Molecular Sieve, Halloysite, Sepiolite and Expanded Clay as a Tool in Reducing the Content of Trace Elements in Helianthus annuus L. on Copper-Contaminated Soil
Abstract
:1. Introduction
2. Material and Methods
2.1. Methodology of the Plant Growing Trials
2.2. Physicochemical and Chemical Analyses of Soil and Plants
2.3. Statistical Analysis
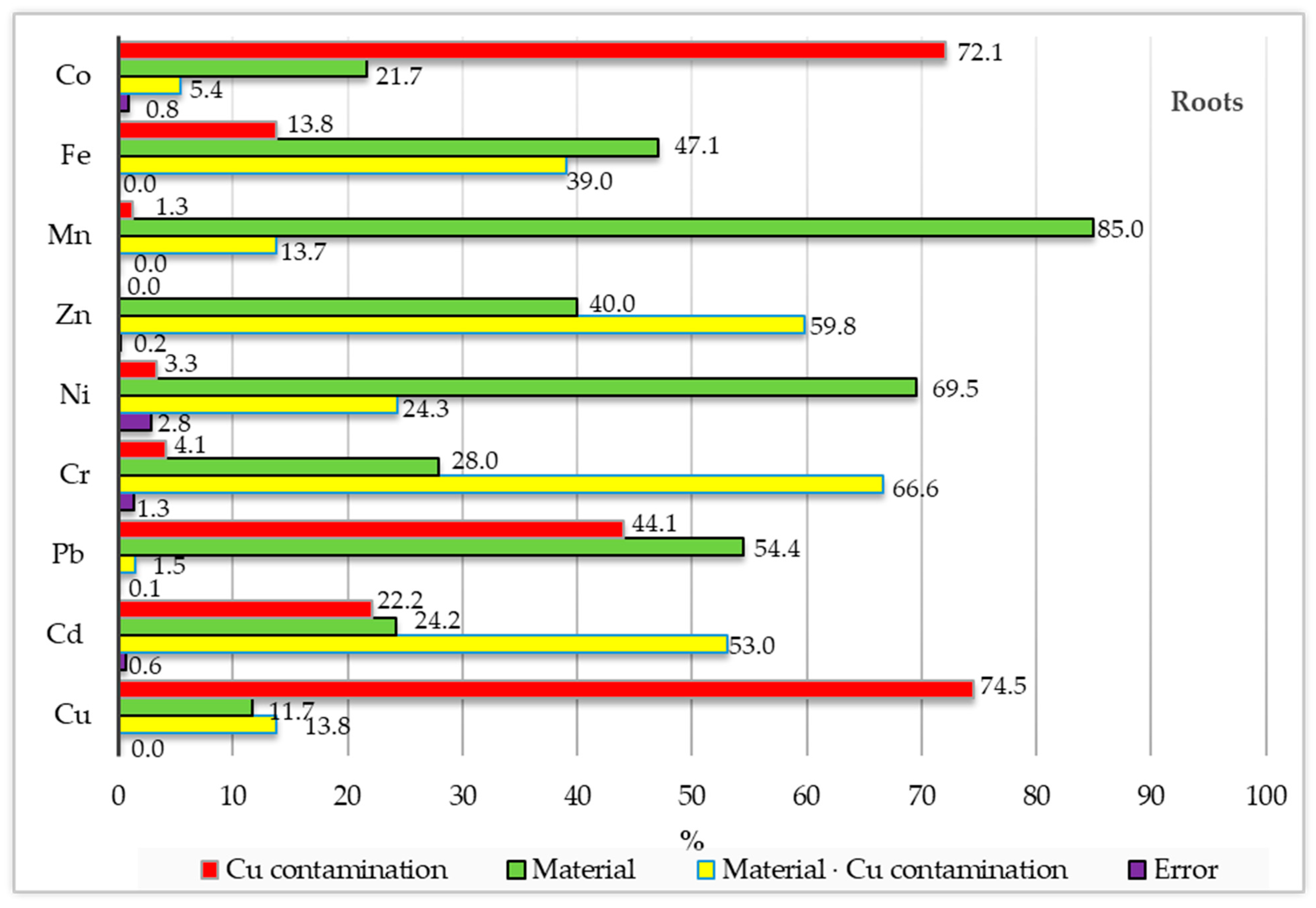
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- El-Shahawi, M.S.; Hamza, A.; Bashammakhb, A.S.; Al-Saggaf, W.T. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Riek, W.; Russ, A.; Marx, M. Concentrations of inorganic and organic pollutants in forest soils as an archive of anthropogenic inputs in the State of Brandenburg, Germany. Appl. Sci. 2021, 11, 1189. [Google Scholar] [CrossRef]
- Garaiyurrebaso, O.; Garbisu, C.; Blanco, F.; Lanzén, A.; Martín, I.; Epelde, L.; Becerril, J.M.; Jechalke, S.; Smalla, K.; Grohmann, E.; et al. Long-term effects of aided phytostabilisation on microbial communities of metal-contaminated mine soil. FEMS Microbiol. Ecol. 2017, 93, fiw252. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of a zeolite and molecular sieve to restore homeostasis of soil contaminated with cobalt. Minerals 2020, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 60, 101–130. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, I.; Ahmad, F.; Sharif, A.; Altaf, A.R.; Teng, H. Heavy metals assessment in water, soil, vegetables and their associated health risks via consumption of vegetables, District Kasur, Pakistan. SN Appl. Sci. 2021, 3, 552. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soil and Plants, 4th ed.; CRS Press, Taylor and Francis Group: Boca Raton, FL, USA, 2010; 548p. [Google Scholar] [CrossRef]
- Napoli, M.; Cecchi, S.; Grassi, C.; Baldi, A.; Zanchi, C.A.; Orlandini, S. Phytoextraction of copper from a contaminated soil using arable and vegetable crops. Chemosphere 2019, 219, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Pandita, S.; Singh Sidhu, G.P.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef] [PubMed]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Alhasany, A.R.; Noaema, A.H.; Alhmadi, H.B. The role of spraying copper and zinc on the growth and yield of Vicia faba L. IOP Conf. Ser. Mater. Sci. Eng. 2019, 571, 012048. [Google Scholar] [CrossRef] [Green Version]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.; Orgiazzi, A.; Jones, A.; Montanarella, L. Science of the total environment copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Wairich, A.; De Conti, L.; Lamb, T.I.; Keil, R.; Neves, L.O.; Brunetto, G.; Sperotto, R.A.; Ricachenevsky, F.K. Throwing copper around: How plants control uptake, distribution, and accumulation of copper. Agronomy 2022, 12, 994. [Google Scholar] [CrossRef]
- Sharma, P.; Sirhindi, G.; Kumar, A.; Harpreet, S. Consequences of copper treatment on pigeon pea photosynthesis, osmolytes and antioxidants defense. Physiol. Mol. Biol. Plants 2017, 23, 809–816. [Google Scholar] [CrossRef]
- Gowayed, S.M.H.; Almaghrabi, O.A. Effect of copper and cadmium on germination and anatomical structure of leaf and root seedling in maize (Zea mays L.). Aust. J. Basic Appl. Sci. 2013, 7, 548–555. [Google Scholar]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- Operacz, A.; Bigaj, A.; Hap, K.; Kotowski, T. The Effect of sample preparation and measurement techniques on heavy metals concentrations in soil: Case study from Kraków, Poland, Europe. Appl. Sci. 2022, 12, 2137. [Google Scholar] [CrossRef]
- Sadiq, S.; Ghazala, Z.; Chowdhury, A.; Büsselberg, D. Metal toxicity at the synapse: Presynaptic, postsynaptic, and long-term effects. J. Toxicol. 2012, 2012, 132671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Htwe, T.; Onthong, J.; Duangpan, S.; Techato, K.; Chotikarn, P.; Sinutok, S. Effect of copper contamination on plant growth and metal contents in rice plant (Oryza sativa L.). Commun. Soil Sci. Plant Anal. 2020, 51, 2349–2360. [Google Scholar] [CrossRef]
- Apori, O.S.; Hanyabui, E.; Asiamah, Y.J. Remediation technology for copper contaminated Soil: A review. Asian Soil Res. J. 2018, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Żołnowski, A.C.; Wyszkowski, M.; Rolka, E.; Sawicka, M. Mineral materials as a neutralizing agent used on soil contaminated with copper. Materials 2021, 14, 6830. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Potential environmental pollution from copper metallurgy and methods of management. Environ. Res. 2021, 197, 111050. [Google Scholar] [CrossRef]
- Wyszkowski, M. Soil Contamination with copper and its effect on selected soil properties after applying neutralizing substances. Pol. J. Environ. Stud. 2019, 28, 2465–2471. [Google Scholar] [CrossRef]
- Bhatt, P.; Pandey, S.C.; Joshi, S.; Chaudhary, P.; Pathak, V.M.; Huang, Y.; Wu, X.; Zhou, Z.; Chen, S. Nanobioremediation: A sustainable approach for the removal of toxic pollutants from the environment. J. Hazard. Mater. 2022, 427, 128033. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Dagan, R.; Bitton, G. Toxicological approach for assessing the heavy metal binding capacity of soils. Soil Sediment Contam. 2007, 16, 451–458. [Google Scholar] [CrossRef]
- Czaban, J.; Siebielec, G. Effects of bentonite on sandy soil chemistry in a long-term plot experiment (II); Effect on pH, CEC, and macro- and micronutrients. Pol. J. Environ. Stud. 2013, 22, 1669–1676. [Google Scholar]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Inazumi, S.; Shishido, K.I.; Nontananandh, S.; Moriiwa, K. Remediation of heavy metals polluted soil using metal insolubilizing materials. J. Environ. Prot. 2018, 9, 770–789. [Google Scholar] [CrossRef] [Green Version]
- Ukalska-Jaruga, A.; Siebielec, G.; Siebielec, S.; Pecio, M. The effect of soil amendments on trace elements’ bioavailability and toxicity to earthworms in contaminated soils. Appl. Sci. 2022, 12, 6280. [Google Scholar] [CrossRef]
- Nwachukwu, O.I.; Pulford, I.D. Comparative effectiveness of selected adsorbant materials as potential amendments for the remediation of lead-, copper- and zinc-contaminated soil. Soil Use Manag. 2008, 24, 199–207. [Google Scholar] [CrossRef]
- Kumpiene, J. Trace elements immobilization in soil using amendments. In Trace Elements in Soil; Hooda, P.S., Ed.; John Wiley and Sons, Ltd.: London, UK, 2010; pp. 353–379. [Google Scholar] [CrossRef]
- Żołnowski, A.C.; Wyszkowski, M. Mineral neutralizers as a tool for improving the properties of soil contaminated with copper. Minerals 2022, 12, 895. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Gonzalez-Núnez, R.; Alba, M.D.; Orta, M.M.; Vidal, M.; Rigol, A. Remediation of metal-contaminated soils with the addition of materials—Part I: Characterization and viability studies for the selection of non-hazardous waste materials and silicates. Chemosphere 2011, 85, 1511–1517. [Google Scholar] [CrossRef]
- Khati, P.; Bhatt, P.; Kumar, R.; Sharma, A. Effect of nanozeolite and plant growth promoting rhizobacteria on maize. 3 Biotech 2018, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, R.; Sas-Nowosielska, A.; Małkowski, E.; Japenga, J.; Kuperberg, J.M.; Pogrzeba, M.; Krzyżak, J. The use of indigenous plant species and calcium phosphate for the stabilization of highly metal-polluted sites in southern Poland. Plant Soil 2005, 273, 291–305. [Google Scholar] [CrossRef]
- Kosiorek, M.; Wyszkowski, M. Remediation of cobalt-contaminated soil using manure, clay, charcoal, zeolite, calcium oxide, main crop (Hordeum vulgare L.), and after-crop (Synapis alba L.). Minerals 2020, 10, 429. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N. Role of different material amendments in shaping the content of heavy metals in maize (Zea mays L.) on soil polluted with petrol. Materials 2022, 15, 2623. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Kim, K.R.; Park, J.S.; Kim, M.S.; Koo, N.; Lee, S.H.; Lee, J.S.; Lee, S.S.; Ok, Y.S.; Kim, J.-G. Changes in heavy metal phytoavailability by application of immobilizing agents and soil cover in the upland soil nearby abandoned mining area and subsequent metal uptake by red pepper. Korean J. Soil. Sci. Fert. 2010, 43, 864–871. [Google Scholar]
- Liang, X.; Han, J.; Xu, Y.; Sun, Y.; Wang, L.; Tan, X. In situ field-scale remediation of Cd polluted paddy soil using sepiolite and palygorskite. Geoderma 2014, 235–236, 9–18. [Google Scholar] [CrossRef]
- Tekin, N.; Dinçer, A.; Demirbaş, O.; Alkan, M. Adsorption of cationic polyacrylamide onto sepiolite. J. Hazard Mater. 2006, 134, 211–219. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, A.; Chaudhary, P.; Khati, P. Management of plant vigor and soil health using two agriusable nanocompounds and plant growth promotory rhizobacteria in Fenugreek. 3 Biotech 2020, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Galamboš, M.; Suchánek, P.; Rosskopfová, O. Sorption of anthropogenic adionuclides on natural and synthetic inorganic sorbents. J. Radioanal. Nucl. Chem. 2012, 293, 613–633. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, H.; Lin, J.; Lin, Z.; Sun, J. Zeolite A synthesized from alkaline assisted pre-activated halloysite for efficient heavy metal removal in polluted river water and industrial wastewater. J. Environ. Sci. 2017, 56, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Levis, S.R.; Deasy, P.B. Characterisation of halloysite for use as a microtubular drug delivery system. Int. J. Pharm. 2002, 243, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Mittal, A.; Usman, M.; Mittal, J.; Yu, G.; Núñez-Delgado, A.; Kornaros, M. A review on halloysite-based adsorbents to remove pollutants in water and wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar] [CrossRef]
- Massaro, M.; Noto, R.; Riela, S. Halloysite nanotubes: Smart nanomaterials in catalysis. Catalysts 2022, 12, 149. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Minerals 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Rashad, A.M. Lightweight expanded clay aggregate as a building material—An overview. Constr. Build. Mater. 2018, 170, 757–775. [Google Scholar] [CrossRef]
- Zheng, W.; He, D.; Li, H.; Wang, F.; Yang, Y.; Zhang, J. The preparation and properties of a shell structure ceramsite. Materials 2020, 13, 1009. [Google Scholar] [CrossRef] [Green Version]
- Boudaghpour, S.; Hashemi, S. A study on light expanded clay aggregate (LECA) in a geotechnical view and its application on greenhouse and greenroof cultivation. Int. J. Geol. 2008, 4, 59–63. [Google Scholar]
- Latosińska, J.; Żygadło, M.; Czapik, P. The influence of sewage sludge content and sintering temperature on selected properties of lightweight expanded clay aggregate. Materials 2021, 14, 3363. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources; Reports No. 106; FAO: Rome, Italy, 2015; p. 192. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 18 November 2022).
- Keeling, J.L. The Mineralogy, Geology and Occurrences of Halloysite; Apple Academic Press: Oakville, ON, Canada, 2015; Available online: https://www.researchgate.net/publication/272481304_The_mineralogy_geology_and_occurrences_of_halloysite (accessed on 11 January 2023).
- Sun, Y.; Sun, G.; Xu, Y.; Liu, W.; Liang, X.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium contaminated soils. J. Environ. Manag. 2016, 166, 204–210. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Liu, L.; Ulhassan, Z.; He, Z.; Yang, X. Sepiolite clay: A review of its applications to immobilize toxic metals in contaminated soils and its implications in soil–plant system. Environ. Technol. Innov. 2021, 23, 101598. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the usefulness of sorbents in the remediation of soil exposed to the pressure of cadmium and cobalt. Materials 2022, 15, 5738. [Google Scholar] [CrossRef] [PubMed]
- Kalyvas, G.; Bilias, F.; Gasparatos, D.; Zafeiriou, I.; Eissa, R.; Karamountzou, E.; Massas, I. Enhanced As, Pb and Zn uptake by Helianthus annuus from a heavily contaminated mining soil amended with EDTA and olive mill wastewater due to increased element mobilization, as verified by sequential extraction schemes. Environments 2022, 9, 61. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/documents/card/en/c/cb4477en/ (accessed on 21 May 2022).
- Hussain, M.; Shah, S.N.A.; Naeem, M.; Farooq, S.; Jabran, K.; Alfarraj, S. Impact of different mulching treatments on weed flora and productivity of maize (Zea mays L.) and sunflower (Helianthus annuus L.). PLoS ONE 2022, 17, e0266756. [Google Scholar] [CrossRef] [PubMed]
- Iram, S.; Tariq, I.; Ahmad, K.S.; Jaffri, S.B. Helianthus annuus based biodiesel production from seed oil garnered from a phytoremediated terrain. Int. J. Ambient. Energy 2022, 43, 1763–1771. [Google Scholar] [CrossRef]
- PN-R-04032; Soil and Mineral Materials—Sampling and Determination of Particle Size Distribution. Polish Committee for Standardization: Warsaw, Poland, 1998; pp. 1–12.
- ISO 10390; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- Klute, A. Methods of Soil Analysis. Part 3. Chemical Methods (Agronomy 9); Norman, A.G., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1201–1230. [Google Scholar]
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. ISO: Geneva, Switzerland, 1995.
- Nelson, D.; Sommers, L. Total carbon, organic carbon, and organic matter. In Method of Soil Analysis: Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W. Untersuchungen über die chemische bodenanalyse als grundlage für die beurteilung des nährstoffzustandes der böden. II. Chemische extractionsmethoden zur phospor- und kaliumbestimmung. Ann. Royal Agricult. Coll. Swed. 1960, 26, 199–215. [Google Scholar]
- Schlichting, E.; Blume, H.; Stahr, K. Bodenkundliches Praktikum; Pareys studientexte 81; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1995. [Google Scholar]
- ISO 11047; Soil Quality—Determination of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc—Flame and Electrothermal Atomic Absorption Spectrometric Methods. International Organization for Standardization: Geneva, Switzerland, 1998.
- US-EPA Method 3051A. Microwave Assisted acid Digestion of Sediment, Sludges, Soils, and Oils; United States Environmental Protection Agency: Washington, DC, USA, 2007; pp. 1–30. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf (accessed on 20 December 2022).
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analysis and Evaluation of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 1–334. [Google Scholar]
- Tibco Software Inc. Statistica Version 13.3; Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA. 2021. Available online: http://statistica.io (accessed on 4 January 2023).
- Wyszkowski, M.; Brodowska, M.S. Phytoextraction with maize of soil contaminated with copper after application of mineral and organic amendments. Agronomy 2020, 10, 1597. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Zhang, W.J.; Li, W.; Lin, Z.P. The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 2009, 75, 1468–1476. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zuo, Y.B.; Wang, B.R.; Li, J.M.; Ma, Y.B. Toxicity and accumulation of copper and nickel in maize plants cropped on calcareous and acidic field soils. Plant Soil 2010, 333, 365–373. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, 13203. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valenta, A.J.M.; Durães, L. Heavy metals in Iberian soils: Removal by current adsorbents/amendments and prospective for aerogels. Adv. Colloid. Interfac. 2016, 237, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-Y.; Shao, H.-B.; Li, H.; Shao, M.-A.; Du, S. Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J. Hazard. Mater. 2009, 170, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M.; et al. Zeolites enhance soil health, crop productivity and environmental safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Huang-Ping, C.; Shu-Hao, C. Adsorption characteristics of both cationic and oxyanionic metal ions on hexadecyltrimethylammonium bromide-modified NaY zeolite. Chem. Eng. J. 2012, 193–194, 283–289. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Ahmad, I.; Abdul Qadir, A.; Murtaza, G.; Rafiq, S.; Jamal, A.; Zeeshan, N.; Murtaza, B.; Javed, W.; Radicetti, E.; et al. Zeolite-assisted immobilization and health risks of potentially toxic elements in wastewater-irrigated soil under brinjal (Solanum melongena) cultivation. Agronomy 2022, 12, 2433. [Google Scholar] [CrossRef]
- Chen, Z.S.; Lee, G.J.; Liu, J.C. The effects of chemical remediation treatments on the extractability and speciation of cadmium and lead in contaminated soils. Chemosphere 2000, 41, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; García-Romero, E. Variability of the surface properties of sepiolite. Appl. Clay Sci. 2012, 67–68, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Abad-Valle, P.; Álvarez-Ayuso, E.; Murciego, A.; Pellitero, E. Assessment of the use of sepiolite amendment to restore heavy metal polluted mine soil. Geoderma 2016, 280, 57–66. [Google Scholar] [CrossRef]
- Lin, D.S.; Liu, Y.; Xu, Y.M.; Zhou, Q.X.; Sun, G.H. Effects of sepiolite on the immobilization of cadmium and zinc in soil. Acta Sci. Nat. Univ. Pekin. 2010, 46, 346–350. [Google Scholar]
- Liang, X.F.; Xu, Y.M.; Wang, L.; Su, G.H.; Qin, X.; Sun, Y. In-situ immobilization of cadmium and lead in a contaminated agricultural fields by adding natural clays combined with phosphate fertilizer. Acta Sci. Circumstantiae 2011, 31, 1011–1018. [Google Scholar]
- Sun, Y.; Sun, G.; Xu, Y.; Wang, L.; Liang, X.; Lin, D. Assessment of sepiolite for immobilization of cadmium-contaminated soils. Geoderma 2013, 193–194, 149–155. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, D.; Xu, Y.; Wang, L.; Liang, X.; Shen, Y. Effects of sepiolite on stabilization remediation of heavy metal-contaminated soil and its ecological evaluation. Front. Environ. Sci. Eng. 2016, 10, 85–92. [Google Scholar] [CrossRef]
- Borisover, M.; Davis, J.A. Adsorption of inorganic and organic solutes by clay minerals. Dev. Clay Sci. 2015, 6, 33–70. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]

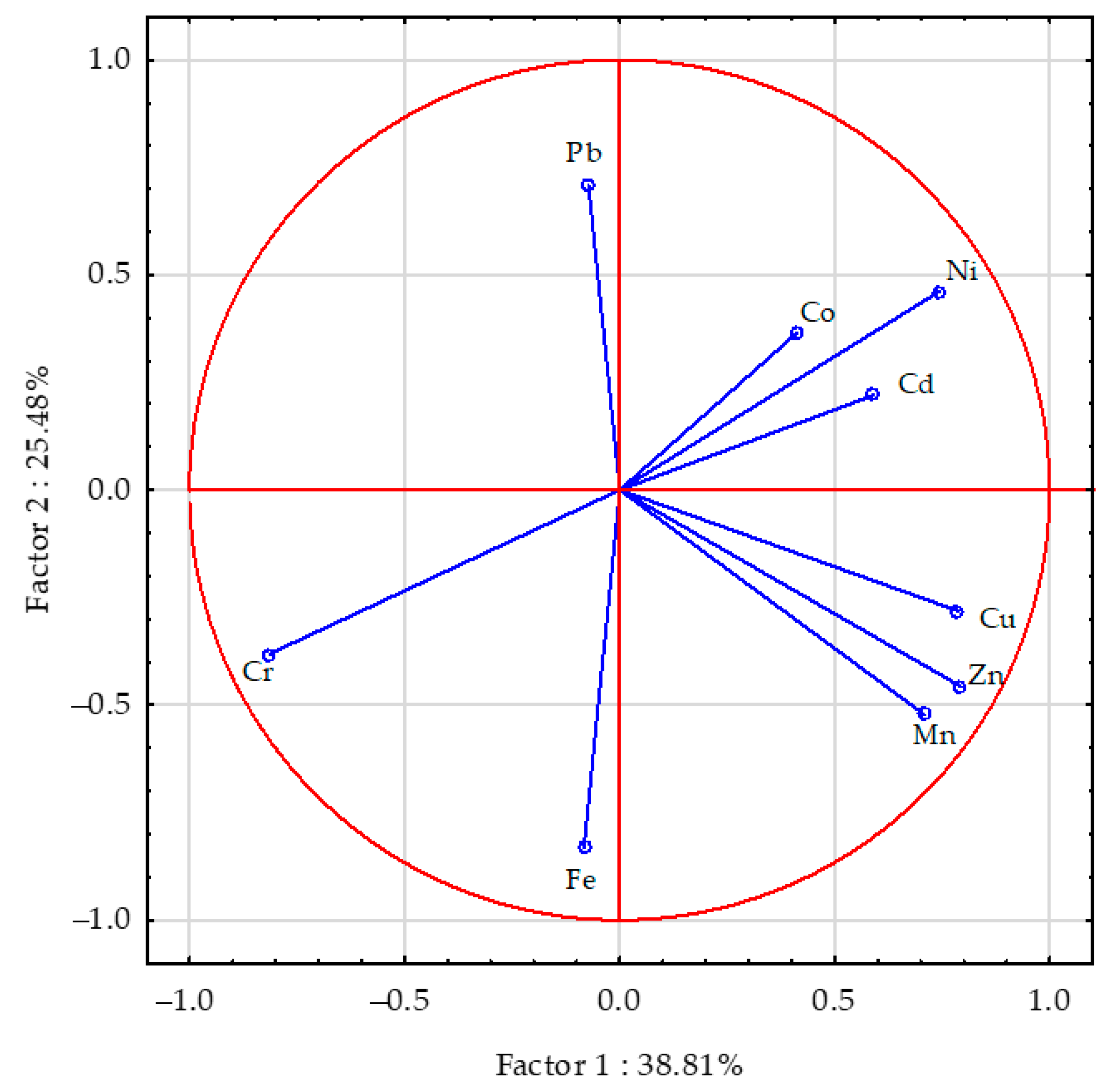

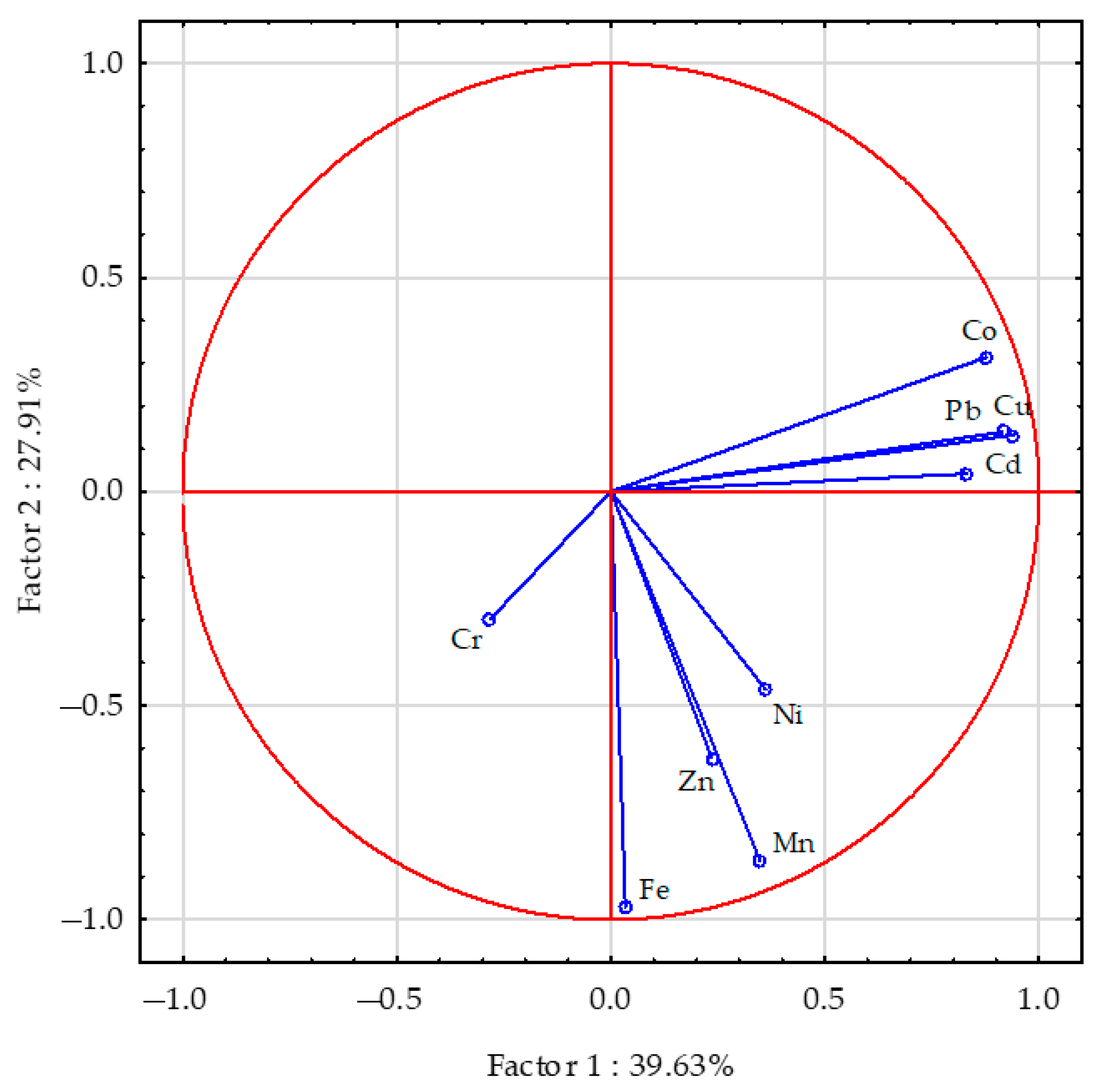
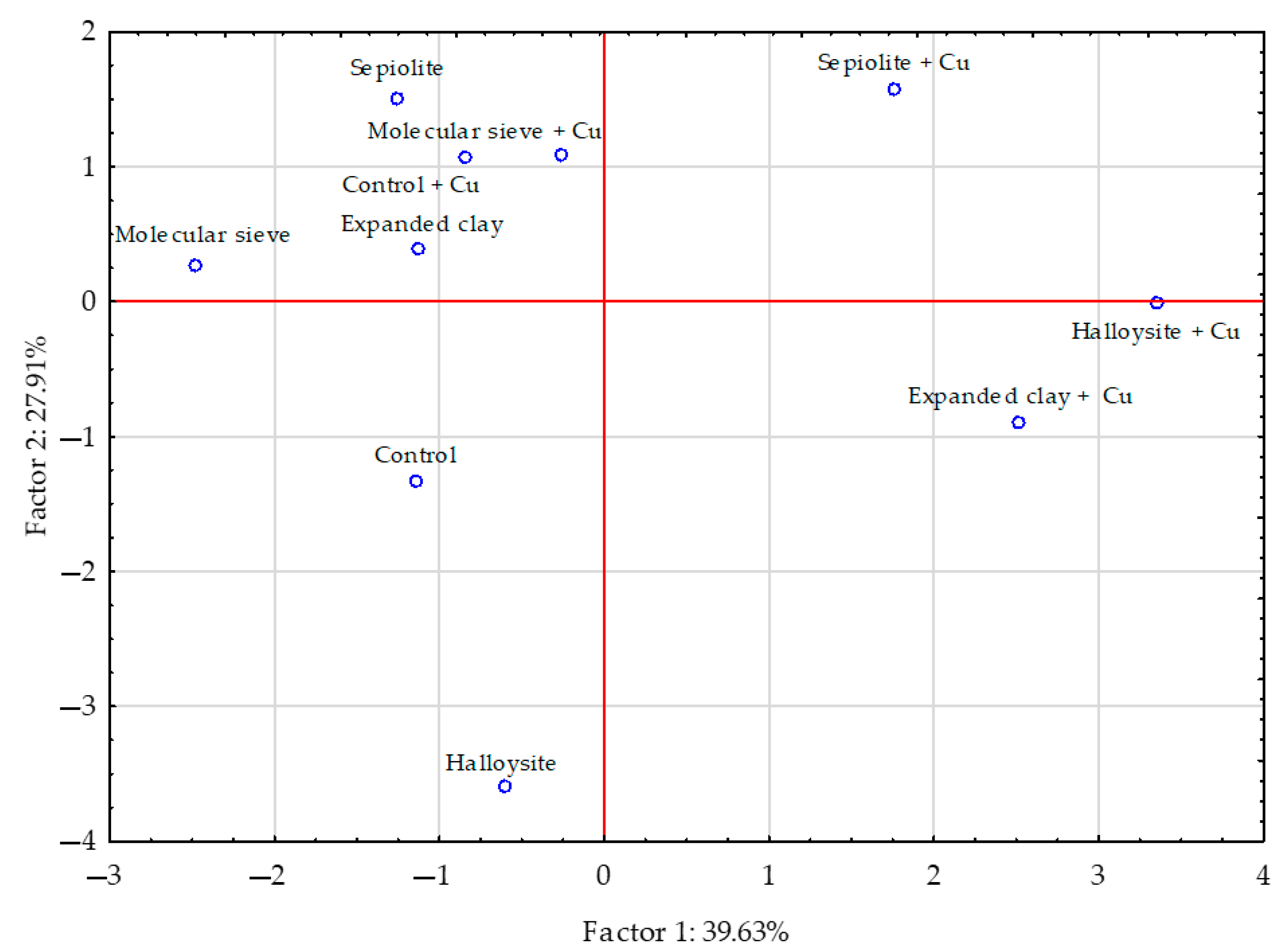
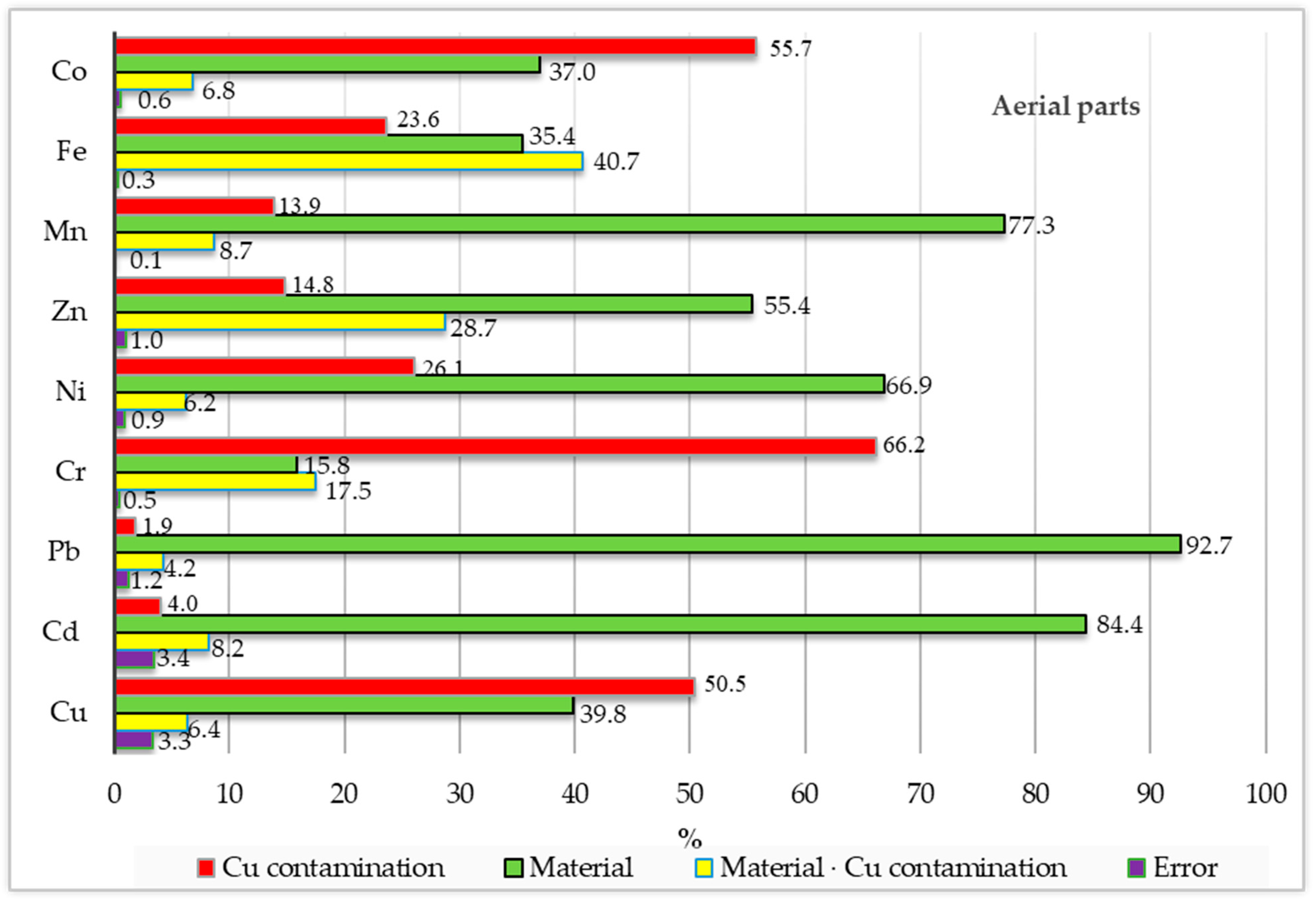
| Object | Aerial Parts | Roots | ||||
|---|---|---|---|---|---|---|
| Without Cu | With Cu | % Change with Cu/without Cu | Without Cu | With Cu | % Change with Cu/without Cu | |
| Copper content (mg kg−1 DM) | ||||||
| Control | 1.628 ± 0.017 bc | 2.234 ± 0.124 e | 37.2 | 4.388 ± 0.016 c | 10.720 ± 0.044 d | 144.3 |
| Molecular sieve | 1.486 ± 0.028 ab | 1.936 ± 0.116 d | 30.3 | 2.484 ± 0.024 a | 15.570 ± 0.078 e | 526.8 |
| Halloysite | 1.282 ± 0.010 a | 1.449 ± 0.028 ab | 13.0 | 3.611 ± 0.045 b | 27.737 ± 0.026 g | 668.1 |
| Sepiolite | 1.430 ± 0.003 ab | 1.792 ± 0.025 cd | 25.3 | 3.533 ± 0.070 b | 19.576 ± 0.062 f | 454.1 |
| Expanded clay | 1.501 ± 0.043 ab | 2.012 ± 0.076 de | 34.0 | 2.651 ± 0.074 a | 29.419 ± 0.041 h | 1009.7 |
| Average | 1.465 A | 1.885 B | 28.6 | 3.333 A | 20.604 B | 518.1 |
| Cadmium content (mg kg−1 DM) | ||||||
| Control | 0.013 ± 0.002 cd | 0.011 ± 0.001 bc | −15.4 | 0.014 ± 0.001 b | 0.006 ± 0.001 a | −57.1 |
| Molecular sieve | 0.009 ± 0.001 ab | 0.009 ± 0.001 ab | 0.0 | 0.007 ± 0.001 a | 0.007 ± 0.001 a | 0.0 |
| Halloysite | 0.006 ± 0.001 a | 0.009 ± 0.001 ab | 50.0 | 0.005 ± 0.001 a | 0.032 ± 0.001 d | 540.0 |
| Sepiolite | 0.016 ± 0.001 d | 0.022 ± 0.001 e | 37.5 | 0.006 ± 0.001 a | 0.018 ± 0.001 c | 200.0 |
| Expanded clay | 0.014 ± 0.001 cd | 0.017 ± 0.001 d | 21.4 | 0.012 ± 0.001 b | 0.017 ± 0.001 c | 41.7 |
| Average | 0.012 A | 0.014 B | 17.2 | 0.009 A | 0.016 B | 81.8 |
| Lead content (mg kg−1 DM) | ||||||
| Control | 0.174 ± 0.005 a | 0.214 ± 0.030 a | 23.0 | 0.128 ± 0.006 b | 0.346 ± 0.001 c | 170.3 |
| Molecular sieve | 0.197 ± 0.002 a | 0.211 ± 0.018 a | 7.1 | 0.099 ± 0.011 a | 0.351 ± 0.002 c | 254.5 |
| Halloysite | 0.388 ± 0.011 d | 0.477 ± 0.007 e | 22.9 | 0.408 ± 0.002 d | 0.749 ± 0.003 g | 83.6 |
| Sepiolite | 0.337 ± 0.009 c | 0.316 ± 0.004 c | −6.2 | 0.439 ± 0.008 e | 0.765 ± 0.010 g | 74.3 |
| Expanded clay | 0.261 ± 0.001 b | 0.264 ± 0.005 b | 1.1 | 0.337 ± 0.008 c | 0.697 ± 0.010 f | 106.8 |
| Average | 0.271 A | 0.296 B | 9.2 | 0.282 A | 0.582 B | 106.1 |
| Object | Aerial Parts | Roots | ||||
|---|---|---|---|---|---|---|
| Without Cu | With Cu | % Change with Cu/without Cu | Without Cu | With Cu | % Change with Cu/without Cu | |
| Chromium content (mg kg−1 DM) | ||||||
| Control | 2.271 ± 0.090 d | 1.844 ± 0.023 c | −18.8 | 1.825 ± 0.069 a | 4.670 ± 0.081 d | 155.9 |
| Molecular sieve | 3.491 ± 0.052 g | 1.435 ± 0.012 b | −58.9 | 3.913 ± 0.070 c | 3.992 ± 0.014 cd | 2.0 |
| Halloysite | 2.799 ± 0.081 e | 1.049 ± 0.001 a | −62.5 | 6.187 ± 0.064 e | 2.996 ± 0.074 b | −51.6 |
| Sepiolite | 3.118 ± 0.069 f | 0.877 ± 0.038 a | −71.9 | 2.503 ± 0.056 ab | 3.052 ± 0.028 b | 21.9 |
| Expanded clay | 1.715 ± 0.040 c | 1.027 ± 0.056 a | −40.1 | 6.292 ± 0.023 e | 3.137 ± 0.090 b | −50.1 |
| Average | 2.679 B | 1.246 A | −53.5 | 4.144 B | 3.569 A | −13.9 |
| Nickel content (mg kg−1 DM) | ||||||
| Control | 2.877 ± 0.041 b | 3.130 ± 0.024 c | 8.8 | 2.600 ± 0.063 ab | 3.035 ± 0.081 b | 16.7 |
| Molecular sieve | 2.485 ± 0.042 a | 2.878 ± 0.045 b | 15.8 | 2.377 ± 0.028 a | 2.998 ± 0.080 b | 26.1 |
| Halloysite | 2.343 ± 0.025 a | 3.290 ± 0.077 cd | 40.4 | 3.729 ± 0.054 c | 2.821 ± 0.070 ab | −24.3 |
| Sepiolite | 3.415 ± 0.020 d | 4.102 ± 0.078 f | 20.1 | 2.610 ± 0.077 ab | 2.752 ± 0.049 ab | 5.4 |
| Expanded clay | 3.380 ± 0.041 d | 3.727 ± 0.076 e | 10.3 | 3.618 ± 0.088 c | 4.463 ± 0.082 d | 23.4 |
| Average | 2.900 A | 3.425 B | 18.1 | 2.987 A | 3.214 B | 7.6 |
| Zinc content (mg kg−1 DM) | ||||||
| Control | 28.32 ± 0.26 d | 32.25 ± 0.14 e | 13.9 | 110.77 ± 1.96 g | 48.09 ± 0.31 c | −56.6 |
| Molecular sieve | 23.18 ± 0.05 a | 25.11 ± 0.36 bc | 8.3 | 38.31 ± 1.67 b | 46.18 ± 1.04 c | 20.5 |
| Halloysite | 28.22 ± 0.08 d | 24.80 ± 0.10 b | −12.1 | 71.40 ± 0.05 f | 61.51 ± 0.10 e | −13.9 |
| Sepiolite | 25.64 ± 0.30 bc | 28.53 ± 0.22 d | 11.3 | 29.18 ± 0.18 a | 56.50 ± 0.05 d | 93.6 |
| Expanded clay | 26.38 ± 0.06 c | 32.25 ± 0.28 e | 22.3 | 33.41 ± 0.47 a | 70.30 ± 0.74 f | 110.4 |
| Average | 26.35 A | 28.59 B | 8.5 | 56.61 A | 56.52 A | −0.2 |
| Object | Aerial Parts | Roots | ||||
|---|---|---|---|---|---|---|
| Without Cu | With Cu | % Change with Cu/without Cu | Without Cu | With Cu | % Change with Cu/without Cu | |
| Manganese content (mg kg−1 DM) | ||||||
| Control | 29.49 ± 0.14 f | 32.11 ± 0.43 g | 8.9 | 55.19 ± 0.61 d | 22.61 ± 0.07 a | −59.0 |
| Molecular sieve | 15.74 ± 0.06 a | 18.06 ± 0.09 b | 14.7 | 33.79 ± 0.19 c | 22.95 ± 0.73 a | −32.1 |
| Halloysite | 20.76 ± 0.09 d | 23.86 ± 0.10 e | 14.9 | 134.68 ± 0.77 g | 99.66 ± 0.67 f | −26.0 |
| Sepiolite | 16.37 ± 0.26 a | 19.29 ± 0.10 c | 17.8 | 25.88 ± 0.07 b | 21.85 ± 0.18 a | −15.6 |
| Expanded clay | 21.22 ± 0.04 d | 32.85 ± 0.07 g | 54.8 | 26.52 ± 0.08 b | 67.41 ± 0.63 e | 154.2 |
| Average | 20.72 A | 25.23 B | 21.8 | 55.21 B | 46.90 A | −15.1 |
| Iron content (mg kg−1 DM) | ||||||
| Control | 24.94 ± 0.01 f | 20.34 ± 0.37 c | −18.4 | 1911.71 ± 4.43 g | 458.62 ± 7.31 a | −76.0 |
| Molecular sieve | 18.84 ± 0.09 b | 19.31 ± 0.20 b | 2.5 | 1444.87 ± 7.60 e | 810.06 ± 7.41 cd | −43.9 |
| Halloysite | 23.50 ± 0.16 e | 17.41 ± 0.02 a | −25.9 | 3195.57 ± 6.25 h | 1437.98 ± 2.69 e | −55.0 |
| Sepiolite | 20.20 ± 0.04 c | 17.48 ± 0.07 a | −13.5 | 672.19 ± 4.03 b | 752.27 ± 6.58 c | 11.9 |
| Expanded clay | 20.06 ± 0.05 c | 21.76 ± 0.01 d | 8.5 | 827.13 ± 8.16 d | 1704.58 ± 10.39 f | 106.1 |
| Average | 21.51 B | 19.26 A | −10.5 | 1610.29 B | 1032.70 A | −35.9 |
| Cobalt content (mg kg−1 DM) | ||||||
| Control | 0.037 ± 0.001 a | 0.089 ± 0.004 e | 140.5 | 0.111 ± 0.009 a | 0.186 ± 0.002 c | 67.6 |
| Molecular sieve | 0.040 ± 0.001 ab | 0.093 ± 0.002 e | 132.5 | 0.104 ± 0.001 a | 0.215 ± 0.0052 d | 106.7 |
| Halloysite | 0.093 ± 0.002 e | 0.113 ± 0.002 f | 21.5 | 0.155 ± 0.003 b | 0.264 ± 0.012 e | 70.3 |
| Sepiolite | 0.046 ± 0.004 bc | 0.073 ± 0.002 d | 58.7 | 0.154 ± 0.002 b | 0.256 ± 0.007 e | 66.2 |
| Expanded clay | 0.050 ± 0.001 c | 0.090 ± 0.003 e | 80.0 | 0.168 ± 0.005 bc | 0.216 ± 0.003 d | 28.6 |
| Average | 0.053 A | 0.092 B | 72.2 | 0.138 A | 0.227 B | 64.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowski, M.; Wyszkowska, J.; Kordala, N.; Zaborowska, M. Molecular Sieve, Halloysite, Sepiolite and Expanded Clay as a Tool in Reducing the Content of Trace Elements in Helianthus annuus L. on Copper-Contaminated Soil. Materials 2023, 16, 1827. https://doi.org/10.3390/ma16051827
Wyszkowski M, Wyszkowska J, Kordala N, Zaborowska M. Molecular Sieve, Halloysite, Sepiolite and Expanded Clay as a Tool in Reducing the Content of Trace Elements in Helianthus annuus L. on Copper-Contaminated Soil. Materials. 2023; 16(5):1827. https://doi.org/10.3390/ma16051827
Chicago/Turabian StyleWyszkowski, Mirosław, Jadwiga Wyszkowska, Natalia Kordala, and Magdalena Zaborowska. 2023. "Molecular Sieve, Halloysite, Sepiolite and Expanded Clay as a Tool in Reducing the Content of Trace Elements in Helianthus annuus L. on Copper-Contaminated Soil" Materials 16, no. 5: 1827. https://doi.org/10.3390/ma16051827
APA StyleWyszkowski, M., Wyszkowska, J., Kordala, N., & Zaborowska, M. (2023). Molecular Sieve, Halloysite, Sepiolite and Expanded Clay as a Tool in Reducing the Content of Trace Elements in Helianthus annuus L. on Copper-Contaminated Soil. Materials, 16(5), 1827. https://doi.org/10.3390/ma16051827










