Antioxidant Capacity, Nitrite and Nitrate Content in Beetroot-Based Dietary Supplements
Abstract
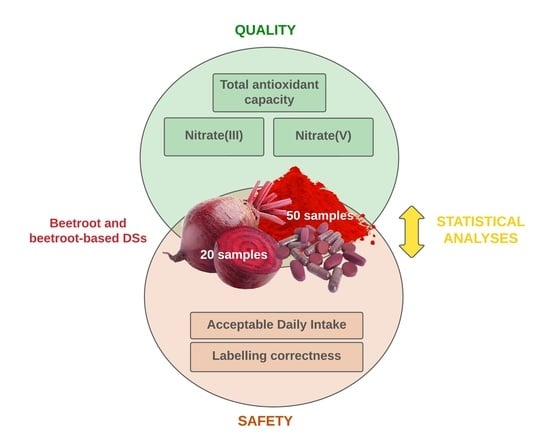
:1. Introduction
2. Materials and Methods
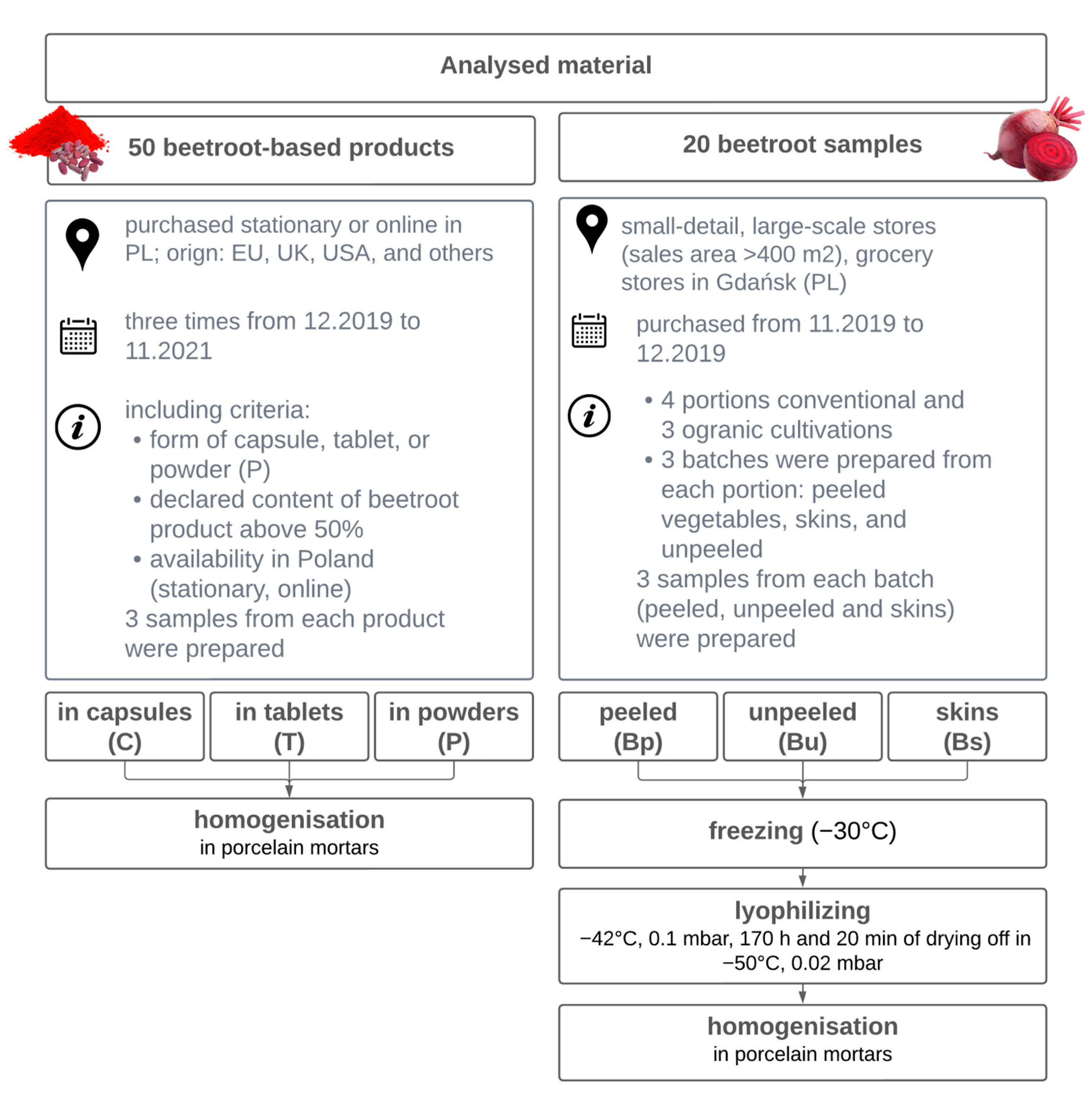
2.1. Materials
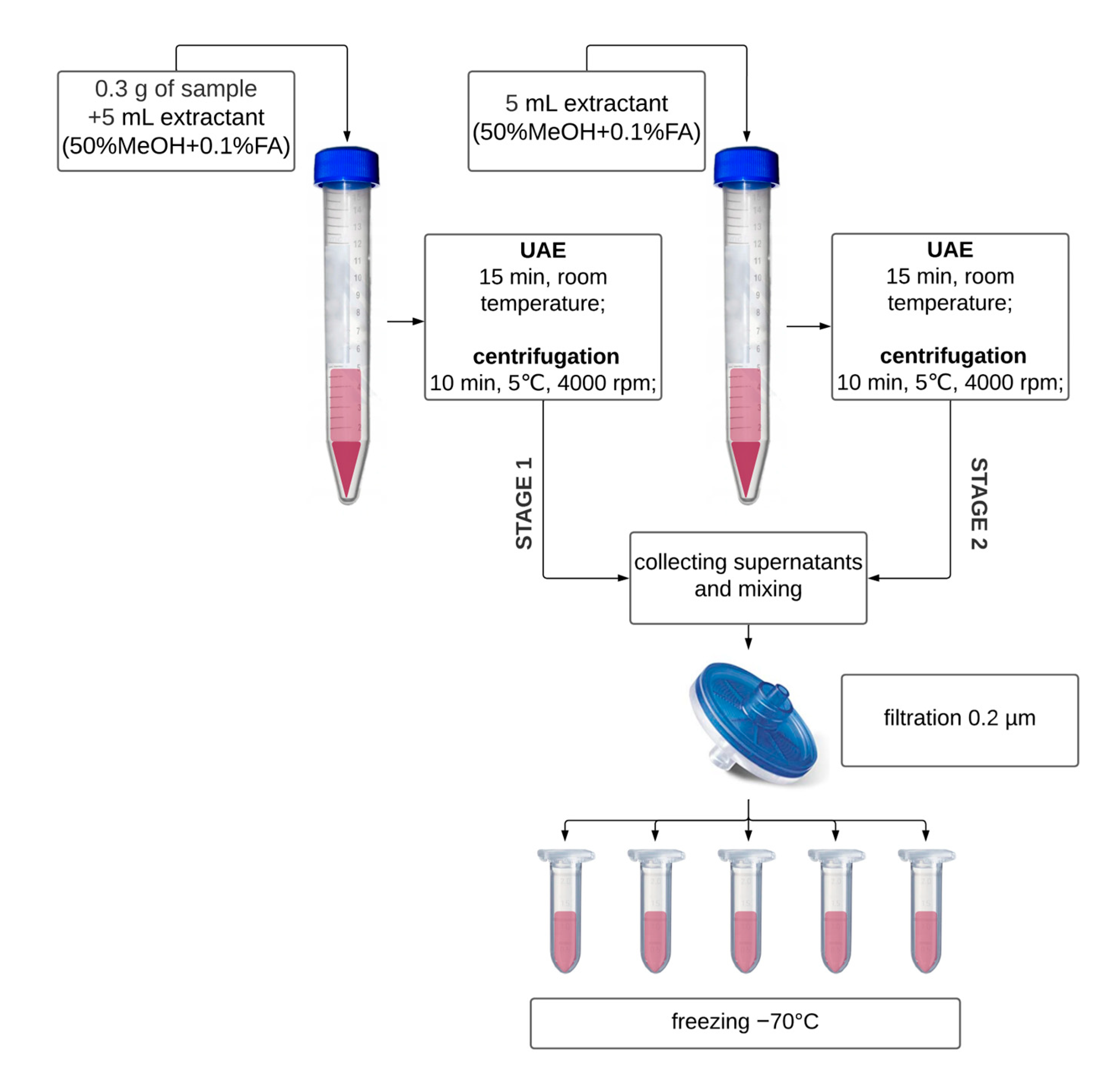
2.1.1. Sample Preparation
2.1.2. Reagents and Standards
2.2. The Total Antioxidant Capacity (TAC) and Total Phenolic Content (TPC)
2.2.1. Extract Preparation for TAC and TPC Determination
2.2.2. TPC Determination
2.2.3. CUPRAC
2.2.4. DPPH
2.3. The Nitrite and Nitrate Determination
2.3.1. Extraction for Nitrites and Nitrates Determination
2.3.2. Nitrites Determination
2.3.3. Nitrates Determination
2.4. Validation
2.5. Labelling Assessment
- Labelling in Polish;
- The name of the food;
- The list of ingredients;
- The net amount of food;
- The date of minimum durability or best-before date;
- The presence of the term “dietary supplement”;
- Indication of the recommended daily portion of the product;
- The presence of a warning regarding not exceeding the recommended daily portion;
- A statement that dietary supplements cannot be used as a substitute for a varied diet;
- A statement that they should be kept out of the reach of small children.
2.6. Statistical Analyses
3. Results
3.1. Total Phenolic Content and the TAC
3.2. Nitrate and Nitrite Content
Health Risk Assessment
3.3. The Correlation between the Antioxidant Potential and the Content of Nitrites and Nitrates in Beetroot and Beetroot–Based Products
3.4. Labelling Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grembecka, M.; Szefer, P.; Dybek, K.; Gurzynska, A. Ocena zawartosci wybranych biopierwiastkow w warzywach. Rocz. Państwowego Zakładu Hig. 2008, 59, 179–186. [Google Scholar]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red beetroot betalains: Perspectives on extraction, processing, and potential health benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef] [PubMed]
- Czapski, J.; Mikołajczyk, K.; Kaczmarek, M. Relationship between antioxidant capacity of red beet juice and contents of its betalain pigments. Polish J. Food Nutr. Sci. 2009, 59, 119–122. [Google Scholar]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, A.G.; Sips, A.J.A.M.; Schothorst, R.C.; Lambers, A.C.; Meulenbelt, J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicol. Lett. 2008, 181, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Bonilla Ocampo, D.A.; Paipilla, A.F.; Marín, E.; Vargas-Molina, S.; Petro, J.L.; Pérez-Idárraga, A. Dietary nitrate from beetroot juice for hypertension: A systematic review. Biomolecules 2018, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Chawla, H.; Parle, M.; Sharma, K.; Yadav, M. Beetroot: A health promoting functional food. Inven. Rapid Nutraceuticals 2016, 1, 0976–3872. [Google Scholar]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Gilchrist, M.; Winyard, P.G.; Fulford, J.; Anning, C.; Shore, A.C.; Benjamin, N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: Development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide-Biol. Chem. 2014, 40, 67–74. [Google Scholar] [CrossRef]
- Shepherd, A.I.; Gilchrist, M.; Winyard, P.G.; Jones, A.M.; Hallmann, E.; Kazimierczak, R.; Rembialkowska, E.; Benjamin, N.; Shore, A.C.; Wilkerson, D.P. Effects of dietary nitrate supplementation on the oxygen cost of exercise and walking performance in individuals with type 2 diabetes: A randomized, double-blind, placebo-controlled crossover trial. Free Radic. Biol. Med. 2015, 86, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Bock, J.M.; Ueda, K.; Feider, A.J.; Hanada, S.; Casey, D.P. Combined inorganic nitrate/nitrite supplementation blunts α-mediated vasoconstriction during exercise in patients with type 2 diabetes. Nitric Oxide 2022, 118, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Feelisch, M.; Björne, H.; Jansson, E.Å.; Weitzberg, E. Cardioprotective effects of vegetables: Is nitrate the answer? Nitric Oxide-Biol. Chem. 2006, 15, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.E.; Treichler, D.P.; Ueda, K.; Bock, J.M.; Casey, D.P. Sodium nitrate supplementation improves blood pressure reactivity in patients with peripheral artery disease. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Azizi, M.; Tahmasbi, W.; Bashiri, P. The effects of consuming 6 weeks of beetroot juice (Beta vulgaris L.) on hematological parameters in female soccer players. J. Kermanshah Univ. Med. Sci. 2018, 22, e82300. [Google Scholar] [CrossRef] [Green Version]
- Ranchal-Sanchez, A.; Diaz-Bernier, V.M.; De La Florida-Villagran, C.A.; Llorente-Cantarero, F.J.; Campos-Perez, J.; Jurado-Castro, J.M. Acute effects of beetroot juice supplements on resistance training: A randomized double-blind crossover. Nutrients 2020, 12, 1912. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Castaño, M.V.; Palau-Salvà, G.; Serra-Payá, N.; Ruiz-Hermosel, M.; Berbell, M.; Viñals, X.; Bataller, M.G.; Carbonell, T.; Vilches-Saez, S.; Cobo, E.P.; et al. Understanding the effects of beetroot juice intake on CrossFit performance by assessing hormonal, metabolic and mechanical response: A randomized, double-blind, crossover design. J. Int. Soc. Sports Nutr. 2020, 17, 56. [Google Scholar] [CrossRef]
- Broeder, C.E.; Flores, V.; Julian, B.; Wojan, F.; Tauber, R.; Schubert, L.; Salacinski, A.; Ivy, J.L. Nitric oxide enhancement supplement containing beet nitrite and nitrate benefits high intensity cycle interval training. Curr. Res. Physiol. 2021, 4, 183–191. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA J. 2017, 15, e04786. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of sodium nitrate (E 251) and potassium nitrate (E 252) as food additives. EFSA J. 2017, 15, e04787. [Google Scholar] [CrossRef]
- Guldiken, B.; Toydemir, G.; Nur Memis, K.; Okur, S.; Boyacioglu, D.; Capanoglu, E. Home-processed red beetroot (Beta vulgaris L.) products: Changes in antioxidant properties and bioaccessibility. Int. J. Mol. Sci. 2016, 17, 858. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of the antioxidant capacity of food products: Methods, applications and limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Bartosz, G. Total antioxidant capacity. Adv. Clin. Chem. 2003, 37, 219–292. [Google Scholar] [PubMed]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; Hall, R.; De Vos, R. Changes in antioxidant and metabolite profiles during production of tomato paste. J. Agric. Food Chem. 2008, 56, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Kujala, T.; Loponen, J.; Pihlaja, K. Betalains and phenolics in red beetroot (Beta vulgaris) peel extracts: Extraction and characterisation. Zeitschrift Fur Naturforsch.-Sect. C J. Biosci. 2001, 56, 343–348. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jimenez, A.R.; Paredes-Lopez, O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability-a review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef] [Green Version]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Kavalcová, P.; Bystrická, J.; Tomáš, J.; Karovičová, J.; Kovarovič, J.; Lenková, M. The content of total polyphenols and antioxidant activity in red beetroot. Potravinarstvo 2015, 9, 77–83. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzynska, K. Determination of selenium species in beetroot juices. Heliyon 2020, 6, e04194. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventós, R.M. Folin-Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications, 1st ed.; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2017; Volume 1, pp. 107–115. [Google Scholar] [CrossRef]
- Agbor, G.A.; Vinson, J.A.; Donnelly, P.E. Folin-Ciocalteau reagent for polyphenolic assay. Int. J. Food Sci. Nutr. Diet. 2014, 3, 147–156. [Google Scholar] [CrossRef]
- Musci, M.; Yao, S. Optimization and validation of Folin–Ciocalteu method for the determination of total polyphenol content of Pu-erh tea. Int. J. Food Sci. Nutr. 2017, 68, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Ahmed, A.R.; Knorr, D.; Smetanska, I. The effect of different processing methods on phenolic acid content and antioxidant activity of red beet. Food Res. Int. 2012, 48, 16–20. [Google Scholar] [CrossRef]
- ISO 6635:1984; Fruits, Vegetables and Derived Products—Determination of Nitrite and Nitrate Content—Molecular Absorption Spectrometric Method. ISO International Organization for Standardization: Geneva, Switzerland, 1984; pp. 1–8.
- Huber, L. Validation of analytical methods and processes. In Pharmaceutical Process Validation; Nash, R.A., Wachter, A.H., Eds.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780203912119. [Google Scholar]
- The Chief Sanitary Inspectorate Register of Products Subject to the Notification of the First Placing on the Market. Available online: https://powiadomienia.gis.gov.pl/ (accessed on 14 November 2022).
- The European Parliament and the Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Off. J. Eur. Union. 2011, 304, pp. 18–63. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:en:PDF (accessed on 25 January 2023).
- The Seym of the Republic of Poland. Act of August 25, 2006 on Food and Nutrition Safety (as Amended). J. Law 2006, 171, 1225. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20061711225/U/D20061225Lj.pdf (accessed on 25 January 2023).
- The Minister of Health. Regulation of the Minister of Health of 9 October 2007 on the composition and labeling of dietary supplements. J. Law 2007, 196, 1425. [Google Scholar]
- The European Parliament and the Council. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2006, 404, 9–25. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1924&from=PL (accessed on 25 January 2023).
- The European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Off. J. Eur. Union. 2012, 136, pp. 1–40. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0432&from=PL (accessed on 25 January 2023).
- European Commission. EU Register of Nutrition and Health Claims Made on Foods (v.3.6). Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=search (accessed on 14 November 2022).
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçǧ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Güçlü, K.; Altun, M.; Özyürek, M.; Karademir, S.E.; Apak, R. Antioxidant capacity of fresh, sun- and sulphited-dried Malatya apricot (Prunus armeniaca) assayed by CUPRAC, ABTS/TEAC and folin methods. Int. J. Food Sci. Technol. 2006, 41, 76–85. [Google Scholar] [CrossRef]
- Gościnna, K.; Czapski, J. Wpływ wielkości i części korzenia buraka ćwikłowego odmiany Bonel na zawartość wybranych związków i aktywność przeciwutleniającą soku. Zesz. Probl. Postępów Nauk Rol. 2018, 588, 149–157. [Google Scholar] [CrossRef]
- Murawa, D.; Banaszkiewicz, T.; Majewska, E.; Błaszczuk, B.; Sulima, J. Zawartość azotanów (III) i (V) w wybranych gatunkach warzyw i ziemniakach dostępnych w handlu w Olsztynie w latach 2003–2004. Bromatol. Chem. Toksykol. 2008, 41, 67–71. [Google Scholar]
- Keller, R.M.; Beaver, L.; Prater, M.C.; Hord, N.G. Dietary nitrate and nitrite concentrations in food patterns and dietary supplements. Nutr. Today 2020, 55, 218–226. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, S.T.J.; Wylie, L.J.; Thompson, C.; Vanhatalo, A.; Jones, A.M. Potential benefits of dietary nitrate ingestion in healthy and clinical populations: A brief review. Eur. J. Sport Sci. 2019, 19, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, D.K.; Bryan, N.S. Sodium nitrite: The “cure” for nitric oxide insufficiency. Meat Sci. 2012, 92, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in cured meats, health risk issues, alternatives to nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, L. Drinking-water nitrate, methemoglobinemia, and global burden of disease: A discussion. Environ. Health Perspect. 2004, 112, 1371–1374. [Google Scholar] [CrossRef] [Green Version]
- Ashor, A.W.; Jajja, A.; Sutyarjoko, A.; Brandt, K.; Qadir, O.; Lara, J.; Siervo, M. Effects of beetroot juice supplementation on microvascular blood flow in older overweight and obese subjects: A pilot randomised controlled study. J. Hum. Hypertens. 2015, 29, 511–513. [Google Scholar] [CrossRef]
- Mills, C.E.; Govoni, V.; Faconti, L.; Casagrande, M.L.; Morant, S.V.; Crickmore, H.; Iqbal, F.; Maskell, P.; Masani, A.; Nanino, E.; et al. A randomised, factorial trial to reduce arterial stiffness independently of blood pressure: Proof of concept? The VaSera trial testing dietary nitrate and spironolactone. Br. J. Clin. Pharmacol. 2020, 86, 891–902. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Blekkenhorst, L.C.; Lewis, J.R.; Prince, R.L.; Devine, A.; Bondonno, N.P.; Bondonno, C.P.; Wood, L.G.; Puddey, I.B.; Ward, N.C.; Croft, K.D.; et al. Nitrate-rich vegetables do not lower blood pressure in individuals with mildly elevated blood pressure: A 4-wk randomized controlled crossover trial. Am. J. Clin. Nutr. 2018, 107, 894–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundqvist, M.L.; Larsen, F.J.; Carlström, M.; Bottai, M.; Pernow, J.; Hellénius, M.L.; Weitzberg, E.; Lundberg, J.O. A randomized clinical trial of the effects of leafy green vegetables and inorganic nitrate on blood pressure. Am. J. Clin. Nutr. 2020, 111, 749–756. [Google Scholar] [CrossRef] [PubMed]

| CUPRAC | FC | DPPH | Nitrates and Nitrites | ||||
|---|---|---|---|---|---|---|---|
| Standard substance | Trolox (TE) | gallic acid (GA) | gallic acid (GA) | sodium nitrite | |||
| Calibration curve equation | y = 17.3x + 0.00234 | y = 0.131x + 0.000874 | y = −0.481x + 0.786 | y = 1.0973x + 0.0037 | |||
| The determination coefficient (R2) | 0.9993 | 0.9991 | 0.9991 | 0.9998 | |||
| Linearity range | 0.0005–0.070 μmol/mL | 0.10–10 μg/mL | 0.13–1 μg/mL | 0.0027–0.6 µg/mL | |||
| LOD | 0.000187 μmol/mL | 0.074 μg/mL | 0.040 μg/mL | 0.009 μg/mL | |||
| LOQ | 0.000562 μmol/mL | 0.22 μg/mL | 0.12 μg/mL | 0.0027 μg/mL | |||
| supplement | lyophilizate | supplement | lyophilizate | gallic acid | supplement | lyophilizate | |
| Precision | 1.7–3.1% | 0.34–1.4% | 1.1–3.9% | 1.1–5.5% | 2.0–4.6% | I stage: 1.26–1.97% II stage: 4.73–4.91% | I stage: 0.22–4.28% II stage: 2.11–4.95% |
| Recovery | 95–115% | 99–109% | 96–106% | 86–105% | 91–113% | I stage: 86.17–93.47%II stage: 99.06–104.07% | I stage: 80.77–94.08%II stage: 93.99–104.72% |
| Beetroot | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Unit | Conventional | Organic | ||||||||||
| n | Mean | SD | Min | Median | Max | n | Mean | SD | Min | Median | Max | ||
| FC | mg GAE/g d.w. | 12 | 15 | 0.702 | 6.8 | 12 | 26 | 8 | 14 | 0.47 | 9.7 | 13 | 34 |
| CUPRAC | µmol TE/g d.w. | 12 | 171 | 11 | 93 | 150 | 365 | 8 | 196 | 7.1 | 123 | 179 | 413 |
| Nitrites | µg/g d.w. | 12 | 2.4 | 0.25 | 0.702 | 1.8 | 7.1 | 8 | 5.1 | 0.298 | 1.96 | 3.7 | 15 |
| Nitrates | mg/kg d.w. | 12 | 4980 | 111 | 2101 | 4912 | 8801 | 8 | 2612 | 88 | 423 | 2509 | 6606 |
| FC | mg GAE/100 g f.w. | 12 | 211 | 16 | 91 | 218 | 595 | 8 | 299 | 13 | 170 | 279 | 794 |
| CUPRAC | µmol TE/100 g f.w. | 12 | 2743 | 233 | 1320 | 2884 | 7988 | 8 | 4064 | 192 | 2464 | 3905 | 9779 |
| Nitrites | µg/100 g f.w. | 12 | 49 | 6.9 | 12 | 38 | 196 | 8 | 109 | 7.5 | 34 | 81 | 293 |
| Nitrates | mg/100 g f.w. | 12 | 90 | 1.97 | 45 | 90 | 153 | 8 | 54 | 1.997 | 11 | 49 | 134 |
| DPPH | % | 12 | 42 | 1.7 | 28 | 40 | 63 | 8 | 43 | 1.3 | 31 | 41 | 64 |
| Beetroot-Based Dietary Supplements | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Unit | Capsules | Tablets | Powders | |||||||||||||||
| n | Mean | SD | Min | Median | Max | n | Mean | SD | Min | Median | Max | n | Mean | SD | Min | Median | Max | ||
| FC | mg GAE/g | 21 | 14 | 1.2 | 1.8 | 6.2 | 41 | 16 | 7.8 | 0.77 | 0.68 | 3.4 | 33 | 12 | 13 | 0.68 | 2.5 | 10 | 61 |
| CUPRAC | µmol TE/g | 21 | 126 | 11 | 13 | 62 | 312 | 16 | 76 | 3.5 | 1.3 | 42 | 278 | 12 | 139 | 5.9 | 27 | 119 | 467 |
| Nitrites | µg/g | 21 | 2.3 | 0.21 | 0.81 | 1.2 | 8.9 | 16 | 1.3 | 0.079 | 0.29 | 1.2 | 2.5 | 12 | 2.9 | 0.25 | 0.95 | 2.4 | 6.36 |
| Nitrates | mg/kg | 21 | 4230 | 155 | 383 | 2373 | 15,186 | 16 | 2099 | 67 | 504 | 1979 | 3746 | 12 | 4161 | 241 | 91 | 2265 | 13,110 |
| FC | mg GAE/d. d. | 21 | 15 | 1.3 | 0.73 | 8.5 | 42 | 16 | 17 | 1.4 | 1.5 | 18 | 41 | 12 | 99 | 9.6 | 4.1 | 92 | 251 |
| CUPRAC | µmol TE/d. d. | 21 | 138 | 10 | 5.2 | 103 | 363 | 16 | 167 | 8.1 | 5.5 | 174 | 350 | 12 | 1228 | 97 | 48 | 1065 | 3520 |
| Nitrites | µg/d. d. | 21 | 3.3 | 0.56 | 0.33 | 1.85 | 23.78 | 16 | 4.2 | 0.36 | 0.21 | 2.6 | 14 | 12 | 28 | 3.4 | 1.7 | 18 | 78 |
| Nitrates | mg/d. d. | 21 | 6.1 | 0.26 | 0.20 | 3.9 | 18 | 16 | 4.6 | 0.35 | 1.3 | 3.8 | 11 | 12 | 43 | 2.7 | 0.90 | 22 | 169 |
| DPPH | % | 21 | 39 | 1.67 | 7.2 | 22 | 90 | 16 | 35 | 2.4 | 4.9 | 17 | 90 | 12 | 37 | 1.3 | 12 | 37 | 90 |
| %ADI for NO2¯ | %ADI for NO3¯ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Product | n | Mean | SD | Min | Median | Max | n | Mean | SD | Min | Median | Max |
| DSs and food products | ||||||||||||
| Tablets | 14 | 0.030 | 0.030 | 0.0015 | 0.018 | 0.098 | 15 | 1.3 | 0.90 | 0.38 | 1.1 | 3.2 |
| Capsules | 18 | 0.023 | 0.039 | 0.0023 | 0.012 | 0.17 | 17 | 1.7 | 1.5 | 0.056 | 0.87 | 5.1 |
| Powders | 11 | 0.203 | 0.18 | 0.012 | 0.13 | 0.55 | 11 | 12 | 16 | 0.26 | 6.2 | 48 |
| Beetroot | ||||||||||||
| Conventional | 12 | 0.35 | 0.35 | 0.087 | 0.27 | 1.4 | 12 | 20.1 | 13 | 1.84 | 16.1 | 42.7 |
| Organic | 8 | 0.78 | 0.62 | 0.24 | 0.58 | 2.1 | 8 | 15 | 11 | 3.02 | 14 | 38 |
| The Analysed Feature of Product Marking | Results |
|---|---|
| Registration in the register of products is subject to notification of first placing on the market [39] | 88% of analysed products were reported to the Register [39] and 12% did not |
| Labelling in Polish | 26% of products had a complete or partial lack of markings in Polish |
| List of ingredients | 5% of the packaging was missing the word “ingredients” before the list of ingredients |
| The net amount of food | 26% of packages did not declare the net weight of the product |
| Date of minimum durability or best-before date | 23% of manufacturers used incorrect wording preceding the date of minimum durability in the labelling |
| The presence of the term “dietary supplement” | 6% of products were not marked with the term “dietary supplement” |
| Indication of the recommended daily portion | 3% of products did not have the recommended daily portion for consumption specified |
| The presence of a warning regarding not exceeding the recommended daily portion | 12% of packages lacked such a warning |
| The statement that DSs cannot be used as a substitute (replacement) for a varied diet | 12% did not include this statement |
| The statement that DSs should be kept out of the reach of small children | 9% did not include this statement |
| The content of vitamins and minerals and other substances with nutritional or other physiological effects present in the dietary supplement in numerical form, calculated per recommended daily portion of the product | 21% of the packaging did not contain the content of vitamins, minerals and other substances per recommended daily portion |
| The information on the content of vitamins and minerals in percentage concerning the reference daily intake (RDI) | 3% of the packaging did not contain information on the content of vitamins and minerals in percentage concerning RDI |
| Labelling may not suggest that the food has effects or properties that it does not have, or attribute to the food the property of preventing or treating disease | 15% of the products suggested that the food had the properties of preventing or treating diseases |
| Health claims | 21% of products contained health claims on the packaging that were not included in the EFSA Health Claims Register or were not allowed to be used due to a lack of scientific evidence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezińska-Rojek, J.; Sagatovych, S.; Malinowska, P.; Gadaj, K.; Prokopowicz, M.; Grembecka, M. Antioxidant Capacity, Nitrite and Nitrate Content in Beetroot-Based Dietary Supplements. Foods 2023, 12, 1017. https://doi.org/10.3390/foods12051017
Brzezińska-Rojek J, Sagatovych S, Malinowska P, Gadaj K, Prokopowicz M, Grembecka M. Antioxidant Capacity, Nitrite and Nitrate Content in Beetroot-Based Dietary Supplements. Foods. 2023; 12(5):1017. https://doi.org/10.3390/foods12051017
Chicago/Turabian StyleBrzezińska-Rojek, Joanna, Svitlana Sagatovych, Paulina Malinowska, Kamila Gadaj, Magdalena Prokopowicz, and Małgorzata Grembecka. 2023. "Antioxidant Capacity, Nitrite and Nitrate Content in Beetroot-Based Dietary Supplements" Foods 12, no. 5: 1017. https://doi.org/10.3390/foods12051017
APA StyleBrzezińska-Rojek, J., Sagatovych, S., Malinowska, P., Gadaj, K., Prokopowicz, M., & Grembecka, M. (2023). Antioxidant Capacity, Nitrite and Nitrate Content in Beetroot-Based Dietary Supplements. Foods, 12(5), 1017. https://doi.org/10.3390/foods12051017