = 17.1 Hz, 1H, CHaHbC(O)N), 4.24 (dd, J = 17.1, 1.3 Hz, 1H, CHaHbC(O)N), 4.46 (dd, J = 11.6, 3.7 Hz, 1H, CHC(O)), 6.30 (s, 1H, CHPh), 7.07–7.03 (m, 1H, Ar-H), 7.10–7.07 (m, 1H, Ar-H), 7.31–7.29 (m, 1H, Ar-H), 7.35–7.32 (m, 2H, Ar-H), 7.43–7.40 (m, 2H, Ar-H), 7.60 (d, J = 7.3 Hz, 1H, Ar-H), 10.2 (s,1H, NH). ESI-MS: m/z 484 (M+ + 2), m/z 482 (M+).
(6S,12S)-2-(4-Amino-butyl)-6-(4-bromo-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7l). Yellow solid. Yield: 34%. mp: 236–238 °C. TLC, Rf: 0.37, (CH2Cl2/CH3OH/TEA, 93:5:2). IR: 3666.41, 3542.31 (NH2), 2930.4 (NH), 1680.2, 1669.6 (CO). 1H-NMR (Acetone-d6): δ 1.62–1.54 (m, 2H, CH2CH2), 1.69–1.63 (m, 2H, CH2CH2), 3.11 (ddd, J = 15.9, 11.6, 1.2 Hz, 1H, CHaHb), 3.21 (t, J = 6.7 Hz, 1H, CH2NH2), 3.44 (dt, J = 13.7, 7.0 Hz, 1H, NCHaHbCH2), 3.70–3.60 (m, 2H, NCHaHbCH2, CHaHb), 3.92 (d, J = 17.1 Hz, 1H, CHaHbC(O)N), 4.24 (dd, J = 17.0, 1.3 Hz, 1H, CHaHbC(O)N), 4.44 (dd, J = 11.5, 4.6 Hz, 1H, CHC(O)), 6.34 (s, 1H, CHPh), 7.06–7.02 (m, 1H, Ar-H), 7.10–7.06 (m, 1H, Ar-H), 7.33–7.31 (m, 1H, Ar-H), 7.37–7.34 (m, 2H, Ar-H), 7.41–7.37 (m, 2H, Ar-H), 7.59–7.57 (m, 1H, Ar-H). ESI-MS: m/z 483 (M+ + 2), m/z 481 (M+).
(6R,12S)-6-(4-Bromo-phenyl)-2-(2-hydroxy-ethyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7m). Yellow solid. Yield: 45%. mp: 206–208 °C. TLC, Rf: 0.38, (CH2Cl2/CH3OH, 97:3). IR: 3056.91 (OH), 2920.61 (NH), 1656.8, 1650.29 (CO). 1H-NMR (DMSO-d6): δ 3.00 (dd, J = 15.1, 12.1 Hz, 1H, CHaHb), 3.38 (dd, J = 8.9, 5.5 Hz, 2H, CH2OH), 3.55 (t, J = 5.4 Hz, 2H, NCHaHbCH2), 4.05 (dd, J = 11.7, 4.1 Hz, 1H, CHC(O)), 4.14 (d, J = 17.8 Hz, 1H, CHaHbC(O)N), 4.33 (d, J = 17.7 Hz, 1H, CHaHbC(O)N), 6.89 (s,1H, CHPh), 7.03 (t, J = 7.4 Hz, 1H, Ar-H), 7.12 (t, J = 7.5 Hz, 1H, Ar-H), 7.18 (d, J = 8.5 Hz, 2H, Ar-H), 7.33 (d, J = 8.0 Hz, 1H, Ar-H), 7.58 (d, J = 8.3 Hz, 2H, Ar-H), 7.53 (d, J = 7.7 Hz, 1H, Ar-H), 11.05 (s, 1H, NH). ESI-MS: m/z 456 (M+ + 2), m/z 454 (M+).
(6R,12S)-2-(2-Amino-ethyl)-6-(4-bromo-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7n). Yellow solid. Yield: 34%. mp: 206–210 °C. TLC, Rf: 0.49, (CH2Cl2/CH3OH/TEA, 95:4:1). IR: 3733.97, 3662.21 (NH2), 2968 (NH), 1657.8, 1650 (CO). 1H-NMR (DMSO-d6): δ 3.08–2.99 (m, 1H,CHaHb), 2.72 (t, J = 6.4 Hz, 2H, NCHaHbCH2), 3.41 (dd, J = 13.2, 6.7 Hz, 2H, CH2NH2), 4.14–3.99 (m, 2H, CHaHbC(O)N, CHC(O)), 4.34 (d, J = 17.7 Hz, 1H, CHaHbC(O)N), 6.89 (s, 1H, CHPh), 7.03 (t, J = 7.4 Hz, 1H, Ar-H), 7.12 (t, J = 7.6 Hz, 1H, Ar-H), 7.18 (d, J = 8.1 Hz, 2H, Ar-H), 7.33 (d, J = 8.1 Hz, 1H, Ar-H), 7.53 (d, J = 7.9 Hz, 1H, Ar-H), 7.58 (d, J = 7.7 Hz, 2H, Ar-H), 11.06 (s, 1H, NH). ESI-MS: m/z 455 (M+ + 2), m/z 453 (M+).
(6R,12S)-6-(4-Bromo-phenyl)-2-(4-hydroxy-butyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7o). Yellow solid. Yield: 30%. mp: 205–208 °C. TLC, Rf: 0.32, (CH2Cl2/CH3OH, 97:3). IR: 3100.62 (OH), 2920(NH), 1671.8, 1660.9 (CO). 1H-NMR (DMSO-d6): δ 1.44–1.33 (m, 2H, CH2CH2), 1.60–1.49 (m, 2H, CH2CH2), 2.99 (dd, J = 14.9, 12.3 Hz, 1H, CHaHb), 3.15 (dd, J = 13.3, 7.0 Hz, 1H, NCHaHbCH2), 3.40 (t, J = 6.0 Hz, 2H, CH2OH), 3.48 (dd, J = 13.8, 7.1 Hz, 1H, NCHaHbCH2), 4.06–3.98 (m, 2H, CHaHbC(O)N, CHC(O)), 4.30 (d, J = 17.8 Hz, 1H, CHaHbC(O)N), 6.88 (s, 1H, CHPh), 7.03 (t, J = 7.4 Hz, 1H, Ar-H), 7.11 (d, J = 8.0 Hz, 1H, Ar-H), 7.17 (d, J = 8.4 Hz, 2H, Ar-H), 7.33 (d, J = 8.1 Hz, 1H, Ar-H), 7.53 (d, J = 8.0 Hz, 1H, Ar-H), 7.58 (d, J = 8.2 Hz, 2H, Ar-H), 11.06 (s, 1H, NH). ESI-MS: m/z 484 (M+ + 2), m/z 482 (M+).
(6R,12S)-2-(4-Amino-butyl)-6-(4-bromo-phenyl)-2,3,6,7,12,12a-hexahydro-pyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (7p). Yellow solid. Yield: 36%. mp: 210–212 °C. TLC, Rf: 0.37, (CH2Cl2/CH3OH/TEA, 93:5:2). IR: 3666.41, 3542.31 (NH2), 2930.4 (NH), 1677.5, 1668.9 (CO). 1H-NMR (DMSO-d6): δ 1.37–1.26 (m, 2H, CH2CH2), 1.58–1.49 (m, 2H, CH2CH2), 2.54 (t, J = 6.9 Hz, 2H, CH2NH2), 2.99 (dd, J = 14.5, 12.2 Hz, 1H, CHaHb), 3.28 (dd, J = 15.4, 4.2 Hz, 1H, CHaHb), 3.52–3.41 (m, 1H, NCHaHbCH2), 4.08–3.99 (m, 2H, CHaHbC(O)N, CHC(O)), 4.29 (d, J = 17.3 Hz, 1H, CHaHbC(O)N), 6.88 (s, 1H, CHPh), 7.06–7.00 (m, 1H, Ar-H), 7.15–7.09 (m, 1H, Ar-H), 7.17 (d, J = 8.4 Hz, 2H, Ar-H), 7.33 (d, J = 8.0 Hz, 1H, Ar-H), 7.53 (d, J = 7.6 Hz, 1H, Ar-H), 7.60–7.56 (m, 2H, Ar-H), 11.07 (s, 1H, NH). ESI-MS: m/z 483 (M+ + 2), m/z 481 (M+).