Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics
Abstract
1. Introduction
2. Phytochemistry
2.1. Phenylethanoid Glycosides
2.2. Lignans
2.3. Aliphatic C6-C2 Alcohols
2.4. Iridoids, Diterpenoids and Triterpenoids
2.5. Sterols
2.6. Flavonoids
2.7. Volatiles
2.8. Alkaloids
2.9. Others
3. Quality Control
4. Pharmacology
4.1. Anti-Inflammatory Effect
4.2. Antibacterial Effect
4.3. Antiviral Effect
4.4. Antioxidant Effect
4.5. Neuroprotective Effect
4.6. Antitumor Effect
4.7. Hepatoprotective Effect
4.8. Cardiovascular Protective Effect
4.9. Others
5. Pharmacokinetics
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Xu, L.G.; Huang, R.Q. Textual research on “Lianqiao” whose other name be “Lanhua” (cymbidium) in Shen Nong’s Herbal. Lishizhen Med. Mater. Med. Res. 2000, 11, 358–359. [Google Scholar]
- International Pharmacopoeia Commission. Pharmacopoeia Commission of the People’s Republic of China. In Pharmacopoeia of People’s Republic of China; Chemical Industry Press: Beijing, China, 2015; Volume 1, pp. 1–1750. ISBN 978-7-5067-7337-9. [Google Scholar]
- The Japan Drug Editional Commission of Administration. Japanese Pharmacopoeia; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2011; pp. 1–2131.
- Korea Food and Drug Administration. South Korean Pharmacopoeia; Monografs Part II; Ministry of Health and Welfare: Se jong, Korean, 2015; pp. 1004–1241.
- Chen, H.Y.; Lin, Y.H.; Huang, J.W.; Chen, Y.C. Chinese herbal medicine network and core treatments for allergic skin diseases: Implications from a nationwide database. J. Ethnopharmacol. 2015, 168, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.P.; Zhang, F.S.; Li, Z.Y.; Qin, X.M.; Zhang, L.W. Comparison of Fruits of Forsythia suspensa at Two Different Maturation Stages by NMR-Based Metabolomics. Molecules 2015, 20, 10065–10081. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.L.; Ding, R.B.; Zou, L.D.; Zhang, C.; Wang, K.; Liu, F.; Li, P.; Chen, M.W.; Wan, J.B.; Su, H.X.; et al. Forsythiae Fructus Inhibits B16 Melanoma Growth Involving MAPKs/Nrf2/HO-1 Mediated Anti-Oxidation and Anti-Inflammation. Am. J. Chin. Med. 2016, 44, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Xu, H.F.; Huang, H. Effects of the extract of Forsythia suspensa on influenza A H1N1 infection in vitro. J. Med. Plants Res. 2010, 4, 1455–1458. [Google Scholar]
- Zuo, T. Study on the Mechanism of Forsythia Suspensa Preventing Vomiting Effect on Chemotherapy Mice. China J. Chin. Med. 2015, 30, 1400–1404. [Google Scholar]
- Feng, Q.; Xia, W.K.; Wang, X.Z.; Song, H.Y.; Yao, J.C. Protective effects of phillygenin against CCl4 induced hepatic injury in rat. Chin. Pharmacol. Bull. 2015, 31, 426–430. [Google Scholar]
- Zhang, F.; Yang, Y.N.; Song, X.Y.; Shao, S.Y.; Feng, Z.M.; Jiang, J.S.; Li, L.; Chen, N.H.; Zhang, P.C. Forsythoneosides A–D, Neuroprotective Phenethanoid and Flavone Glycoside Heterodimers from the Fruits of Forsythia suspense. J. Nat. Prod. 2015, 78, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.Q.; Li, P.; Shi, W.D.; Bai, S.P. The effects and mechanism of Forsythia suspensa on Atherosclerosis Rats Model. Pharm. Clin. Chin. Mater. Med. 2016, 32, 28–33. [Google Scholar]
- Zhang, Q.; Jia, C.H.; Xu, H.Y.; Wang, Y.F.; Zhang, M.L.; Huo, C.H.; Shi, Q.W.; Yu, S.H. Chemical Constituents of Plants from the Genus Forsythia. Mini Rev. Org. Chem. 2012, 9, 303–318. [Google Scholar]
- Yan, X.J.; Bai, X.Y.; Liu, Q.B.; Liu, S.; Gao, P.Y.; Li, L.Z.; Song, S.J. Two new glycosides from the fruits of Forsythia suspense. J. Asian Nat. Prod. Res. 2014, 16, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.X.; Xia, Y.G.; Yang, B.Y.; Liang, J.; Zhang, Q.B.; Li, G.Y. A New Caffeoyl Phenylethanoid Glycoside from the Unripe Fruits of Forsythia suspensa. Chin. J. Nat. Med. 2009, 7, 278–282. [Google Scholar] [CrossRef]
- Ming, D.S.; Yu, D.Q.; Yu, S.S. Two New Caffeyol Glycosides from Forsythia suspensa. J. Asian Nat. Prod. Res. 1999, 1, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.N.; Zhuang, H.; Kokot, S. A High Performance Liquid Chromatography and Electrospray Ionization Mass Spectrometry Method for the Analysis of the Natural Medicine, Forsythia Suspensa. Anal. Lett. 2014, 47, 102–116. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q.; Shi, X.W.; Zhang, X.W.; Sheng, X.N.; Zhang, L.T. Simultaneous Quantification of 14 Bioactive Constituents in Forsythia Suspensa by Liquid Chromatography-Electrospray Ionization-Mass Spectrometry. Phytochem. Anal. 2010, 21, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Guo, H. Studies on the Chemical Constituents of Forsythia suspensa. Ph.D. Thesis, Peking University, Beijing, China, May 2006. [Google Scholar]
- Endo, K.; Hikino, H. Structures of rengyol, rengyoxide, and rengyolone, new cyclohexylethane derivatives from Forsythia suspensa fruits. Can. J. Chem. 1984, 62, 2011–2014. [Google Scholar] [CrossRef]
- Wang, F.N.; Ma, Z.Q.; Liu, Y.; Guo, Y.Z.; Gu, Z.W. New phenylethanoid glycosides from the fruits of Forsythia suspensa (Thunb.) Vahl. Molecules 2009, 14, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, A.H.; Ye, M.; Yang, M.; Guo, D.A. Characterization of phenolic compounds in the fruits of Forsythia suspensa by high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spctrom. 2007, 21, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dai, Y.; Zhang, S.X.; Duan, Y.H.; Liu, M.L.; Chen, L.Y.; Yao, X.S. Quinoid glycosides from Forsythia suspensa. Phytochemistry 2014, 104, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dai, Y.; Duan, Y.H.; Liu, M.L.; Yao, X.S. A new lignan glycoside from Forsythia suspensa. Chin. J. Nat. Med. 2014, 12, 697–699. [Google Scholar] [CrossRef]
- Xia, Y.G.; Yang, B.Y.; Liang, L.; Kuang, H.X. Caffeoyl Phenylethanoid Glycosides from Unripe Fruits of Forsythia Suspensa. Chem. Nat. Compd. 2015, 51, 656–659. [Google Scholar] [CrossRef]
- Liu, D.L.; Zhang, Y.; Xu, S.X.; Xu, Y.; Wang, Z.X. Phenylethanoid Glycosides from Forsythia suspensa Vahl. J. Chin. Pharm. Sci. 1998, 7, 103–106. [Google Scholar]
- Qu, H.H.; Zhang, Y.M.; Chai, X.Y.; Sun, W.J. Isoforsythiaside, an antioxidant and antibacterial phenylethanoid glycoside isolated from Forsythia suspensa. Bioorg. Chem. 2012, 40, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.X.; Xia, Y.G.; Liang, J.; Yang, B.Y.; Wang, Q.H. Lianqiaoxinoside B, a novel caffeoyl phenylethanoid glycoside from Forsythia suspensa. Molecules 2011, 16, 5674–5681. [Google Scholar] [CrossRef] [PubMed]
- Seya, K.; Endo, K.; Hikino, H. Structures of rengyosides A, B, and C, three glucosides of Forsythia suspensa fruits. Phytochemistry 1989, 28, 1495–1498. [Google Scholar] [CrossRef]
- Yan, X.J.; Wen, J.; Xiang, Z.; Cai, D.; Lv, C.N.; Zhao, Y.; Qu, Z.Y.; Liu, Y.J.; Qu, J.L. Two new phenolic acids from the fruits of Forsythia suspense. J. Asian Nat. Prod. Res. 2016, 19, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Yu, S.H.; Huo, H.L.; Li, F.G.; Sheng, N.; Zhang, L.T. Hplc-Esi-Ms/Ms Quantitative Method for Simultaneous Analysis of Five Bioactive Constituents of Forsythia Suspensa in Rat Bile After. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 44–60. [Google Scholar]
- Chang, M.J.; Hung, T.M.; Min, B.S.; Kim, J.C.; Woo, M.H.; Choi, J.S.; Lee, H.K.; Bae, K.H. Lignans from the fruits of Forsythia suspensa (Thunb.) Vahl protect high-density lipoprotein during oxidative stress. Biosci. Biotechnol. Biochem. 2008, 72, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhou, G.A.; Liu, M.W. Chemical Constituents from Forsythia suspense. Chin. J. Nat. Med. 2008, 6, 235–236. [Google Scholar] [CrossRef]
- Yan, X.J.; Peng, Y.; Liu, Z.X.; Wen, J.; Liu, Q.B.; Li, L.Z.; Song, S.J. Three new lignan glycosides from the fruits of Forsythia suspense. J. Asian Nat. Prod. Res. 2014, 16, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Xu, S.X.; Wang, W.F. A Novel Lignan Glucoside from Forsythia suspensa Vahl. J. Chin. Pharm. Sci. 1998, 7, 49–51. [Google Scholar]
- Feng, W.S.; Li, K.K.; Zheng, X.K. Studies on Chemical constituents in Forsythia suspensa (Thunb.) Vahl. Chin. Pharm. J. 2009, 44, 490–492. [Google Scholar]
- Piao, X.L.; Jang, M.H.; Cui, J.; Piao, X.S. Lignans from the fruits of Forsythia suspensa. Bioorg. Med. Chem. Lett. 2008, 18, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Hung, H.Y.; Nian, C.W.; Hwang, T.L.; Cheng, J.C.; Kuo, D.H.; Lee, E.J.; Tai, S.H.; Wu, T.S. Chemical Constituents and Anti-inflammatory Principles from the Fruits of Forsythia suspensa. J. Nat. Prod. 2016, 12, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, Y.; Suzuki, E.; Mitsuhashi, T. Naturally occurring antioxidant. (I). Isolation and determination of natural phenolic antioxidants from Forsythia suspensa Vahl. In Tokyo Gakugei Daigaku Kiyo, Dai-4-bumon: Sugaku, Shizen Kagaku; Tokyo Gakugei Daigaku: Tokyo, Japan, 1981; Volume 33, pp. 119–123. [Google Scholar]
- Endo, K.; Seya, K.; Hikino, H. Stereostructure of rengyol and isorengyol, phenylethanoids of Forsythia suspensa. Tetrahedron 1987, 43, 2681–2688. [Google Scholar] [CrossRef]
- Endo, K.; Seya, K.; Hikino, H. Structure and enantioselective synthesis of suspenol, a new polyol of Forsythia suspensa. In Proceedings of the Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, Sapporo, Japan, 26–28 August 1987; Volume 29, pp. 660–667. [Google Scholar]
- Wang, W.F.; Liu, D.L.; Xu, S.X.; Xiao, F.H. Rengyolester isolated from Forsythia suspensa Vahl. J. Shenyang Pharm. Univ. 1999, 16, 138. [Google Scholar]
- Zhang, G.G.; Song, S.J.; Ren, J.; Xu, S.X. A New Compound from Forsythia suspensa (Thunb.) Vahl with Antiviral Effect on RSV. J. Herb. Pharmacother. 2002, 2, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, S.J.; Zhang, G.G.; Xu, S.X. A new compound from the fruit of Forsythia suspensa (Thunb.) Vahl. J. Shenyang Pharm. Univ. 2003, 20, 48–49. [Google Scholar]
- Dong, S.M. New quinoid glycosides from Forsythia suspensa. J. Nat. Prod. 1998, 61, 377–379. [Google Scholar]
- Wang, Y.Z.; Ma, Q.G.; Zheng, X.K.; Feng, W.S. A new forsythenside from Forsythia suspensa. Chin. Chem. Lett. 2008, 19, 1234–1236. [Google Scholar] [CrossRef]
- Xue, J.; Xie, L.; Liu, B.R.; Yu, L.X. Triterpenoids from the Fruits of Forsythia suspensa. Chin. J. Nat. Med. 2010, 8, 414–418. [Google Scholar] [CrossRef]
- Kuo, P.C.; Chen, G.F.; Yang, M.L.; Lin, Y.H.; Peng, C.C. Chemical Constituents from the Fruits of Forsythia suspensa and Their Antimicrobial Activity. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar]
- Ming, D.S.; Yu, D.Q.; Yu, S.S.; Liu, J.; He, C.H. A new furofuran mono-lactone from Forsythia suspensa. J. Asian Nat. Prod. Res. 1999, 1, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Rouf, A.S.S.; Ozaki, Y.; Rashid, M.A.; Rui, J. Dammarane derivatives from the dried fruits of Forsythia suspensa. Phytochemistry 2001, 56, 815–818. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, B. Study on the apoptotic induction mechanism of triterpenes isolated from Forsythia suspense in human gastric cancer cell line SGC-7901. Chin. J. Clin. Pharmacol. Ther. 2010, 15, 851–855. [Google Scholar]
- Shin, S.J.; Park, C.E.; Baek, N.I.; Chung, I.S.; Park, C.H. Betulinic and oleanolic acids isolated from Forsythia suspensa VAHL inhibit urease activity of Helicobacter pylori. Biotechnol. Bioprocess Eng. 2009, 14, 140–145. [Google Scholar] [CrossRef]
- Yin, J.; Guo, L.G. Modern Research and Clinical Application of Chinese Medicine (I); Academy Press: Beijing, China, 1993; pp. 356–358. [Google Scholar]
- Lee, J.S.; Min, B.S.; Bae, K.H. Cytotoxic Constituents from the Forsythiae Fructus against L1210 and HL60 cells. Yakhak Hoeji 1996, 40, 462–467. [Google Scholar]
- Shi, J.M.; Sun, J.; Zhang, G.D.; Yu, L.X.; Qian, X.P.; Liu, B.R. Inhibitory effects of Ambrolic Acid on cell proliferation in human gastric carcinoma cell line SGC-7901. Acta Univ. Med. Nanjing Nat. Sci. 2009, 29, 445–449. [Google Scholar]
- Chen, Y.J.; Xiang, J.; Xu, M.J.; Tao, L.; Gu, W. Studies on Chemical Constituents of Forsythia suspensa (Thunb.) Vahl. Chin. J. Chin. Mater. Med. 1999, 24, 296. [Google Scholar]
- Guo, Q.; Wang, Z.M.; Lin, L.M.; Xia, B.H.; Deng, X.L. Researches on chemical constituents in medicinal plants in genus Forsythia. Chin. J. Exp. Tradit. Med. Form. 2009, 15, 74–79. [Google Scholar]
- Qu, J.L.; Yan, X.J.; Li, C.Y.; Wen, J.; Lu, C.N.; Ren, J.G.; Peng, Y.; Song, S.J. Comparative Evaluation of Raw and Ripe Fruits of Forsythia suspensa by HPLC–ESI-MS/MS Analysis and Anti-Microbial Assay. J. Chromatogr. Sci. 2017, 55, 451–458. [Google Scholar] [PubMed]
- Bai, Y.; Li, J.; Liu, W.; Jiao, X.C.; He, J.; Liu, J.; Ma, L.; Gao, X.M.; Chang, Y.X. Pharmacokinetic of 5 components after oral administration of Fructus Forsythiae by HPLC-MS/MS and the effects of harvest time and administration times. J. Chromatogr. B 2015, 993–994, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, S.J.; Xu, S.X.; Fu, X.H. Study on the chemical constituents of the fruits of Forsythia suspensa (Thunb.) Vahl. J. Shenyang Pharm. Univ. 2003, 20, 101–103. [Google Scholar]
- Lee, H.W.; Lee, H.S. Acaricidal Abilities and Chemical Composition of Forsythia suspense Fruit Oil against Storage and Pyroglyphid Mites. J. Appl. Biol. Chem. 2015, 58, 105–108. [Google Scholar] [CrossRef]
- Yang, J.J.; Wei, H.M.; Teng, X.N.; Zhang, H.Q.; Shi, Y.H. Dynamic Ultrasonic Nebulisation Extraction Coupled with Headspace Ionic Liquid-based Single-drop Microextraction for the Analysis of the Essential Oil in Forsythia suspensa. Phytochem. Anal. 2014, 25, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.J.; Sun, S.; Song, D.Q.; Sun, Y.; Zhang, Y.P.; Liu, H.; Zhang, H.Q.; Yu, A.M. Rapid Extraction of Essential Oil from Dried Cinnamomum cassia Presl and Forsythia suspensa (Thunb.) Vahl by Ionic Liquid Microwave Extraction. Chin. J. Chem. 2010, 28, 2513–2519. [Google Scholar] [CrossRef]
- Jiao, J.; Fu, Y.J.; Zu, Y.J.; Luo, M.; Wang, W.; Zhang, L.; Li, J. Enzyme-assisted microwave hydro-distillation essential oil from Fructus forsythia, chemical constituents, and its antimicrobial and antioxidant activities. Food Chem. 2012, 134, 235–243. [Google Scholar] [CrossRef]
- Sun, Y.N.; Ban, R.M.; Deng, Y.H.; Wang, Z.; Ni, Y. Comparative Study on the Chemical Constitutions of Volatile Oli in Forsythia suspensa and Old F. suspensa. China Pharm. 2016, 27, 2087–2089. [Google Scholar]
- Wei, S.; Wu, T.; Li, M.; Zhang, S.R. Analysis of Major Components and Antibacterial Activity of Volatile Oil from Forsythiae Fructus in Different Origins. Chin. J. Exp. Tradit. Med. Form. 2016, 22, 69–74. [Google Scholar]
- Dai, S.J.; Ren, Y.; Shen, L.; Zhang, D.W. New alkaloids from Forsythia suspensa and their anti-inflammatory activities. Planta Med. 2009, 75, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.M.; Sun, R.X.; Liu, C.; Zhang, R.P.; Shi, J.G. Simultaneous Structural Identification of Constituents in Active Herbal Extract of Forsythia Suspensa Using Nuclear Magnetic Resonance/Liquid Chromatography-Mass Spectrometry Parallel Dynamic Spectroscopy. Chin. J. Anal. Chem. 2011, 39, 323–329. [Google Scholar]
- Cai, Q.; Liu, Y.Q.; Feng, X. Studies on the Chemical Constituents from the Seed of Forsythia suspense. J. Chin. Med. Mater. 2009, 32, 1691–1693. [Google Scholar]
- Ming, D.S. Studies on the Chemical Constituents and Pharmacological Activities on Forsythia suspensa and Valeriana jatamansi Jones. Ph.D. Thesis, Peking Union Medical College, Beijing, China, May 1998. [Google Scholar]
- Chen, X.; Beutler, J.A.; McCloud, T.G.; Loehfelm, A.; Yang, L.; Dong, H.; Chertov, O.Y.; Salcedo, R.; Oppenheim, J.J.; Howard, O.M.Z. Tannic Acid Is an Inhibitor of CXCL12 (SDF-1)/CXCR4 with Antiangiogenic Activity. Clin. Cancer Res. 2003, 9, 3115–3123. [Google Scholar] [PubMed]
- Sun, W.J.; Sheng, J.G. Concise Manual of Natural Active Ingredients; Chinese Medical Science and Technology Press: Beijing, China, 1998. [Google Scholar]
- Wang, S.C.; Shi, S.S.; Lian, H.; Zhu, C.; Wang, H.J.; Liu, R.M.; Bligh, S.W.A. Structural Features and Anti-Complement Activity of an Acidic Polysaccharide from Forsythia suspensa. J. Glycom. Lipidom. 2016, 2, 1–8. [Google Scholar]
- Wang, Y.Q.; Guo, Z.M.; Jin, Y.; Zhang, X.L.; Li, W.; Liang, X.M. Selective enrichment with “click oligo (ethylene glycol)” column and TOF–MS characterization of simple phenylpropanoids in the fruits of Forsythia Suspensa. J. Sep. Sci. 2009, 32, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.X.; Zhang, N.; Lu, Z.B. The chemical constituents of green Forsythia suspensa. Inform. Tradit. Chin. Med. 1985, 8, 25. [Google Scholar]
- Cheng, G.D.; Zhao, Y.L.; Li, H.; Wu, Y.; Li, X.X.; Han, Q.; Dai, C.S.; Li, Y.H. Forsythiaside attenuates lipopolysaccharide-induced inflammatory responses in the bursa of Fabricius of chickens by downregulating the NF-κB signaling pathway. Exp. Ther. Med. 2014, 7, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Pang, P.; Zheng, K.; Nie, J.; Xu, H.C.; Wu, S.Z.; Chen, J.; Chen, X.Y. Forsythoside A Controls Influenza A Virus Infection and Improves the Prognosis by Inhibiting Virus Replication in Mice. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Q.; Yu, F.; Huang, H.; Li, P.; Zhu, J.; He, X. Comprehensive Characterization and Quantification of Phillyrin in the Fruits of Forsythia suspensa and Its Medicinal Preparations by Liquid Chromatography–Ion Trap Mass Spectrometry. Acta Chromatogr. 2016, 28, 145–157. [Google Scholar] [CrossRef]
- Lei, J.L.; Li, Y.T.; Nie, H.S.; Du, S.Q.; Li, F. Determination of Phillyrin in Fructus Forsythiae from Different Habitats by HPLC. Chin. Artic. Tradit. Chin. Med. 2012, 30. [Google Scholar] [CrossRef]
- Yan, Y.L.; Liu, M.J.; Yan, H.R.; Li, X.; Xu, J.H.; Yang, J.X. Determination of phillyrin and forsythosideA in Forsythia suspensa from different localities by HPLC. China Pharm. 2015, 26, 37–39. [Google Scholar]
- Xia, H.; Liu, F.Q.; Zhou, Y.P.; Zhang, S.L.; Zhou, X.; Han, J. Simultaneous Determination of Phillyrin and Forsythoside A in Forsythiae Fructus by HPLC. Pharm. J. Chin. PLA 2014, 30, 60–62. [Google Scholar]
- Li, X.J.; Zhang, Y.P.; Yuan, Z.B. Determination of Rutin and Forsythin in Fruit of Forsythia Suspensa (Thunb.) Vahl by Capillary Electrophoresis-Electrochemical Detection. Chromatographia 2002, 56, 171–174. [Google Scholar] [CrossRef]
- Zhang, S.R.; Pei, X.P.; Yan, Y.; Wang, J.J. Content Comparison of Active Components in the Fruit and Folium of Forsysia suspensa in Different Harvesting Time. Chin. Pharm. 2011, 22, 2940–2942. [Google Scholar]
- Ye, L.H.; Gong, X.H.; Li, Y.X.; Peng, C. Comparison of the contents of multiple components derived from qingqiao and laoqiao. Pharm. Clin. Chin. Mater. Med. 2013, 4, 6–8. [Google Scholar]
- Xia, B.H.; Zhu, J.J.; Wang, Z.M.; Lin, L.M.; Gao, H.M. Quantitative determination of forsythiaside in Forsythia suspensa. Chin. J. Chin. Mater. Med. 2010, 35, 2110–2112. [Google Scholar]
- Qu, H.H.; Li, B.X.; Li, X.; Tu, G.Z.; Lü, J.; Sun, W.J. Qualitative and quantitative analyses of three bioactive compounds in different parts of Forsythia suspense by high-performance liquid chromatography-electrospray ionization-mass spectrometry. Microchem. J. 2008, 89, 159–164. [Google Scholar] [CrossRef]
- Xia, Y.G.; Yang, B.Y.; Wang, Q.H.; Liang, J.; Wei, Y.H.; Yu, H.D.; Zhang, Q.B.; Kuang, H.X. Quantitative analysis and chromatographic fingerprinting for the quality evaluation of Forsythia suspensa extract by HPLC coupled with photodiode array detector. J. Sep. Sci. 2009, 32, 4113–4125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J. Studies on the main chemical constituents of Qinqiao and Laoqiao, And the Activity of Phillyrin and Forsythiaside A. Ph.D. Thesis, Shanxi University of Chinese Medicine, Tai Yuan, Shanxi, China, June 2015. [Google Scholar]
- Fu, Y.F.; Li, Q.; Bi, K.S. Determination of seven components in Forsythia suspensa by RP-HPLC. Chin. Tradit. Herb. Drugs 2013, 44, 1043–1046. [Google Scholar]
- Guo, H.; Liu, A.H.; Li, L.; Guo, D.A. Simultaneous determination of 12 major constituents in Forsythia suspensa by high performance liquid chromatography-DAD method. J. Pharm. Biomed. Anal. 2007, 43, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.R.; Pei, X.L.; Wang, H.Y. Comparison of the Contents of α-pinene and β-pinene in Volatile Oil of Forsythia suspensa in Different Harvest Periods. Chin. Pharm. 2013, 24, 4469–4471. [Google Scholar]
- Zhao, P.F.; Piao, X.S.; Pan, L.; Zeng, Z.K.; Li, Q.Y.; Xu, X.; Wang, H.L. Forsythia suspensa extract attenuates lipopolysaccharide-induced inflammatory liver injury in rats via promoting antioxidant defense mechanisms. Anim. Sci. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lim, C.; Kim, H.; Cho, S. A Study of the Anti-Inflammatory Effects of the Ethyl Acetate Fraction of the Methanol Extract of Forsythiae Fructus. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 102–113. [Google Scholar] [PubMed]
- Sohn, S.H.; Ko, E.; Kim, Y.; Shin, M.; Hong, M.; Bae, H. Genomewide expression profile of Forsythia suspensa on lipopolysaccaride-induced activation in microglial cells. Mol. Cell. Toxicol. 2008, 4, 113–123. [Google Scholar]
- Wang, Y.; Zhao, H.F.; Lin, C.X.; Ren, J.; Ye, Y.Y.; Ji, Z.H.; Zhang, S.Z. The inhibitory effect of forsythin on inflammation in LPS-induced BV2 microglia cells. J. Apoplexy Nervous Dis. 2016, 33, 338–341. [Google Scholar]
- Hao, Y.; Li, D.F.; Piao, X.L.; Piao, X.S. Forsythia suspensa extract alleviates hypersensitivity induced by soybean β-conglycinin in weaned piglets. J. Ethnopharmacol. 2010, 128, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Yoon, T.; Jang, S.; Ho, K.K. Forsythia suspensa Suppresses House Dust Mite Extract-Induced Atopic Dermatitis in NC/Nga Mice. PLoS ONE 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Luo, L.; Gong, X.H.; Li, Y.; Zhao, M.J.; Zhang, R.Q.; Li, Y.X. Effect of Forsythia Extract on Paw Edema Induced by Carrageenan and Fresh Egg White in Rats. Liaoning Tradit. Chin. Med. Mag. 2016, 43, 2200–2202. [Google Scholar]
- Guo, J.; Shen, Y.J.; Xie, Y.H. The experimental study inflammation of Essential Oil from Fructus forsythia. Sichuan Physiol. Sci. Mag. 2005, 27, 136–137. [Google Scholar]
- Pan, C.W.; Zhou, G.Y.; Chen, W.L.; Zhuge, L.; Jin, L.X.; Zheng, Y.; Lin, W.; Pan, Z.Z. Protective effect of forsythiaside A on lipopolysaccharide/d-galactosamine-induced liver injury. Int. Immunopharmacol. 2015, 26, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.L.; Cao, X.; Li, N.; Xu, Y.M.; Wu, Q.Y.; Bai, J.; Yin, Z.M.; Luo, L.; Lan, L. Forsythin inhibits lipopolysaccharide-induced inflammation by suppressing JAK-STAT and p38 MAPK signalings and ROS production. Inflamm. Res. 2014, 63, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Y. Experimental Study on The Effect of Anti-endotoxin of Forsythoside A through HMGB1/TLR4/NF-κB Signaling Pathway. Ph.D. Thesis, Nanchang University, Nanchang, China, June 2016. [Google Scholar]
- Zhong, W.T.; Wu, Y.C.; Xie, X.X.; Zhou, X.; Wei, M.M.; Soromou, L.W.; Ci, X.X.; Wang, D.C. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-κB activation in acute lung injury mice. Fitoterapia 2013, 90, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.H.; Lee, C.K.; Ha, N.J.; Yim, D.; Kim, K. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways. J. Inflamm. 2011, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Zhang, L.W. Anti-inflammatory Activity of Forsythia suspensa Extract on Human Airway Epithelial Cells Inflammation Model. Nat. Prod. Res. Dev. 2015, 27, 1248–1253. [Google Scholar]
- Sung, Y.Y.; Lee, A.Y.; Kim, H.K. Forsythia suspensa fruit extracts and the constituent matairesinol confer anti-allergic effects in an allergic dermatitis mouse model. J. Ethnopharmacol. 2016, 187, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, F.; Ma, R.; Hu, X.P. Forsythiaside inhibits cigarette smoke-induced lung inflammation by activation of Nrf2 and inhibition of NF-κB. Int. Immunopharmacol. 2015, 28, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Coon, T.A.; McKelvey, A.C.; Weathington, N.M.; Birru, R.L.; Lear, T.; Leikauf, G.D.; Chen, B.B. Novel PDE4 Inhibitors Derived from Chinese Medicine Forsythia. PLoS ONE 2014, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Lee, J.Y.; Kim, C.J. Anti-inflammatory activity of arctigenin from Forsythiae Fructus. J. Ethnopharmacol. 2008, 116, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.F.; Lin, C.X.; Ren, J.; Zhang, S.Z. Forsythiaside A Exhibits Anti-inflammatory Effects in LPS-Stimulated BV2 Microglia Cells Through Activation of Nrf2/HO-1 Signaling Pathway. Neurochem. Res. 2016, 41, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Wang, X.X.; Liu, D. Inhibitory effect of forsythin on the inflammatory responses of monocyte-macrophage induced by Staphylococcus aureus. J. Xinxiang Med. Univ. 2016, 6, 466–468. [Google Scholar]
- Guo, N.; Gai, Q.Y.; Jiao, J.; Wang, W.; Zu, Y.G.; Fu, Y.J. Antibacterial Activity of Fructus forsythia Essential Oil and the Application of EO-Loaded Nanoparticles to Food-Borne Pathogens. Foods 2016, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.M.; He, Y.; Wang, S.W.; Wang, J.B.; Xie, Y.H. Experimental stydy on the antibacterial activity of the Essential Oil from Forsythiae Fructus in vitro. Inner Monog. Med. 2011, 15, 99–100. [Google Scholar]
- Li, H.; Dai, X.H.; Tian, W.L.; Zhang, H.L. Chinese herbal medicine Fructus forsythia extract inhibits Staphylococcus aureus alpha-hemolysin secretion activity. Chin. J. Vet. Sci. 2013, 33, 404–408. [Google Scholar]
- Han, X.; Piao, X.S.; Zhang, H.Y.; Li, P.F.; Yi, J.Q.; Zhang, Q.; Li, P. Forsythia suspensa Extract Has the Potential to Substitute Antibiotic in Broiler Chicken. Asian Australas. J. Anim. Sci. 2012, 25, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Mu, X.P.; Bo, C.P.; Zhang, D.C.; Deng, W.Y. Effect of Forsythia suspensa on the adeb gene of the active efflux system of multidrug-resistant Acinetobacter baumannii. J. Pathog. Biol. 2016, 6, 111–114. [Google Scholar]
- Ko, H.C.; Wei, B.L.; Chiou, W.F. Dual regulatory effect of plant extracts of Forsythia suspense on RANTES and MCP-1 secretion in influenza A virus-infected human bronchial epithelial cells. J. Ethnopharmacol. 2005, 102, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.Y.; Li, Q.J.; Zhang, H.M.; Zhang, X.J.; Shi, P.H.; Zhang, X.J.; Yang, J.; Zhou, Z.; Wang, S.Q. Protective effects of phillyrin against influenza A virus in vivo. Arch. Pharm. Res. 2016, 39, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.J.; Zhang, Q.; Wang, N.R.; Yang, B.; He, S.Q.; Sun, J. Effect of Phillyrin on Gene Expression of Influenza A Virus Nucleoprotein. Res. Tradit. Chin. Med. West. Med. 2012, 15, 2082–2084. [Google Scholar]
- Yang, M.; Lu, Y.; Ma, Y.Y.; Wu, G.Y.; Beier, R.R.; Hou, X.L.; Wu, G.J. Inhibition of porcine reproductive and respiratory syndrome virus in vitro by forsythoside A. Int. J. Pharmacol. 2015, 11, 394–399. [Google Scholar]
- Li, H.W.; Wu, J.F.; Zhang, Z.W.; Ma, Y.Y.; Liao, F.F.; Zhang, Y.; Wu, G.J. Forsythoside A inhibits the avian infectious bronchitis virus in cell culture. Phytother. Res. 2011, 25, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, B.H.; Yang, X.L.; Lv, A.; Zhang, Z.C.; Gong, P.; Li, D.Y.; Hou, X.L. Effect of forsythoside A on expression of intracelluar receptors and antiviral gene in IBV-infected cells. J. Beijing Univ. Agric. 2017, 32, 37–41. [Google Scholar]
- Huang, C.Y.; Huang, T.Z.; Zhang, H.; Cheng, S.J.; Qi, L.Y.; Xiao, G.; Huang, S.Y. Study on Optimization of Orthogonal Design of Enzyme Extraction Craft and Antioxidant Activity of Forsythia suspense Polysaccharide. Chem. World 2017, 1, 38–42. [Google Scholar]
- Lu, T.; Piao, X.L.; Zhang, Q.; Wang, D.; Piao, X.S.; Kim, S.W. Protective effects of Forsythia suspensa extract against oxidative stress induced by diquat in rats. Food Chem. Toxicol. 2010, 48, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Zheng, H.J.; Ling, X.L.; Liang, H.Y.; Huang, C.J.; Huang, S.Y. Study of Antioxidant Property of the Extract from Forsythia suspense Ethyl Acetate. Chin. Wild Plant Res. 2017, 36, 15–17. [Google Scholar]
- Piao, X.L.; Cho, E.J.; Jang, M.H.; Cui, J. Cytoprotective effect of lignans from Forsythia suspensa against peroxynitrite-induced LLC-PK1 cell damage. Phytother. Res. 2009, 23, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.Y.; Liu, M.J.; Yan, H.R.; Li, X.; Xu, J.H.; Yang, J.X. Study on Anti-aging Effects of Phillyrin on Aging Model Mice. Chin. Pharm. 2015, 26, 37–39. [Google Scholar]
- Hao, P.F.; Piao, X.S.; Zeng, Z.K.; Li, P.; Xu, X.; Wang, H.L. Effect of Forsythia suspensa extract and chito-oligosaccharide alone or in combination on performance, intestinal barrier function, antioxidant capacity and immune characteristics of weaned piglets. Anim. Sci. J. 2016. [Google Scholar] [CrossRef]
- Zeng, Z.K.; Li, Q.Y.; Piao, X.S.; Liu, J.D.; Zhao, P.F.; Xu, X.; Zhang, S.; Niu, S. Forsythia suspensa extract attenuates corticosterone-induced growth inhibition, oxidative injury, and immune depression in broilers. Poult. Sci. 2014, 93, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Piao, X.S.; Zhang, Q.; Li, P.; Yi, J.Q.; Liu, J.D.; Li, Q.Y.; Wang, G.Q. The effect of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poult. Sci. 2013, 92, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, S.Y.; Song, X.Y.; Xia, C.Y.; Yang, Y.N.; Zhang, P.C.; Chen, N.H. Protective effects of Forsythia suspense extract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. Neuro Toxicol. 2016, 52, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.P.; Tian, Y.J. Ameliorative Effect and Its Mechanism of Forsythiaside on Learning and Memory of Composite Alzheimer’s Disease Model Mice. J. Int. Transl. Med. 2016, 4, 51–57. [Google Scholar]
- Wang, H.M.; Wang, L.W.; Liu, X.M.; Li, C.L.; Xu, S.P.; Farooq, A.D. Neuroprotective effects of forsythiaside on learning and memory deficits in senescence-accelerated mouse prone (SAMP8) mice. Pharmacol. Biochem. Behav. 2013, 105, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Sun, X.P.; Wang, Y.H.; Wang, L.W.; Liu, X.M. Effect of Forsythiaside on Scopolamine-induced Learning and Memory Impairment in Mice. Chin. J. Exp. Tradit. Med. Form. 2016, 22, 177–181. [Google Scholar]
- Kim, J.M.; Kim, S.; Kim, D.H.; Lee, C.H.; Park, S.J.; Jung, J.W.; Ko, K.H.; Cheong, J.H.; Lee, S.H.; Ryu, J.H. Neuroprotective effect of forsythiaside against transient cerebral global ischemia in gerbil. Eur. J. Pharmacol. 2011, 660, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.X.; Zhang, L.W.; Du, H.Z. Improvement of Forsythoside A on Neuroinflammation Iuduced by Aβ25–35 Oligomer. Nat. Sci. Educ. 2016, 39, 631–638. [Google Scholar]
- Sun, X.P.; Wang, Y.H.; Wang, L.W.; Qin, C.; Liu, X.M. Neuroprotective Effects of Forsythiaside on Glutamate, Low-glucose and Low Serum, Aβ25–35-induced Neurotoxicity in PC 12 Cell. Chin. J. Exp. Tradit. Med. Form. 2013, 19, 197–200. [Google Scholar]
- Zhang, M.R.; Wei, S.R.; Wu, Y.C.; Sun, F.L.; Ai, H.J.; Zhang, L.; Wang, W. Effects of Phillyrin on MPP+-induced Injury in SH-SY5Y Neuroblastoma Cells. Acta Neuropharmacol. 2011, 1, 12–15. [Google Scholar]
- Qu, X.; Li, X.; Cai, P.P.; Shang, X.Y.; Guo, D.B.; You, N.; Li, H.Y. In vitro induction of apoptosis caused by bioactive compounds extracted from Forsythia suspensa and its mechanisim in HeLa cells. Chin. J. Public Health 2013, 29, 397–399. [Google Scholar]
- Cai, P.P.; Li, X.; Qu, X.; Shang, X.Y.; Li, Y.K.; Li, H.Y. In vitro induction of apoptosis by the forsythia ethanol extract LQ-4 in human cervical cancer Hela cells. Chin. J. Clin. 2015, 7, 9235–9238. [Google Scholar]
- Guo, D.B.; Li, X.; Pu, Y.A.; You, N.; Zhong, E.D.; Cai, P.P.; Qu, X.; Li, H.Y. Research on forsythia anti-tumor component (LQ-4) effect on apoptosis of SGC-7901 cells in vitro. Chin. J. Clin. 2011, 5, 4345–4349. [Google Scholar]
- Zheng, M.; Jiang, Z.M. Effects of phillyrin on VEGF and endostatin expression in Lewis lung carcinoma. Chin. J. Pathophysiol. 2016, 32, 167–171. [Google Scholar]
- Wang, C.L.; Yan, H.T.; Liu, B.R. Effects of antiproliferation and radiosensitivity on PC-3 cell of prostate cancer induced by triterpenes component. Shangdong Med. 2011, 51, 25–27. [Google Scholar]
- Wang, E.L.; Yao, J.C.; Liu, Z. Effect of forsythiaside on immunological hepatic fibrosis of rats. Drug Eval. Res. 2015, 38, 161–164. [Google Scholar]
- Liu, Y.H.; Qi, Z.L.; Xu, G.X.; He, L.; Yang, J.H. Protective effect of forsythin on alcoholic liver injury. Chin. Clin. Pharmacol. Ther. 2016, 21, 6–9. [Google Scholar]
- Fan, X.B.; Li, W.X.; Chen, B.H.; Xiong, Z.Y.; Duan, J.M. Effect of Forsythia suspensa on expression of NF-κB and Foxp3 during liver injury in rats with severe acute pancreatitis. J. Clin. Hepatol. 2013, 29, 503–507. [Google Scholar]
- Zhang, Y.Y.; Feng, F.; Chen, T.; Li, Z.W.; Shen, Q.W.W. Antidiabetic and antihyperlipidemic activities of Forsythia suspensa (Thunb.) Vahl (fruit) in streptozotocin-induced diabetes mice. J. Ethnopharmacol. 2016, 192, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Nagai, M. Vasorelaxant effects of forsythiaside from the fruits of Forsythia suspensa. Yakugaku Zasshi 2015, 125, 219–224. [Google Scholar] [CrossRef]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Hwang, Y.P.; Na, M.K.; Jeong, H.G. Phillyrin attenuates high glucose-induced lipid accumulation in human HepG2 hepatocytes through the activation of LKB1/AMP-activated protein kinase-dependent signaling. Food Chem. 2013, 136, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Zhang, L.L.; Guo, Y.L.; Lin, D.P. Phillyrin, a Natural Ligands, Attenuates Tumor Necrosis Factor α-Mediated Insulin Resistanceand Lipolytic Acceleration in 3T3-L1 Adipocytes. Nat. Planta Med. 2014, 80, 880–886. [Google Scholar]
- Xiao, H.B.; Sui, G.G.; Lu, X.Y. Phillyrin lowers body weight in obese mice via the modulation of PPAR/-ANGPTL 4 pathway. Obes. Res. Clin. Pract. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Park, S.Y.; Song, H.G.; Hwang, E.; Lee, D.G.; Yi, T.H. The Androgenic Alopecia Protective Effects of Forsythiaside A and the Molecular Regulation in a Mouse Model. Phytother. Res. 2015, 29, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R. Effect of Forsythiaside A on Immune Regulation in Endotoxemia Mice and Mechanism of Action. Chin. Med. Guide 2016, 22, 57–60. [Google Scholar]
- Su, H.C.; Wang, H.Y.; Liu, C.L.; Kong, X.Y.; Lin, N. Effect of Forsythiaside A on Temperature and Expression of TRPA1 in Mice with Yeast Induced Pyrexia. Chin. J. Exp. Tradit. Med. Form. 2016, 22, 134–138. [Google Scholar]
- Meng, X.L.; Guo, Y.L.; Su, C.F.; Huang, H.Y.; Gui, X.J.; Li, X.L. Discussion of Inhibitory of Forsythoside A on Efflux Function and Mechanism of P-glycoprotein in Caco-2 Cell Membrane. Chin. J. Exp. Tradit. Med. Form. 2015, 21, 5–8. [Google Scholar]
- Lin, Y.H.; Chen, Y.C.; Hu, S.; Chen, H.Y.; Chen, J.L.; Yang, S.H. Identifying core herbal treatments for urticaria using Taiwan’s nationwide prescription database. J. Ethnopharmacol. 2013, 148, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, Y.H.; Chen, Y.C. Identifying Chinese herbal medicine network for treating acne: Implications from a nationwide database. J. Ethnopharmacol. 2016, 179, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.N.; Pan, R.L.; Liao, Y.H.; Chen, Y.; Tang, J.T.; Chang, Q. An LC-MS/MS method for determination of forsythiaside in rat plasma and application to a pharmacokinetic study. J. Chromatogr. B. 2010, 878, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, X.Y.; Guo, J.H.; Li, W.; Ma, X.H.; Zhu, Y.H. Pharmacokinetic study of unbound forsythiaside in rat blood and bile by microdialysis coupled with HPLC method. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Ye, L.H.; Jiang, X.H.; Peng, C. Assessment and modulation of phillyrin absorption by P-gp using Caco-2 cells and MDR1-MDCKII cells. Eur. J. Drug Metab. Pharmacokinet. 2011, 36, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Zhang, X.X.; Jia, P.P.; Zhang, Y.F.; Tang, S.W.; Wang, H.T.; Li, S.; Yu, X.L.; Li, Y.F.; Zhang, L.T. Metabolic profile of phillyrin in rats obtained by UPLC-Q-TOF-MS. Biomed. Chromatogr. 2016, 30, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.H.; Li, Y.X.; Peng, C.; Gong, X.H.; Zheng, X.G. Determination of phillygenin in rat plasma by high-performance liquid chromatography and its application to pharmacokinetic studies. Eur. J. Drug. Metab. Pharmacokinet. 2013, 38, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Liang, X.L.; Feng, L.Y.; Liu, D.; Qin, M.N.; Liu, S.; Liu, G.F.; Dong, M. Effects of phillyrin and forsythoside A on rat cytochrome P450 activities in vivo and in vitro. Xenobiotica 2017, 47, 297–303. [Google Scholar] [PubMed]
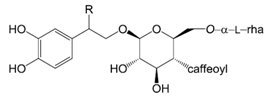 | Compounds | R |
| Forsythoside A (1) | H | |
| Forsythoside C (Suspensaside, 2) | OH | |
| (R)-Suspensaside (3) | β-OH | |
| (S)-Suspensaside (4) | α-OH | |
| (S)-Suspensaside methyl ether (5) | α-OCH3 | |
| Suspensaside B (6) | OC4H9 | |
| (R)-Forsythoside J (7) |  | |
| (S)-Forsythoside J (8) |  | |
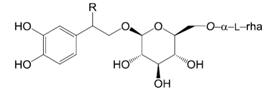 | Compounds | R |
| Forsythoside D (9) | OH | |
| Forsythoside E (10) | H | |
| β-Methoxyforsythoside E (11) | OCH3 |
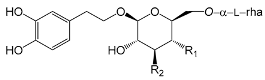 | Compounds | R1 | R2 |
| Iso-forsythoside A/Forsythoside I/Lianqiaoxinside A (12) | OH | caffeoyl | |
| ForsythosideA-4’O-β-d-glucopyranoside (13) | (4’O-β-d-glu) caffeoyl | OH | |
| Forsythenside K (14) | coumaroyl | OH | |
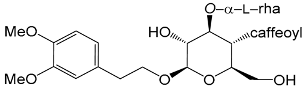
| Poliumoside (15) | caffeoyl | O-β-l-rha |
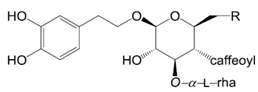 | Compounds | R |
| Acteoside (16) | OH | |
| Forsythoside B (17) | O-api | |
| Forsythoside G (18) | 2-O-methyl-api | |
| Forsythoside F (19) | O-β-d-xyl | |
| Angoroside A (20) | O-arabinose | |
 | Compounds | R |
| Calceolarioside C (21) | H | |
| (S)-β-hydroxycalceolarioside C (22) | α-OH | |
| (R)-β-hydroxycalceolarioside C (23) | β-OH | |
| (S)-β-methoxycalceolarioside C (24) | α-OCH3 | |
| (R)-β-methoxycalceolarioside C (25) | β-OCH3 |
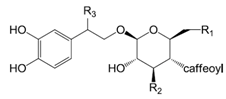 | Compounds | R1 | R2 | R3 |
| Calceolarioside A (26) | OH | OH | H | |
| Derhamnosyl suspensaside (27) | OH | OH | OH | |
| β-methoxylacteoside (28) | OH | O-α-l-rha | OCH3 | |
| Caffeoyl calceolarioside C (29) | O-β-d-glc | O-api | H | |
| Isoforsythiaside (30) | O-β-l-rha | OH | H |
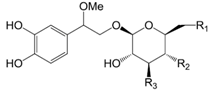 | Compounds | R1 | R2 | R3 |
| β-Methoxyferruginoside B (31) | O-β-d-glc | OH | OH | |
| β-Methoxylipedoside A (32) | OH | coumaroyl | O-α-l-rha |
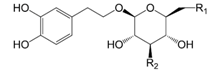 | Compounds | R1 | R2 |
| Calceolarioside B (33) | caffeoyl | OH | |
| Lianqiaoxinoside C (34) | O-β-d-xyl | caffeoyl | |
| Plantainoside A (35) | OH | caffeoyl |
 | Compounds | R |
| Forsythoside J (36) | O-β-d-xyl | |
| Plantainoside B (37) | OH | |
| Forsythoside H (38) | O-α-l-rha |
 | Compounds | R1 | R2 | R3 |
| Suspensaside A (39) | OH | caffeoyl | O-α-l-rha | |
| Suspensaside A isomer (40) | OH | caffeoyl | coumaroyl | |
| Demethylsuspensaside A (41) | OH | caffeoyl | O-xyl/O-api | |
| Suspensaside C (42) | OH | OH | O-α-l-rha | |
| Lianqiaoxinoside B (43) | caffeoyl | OH | O-α-l-rha |
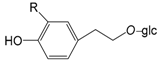 | Salidroside R = H (44) |
| 3,4-Dihydroxyphenylethyl-8-O-β-d-glucopyranoside R = OH (45) | |
 | Forsythiayanoside C (46) |
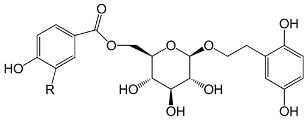 | 2-(2,5-Dihydroxyphenyl)-ethyl-O-(6-O-p-hydroxybenzoyl)-d-glucopyranoside R = H (47) |
| 2-(2,5-Dihydroxyphenyl)-ethyl-O-(6-ovanilloyl)-d-glucopyranoside R = OCH3 (48) | |
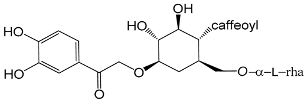 | 2-(3,4-Dihydroxyphenyl)-2-oxo-ethyl-O-l-rhamnopyranosyl-(16)-(4-O-caffeoyl)-d-glucopyranoside. (49) |
 | Brachynoside (50) |
 | Phenethyl alcohol β-d-xylopyranosyl-(1→6)-β-d-glucopyranoside (51) |
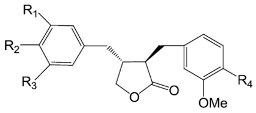 | Compounds | R1 | R2 | R3 | R4 |
| Arctigenin (52) | OCH3 | OCH3 | H | OH | |
| Arctiin (53) | OCH3 | OCH3 | H | O-glu | |
| Matairesinoside (54) | OCH3 | OH | H | O-glu | |
| Matairesinol (55) | OCH3 | OH | H | OH | |
| 2′,5′-Dihydroxy-4′′-caffeoyl matairesinol (56) | OCH3 | OH | OH | caffeoyl | |
| 3′,4′,5′-Trihydroxy-3′′-methoxy-4′′-caffeoyl lignan (57) | OH | OH | OH | caffeoyl | |
| Matairesinol-4′-O-glucoside (58) | OCH3 | O-β-d-glc | H | OH |
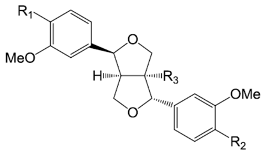 | Compounds | R1 | R2 | R3 |
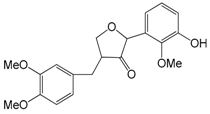
| Phillygenin (59) | OCH3 | OH | H | |
| Phillyrin (forsythin 60) | OCH3 | O-β-d-glc | H | |
| Caffeoyl phillygenin (61) | OCH3 | caffeoyl | H | |
| (+)-Epipinoresinol (62) | OH | OH | H | |
| 7′-Epi-8-hydroxypinoresinol (63) | OH | OH | OH | |
| (+) Epipinoresinol-4-β-d-glucoside (64) | OH | O-β-d-glc | H | |
| (+)-8-Hydroxyepipinoresinol-4-O-β-d-glucopyranoside (65) | OH | O-β-d-glc | OH | |
| (+) epipinoresinol-4′-β-d-glucoside (66) | O-β-d-glc | OH | H | |
| forsythialanside E (67) | O-β-d-glc | OH | OH | |
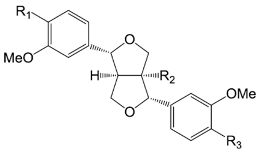 | Compounds | R1 | R2 | R3 |
| (+) Pinoresinol (68) | OH | H | OH | |
| (+) Pinoresinol-β-d-glucoside (69) | O-β-d-glc | H | OH | |
| (+) Pinoresinol monomethyl ether-β-d-glucoside (70) | O-β-d-glc | H | OCH3 | |
| Pinoresinol diglucoside (71) | O-β-d-glc | H | O-β-d-glc | |
| Caffeoyl pinoresinol (72) | caffeoyl | H | OH | |
| (+)-1-Hydroxypinordsinol (73) | OH | OH | OH | |
| (+)-1-Hydroxypinordsinol-4′-O-β-d-glucoside (74) | OH | OH | O-β-d-glc | |
| (+)-1-Hydroxypinordsinol-4′′-O-β-d-glucoside (75) | O-β-d-glc | OH | OH | |
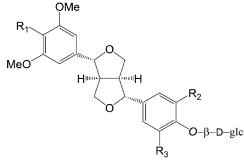 | Compounds | R1 | R2 | R3 |
| 3′,4′,5′-Trimethoxyl-4′′-hydroxyllignan O-glucoside (76) | OCH3 | H | H | |
| Syringaresinol-4-O-β-d-glucoside (77) | OH | OCH3 | OCH3 |
 | Compounds | R1 | R2 | R3 |
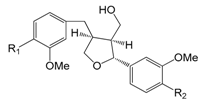
| Isolariciresinol (78) | H | H | H | |
| Isolariciresinol-4-O-β-d-glucopyranoside (79) | H | H | O-β-d-glc | |
| Isolariciresinol-9′-O-β-d-glucopyranoside (80) | H | O-β-d-glc | H | |
| Isoolivil (81) | OH | H | H | |
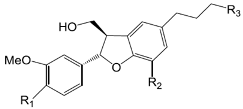 | Compounds | R1 | R2 | R3 |
| Cedrusin (82) | OH | OH | OH | |
| Glochidioboside (83) | OH | OCH3 | O-glc | |
| Forsythialanside C (84) | O-glc | OCH3 | O-rha | |
| Forsythialanside D (85) | O-rha | OCH3 | O-rha | |
| Dihydrodehydrodiconiferyl alcohol-4-O-β-d-glucoside (86) | O-glc | OCH3 | OH |
 | Icariside E4 (87) |
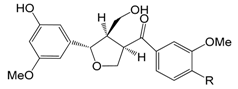 | Forsythialan A R = OH (88) |
| Forsythialan B R = OMe (89) | |
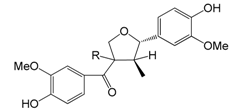 | rel-(7R,8′R,8S)-Forsythialan C R = β-H (90) |
| rel-(7R,8′R,8R)-Forsythialan C R = α-H (91) | |
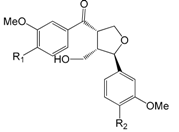 | Forsythialanside A R1 = OMe R2 = O-glc (92) |
| Forsythialanside B R1 = O-glc R2 = OH (93) | |
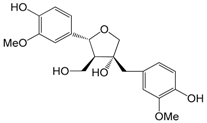 | Olivil (94) |
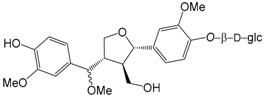 | Forsythiayanoside B (95) |
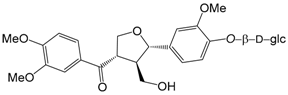 | Forsythiayanoside A (96) |
 | 3-Furanone-2-(3-methoxy-4-hydroxyphenyl)-4-veratryl (97) |
 | Lariciresinol R1 = OH R2 = OH (98) |
| Lariciresinol-4-O-β-d-glucoside R1 = OH R2 = O-β-d-glc (99) | |
| Lariciresinol-4′-O-β-d-glucoside R1 = O-β-d-glc R2 = OH (100) | |
 | Benzenebutanoic acid (101) |
 | Compounds | R1 | R2 | R3 | R4 |
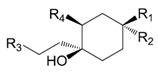
| Isorengyol (102) | H | OH | OH | H | |
| Rengyol (103) | OH | H | OH | H | |
| Suspenol (104) | OH | H | OH | OH | |
| Rengyolester (105) | OH | H | 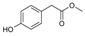 | OH | |
| Rengyoside A (106) | OH | H | O-β-d-glc | H | |
| Rengyoside C (107) | OH | H | 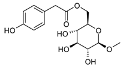 | H |
 | Rengynic acid R = OH (108) |
| Rengynic acid-1′-O-β-d-glucopyranoside R = O-β-d-glc (109) | |
 | Rengyolone (halleridone) (110) |
 | Rengyoxide R = OH (111) |
| Rengyoside B R = O-β-d-glc (112) | |
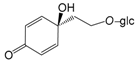 | Cornoside (113) |
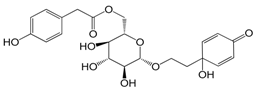 | Forsythenside A (114) |
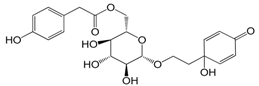 | Forsythenside B (115) |
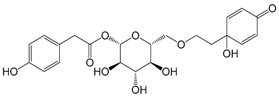 | Forsythenside F (116) |
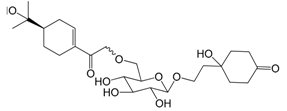 | Forsythenside H (117) |
 | Forsythenside G (118) |
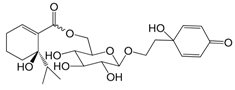 | Forsythenside I (119) |
 | Forsythenside J (120) |
 | Adoxosidic acid (121) |
 | Adoxosidic acid 10-p-hydroxyphenylacetate (122) |
 | 3β-Hydroxylabda-8(17), 13E-dien-15-oic acid (123) |
 | 3β-Hydroxyanticopalic acid (124) |
 | Agatholic acid (125) |
 | 3-Oxoanticopalic acid (126) |
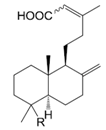 | Compounds | R |
| 19-Hydroxylabda-8(17),13(Z)-dien-15-oic acid (127) |  | |
| 19-Hydroxylabda-8(17),13(E)dien-15-oic acid (128) | 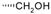 | |
| 19-Formyllabda-8(17),13(E)-dien-15-oic acid (129) | 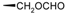 | |
| 19-Formyllabda-8(17),13(Z)-dien-15-oic acid (130) |  | |
| Labda-8(17),13(Z)-dien-15,18-dioic acid (131) | 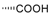 |
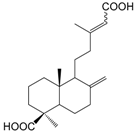 | Labda-8(17),13(Z)-diene-15,19dioic acid (132) |
| Labda-8(17),13(E)-diene-15,19-dioic acid (133) | |
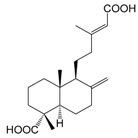 | Dehydropinifolic acid (134) |
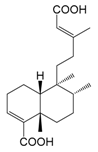 | Haplopappic acid (135) |
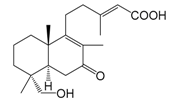 | 18-Hydroxy-7-oxolabda-8(9),13(E)-dien-15-oic acid (136) |
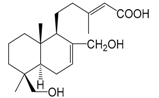 | 19-Dihydroxylabda-7(8),13(E)-dien-15-oic acid (137) |
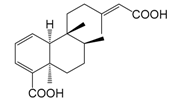 | Forsythidin A (138) |
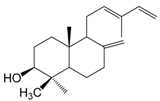 | 3β-Hydroxy-12,13(E)-biformene (139) |
| 3β-Hydroxy-12,13(Z)-biformene (140) | |
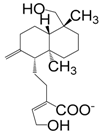 | 19-Hydroxy-8(17)(E)-13-labdadien-15-oate (141) |
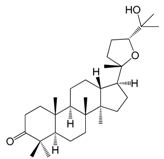 | Ocotillone (142) |
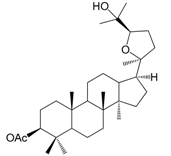 | Ocotillol acetate (143) |
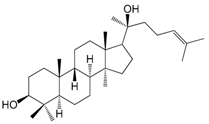 | Garcinielliptone Q (144) |
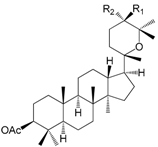 | 3β-Acetyl-20,25-epoxydammarane-24α-ol R1 = H,R2 = OH (145) |
| 3β-Acetyl-20,25-epoxydammarane-24β-ol R1 = OH,R2 = H (146) | |
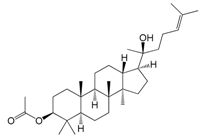 | Dammar-24-en-3β-acetoxy-20-ol (147) |
 | 3β-Acetoxy-25-methoxydammar-23-en-20β-ol (148) |
 | 3β-Acetoxy-20S,24R-dammarane-25-ene-24-hydroperoxy-20-ol (149) |
 | Cabralea lactone 3-acetate (150) |
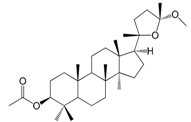 | Cabralea lactone 3-acetate 24-methyl ether (151) |
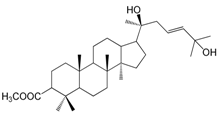 | 3-Acetylisofouquierol (152) |
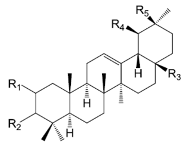 | Compounds | R1 | R2 | R3 | R4 | R5 |
| Oleanolic acid (153) | H | β-OH | COOH | H | Me | |
| 3β-Acetyloleanolic acid (154) | H | β-OAc | COOH | H | Me | |
| β-Amyrin acetate (155) | H | β-OAc | Me | H | Me | |
| Ursolic acid (156) | H | β-OH | COOH | Me | H | |
| 2α,3α-Hydroxyursolic acid (157) | α-OH | α-OH | COOH | Me | H |
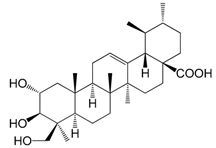 | 2α,23-Hydroxyursolic acid (158) |
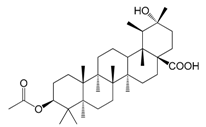 | 3β-Acetoxy-20α-hydroxyursan-28-oic acid (159) |
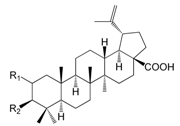 | Betulinic acid R1 = H R2 = OH (160) |
| 3β-Acetylbetulinic acid R1 = H R2 = OAc (161) | |
| 2α-Hydroxybetulinic acid R1 = α-OH R2 = OH (162) | |
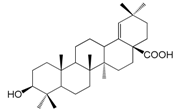 | Ambrolic acid (163) |
 | Morolic acid (164) |
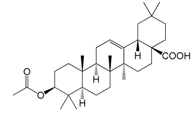 | 3β-Acetoxyolean-12-en-28-oic acid (165) |
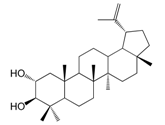 | Alphitolic acid (166) |
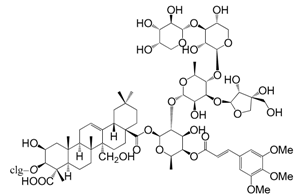 | Onjisaponin F (167) |
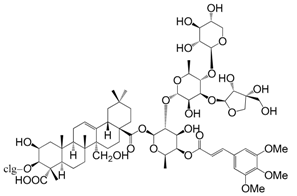 | Onjisaponin G (168) |
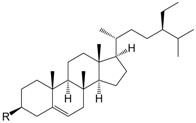 | β-Sitosterol R = OH (169) |
| Daueosterol R = O-β-d-glc (170) | |
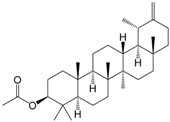 | Taraxasterol acetate (171) |
 | Stigmasterol (172) |
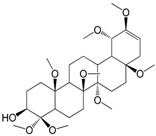 | ψ-Taraxasterol (173) |
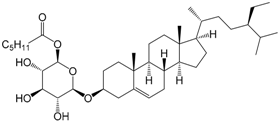 | (6′-O-Palmitoyl)-sitosterol-3-O-β-d-glucoside (174) |
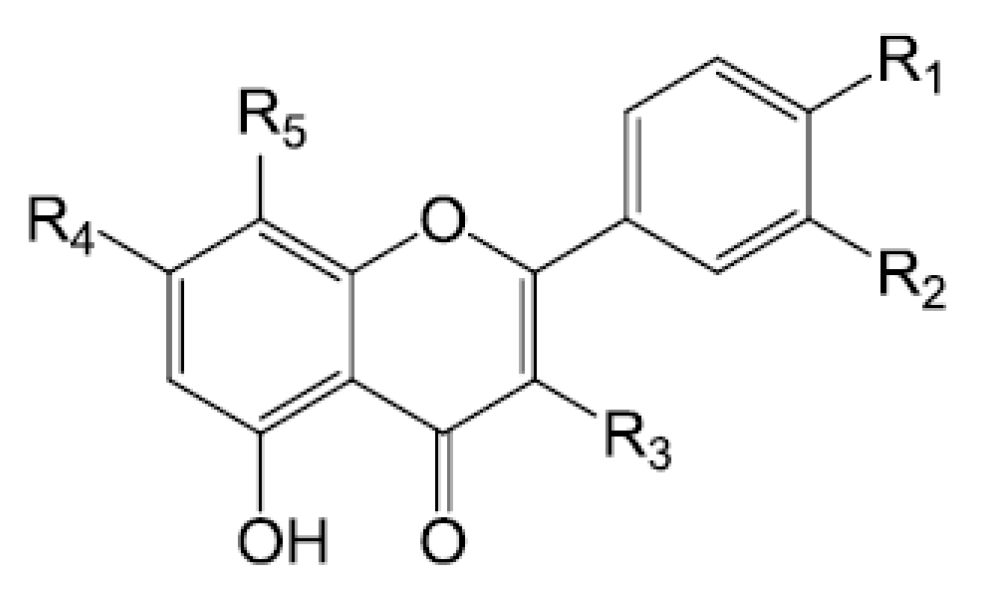
| Compounds | R1 | R2 | R3 | R4 | R5 |
| Rutin (175) | OH | OH | O-β-d-glc-O-α-l-rha | OH | H |
| Quercetin (177) | OH | OH | OH | OH | H |
| Isorhamnetin (180) | OCH3 | OH | OH | OH | H |
| Kaempferfol (181) | OH | H | OH | OH | H |
| Hyperin (182) | OH | OH | O-β-d-gal | OH | H |
| Kaempferol-3-O-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside (185) | OH | H | O-β-d-glc | O-α-l-rha | H |
| Kaempferol-3-O-β-d-(2″-O-β-d-glucopyranosyl-6″O-α-l-rhamnopyranosyl)glucopyranoside (186) | OH | H | O-β-d-(2″-O-β-d-glc-6″O-α-l-rha)glc | OH | H |
| Wogonin-7-O-glcoside (187) | H | H | H | O-β-d-glc | OMe |
| Baicalin (188) | H | H | H | O-glc | OH |
 | Hesperidin (190) |
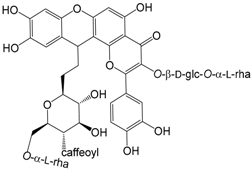 | Forsythoneoside A 7′R (191) |
| Forsythoneoside B 7′S (192) | |
 | Forsythoneoside C M configuration (193) |
| Forsythoneoside D P configuration (194) |
 | Rutaecarpine (246) |
 | Suspensine A (247) |
 | (−)-Egenine R = OH (248) |
| (−)-7′-O-Methylegenine R = OMe (249) | |
 | (−)-Bicuculline (250) |
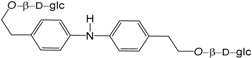 | Bis-2-(4-aminophenyl) ethyl-β-d-glucopyranoside (251) |
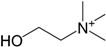 | Choline (252) |
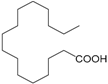 | Palmitic acid (253) |
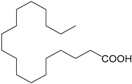 | Stearic acid (254) |
 | Succinic acid (255) |
 | Suspenolic acid (256) |
 | 2-Furancarboxylic acid (257) |
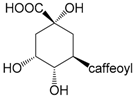 | Chlorogenic acid (258) |
 | Anchoic acid (259) |
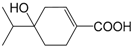 | 4-Hydroxy-4-isopropylcyclohex-1-enecarboxylic acid (260) |
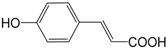 | p-Coumaric acid (261) |
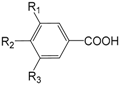 | Compounds | R1 | R2 | R3 |
| Protocatechuic acid (262) | H | OH | OH | |
| Vanillic acid (263) | H | OH | OMe | |
| p-Hydroxybenzoic acid (264) | H | OH | H | |
| Benzoic acid (265) | H | H | H | |
| 3,4-Dimethoxybenzoic acid (266) | H | OMe | OMe | |
| Syringic acid (267) | OMe | OH | OMe |
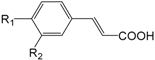 | Compounds | R1 | R2 |
| Caffeic acid (268) | OH | OH | |
| trans-Coumaric acid (269) | OH | H | |
| trans-Ferulic acid (270) | OH | OMe |
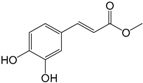 | Caffeic acid methyl ester (271) |
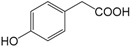 | p-Hydroxybenzylacetic acid (272) |
 | Tannic acid (273) |
 | Gallic acid (274) |
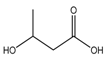 | 3-Hydroxybutyric acid (275) |
 | Acetic acid (276) |
 | Pyruvic acid (277) |
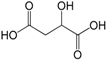 | Malic acid (278) |
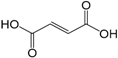 | Fumaric acid (279) |
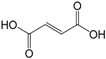
 | Formic acid (280) |
 | Isoleucine (281) |
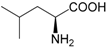 | Leucine (282) |
 | Valine (283) |
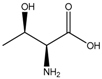 | Threonine (284) |
 | Alanine (285) |
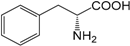 | Phenylalanine (286) |
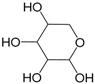 | β-Xylose (287) |
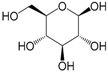 | β-Glucose (288) |
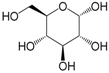 | α-Glucose (289) |
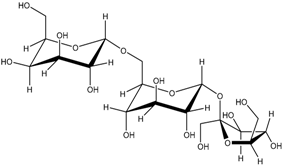 | Raffinose (290) |
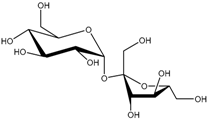 | Sucrose (291) |
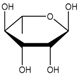 | l-Rhamnose (292) |
 | Lactose (293) |
 | Erythritol (294) |
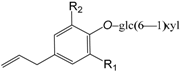 | Forsythenside L R1 = H R2 = OH (295) |
| Sasanquin R1 = OMe R2 = H (296) | |
 | Forsythiayanoside D (297) |
 | (6S,9R)-Roseoside (298) |
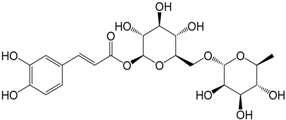 | Swertiamacroside (299) |
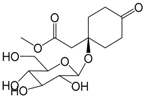 | 2,3,5,6-Tetrahydro-jacaranone-4-O-β-d-glucopyranoside (300) |
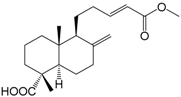 | Labda-8(17),13E-dien-15,18-dioic acid 15-methyl ester (301) |
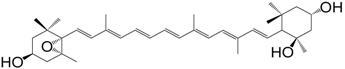 | β-Carotene-5,6-epoxide (302) |
 | Mutatochrome (303) |
 | Neoxanthin (304) |
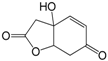 | 1-Oxo-4-hydroxy-2(3)-en-4ethylcyclohexa-5,8-olide (305) |
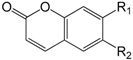 | Esculetin R1 = OH,R2 = OH (306) |
| 6,7-Dimethoxycoumarin R1 = OMe, R2 = OMe (307) | |
 | Hydroxytyrosol R = OH (308) |
| p-Tyrosol R = H (309) | |
 | 4-Hydroxybenylacetic acid methyl ester (310) |
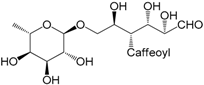 | 4-Caffeoylrutinose (312) |
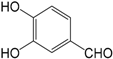 | Protocatechualdehyde (313) |
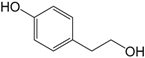 | p-Hydroxyphenylethanol (314) |
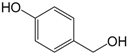 | p-Hydroxybenzylalcohol (315) |
 | n-Hentriacontane (316) |
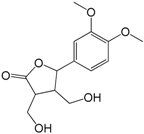 | 2,3-Dihydroxymethyl-4-(3′,4′-dimethoxyphenyl)-γ-butyrolactone (317) |
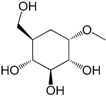 | Methyl-α-d-glucopyranoside (318) |
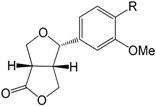 | Forsythenin R = OMe (319) |
| 4-O-Demethylforsythenin R = OH (320) | |
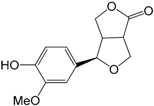 | Salicifoliol (321) |
| NO. | Compound Name | Source | Reference | |
|---|---|---|---|---|
| Phenylethanoid Glycosides | ||||
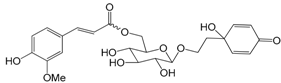
| 1 | forsythoside A (forsythiaside) | UFF, RFF | [6,14,15] | |
| 2 | forsythoside C (suspensaside) | RFF | [6,16] | |
| 3 | (R)-suspensaside | UFF | [17,18] | |
| 4 | (S)-suspensaside | UFF | [17,18] | |
| 5 | (S)-suspensaside methyl ether | N.M. | [18] | |
| 6 | suspensaside B | N.M. | [16] | |
| 7 | (R)-forsythoside J | N.M. | [19] | |
| 8 | (S)-forsythoside J | N.M. | [19] | |
| 9 | forsythoside D | N.M. | [20] | |
| 10 | forsythoside E | UFF | [20,21] | |
| 11 | β-methoxyforsythoside E | N.M. | [22] | |
| 12 | iso-forsythoside A/forsythoside I/lianqiaoxinside A | UFF | [15,17,21] | |
| 13 | forsythoside A 4′-O-β-d-glucopyranoside | N.M. | [11] | |
| 14 | forsythenside K (lipedoside A) | N.M. | [22,23] | |
| 15 | poliumoside | N.M. | [11] | |
| 16 | acteoside | N.M. | [22] | |
| 17 | forsythoside B | UFF | [17,22] | |
| 18 | forsythoside G | N.M. | [22] | |
| 19 | forsythoside F | UFF | [21,24] | |
| 20 | angoroside A | N.M. | [11] | |
| 21 | calceolarioside C | UFF | [25] | |
| 22 | (S)-β-hydroxycalceolarioside C | N.M. | [22] | |
| 23 | (R)-β-hydroxycalceolarioside C | N.M. | [22] | |
| 24 | (S)-β-methoxycalceolarioside C | N.M. | [22] | |
| 25 | (R)-β-methoxycalceolarioside C | N.M. | [22] | |
| 26 | calceolarioside A | N.M. | [26] | |
| 27 | derhamnosyl suspensaside | N.M. | [22] | |
| 28 | β-methoxyacteoside | N.M. | [22] | |
| 29 | caffeoyl calceolarioside C | N.M. | [22] | |
| 30 | isoforsythiaside | N.M. | [27] | |
| 31 | β-methoxylferruginoside B | N.M. | [22] | |
| 32 | β-methoxylipedoside A | N.M. | [22] | |
| 33 | calceolarioside B | UFF | [21] | |
| 34 | lianqiaoxinoside C | UFF | [25] | |
| 35 | plantainoside A | N.M. | [24] | |
| 36 | forsythoside J | UFF | [21] | |
| 37 | plantainoside B | N.M. | [24] | |
| 38 | forsythoside H | UFF | [21,24,28] | |
| 39 | suspensaside A | N.M. | [16,22] | |
| 40 | suspensaside A isomer | N.M. | [22] | |
| 41 | demethyl suspensaside A | N.M. | [22] | |
| 42 | suspensaside C | N.M. | [14] | |
| 43 | lianqiaoxinoside B | UFF | [28] | |
| 44 | salidroside | N.M. | [29] | |
| 45 | 3,4-dihydroxyphenylethyl-8-O-β-d-glucopyranoside | UFF | [17] | |
| 46 | forsythiayanoside C | UFF | [30] | |
| 47 | 2-(2,5-dihydroxyphenyl)-ethyl-O-(6-O-p-hydroxybenzoyl)-β-d-glucopyranoside | N.M. | [11] | |
| 48 | 2-(2,5-dihydroxyphenyl)-ethyl-O-(6-O-vanilloyl)-β-d-glucopyranoside | N.M. | [11] | |
| 49 | 2-(3,4-dihydroxyphenyl)-2-oxo-ethyl-O-α-l-rhamnopyranosyl-(1→6)-(4-O-caffeoyl)-β-d-glucopyranoside | N.M. | [11] | |
| 50 | brachynoside | N.M. | [22] | |
| 51 | phenethyl alcohol β-d-xylopyranosyl-(1→6)-β-d-glucopyranoside | UFF | [21] | |
| Lignans | ||||
| 52 | arctigenin | UFF | [17,22] | |
| 53 | arctiin | UFF | [17,22] | |
| 54 | matairesinoside | N.M. | [22] | |
| 55 | matairesinol | UFF | [17,22] | |
| 56 | 2′,5′-dihydroxy-4′′-caffeoyl matairesinol | N.M. | [22] | |
| 57 | 3′,4′,5′-trihydroxy-3′′-methoxyl-4′′-caffeoyl lignan | N.M. | [22] | |
| 58 | matairesinol-4′-O-glucoside | N.M. | [31] | |
| 59 | phillygenin | UFF, RFF | [15,32,33] | |
| 60 | phillyrin (forsythin) | UFF, RFF | [6,17,33] | |
| 61 | caffeoyl phillygenin | N.M. | [22] | |
| 62 | (+) epipinoresinol | RFF | [33] | |
| 63 | 7′-epi-8-hydroxypinoresinol | N.M. | [32] | |
| 64 | (+) epipinoresinol-4-O-β-d-glucoside | N.M. | [34] | |
| 65 | (+)-8-hydroxyepipinoresinol-4-O-β-d-glucopyranoside | N.M. | [34] | |
| 66 | (+) epipinoresinol-4′-O-β-d-glucoside | N.M. | [34] | |
| 67 | forsythialanside E | N.M. | [24] | |
| 68 | pinoresinol | N.M. | [32] | |
| 69 | (+) pinoresinol-β-d-glucoside | N.M. | [35] | |
| 70 | (+) pinoresinol monomethyl ether-β-d-glucoside | N.M. | [35] | |
| 71 | pinoresinol diglucoside | N.M. | [22] | |
| 72 | caffeoyl pinoresinol | N.M. | [22] | |
| 73 | (+)-1-hydroxypinordsinol/8-hydroxypinoresinol | N.M. | [19,32] | |
| 74 | (+)-1-hydroxypinordsinol-4′-O-β-d-glucoside | N.M. | [19] | |
| 75 | (+)-1-hydroxypinordsinol-4′-O-β-d-glucoside | N.M. | [19] | |
| 76 | 3′,4′,5′-trimethoxy-4′′-hydroxyllignan O-glucoside | N.M. | [22] | |
| 77 | syringaresinol-4-O-β-d-glucoside | N.M. | [23] | |
| 78 | isolariciresinol | UFF, RFF | [15,33,36] | |
| 79 | isolariciresinol-4-O-β-d-glucopyranoside | RFF | [36] | |
| 80 | isolariciresinol-9′-O-β-d-glucopyranoside | RFF | [36] | |
| 81 | isoolivil | RFF | [36] | |
| 82 | cedrusin | N.M. | [32] | |
| 83 | glochidioboside | N.M. | [34] | |
| 84 | forsythialanside C | N.M. | [23] | |
| 85 | forsythialanside D | N.M. | [23] | |
| 86 | dihydrodehydrodiconiferyl alcohol-4-O-β-d-glucoside | N.M. | [23] | |
| 87 | icariside E4 | N.M. | [23] | |
| 88 | forsythialan A | N.M. | [37] | |
| 89 | forsythialan B | N.M. | [37] | |
| 90 | rel-(7R,8′R,8S)-forsythialan C | N.M. | [38] | |
| 91 | rel-(7R,8′R,8R)-forsythialan C | N.M. | [38] | |
| 92 | forsythialanside A | N.M. | [23] | |
| 93 | forsythialanside B | N.M. | [23] | |
| 94 | olivil | UFF | [17,32] | |
| 95 | forsythiayanoside B | N.M. | [34] | |
| 96 | forsythiayanoside A | N.M. | [34] | |
| 97 | 3-furanone-2-(3-methoxy-4-hydroxyphenyl)-4-veratryl | N.M. | [22] | |
| 98 | lariciresinol | N.M. | [32] | |
| 99 | lariciresinol-4-O-β-d-glucoside | N.M. | [24] | |
| 100 | lariciresinol-4′-O-β-d-glucoside | N.M. | [24] | |
| 101 | benzenebutanoic acid | N.M | [39] | |
| Aliphatic C6-C2 alcohols | ||||
| 102 | isorengyol | N.M. | [40] | |
| 103 | rengyol | UFF | [6,20,40] | |
| 104 | suspenol | N.M. | [41] | |
| 105 | rengyolester | N.M. | [42] | |
| 106 | rengyoside A | N.M. | [29] | |
| 107 | rengyoside C | N.M. | [29] | |
| 108 | rengynic acid | N.M. | [14,43] | |
| 109 | rengynic acid-1′-O-β-d-glucopyranoside | N.M. | [44] | |
| 110 | rengyolone (halleridone) | N.M. | [20,29] | |
| 111 | rengyoxide | N.M. | [20] | |
| 112 | rengyoside B | N.M. | [29] | |
| 113 | cornoside | RFF | [6,23] | |
| 114 | forsythenside A | N.M. | [23,45] | |
| 115 | forsythenside B | N.M. | [45] | |
| 116 | forsythenside F | N.M. | [46] | |
| 117 | forsythenside H | N.M. | [23] | |
| 118 | forsythenside G | N.M. | [23] | |
| 119 | forsythenside I | N.M. | [23] | |
| 120 | forsythenside J | N.M. | [23] | |
| Iridoids | ||||
| 121 | adoxosidic acid | UFF, RFF | [6] | |
| 122 | adoxosidic acid 10-p-hydroxyphenylacetate | N.M. | [38] | |
| Diterpenoids | ||||
| 123 | 3β-hydroxylabda-8(17), 13(E)-dien-15-oic acid | N.M. | [47] | |
| 124 | 3β-hydroxyanticopalic acid | N.M. | [48] | |
| 125 | agatholic acid | N.M. | [48] | |
| 126 | 3-oxoanticopalic acid | N.M. | [38] | |
| 127 | 19-hydroxylabda-8(17),13(Z)-dien-15-oic acid | N.M. | [38] | |
| 128 | 19-hydroxylabda-8(17),13(E)dien-15-oic acid | N.M. | [38] | |
| 129 | 19-formyllabda-8(17),13(E)-dien-15-oic acid | N.M. | [38] | |
| 130 | 19-formyllabda-8(17),13(Z)-dien-15-oic acid | N.M. | [38] | |
| 131 | labda-8(17),13(Z)-dien-15,18-dioic acid | N.M. | [38] | |
| 132 | labda-8(17),13(Z)-diene-15,19dioic acid | N.M. | [38] | |
| 133 | labda-8(17),13(E)-diene-15,19-dioic acid | N.M. | [38] | |
| 134 | dehydropinifolic acid | N.M. | [38] | |
| 135 | haplopappic acid | N.M. | [38] | |
| 136 | 18-hydroxy-7-oxolabda-8(9),13(E)-dien-15-oic acid | N.M. | [38] | |
| 137 | 17,19-dihydroxylabda-7(8),13(E)-dien-15-oic acid | N.M. | [38] | |
| 138 | forsythidin A | N.M. | [38] | |
| 139 | 3β-hydroxy-12,13(E)-biformene | N.M. | [38] | |
| 140 | 3β-hydroxy-12,13(Z)-biformene | N.M. | [38] | |
| 141 | 19-hydroxy-8(17)(E)-13-labdadien-15-oate | N.M. | [38] | |
| Triterpenoids | ||||
| 142 | ocotillone | N.M. | [49] | |
| 143 | ocotillol monoacetate | N.M. | [49] | |
| 144 | garcinielliptone Q | N.M. | [38] | |
| 145 | 3β-acetyl-20,25-epoxydammarane-24α-ol | N.M. | [50] | |
| 146 | 3β-acetyl-20,25-epoxydammarane-24β-ol | N.M. | [50] | |
| 147 | dammar-24-en-3β-acetoxy-20-ol | N.M. | [38,47,51] | |
| 148 | 3β-acetoxy-25methoxydammar-23-en-20β-ol | N.M. | [38] | |
| 149 | 3β-acetoxyl-20S,24R-dammarane-25-ene-24-hydroperoxy-20-ol | N.M. | [47] | |
| 150 | cabralea lactone 3-acetate | N.M. | [47] | |
| 151 | cabralea lactone 3-acetate 24-methyl ether | N.M. | [38] | |
| 152 | 3-acetylisofouquierol | N.M. | [47] | |
| 153 | oleanolic acid | RFF | [33,52] | |
| 154 | 3β-acetyloleanolic acid | N.M. | [48] | |
| 155 | β-amyrin acetate | N.M. | [47] | |
| 156 | ursolic acid | RFF | [33] | |
| 157 | 2α,3α-hydroxyursolic acid | N.M. | [53] | |
| 158 | 2α,23-hydroxyursolic acid | RFF | [33] | |
| 159 | 3β-acetoxy-20α-hydroxyursan-28-oic acid | N.M. | [48] | |
| 160 | betulinic acid | RFF | [33,52] | |
| 161 | 3β-acetylbetulinic acid | N.M. | [54] | |
| 162 | 2α-hydroxybetulinic acid | RFF | [33] | |
| 163 | ambrolic acid | N.M. | [51,55] | |
| 164 | morolic acid | N.M. | [47] | |
| 165 | 3β-acetoxyolean-12-en-28-oic acid | N.M. | [38] | |
| 166 | alphitolic acid | N.M. | [38] | |
| 167 | onjisaponin F | N.M. | [53] | |
| 168 | onjisaponin G | N.M. | [53] | |
| Sterols | ||||
| 169 | β-sitosterol | N.M. | [56] | |
| 170 | daucosterol | N.M. | [57] | |
| 171 | taraxasterol acetate | N.M. | [48] | |
| 172 | stigmasterol | N.M. | [48] | |
| 173 | ψ-taraxasterol | N.M. | [48] | |
| 174 | (6′-O-palmitoyl)-sitosterol-3-O-β-d-glucoside | N.M. | [49] | |
| Flavonoids | ||||
| 175 | rutin | UFF, RFF | [6,22,58] | |
| 176 | rutin-O-hexoside | N.M. | [22] | |
| 177 | quercetin | UFF, RFF | [58] | |
| 178 | quercetin-O-rhamnosyl hexoside | N.M. | [22] | |
| 179 | trimethoxyquercetin-O-feruloyl rhamnoside | N.M. | [22] | |
| 180 | isorhamnetin | N.M | [59] | |
| 181 | kaempferfol | N.M. | [22] | |
| 182 | hyperin | N.M. | [18] | |
| 183 | kaempferol dirhamnoside | N.M. | [22] | |
| 184 | kaempferol-O-rhamnosylhexoside | N.M. | [22] | |
| 185 | kaempferol-3-O-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside | N.M. | [11] | |
| 186 | kaempferol-3-O-β-d-(2″-O-β-d-glucopyranosyl-6″O-α-l-rhamno-pyranosyl)glucopyranoside | N.M. | [11] | |
| 187 | wogonin-7-O-glcoside | N.M. | [60] | |
| 188 | baicalin | UFF, RFF | [58] | |
| 189 | hesperidin | N.M. | [18] | |
| 190 | forsythoneoside A | N.M. | [11] | |
| 191 | forsythoneoside B | N.M. | [11] | |
| 192 | forsythoneoside C | N.M. | [11] | |
| 193 | forsythoneoside D | N.M. | [11] | |
| Volatiles | ||||
| 194 | β-pinene | N.M. | [61] | |
| 195 | myrtenol | N.M. | [61] | |
| 196 | (+)-α-pinene | N.M. | [61] | |
| 197 | (−)-trans-pinocarveol | N.M. | [61] | |
| 198 | sabinene | N.M. | [61] | |
| 199 | pinocarvone | N.M. | [61] | |
| 200 | (−)-terpinen-4-ol | N.M. | [61] | |
| 201 | dipentene | N.M. | [61] | |
| 202 | camphene | N.M. | [61] | |
| 203 | myrcene | N.M. | [61] | |
| 204 | α-terpinene | N.M. | [61] | |
| 205 | O-cymene | N.M. | [61] | |
| 206 | eucalyptol (1,8-cineole) | N.M. | [61] | |
| 207 | γ-terpinene | N.M. | [61] | |
| 208 | campholenic aldehyde | N.M. | [61] | |
| 209 | (S)-cis-verbenol | N.M. | [61] | |
| 210 | 2,5-cyclooctadien-1-ol | N.M. | [61] | |
| 211 | (1S)-(−)-verbenone | N.M. | [61] | |
| 212 | α-pinene | N.M. | [61] | |
| 213 | β-phellandrene | N.M. | [62] | |
| 214 | (+)-carene | N.M. | [62] | |
| 215 | α-terpinolene | N.M. | [62] | |
| 216 | 1,4-cyclohexadiene | N.M. | [62] | |
| 217 | 4-carvomenthenol | N.M. | [62] | |
| 218 | (±)-α-terpinel | N.M. | [62] | |
| 219 | (−)-myrtenal | N.M. | [62] | |
| 220 | 2-methyl-5-(1-methylethenyl)cyclohexanol | N.M. | [62] | |
| 221 | estragole | N.M. | [62] | |
| 222 | 1-hexanol | N.M. | [63] | |
| 223 | (−)-β-pinene | N.M. | [63] | |
| 224 | (+)-4-carene | N.M. | [63] | |
| 225 | linalool | N.M. | [64] | |
| 226 | trans-carveol | N.M. | [64] | |
| 227 | p-cymen-8-ol | N.M. | [64] | |
| 228 | trans-nerolidol | N.M. | [64] | |
| 229 | camphor | N.M. | [64] | |
| 230 | β-ocimene | N.M. | [64] | |
| 231 | germacrene D | UFF | [65] | |
| 232 | α-cubebene | UFF | [65] | |
| 233 | bornyl acetate | UFF | [65] | |
| 234 | cis-piperitol | UFF | [65] | |
| 235 | α-pinocarvone | UFF | [65] | |
| 236 | α-terpineol | UFF | [65] | |
| 237 | ocimene | UFF | [62,65] | |
| 238 | α-phellandrene | UFF | [65] | |
| 239 | nutmeg aldehyde | RFF | [65] | |
| 240 | (-)-alloaromadendren | RFF | [65] | |
| 241 | cumene formaldehyde | RFF | [65] | |
| 242 | 3-cyclohexene-1-methanol | RFF | [65] | |
| 243 | 4-methylene-1-cyclohexanone | RFF | [65] | |
| 244 | p-cymene | UFF | [66] | |
| 245 | limonene | UFF | [66] | |
| Alkaloids | ||||
| 246 | rutaecarpine | N.M. | [57] | |
| 247 | suspensine A | UFF | [67] | |
| 248 | (−)-egenine | UFF | [67] | |
| 249 | (−)-7′-O-methylegenine | UFF | [67] | |
| 250 | (−)-bicuculline | UFF | [67] | |
| 251 | bis-2-(4-aminophenyl)ethyl-β-d-glucopyranoside | N.M. | [68] | |
| 252 | choline | UFF, RFF | [6] | |
| Organic acids | ||||
| 253 | palmitic acid | N.M. | [56] | |
| 254 | stearic acid | N.M. | [56] | |
| 255 | succinic acid | UFF, RFF | [6] | |
| 256 | suspenolic acid | N.M. | [45] | |
| 257 | 2-furancarboxylic acid | N.M. | [48] | |
| 258 | chlorogenic acid | N.M. | [18] | |
| 259 | anchoic acid | UFF, RFF | [58] | |
| 260 | 4-hydroxy-4-isopropylcyclohex-1-enecarboxylic acid | UFF, RFF | [58] | |
| 261 | p-coumaric acid | UFF, RFF | [58] | |
| 262 | protocatechuic acid | seeds | [69] | |
| 263 | vanillic acid | N.M. | [70] | |
| 264 | p-hydroxybenzoic acid | N.M. | [48] | |
| 265 | benzoic acid | N.M. | [48] | |
| 266 | 3,4-dimethoxybenzoic acid | N.M. | [48] | |
| 267 | syringic acid | N.M. | [48] | |
| 268 | caffeic acid | N.M. | [70] | |
| 269 | trans-coumaric acid | N.M. | [48] | |
| 270 | trans-ferulic acid | N.M. | [48] | |
| 271 | caffeic acid methyl ester | RFF | [36] | |
| 272 | p-hydroxybenylacetic acid | N.M. | [70] | |
| 273 | tannic acid | N.M. | [71] | |
| 274 | gallic acid | RFF | [6] | |
| 275 | 3-hydroxybutyric acid | UFF | [6] | |
| 276 | acetic acid | UFF, RFF | [6] | |
| 277 | pyruvic acid | UFF, RFF | [6] | |
| 278 | malic acid | UFF, RFF | [6] | |
| 279 | fumaric acid | UFF | [6] | |
| 280 | formic acid | UFF | [6] | |
| Amino acids | ||||
| 281 | isoleucine | UFF | [6] | |
| 282 | leucine | UFF | [6] | |
| 283 | valine | UFF, RFF | [6] | |
| 284 | threonine | UFF | [6] | |
| 285 | alanine | UFF | [6] | |
| 286 | phenylalanine | RFF | [6] | |
| Sugar derivatives | ||||
| 287 | β-xylose | UFF, RFF | [6] | |
| 288 | β-glucose | UFF | [6] | |
| 289 | α-glucose | UFF, RFF | [6] | |
| 290 | raffinose | UFF | [6] | |
| 291 | sucrose | RFF | [6] | |
| 292 | l-rhamnose | RFF | [36] | |
| 293 | lactose | N.M. | [72] | |
| 294 | erythritol | N.M. | [60] | |
| 295 | [4]-α-d-GalpA-(1→2]7-[4]-α-d-GalpA-(1→2)-α-l-Rhap-(1→2]2 | N.M. | [73] | |
| Allylbenzene glycosides | ||||
| 296 | forsythenside L | N.M. | [23] | |
| 297 | sasanquin | N.M. | [23] | |
| Other compounds | ||||
| 298 | forsythiayanoside D | UFF | [30] | |
| 299 | (6S,9R)-roseoside | N.M. | [48] | |
| 300 | swertiamacroside | N.M. | [74] | |
| 301 | 2,3,5,6-tetrahydrojacaranone-4-O-β-d-glucopyranoside | N.M. | [14] | |
| 302 | labda-8(17),13(E)-dien-15,18-dioic acid 15-methyl ester | N.M. | [48] | |
| 303 | β-carotene-5,6-epoxide | N.M. | [72] | |
| 304 | mutatochrome | N.M. | [72] | |
| 305 | neoxanthin | N.M. | [72] | |
| 306 | 1-oxo-4-hydroxy-2(3)-en-4-ethylcyclohexa-5,8-olide | N.M. | [38] | |
| 307 | esculetin | N.M. | [48] | |
| 308 | 6,7-dimethoxycouma | N.M. | [53] | |
| 309 | hydroxytyrosol | N.M. | [48] | |
| 310 | p-tyrosol | N.M. | [48] | |
| 311 | 4-hydroxybenylacetic acid methyl ester | RFF | [36] | |
| 312 | 4-caffeoylrutinose | N.M. | [20] | |
| 313 | protocatechualdehyde | N.M. | [48] | |
| 314 | p-hydroxyphenylethanol | UFF, RFF | [58] | |
| 315 | p-hydroxybenzylalcohol | UFF, RFF | [58] | |
| 316 | n-hentriacontane | UFF | [75] | |
| 317 | 2,3-dihydroxymethyl-4-(3′,4′-dimethoxyphenyl)-γ-butyrolactone | N.M. | [57] | |
| 318 | methyl-α-d-glucopyranoside | N.M. | [48] | |
| 319 | forsythenin | N.M. | [49] | |
| 320 | 4-O-demethylforsythenin | N.M. | [38] | |
| 321 | salicifoliol | N.M. | [38] | |
| Analytes | Method | Results | Reference |
|---|---|---|---|
| Phillyrin | LC-MS | The contents of phillyrin in Forsythiae Fructus and three medicinal preparations (Xiao′erqingyan granules, Niuhuangshangqing pills, Yinqiao tablets) were 1.30, 0.48, 3.36, 0.35 mg/g, respectively | [78] |
| Phillyrin | HPLC | The contents of phillyrin in Forsythiae Fructus from ten habitats were from 0.72 to 3.54 mg/g, indicating the influence of habitat on the quality of Forsythiae Fructus. | [79] |
| Phillyrin Forsythoside A | HPLC | In four batches of UFF, the contents of phillyrin and forsythoside A were 0.73–2.16% and 0.85–1.56%, respectively. In eleven batches of RFF, the contents of phillyrin and forsythoside A were 0.57–2.50% and 0.33–0.76%, respectively. | [80] |
| Phillyrin, Forsythoside A | HPLC | The contents of phillyrin and forsythoside A from three batches were 3.08–4.35 mg/g and 15.89–20.76 mg/g, respectively. | [81] |
| Rutin Forsythin | CE-ED | The contents of rutin and forsythin in Forsythiae Fructus were 2.03 mg/g and 2.95 mg/g, respectively. | [82] |
| Forsythoside A Rutin Phillyrin | HPLC | In UFF from different harvesting times, the contents of forsythoside A, rutin and phillyrin were 3.87–8.72%, 0.05–0.36% and 0.10–0.63%, respectively, which reached a peak in early July. | [83] |
| Forsythoside A, Phillyrin, Phillygenin | HPLC | In three batches of UFF, the average contents of forsythoside A, phillyrin and phillygenin were 3.3385, 0.2934 and 0.4873 mg/g, respectively. In the RFF, the average contents were 0.3129, 0.2228 and 0.9258 mg/g, respectively. | [84] |
| Rutin Forsythoside A Phillyrin | HPLC-PDA | The contents of rutin, forsythoside A and phillyrin in three batches of RFF were linear in the range of 0.1–2.0, 0.12–2.4 and 0.05–1.0 μg/g, respectively. | [85] |
| Forsythoside A Rutin Forsythin | HPLC-ESI-MS | In UFF, the contents of forsythoside A, rutin and forsythin were 3.783%, 0.105% and 0.365%, respectively. In RFF, the contents were 0.257%, 0.167% and 0.043%, respectively. | [86] |
| (+)-Pinoresinol-β-d-glucoside, Forsythoside A, Phillyrin Phillygenin | HPLC-PDA | In nineteen batches of UFF, the contents of (+)-pinoresinol-β-d-glucoside, forsythoside A, phillyrin and phillygenin were 3.95–6.14%, 9.15–15.71%, 0.80–1.64% and 0.70–2.10%, respectively. In nineteen batches of RFF, the contents were 3.76–5.55%, 5.91–10.59%, 0.45–1.27% and 1.40–2.00%, respectively. Apart from the harvest times, the plant origins, manufacturing methods and storage conditions also played a role in the variation of the contents of the active components. | [87] |
| Total flavonoids, Forsythoside A , Rutin, Quercetin | HPLC | In UFF, the contents of total flavonoids, forsythin, forsythoside A, rutin and quercetin were 1.362%, 29.95 ± 0.06 mg/g, 64.0325 ± 0.03 mg/g, 2.6075 ± 0.02 mg/g and almost 0 mg/g, respectively. In RFF, the contents of them were 1.099%, 22.975 ± 0.04 mg/g, 58.3325 ± 0.03 mg/g, 0.57075 ± 0.01 mg/g and 0.0209 ± 0.07 mg/g, respectively. | [88] |
| Cafferic acid, Forsythoside A, Forsythoside B, Rutin, Hyperin, Forsythin Arctigenin | RP-HPLC | The contents of cafferic acid, forsythoside A, forsythoside B, rutin, hyperin, forsythin and arctigenin in Forsythiae Fructus from six origins were 3.377–7.457 mg/g, 14.06–88.00 mg/g, 1.325–3.196 mg/g, 0.2682–3.1470 mg/g, 0.4109–0.7008 mg/g, 2.128–5.226 mg/g and 0.7437–3.6720 mg/g, respectively. | [89] |
| Chlorogenic acid, R-suspensaside, S-suspensaside, S-suspensaside methyl ether, Forsythoside, (+)-Pinoresinol-β-d-glucoside, (+)-Epipinoresinol-4′-O-glucoside, Matairesinol-4′-O-glucoside, rutin, Hesperidin, Hyperin, Phillyrin, Phillygenin, (+)-Epipinoresinol | LC-ESI-MS | The fourteen compounds from twelve batches of Forsythiae Fructus from nine regions were quantified and were present at 0.0004–0.0068%, 0.0098–0.0795%, 0.0167–0.1482%, 0.0100–0.4904%, 0.2076–0.8693%, 0.0086–0.2044%, 0.0073–0.1720%, 0.0070–0.0724%, 0.0742–0.2226%, 0.0041–0.0257%, 0.0010–0.0059%, 0.0200–0.4236%, 0.0448–0.1020% and 0.0024–0.1231%, respectively. | [18] |
| R-suspensaside, S-suspensaside methyl ether, (+)-Pinoresinol-β-d-glucoside, Forsythoside A, (+)-Epipinoresinol-4′-O-glucoside, Suspensaside A, Rutin, Phillyrin, Pinoresinol, (+)-Epipinoresinol and Phillygenin | HPLC-DAD | The levels of twelve constituents varied from 16.86 to 74.55 mg/g; rutin is the most stable, with only three-fold variation in the detected thirty-three samples. As the main compound, the contents of forsythoside A ranged from 5.15 to 55.78 mg/g. | [90] |
| Forsythoside E, Forsythoside A , Suspensaside A, Rutin, Baicalin, Quercetin, Phillyrin, (+)-Epipinoresinol, (+)-Pinoresinol-4-O-β-d-glucoside (+)-Epipinoresinol-4-O-β-d-glucoside, Chlorogenic acid, p-Hydroxybenzoic acid, p-Coumaric acid, Anchoic acid 4-Hydroxy-4-isopropylcyclohex-1-enecarboxylic acid, p-Hydroxyphenyl-ethanol, p-Hydroxybenzylalcohol | HPLC–ESI-MS/MS | In the UFF, the contents of forsythoside A, phillyrin, (+)-epipinoresinol, (+)-epipinoresinol-4-O-β-d-glucoside, (+)-pinoresinol-4-O-β-d-glucoside were 31.1–41.7, 10.8–12.7, 11.1–21.0, 9.1–16.4, 5.2–14.4 mg/g, respectively. In the RFF, the contents of them were 6.7–8.5, 0.8–5.4, 1.6–6.4, 2.2–5.8, 1.2–4.8 mg/g, respectively. Moreover, total contents of flavonoids in the UFF were higher than in the RFF, while those of phenolic acids were on the contrary. Contents of the aliphatic acids and terpenoids were not significantly different between the UFF and the RFF. | [58] |
| α-pinene, Camphene, β-Pinene, Myrcene, p-Cymene, Limonene α-Terpineol | GC | In the UFF from sixteen batches, the contents of α-pinene, camphene, β-pinene, myrcene, p-cymene, limonene and α-terpineol were 0.102–0.337%, 0.004–0.018%, 0.342–1.024%, 0.008–0.024%, 0.006–0.032%, 0.003–0.029% and 0.003–0.017%, respectively. | [66] |
| α-Pinene β-Pinene | GC | In the UFF, the contents of α-pinene and β-pinene were 0.192–0.300% and 0.556–0.934%, while the contents of them were 0.075% and 0.240% in the RFF. | [91] |
| (+)-Pinoresinol-β-d-glucoside, Matairesinol-4′-O-glucoside, Hyperin, Phillyrin, Phillygenin | HPLC-ESI-MS/MS | The contents of (+)-pinoresinol-β-d-glucoside, matairesinol-4′-O-glucoside, hyperin, phillyrin and phillygenin in the 75% methanol extract of Forsythiae Fructus were 227.00, 70.80, 2.67, 225.20 and 106.10 mg/mL, respectively. | [31] |
| Models | Constituent/Extract | Mechanism | Reference |
|---|---|---|---|
| Anti-inflammatory Activity | |||
| LPS-induced liver injury in rats | Ethanol extract | The extract inhibited generation of ROS, MDA, TNF-α, IL-1β and IL-6 in serum and liver via activation of Nrf2-mediated antioxidation and inhibition of NF-κB-mediated inflammatory response. | [92] |
| LPS-stimulated RAW 264.7 cells | Ethyl acetate fraction of the ethanol extract | The extract at 12.5–200 μg/mL inhibited expression of COX-2, thus decreasing the levels of ROS, NO and PGE2 does-dependently. | [93] |
| LPS-stimulated BV-2 microglial cells | Aqueous extract Forsythin | The extract at 1 μg/mL inhibited the MAPK pathway and down-regulated NO biosynthesis-related genes. Forsythin at 50–200 μg/mL significantly suppressed the production of NO and decreased iNOS and TRL4 protein expression in a dose dependent manner. | [94,95] |
| Soybean β-conglycinin-stimulated weaned piglets | Methanol extract | The methanol extract (100 mg/kg) reduced the levels of anaphylactic antibodies, mast cell degranulation, histamine release, T lymphocyte proliferation and IL-4 synthesis and improved intestinal microbial flora. | [96] |
| Dermatophagoides farinae-induced atopic dermatitis in NC/Nga mice | Ethanol extract Forsythoside A, Phillyrin, Pinoresinol, Phylligenin | The extract (25, 50, 100, 200 and 400 μg/mL) suppressed expression of chemokines (TARC, MDC and RANTES), adhension molecules (ICAM-1 and VCAM-1) and inflammatory factors (TNF-α and IL-4) in ear tissues. It could also inhibit the production of chemokines in keratinocytes. Further study revealed that forsythoside A, phillyrin, pinoresinol and phylligenin may be the active constituents for the therapy of atopic dermatitis. | [97] |
| Carrageenan-induced rats | Ethanol extract | The extract (5 g/kg) alleviated carrageenan-induced paw edema in rats, probably by increasing the production of COX-2 and decreasing the expression of PGE2, PGD2, 6-keto-PGF1α and TXB2. | [98] |
| Xylene-stimulated mice Acetic acid-stimulated mice Carrageenan-induced rats Oleic acid-stimulated rats | Volatiles | Volatiles inhibited the ear-swelling induced by xylene at 0.12 and 0.24 mL/kg, withstood the hyperfunction of celiac capillary permeability induced by acetic acid at 0.24 mL/kg, alleviated rats paw edema induced by carrageenan at 0.12 and 0.24 mL/kg, inhibited pleuritis induced by carrageenan at 0.24 mL/kg and decreased acute lung injury induced by oleic acid at 0.12 and 0.24 mL/kg. | [99] |
| Anti-inflammatory Activity | |||
| LPS/D-galactosamine-induced acute liver injury mice | Forsythoside A | Forsythoside A (15, 30 and 60 mg/kg) decreased the serum levels of ALT, AST and TNF-α, increased expression of Nrf2 and heme oxygenase-1 and inhibited NF-κB activation, thus protecting against LPS/D-galactosamine-induced acute liver injury. | [100] |
| LPS-stimulated RAW264.7 cells | Forsythin | Forsythin (25, 50, 100, 150 and 200 μg/mL) inhibited the production of ROS, IL-6, IL-1β, TNF-α, NO, PGE2, iNOS and COX-2 in a dose dependent manner by suppressing JAK-STAT and p38 MAPK signaling pathway. | [101] |
| LPS-stimulated RAW264.7 cells | Forsythoside A | Treatment with forsythoside A in LPS-stimulated RAW264.7 cells reduced the secretion of TNF-α, IL-6 and NO via inhibition of HMGB1/TLR4/NF-κB pathaway. | [102] |
| LPS-induced acute lung injury male BALB/c mice | Phillyrin | Phillyrin (20 mg/kg) pretreatment significantly decreased the production of IL-1β, IL-6, TNF-α and the concentration of myeloperoxidase in lung tissues via inhibition of MAPK and NF-κB pathways. | [103] |
| LPS-stimulated RAW264.7 cells | Arctiin | Arctiin (12.5, 25, 50 and 100 μg/mL) inhibited NF-κB pathway, thus reducing the production of IL-1β, IL-6, TNF-α and PGE2 in a dose dependent manner, as well as expression of co-stimulatory molecules (B7-1 and B7-2). | [104] |
| LPS-stimulated BEAS-2B cells | 90% Forsythoside A extracts | Forsythoside A extracts (25, 50 and 100 μg/mL) significantly reduced the production of NO in a dose-dependent manner and the level of intracellular ROS in a dose-effect manner. | [105] |
| Bursa of Fabricius of chickens | Forsythoside A | Forsythoside A (30 and 60 mg/kg) suppressed the NF-κB-iNOS-NO signaling pathway to reduce the production of IL-6, IL-1β, TNF-α and COX-2. | [76] |
| Allergic dermatitis in NC/Nga mice | Ethanol extract Matairesinol | In vitro, the Forsythiae Fructus ethanol extracts at 200 μg/mL inhibited histamine to release from mast cells. Further study revealed that matairesinol suppressed inflammatory cell infiltration, IL-4 and IFN-γ mRNA expression and lowered IgE levels in vivo. | [106] |
| Anti-inflammatory Activity | |||
| COPD mice | Forsythoside A | Forsythoside A (15, 30 and 60 mg/kg) suppressed the production of IL-1β, IL-6, TNF-α and NO and reversed cigarette smoke induced GSH/GSSG ratio, which were related to activation of Nrf2 dose-dependently and inhibition of NF-κB. | [107] |
| Male C57LB/6 mice | Forsythin | As a selective inhibitor of PDE4, forsythin significantly decreased the levels of IL-1β, IL-6 and TNF in LPS/H1N1 influenza-induced lung injury and sepsis in vivo. Moreover, authors took it as a lead compound and developed three other PDE4 inhibitors with higher activities. | [108] |
| Male Sprague-Dawley rats RAW 264.7 cells | Arctigenin | Arctigenin (0.1–1.0 mg/ear) significantly decreased myeloperoxidase and eosinophil peroxidase activities in the arachidonic acid (AA) induced edematous tissues homogenate and silica-induced ROS production in the RAW 264.7 cell line at 0.1–10 μM, probably by inhibiting the release or production of AA metabolites and free radicals. | [109] |
| LPS-stimulated BV2 microglia cells nd primary microglia cells | Forsythoside A | Forsythoside A at 2.5, 5 and 10 μg/mL inhibited the production of TNF-α, IL-1β, NO and PGE2 via inhibiting NF-κB and activating Nrf2/HO-1 signaling pathway. | [110] |
| PAF-stimulated rat polymorphonuclear leukocytes | Suspensine A, 7′-O-methylegenine, (−)-Egenine, (−)-Bicuculline | The four alkaloids at 10 μM inhibited the release of β-glucuronidase from polymorphonuclear leukocytes of rats with the rates of 39.6%, 37.7%, 36.5% and 34.8%, respectively. | [67] |
| Staphylococcus aureus (S. aureus)-stimulated monocyte-macrophage | Forsythin | Forsythin at 50 mg/L significantly decreased expression of IL-8, TNF-α, IL-6 and at 100 mg/L also decreased expression of macrophage colony stimulating factor-1 (MCSF-1) dose-dependently. | [111] |
| Antibacterial Activity | |||
| Escherichia coli (E. coli) Staphylococcus aureus (S. aureus) | Essential oil | The essential oil changed the permeability and integrity of the cell membrane, leading to leakage of nucleic acids and proteins with MIC values of 3.13 and 1.56 mg/mL for E. coli and S. aureus, respectively. | [112] |
| Pneumococcus, Escherichia coli (E. coli), S. aureus, Haemophilus influenza, a beta-group Streptococcus, Yersinia enterocxolitica, Klebsiella pneumonia, F‘s dysentery bacillus, Salmonella typhi, Pseudomonas aeruginosa | Essential oil | The essential oil showed antibacterial activity against these ten bacteria. Particularly, β-pinene and the oil after chromatography showed a better inhibitory effect on the other bacteria, except Yersinia enterocolitica and Klebsiella pneumonia. | [113] |
| Escherichia coli (E. coli) (BCRC-11634) | 3β-Acetoxyl-20α-hydroxyursan-28-oic acid β-Amyrin acetate, Betulinic acid ψ-Taraxasterol, 3β-Hydroxyanticopalic acid Agatholic acid, Phillyrin | The seven compounds showed antibacterial effect with MIC values of 4.55, 5.00, 1.20, 1.20, 3.42, 2.62 and 3.94 mg/mL, respectively. | [48] |
| Staphylococcus aureus (S. aureus) | Ethanol extract | The extract inhibited secretion of α-hemolysin in the range of 16–128 mg/L dose-dependently. | [114] |
| Escherichia coli (E. coli), Pseudomonas aeruginosa, Staphylococcus aureus (S. aureus) | Isoforsythoside A Forsythoside A | The MIC of isoforsythoside A for E. coli, Pseudomonas aeruginosa and S. aureus were 40.83, 40.83 and 81.66 μg/mL, respectively, and those of forsythoside A were 38.33, 38.33 and 76.67 μg/mL, respectively. | [27] |
| Esherichia coli (E. coli) K88, Staphylococcus aureus (S. aureus) Salmonella enteric 34R99 | Methanol extract | The Forsythiae Fructus methanol extracts protected against E. coli K88, S. aureus and Salmonella enteric 34R99 with minimum concentrations of 25.00, 12.50 and 1.56 mg/mL, respectively. | [115] |
| Helicobacter pylori | Betulinic acid Oleanolic acid | The Forsythiae Fructus ethanol extracts strongly (82%) inhibited urease activity of Helicobacter pylori. Further study revealed that the active compounds were betulinic acid and oleanolic acid. | [52] |
| Acinetobacter baumannii | Aqueous extract | The aqueous decoction of Forsythiae Fructus inhibited the active efflux pump and induced mutations in the nucleotide sequence of the adeb gene at 2.5 and 5 mg/mL. | [116] |
| Antiviral Activity | |||
| H1N1-infected MDCK cells | 80% Ethanol extract | The 80% ethanol extract of Forsythiae Fructus exhibited an inhibitory effect on H1N1 in a dose-dependent manner at the concentration of 1:512 to 1:8192 mg/mL. | [8] |
| H1N1-infected human bronchial epithelial cell line A549 | 95% Ethanol extract 50% Ethanol extract Aqueous extract | 95% Ethanol extract, 50% ethanol extract and aqueous extract exhibited inhibitory effect on RANTES secretion with IC50 values of 42 ± 6, 117 ± 15 and 232 ± 28 μg/mL, respectively. Moreover, 95% ethanol extract displayed dual regulatory effects on MCP-1 production, while 50% ethanol extract and aqueous extract increased MCP-1 production by 1.4–3.3 and 2.6–3.7 times, respectively. | [117] |
| C57BL/6j mice | Forsythoside A | Forsythoside A (0.4 μg/mL) inhibited influenza A virus replication by suppressing the expression of TLR7, MyD88, TRAF6, IRAK4 and NF-κB p65 mRNA in vivo. | [77] |
| male BALB/C mice | Phillyrin | Phillyrin at a dose of 20 mg/kg/day protected against influenza A shown by the reduction of lung index, viral titers, IL-6 levels, expression of hemagglutinin protein and the alleviated lung tissue damage. | [118] |
| Influenza A transfected-HeLa cells | Phillyrin | Phillyrin significantly decreased the gene expression of IAV nucleoprotein. | [119] |
| PRRSV-infected Marc-145 cells | Forsythoside A | Forsythoside A inhibited porcine reproductive and respiratory syndrome virus (PRRSV) RNA synthesis and promoted secretion of IFN-α. The sterilization rate reached 80% at a concentration of 60 μg/mL. | [120] |
| RSV-infected MDCK cells and Hep-2 cells | Calceolarioside B Forsythoside A | Calceolarioside B and forsythoside A exhibited EC50 values of 3.43 and 6.72 μM for RSV, respectively. | [23] |
| RSV | Rengynic acid | Rengynic acid exhibited an anti-RSV effect with EC50 and MIC values of 9.9 and 41.66 μg/mL, respectively. | [43] |
| IBV-infected primary chicken embryo kidney cells | Forsythoside A | Forsythoside A pretreatment at a dose of 0.64 mM had a direct virucidal effect on IBV, but it had no effect on IBV-infected cells. | [121] |
| IBV-infected HD11 cells | Forsythoside A | Forsythoside A (10 and 20 μM) exhibited an antiviral effect by significantly increasing expression of intracellular receptors (MDA5, LGP2 and NLRC5) and antiviral gene (IRF7, IFN-α, IFN-β) mRNA. | [122] |
| Antioxidant Activity | |||
| DPPH | Isoforsythoside A | Isoforsythoside A exhibited antioxidant activity with an EC50 value of 2.74 μg/mL and Vc exhibited an IC50 of 4.38 μg/mL in the DPPH assay. | [27] |
| DPPH and superoxide anion | Polysaccharides | Forsythiae Fructus polysaccharides showed significant scavenging capacity on the DPPH and superoxide anion with IC50 values of 0.08 and 2.0 mg/mL, respectively. | [123] |
| DPPH in vitro and diquat-stimulated male Sprague Dawley rats in vivo | CH2Cl2 fraction of ethanol extract Forsythoside A Forsythialan A Phillygenin Phillyrin | The CH2Cl2 fraction of ethanol extract (25, 50 and 100 mg/kg) reduced expression of TNF-α, IL-1β, IL-6, MDA and increased the activities of SOD, GSH-Px, GSH. Forsythoside A, forythialan A, phillygenin and phillyrin may be the main active constituents with IC50 values of 10.43 ± 0.15, 29.85 ± 0.43, 53.64 ± 2.70, 351.14 ± 13.15 μg/mL, respectively. | [124] |
| ABTS radical cation | Calceolarioside C | Calceolarioside C scavenged the ABTS radical cation with IC50 values of 22.7 μg/mL and the Vc exhibited an IC50 of 7.2 μg/mL. | [25] |
| ABTS radical cation | Lianqiaoxinoside B Forsythoside H | Lianqiaoxinoside B and forsythoside H scavenged the ABTS radical cation with IC50 values of 15.6 and 17.7 μg/mL, respectively, while Vc exhibited an IC50 of 6.8 μg/mL. | [28] |
| DPPH, Fe3+ and Fe2+ | Ethyl acetate extract | Ethyl acetate extract (1.0 mg/mL) of Forsythiae Fructus exhibited a scavenging rate of 71.39% on the DPPH. It also had a relatively strong ability to reduce Fe3+ and chelate Fe2+. | [125] |
| Peroxynitrite-treated LLC-PK1 cell | Phillygenin 8-Hydroxypinoresinol | Phillygenin and 8-hydroxypinoresinol significantly decreased the leakage of lactate dehydrogenase (LDH) at 10 μΜ and even reverse the LDH release induced by 3-morpholinosydnonimine, an ONOO− generator, at 50 μM. | [126] |
| High-density lipoprotein | Pinoresinol, Phillygenin, 8-Hydroxypinoresinol, 7′-Epi-8-Hydroxypinoresinol, Lariciresinol, Isolariciresinol, Olivil, Cedrusin | The lignans inhibited the generation of thiobarbituric acid-reactive substances in a dose-dependent manner with IC50 values from 8.5 to 18.7 μM and thermo-labile radical initiator-induced lipid peroxidation with IC50 values from 12.1 to 51.1 μM. Among them, pinoresinol and lariciresinol also exerted an inhibitory effect against Cu2+-induced lipid peroxidation of HDL at a concentration of 3 μM. | [32] |
| D-galactose induced aging mice | Phillyrin | A decrease in weight gain rate, spleen index, SOD, GSH-Px and T-AOC activities in serum and liver tissue and an increase in the content of MDA and MAO-B activities in brain tissue were observed after injection of 15 or 45 mg/kg phillyrin. | [127] |
| Antioxidant Activity | |||
| Weaned piglets | Ethanol extract | Dietary supplementation (100 mg/kg) of Forsythiae Fructus ethanol extracts after fourteen days significantly increased glutathione peroxidase activities and serum complement 4 concentration and lowered serum endotoxin and MDA concentration. The oxidative injury disappeared after twenty-eight days. | [128] |
| Corticosterone-treated broilers | Methanol extract | Dietary supplementation (100 mg/kg) of Forsythiae Fructus methanol extract attenuated the decrease of the total antioxidant capacity and SOD activity and increase of serum MDA. | [129] |
| Arbor Acres broilers under high stocking density | Methanol extract | Treatment with Forsythiae Fructus methanol extract (100 mg/kg) increased serum T-AOC and SOD activity and reduced MDA expression. However, no significant differences were found in serum GSH-Px activity. | [130] |
| Neuroprotective Activity | |||
| Rotenone-stimulated PC12 cells and male Sprague-Dawley rats | Ethanol extract | The ethanol extract (50 and 200 mg/kg) exhibited neuroprotective activity by down-regulating protein expression of p-PI3K, p-Akt, p-IκB, p-P65 and cleaving caspase 8, p-p38 and p-JNK. | [131] |
| SAMP8 mice with composite Alzheimer‘s disease | Forsythoside A | Forsythoside A (60, 120 and 240 mg/kg) increased the activity of SOD, ChAT, and GSH-PX inordinately and decreased the content of MDA and NO by varying degrees in a dose-dependent manner. | [132] |
| SAMP8 mice | Forsythoside A | Oral administration of forsythoside A (60, 120 and 240 mg/kg) decreased the levels of IL-1β, NO, MDA and NE and increased the T-SOD and GSH-Px activities and the production of GLU and Ach. | [133] |
| Scopolamine-induced learning and memory impairment in mice | Forsythoside A | Forsythoside A (200 mg/kg) ameliorated scopolamine-induced learning and memory impairment by modulating AchE activity, cAMP expression and p-ERK production and protecting against oxidation. | [134] |
| Gerbils with transient cerebral global ischemia | Forsythoside A | Oral administration of forsythoside A (10 mg/kg) significantly increased the number of viable neurons and decreased degenerating neurons, activated glial cells and the expression of IL-1β and TNF-α, indicating the involvement of anti-inflammatory activities. | [135] |
| Aβ25-35 oligomer-stimulated HT22 cells | Forsythoside A | Forsythoside A (25 μg/mL) significantly decreased production of NO to improve neuroinflammation in Aβ25-35 oligomer-stimulated HT22 cells. | [136] |
| Neuroprotective Activity | |||
| Glutamate or low-glucose and low-serum or Aβ25-35-stimulated PC12 cells | Forsythoside A | Forsythoside A (0.1, 1 and 5 μmol/L) improved proliferation of PC12 cells and significantly reduced cell death in vitro. Moreover, forsythoside A (0.1 and 1 μmol/L) significantly inhibited cell apoptosis induced by Aβ25-35. | [137] |
| MPP+-stimulated SH-SY5Y neuroblastoma cells | Phillyrin | Phillyrin (1, 10 and 100 μmol/L) significantly increased cell viability and reduced leakage of LDH induced by MPP+. | [138] |
| Rotenone-stimulated PC12 cells | Forsythoneoside B Forsythoneoside D | Forsythoneoside B and forsythoneoside D at 0.1 μM inhibited PC12 cell damage induced by rotenone and increased cell viability from 53.9 ± 7.1% to 70.1 ± 4.0% and 67.9 ± 5.2%, respectively. | [11] |
| Anti-tumor Activity | |||
| The murine melanoma B16-F10 cell line and C57BL/6 mice bearing melanoma | Aqueous extract | The aqueous extract inhibited proliferation and angiogenesis of cancer cells, which were closely related to the antioxidant and anti-inflammatory activities via the MAPKs/Nrf2/HO-1 pathway. | [7] |
| HeLa cells | Aqueous extract | The aqueous extract (50 μg/mL) promoted activation of the zymogen of caspase 8 to inhibit proliferation of cells in vitro time-dose-dependently, with IC50 values of 93.74, 33.30 and 22.65 μg/mL for 12, 24 and 48 h, respectively. | [139] |
| HeLa cells | Ethanol extract | In vitro, the ethanol extract (12.5–100 μg/mL) had an inhibitory effect on the proliferation of Hela cells in a time-dose-dependent manner with IC50 values for the 12, 24 and 48 h groups of 97.68, 39.16 and 25.83 μg/mL, respectively. | [140] |
| SGC7901 cells | Aqueous extract | In vitro, the aqueous extract (25–100 μg/mL) inhibited proliferation of SGC7901 cells in a time-dose-dependent manner with IC50 values for the 6, 12 and 24 h of 73.27 ± 3.19, 44.63 ± 2.06 and 35.99 ± 2.43 μg/mL, respectively. | [141] |
| C57BL/6J mice injected with Lewis cells | Phillyrin | Phillyrin (5 and 10 g/kg) significantly inhibited the tumor size and tumor tissue density dose-dependently by decreasing the expression of VEGF and increasing the expression of endostatin. | [142] |
| Anti-tumor Activity | |||
| A549, Colo205, Hep-3B, HL60, and KB cancer cell lines | (+)-8-Hydroxyepipinoresinol-4-O-β-d-glucopyranoside | (+)-8-hydroxyepipinoresinol-4-O-β-d-glucopyranoside showed significant cytotoxicity in A549, Colo205, Hep-3B, HL60 and KB cancer cell lines with IC50 values of 9.48, 7.75, 0.59, 4.06 and 38.38 μM, respectively. | [34] |
| MKN-45, MKN-28, SGC-7901, PNAC-1 and HepG-2 cancer cell lines | Ambrolic acid Dammar-24-en-3β-acetoxy-20-ol | Ambrolic acid inhibited SGC-7901 cells by affecting the S period of DNA synthesis and also reduced the levels of pro-caspase 3, 6, 8, 9 and Bcl-2 proteins and increased the levels of Bax protein to induce cell apoptosis, while dammar-24-en-3β-acetoxy-20-ol only had an inhibitory effect on the cancer cells. | [51,55] |
| PC3 cells of prostate cancer | Dammar-24-en-3β-acetoxy-20-ol | Dammar-24-en-3β-acetoxy-20-ol (6.25–50.0 μg/mL) increased expression of p21, TGF-β and Smad3 and decreased expression of Cyclin D1 and CDC25A to induce cell apoptosis and inhibited the activity of telomerase. Moreover, it affected the radiosensitivity of PC-3 cells of prostate cancer at 25 μg/mL. | [143] |
| Hepatoprotective Activity | |||
| CCl4-induced toxicity in rats | Phillygenin | Phillygenin at 0.15 and 0.5 mg/kg significantly decreased the levels of ALT, AST, total bilirubin, TNF-α and IL-8 in serum and the content of MDA in liver tissue. Meanwhile, it increased the activities of SOD, GSH-Px and GSH. | [10] |
| Bovine serum albumin-induced hepatic fibrosis in rats | Forsythoside A | Forsythoside A alleviated hepatic fibrosis at 0.1, 0.3 and 1.0 mg/kg by decreasing the hydroxyproline content and the levels of layer fibronectin, hyaluronic acid, IV-collagen and procollagen III. | [144] |
| Human normal liver cell lines LO2 | Forsythin | Forsythin reversed nuclear condensation and nuclear fragmentation and decreased expression of apoptosis related proteins (PARP and caspase 3) to prevent alcoholic liver injury does-dependently. | [145] |
| Rats with severe acute pancreatitis | Aqueous extract | The aqueous extract (1.25, 2.5 and 5 g/kg) significantly reduced the serum levels of amylase, ALT and TNF-α in a dose dependent manner and expression of NF-κB mRNA and Foxp3 mRNA in liver tissue. | [146] |
| Cardiovascular Protective Effect | |||
| Streptozotocin-induced diabetic mice | Ethyl acetate extract | Oral administration of the extract (50, 100 and 200 mg/kg) after four weeks significantly decreased the levels of blood glucose, triglyceride, creatinine and so on and increased body weight, insulin secretion and glucose tolerance, which were related to inhibition of glucokinase, phosphorenolpyruvate carboxykinase, insulin-1, insulin-2 and duodenal homeobox factor-1, thus exhibiting antidiabetic and antihyperlipidemic activities. | [147] |
| SD rats with atherosclerosis | Phillyrin | Phillyrin (150 mg/kg) reduced the area of AS plaques and the contents of ICAM-1, VACM-1, IL-1, IL-6 and MDA and increased the contents of NO and SOD, probably by decreaseing expression of sodium hydrogen exchange protein 1 (NHE-1). | [12] |
| Rat aortic rings | Forsythoside A | Forsythoside A inhibited norepinephrine-stimulated vasocontraction by decreasing calcium influx from the extracellular space. | [148] |
| Others | |||
| Cisplatin-treated mice | Aqueous decoction | The aqueous decoction reduced the contents of serum gastrin and promoted gastrointestinal movement at 3, 6 and 12 g/kg, indicating its anti-vomiting activity. | [9] |
| HepG2 cells | Phillyrin | Phillyrin at the concentration of 1, 2.5 and 5 μM induced phosphorylation of LKB1 and activated AMPK, thus reducing expression of SREBP-1c and fatty acid synthase and avoiding accumulation of lipid. | [149] |
| TNF-α-stimulated 3T3-L1 adipocytes | Phillyrin | Phillyrin (40 μM) suppressed activation of I kappaB kinase and N-terminal kinase to attenuate TNF-α-mediated insulin resistance and lipolytic acceleration. | [150] |
| Obese C57BL/6J mice | Phillyrin | Treatment with phillyrin (15 and 45 mg/kg) significantly decreased body weight, the serum levels of TNF-α and leptin and increased expression of PPAR-β/δ, ANGPTL4 and p-AMPK-α. | [151] |
| Dihydrotestosterone-stimulated mice | Forsythoside A | Forsythoside A suppressed apoptosis of hair cells by reducing expression of caspase-9 by 40%, caspase-3 by 53% and increasing the Bcl-2/Bax ratio by 60%. It also retarded the entry into the catagen phase and reduced the expression of TGF-β2 by 75%. | [152] |
| Mice with endotoxemia | Forsythoside A | Forsythoside A (80 mg/kg) enhanced the immune function of mice with endotoxemia, which may be associated with the inhibition of TNF-α and IL-10 secretion and the gene expression of Foxp3. | [153] |
| Yeast-stimulated C57BL/6 mice | Forsythoside A | Forsythoside A (4 and 8 mg/kg) significantly decreased the temperature of mice by up-regulating expression of TRPA1 in the paraventricular nuclei (PN), supraoptic nucleus (SO) and dorsal root ganglion (DRG). | [154] |
| Caco-2 cells | Forsythoside A | Forsythoside A inhibited P-gp ATPase activity to influence the efflux of drugs. | [155] |
| Markers | Methods | Results | Reference |
|---|---|---|---|
| Forsythoside A, Rutin, Phillyrin, Isorhamnetin and Quercetin | HPLC-MS/MS | The t1/2 of forsythoside A, rutin, phillyrin, quercetin and isorhamnetin after single oral administration of UFF extract were 1.91 ± 1.76 h, 1.59 ± 0.92 h, 3.52 ± 4.37 h, 2.70 ± 2.70 h and 6.32 ± 4.69 h, respectively, while those were 4.52 ± 4.77 h, 6.54 ± 8.73 h, 14.74 ± 27.34 h, not detected and not detected after single oral administration of RFF extract. The AUC0–24 h of forsythoside A, rutin and phillyrin were significantly different between sigle oral administration of UFF and RFF extract. | [59] |
| (+)-Pinoresinol-β-d-glucoside, Matairesinol-4′-O-glucoside, Hyperin, Phillyrin, Phillygenin | HPLC-ESI-MS/MS | The average percentages of (+)-pinoresinol-β-d-glucoside, matairesinol-4′-O-glucoside, hyperin, phillyrin and hillygenin excreted in the bile over the dose administered (12 mL/kg body weight) were 0.002%, 0.234%, 0.116%, 0.288%, and 12.700%, respectively. Hyperin was found in plasma, urine and excrement of rat while the others were detected only in bile, indicating lignans of Forsythiae Fructus were excreted mainly via bile. | [31] |
| Forsythoside A | LC-MS/MS | Forsythoside A was rapidly absorbed into the blood with a Tmax of 20.0 min after oral (100 mg/kg) administration, but the Cmax was only 122.2 ± 45.4 ng/mL, indicating a quite low absolute bioavailability with a value of 0.5%. | [158] |
| Forsythoside A | Microdialysis coupled with HPLC | Forsythoside A went through hepatobiliary excretion and the bile-to-blood distribution ratio (AUCbile/AUCblood) was 0.32 ± 0.06 after the intravenous administration of 50 mg/kg. | [159] |
| Phillyrin | UPLC-Q-TOF-MS | A total of thirty-four metabolites of phillyrin were detected in rat bile, urine and feces and M26 was the major one. Phillyrin mainly went through hydrolysis, oxidation and sulfation to transform into the effective forms in vivo. | [161] |
| Phillygenin | HPLC | The elimination half-time (t1/2z) of phillygenin after intravenous administration of 1.4, 2.8 and 5.6 mg/kg were 6.02 ± 1.66, 5.62 ± 0.35 and 5.79 ± 0.81 min, respectively and the AUC (0–∞) were 166.29 ± 18.01, 242.40 ± 7.12 and 332.48 ± 23.98 mg/L min, respectively. All these results suggested the pharmacokinetics of phillygenin followed first-order kinetics. | [162] |
| Phillyrin and Forsythoside A | UHPLC-MS-MS | The t1/2 of caffeine, tolbutamide, metoprolol and dapsone in rats after intraperitoneal administration were 5.86 ± 0.83, 5.87 ± 0.83, 4.67 ± 0.63 and 1.17 ± 0.15 h, respectively. But when given a pretreatment of phillyrin and forsythoside A, the t1/2 of them changed into 4.63 ± 0.56 and 4.15 ± 0.54, 5.56 ± 0.72 and 4.28 ± 0.74, 3.69 ± 0.54 and 4.17 ± 0.27, 1.05 ± 0.15 and 1.02 ± 0.19 h for phillyrin and forsythoside A, respectively, indicating the inductive effect of phillyrin and forsythoside A on CYP. Further study revealed that phillyrin induced rat CYP1A2 and CYP2D1, while forsythoside A induced CYP1A2 and CYP2C11. | [163] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Lu, X.; Tong, X.; Dong, Y.; Tang, L.; Liu, M. Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics. Molecules 2017, 22, 1466. https://doi.org/10.3390/molecules22091466
Dong Z, Lu X, Tong X, Dong Y, Tang L, Liu M. Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics. Molecules. 2017; 22(9):1466. https://doi.org/10.3390/molecules22091466
Chicago/Turabian StyleDong, Zhanglu, Xianyuan Lu, Xueli Tong, Yaqian Dong, Lan Tang, and Menghua Liu. 2017. "Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics" Molecules 22, no. 9: 1466. https://doi.org/10.3390/molecules22091466
APA StyleDong, Z., Lu, X., Tong, X., Dong, Y., Tang, L., & Liu, M. (2017). Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics. Molecules, 22(9), 1466. https://doi.org/10.3390/molecules22091466




