The COVID-19 Vaccination Coverage in ICU Patients with Severe COVID-19 Infection in a Country with Low Vaccination Coverage—A National Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
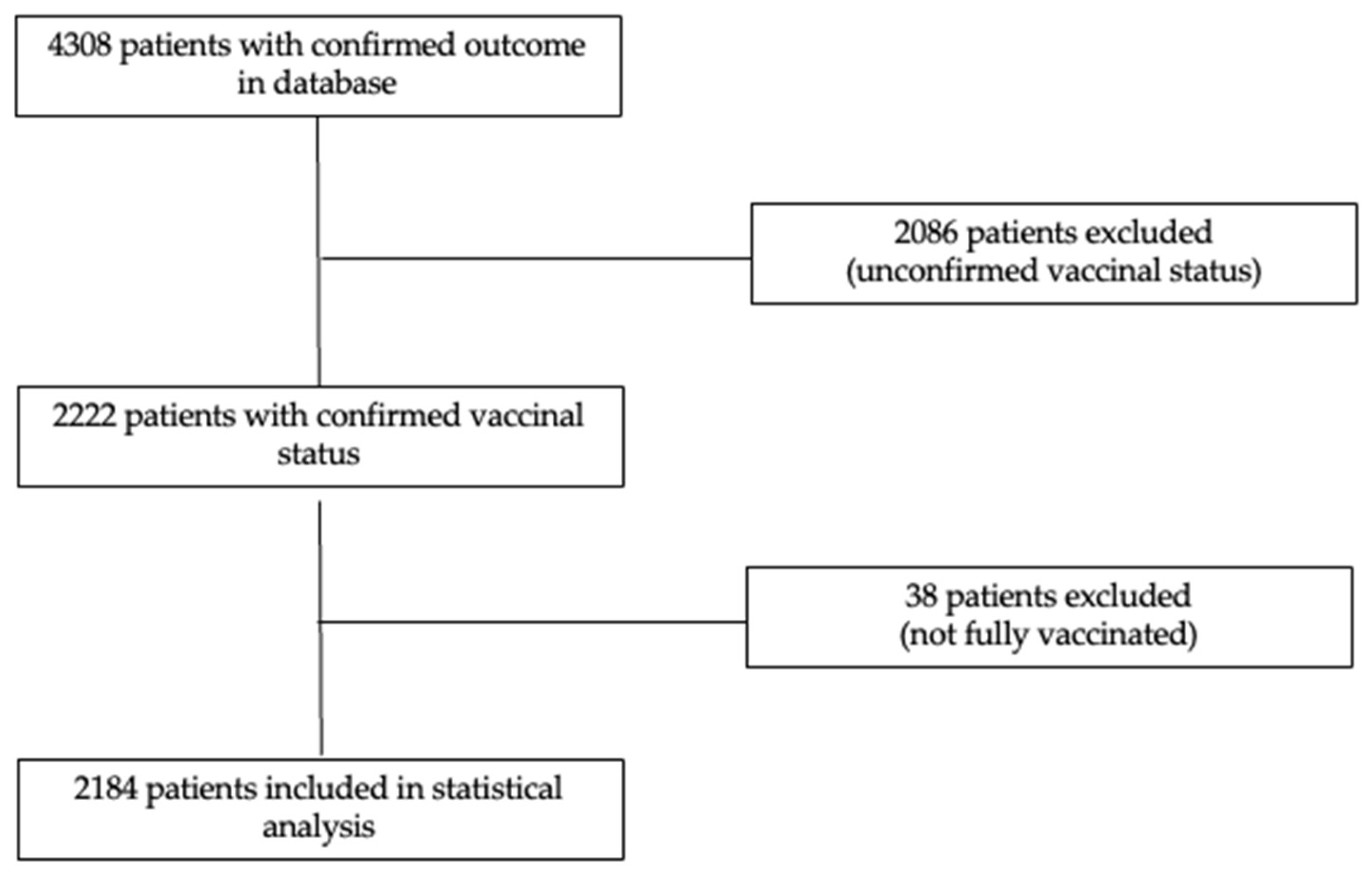
2.1. Patients, Data Collection, and Study Design
2.2. Ethics
2.3. Statistical Analysis
- (1)
- The characteristics of vaccinated and non-vaccinated patients were comparatively presented using appropriate statistical tests (Student, Mann–Whitney U, or Chi-square);
- (2)
- The association between vaccination status and ICU mortality was evaluated using a logistic regression approach. Non-redundant variables with clinical pertinence or statistical significance in univariate logistic regression analyses (p < 0.05) were introduced in the multivariate logistic model. The validity conditions for logistic regression were verified in order to have at least 10 events for each variable included in the multivariate logistic model.
3. Results
3.1. Vaccination Rate in Romanian ICUs
3.2. Characteristics of Fully Vaccinated Patients Admitted in ICU
3.3. Risk Factors for ICU Mortality
4. Discussion
4.1. Relevance of Our Results
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccinetracker.html (accessed on 4 December 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised con-trolled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sikora, D.; Rzymski, P. COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2. Vaccines 2022, 10, 437. [Google Scholar] [CrossRef]
- Grannis, S.J.; Rowley, E.A.; Ong, T.C.; Stenehjem, E.; Klein, N.P.; DeSilva, M.B.; Naleway, A.L.; Natarajan, K.; Thompson, M.G. VISION Network. Interim Estimates of COVID-19 Vaccine Effectiveness Against COVID-19-Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations Among Adults During SARS-CoV-2 B.1.617.2 (Delta) Variant Predom-inance-Nine States, June-August 2021. MMWR Morb. Mortal. Wkly Rep. 2021, 70, 1291–1293. [Google Scholar]
- Chia, P.Y.; Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Chavatte, J.M.; Mak, T.M.; Cui, L.; Kalimuddin, S.; Chia, W.N.; Tan, C.W.; et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: A multicentre cohort study. Clin Microbiol Infect. 2022, 28, 612.e1–612.e7. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. Coronavirus (COVID-19) Vaccinations. 2020. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 28 December 2021).
- Bubenek-Turconi, Ş.I.; Andrei, S.; Văleanu, L.; Ştefan, M.G.; Grigoraş, I.; Copotoiu, S.; Bodolea, C.; Tomescu, D.; Popescu, M.; Filipescu, D.; et al. Clinical characteris-tics and factors associated with ICU mortality during the first year of the SARS-CoV-2 pandemic in Romania: A prospective, cohort, multicentre study of 9000 patients. Eur. J. Anaesthesiol. 2023, 40, 4–12. [Google Scholar] [CrossRef]
- Andrei, S.; Valeanu, L.; Stefan, M.G.; Longrois, D.; Popescu, M.; Stefan, G.; Balan, C.; Arafat, R.; Corneci, D.; Droc, G.; et al. Outcomes of COVID-19 Critically Ill Extremely Elderly Patients: Analysis of a Large, National, Observational Cohort. J. Clin. Med. 2022, 11, 1544. [Google Scholar] [CrossRef]
- Popescu, M.; Ştefan, O.M.; Ştefan, M.; Văleanu, L.; Tomescu, D. ICU-Associated Costs during the Fourth Wave of the COVID-19 Pandemic in a Tertiary Hospital in a Low-Vaccinated Eastern European Country. Int. J. Environ. Res. Public Heal. 2022, 19, 1781. [Google Scholar] [CrossRef]
- Andrei, S.; Isac, S.; Jelea, D.; Martac, C.; Stefan, M.-G.; Cotorogea-Simion, M.; Buzatu, C.G.S.; Ingustu, D.; Abdulkareem, I.; Vasilescu, C.; et al. COVID-19 Pandemic Was Associated with Lower Activity but Not Higher Perioperative Mortality in a Large Eastern European Center. Experiment 2022, 28, e935809. [Google Scholar] [CrossRef]
- COVID 19 Date La Zi—Date Oficiale [Internet]. Available online: https://datelazi.ro/ (accessed on 2 December 2022).
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Raportari Vaccinare COVID. Available online: https://vaccinare-covid.gov.ro/raportari/ (accessed on 17 February 2023).
- Mărcău, F.-C.; Purec, S.; Niculescu, G. Study on the Refusal of Vaccination against COVID-19 in Romania. Vaccines 2022, 10, 261. [Google Scholar] [CrossRef]
- Vianello, A.; Guarnieri, G.; Lionello, F. Unvaccinated COVID-19 patients in the ICU: Views from both sides of the barrier. Pulmonology 2022, 28, 161–163. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043. [Google Scholar] [CrossRef]
- Thygesen, J.H.; Tomlinson, C.; Hollings, S.; Mizani, M.A.; Handy, A.; Akbari, A.; Banerjee, A.; Cooper, J.; Lai, A.G.; Li, K.; et al. Longitudinal Health and Wellbeing COVID-19 National Core Study and the CVD-COVID-UK/COVID-IMPACT Consorti-um. COVID-19 trajectories among 57 million adults in England: A cohort study using electronic health records. Lancet Digit Health 2022, 4, e542–e557. [Google Scholar] [CrossRef]
- Whittaker, R.; Bråthen Kristofferson, A.; Valcarcel Salamanca, B.; Seppälä, E.; Golestani, K.; Kvåle, R.; Watle, S.V.; Buanes, E.A. Length of hospital stay and risk of intensive care admission and in-hospital death among COVID-19 patients in Norway: A regis-ter-based cohort study comparing patients fully vaccinated with an mRNA vaccine to unvaccinated patients. Clin. Microbiol. Infect. 2022, 28, 871–878. [Google Scholar] [CrossRef]
- Sevinc, S.A.; Metin, S.; Basi, N.B.; Ling, J.; Cinar, A.S.; Oba, S. Effectiveness of inactivated SARS-CoV-2 vaccine (CoronaVac) on in-tensive care unit survival. Epidemiol. Infect. 2022, 150, e35. [Google Scholar] [CrossRef]
- Grima, A.A.; Murison, K.R.; Simmons, A.E.; Tuite, A.R.; Fisman, D.N. Relative Virulence of SARS-CoV-2 Among Vaccinated and Unvaccinated Individuals Hospitalized with SARS-CoV-2. Clin. Infect. Dis. 2022, 25, ciac412. [Google Scholar] [CrossRef]
- Modes, M.E.; Directo, M.P.; Melgar, M.; Johnson, L.R.; Yang, H.; Chaudhary, P.; Bartolini, S.; Kho, N.; Noble, P.W.; Isonaka, S.; et al. Clinical Characteristics and Outcomes Among Adults Hospitalized with Laboratory-Confirmed SARS-CoV-2 Infection During Periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) Variant Predominance—One Hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 217–223. [Google Scholar]
- Hilty, M.P.; Keiser, S.; Wendel Garcia, P.D.; Moser, A.; Schuepbach, R.A.; RISC-19-ICU Investigators for Switzerland. mRNA-based SARS-CoV-2 vaccination is associated with reduced ICU admission rate and disease severity in critically ill COVID-19 pa-tients treated in Switzerland. Intensive Care Med. 2022, 48, 362–365. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Abbott, A.; Beesoon, S.; Zuege, D.J.; Wasylak, T.; Manns, B.; Nguyen, T.X. Avoidable intensive care unit resource use and costs of unvaccinated patients with COVID-19: A historical population-based cohort study. Can. J. Anaesth. 2022, 69, 1399–1404. [Google Scholar] [CrossRef]
- Contou, D.; Fraissé, M.; Pajot, O.; Tirolien, J.A.; Mentec, H.; Plantefève, G. Comparison between first and second wave among crit-ically ill COVID-19 patients admitted to a French ICU: No prognostic improvement during the second wave? Crit. Care 2021, 25, 3. [Google Scholar] [CrossRef]
- Juthani, P.V.; Gupta, A.; A Borges, K.; Price, C.C.; I Lee, A.; Won, C.H.; Chun, H.J. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect. Dis. 2021, 21, 1485–1486. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Orenbuch-Harroch, E.; Chowers, M.; Elbaz, M.; Nesher, L.; Stein, M.; Maor, Y.; Cohen, R.; Hussein, K.; Weinberger, M.; et al. BNT162b2 vaccine breakthrough: Clinical charac-teristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin. Microbiol. Infect. 2021, 27, 1652–1657. [Google Scholar] [CrossRef]
- Motos, A.; López-Gavín, A.; Riera, J.; Ceccato, A.; Fernández-Barat, L.; Bermejo-Martin, J.F.; Ferrer, R.; de Gonzalo-Calvo, D.; Menéndez, R.; Pérez-Arnal, R.; et al. Higher frequency of comorbidities in fully vaccinated patients admitted to the ICU due to severe COVID-19: A prospective, multicentre, observational study. Eur. Respir. J. 2021, 59, 2102275. [Google Scholar] [CrossRef]
- Grapsa, E.; Adamos, G.; Andrianopoulos, I.; Tsolaki, V.; Giannakoulis, V.G.; Karavidas, N.; Giannopoulou, V.; Sarri, K.; Mizi, E.; Gavrielatou, E.; et al. Association Between Vaccination Status and Mortality Among Intubated Patients With COVID-19–Related Acute Respiratory Distress Syndrome. JAMA Netw. Open 2022, 5, e2235219–e2235219. [Google Scholar] [CrossRef]
- Seo, W.J.; Kang, J.; Kang, H.K.; Park, S.H.; Koo, H.-K.; Park, H.K.; Lee, S.-S.; Song, J.E.; Kwak, Y.G.; Kang, J. Impact of prior vaccination on clinical outcomes of patients with COVID-19. Emerg. Microbes Infect. 2022, 11, 1316–1324. [Google Scholar] [CrossRef]
- Lytras, T.; Kontopidou, F.; Lambrou, A.; Tsiodras, S. Comparative effectiveness and durability of COVID-19 vaccination against death and severe disease in an ongoing nationwide mass vaccination campaign. J. Med. Virol. 2022, 94, 5044–5050. [Google Scholar] [CrossRef]
- Malli, F.; Lampropoulos, I.C.; Papagiannis, D.; Papathanasiou, I.V.; Daniil, Z.; Gourgoulianis, K.I. Association of SARS-CoV-2 Vac-cinations with SARS-CoV-2 Infections, ICU Admissions and Deaths in Greece. Vaccines 2022, 10, 337. [Google Scholar] [CrossRef]
- Mhawish, H.; Mady, A.; Alaklobi, F.; Aletreby, W.; Asad, T.; Alodat, M.; Alharthy, A.; Abdulrahman, B.; Almahwi, S.; Memish, Z.A. Comparison of severity of immunized versus non-immunized COVID-19 patients admitted to ICU: A prospective observa-tional study. Ann. Med. Surg. 2021, 71, 102951. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, M.; Lai, C.L. COVID-19 Vaccinations: A Comprehensive Review of Their Safety and Efficacy in Special Popula-tions. Vaccines 2021, 9, 1097. [Google Scholar] [CrossRef]
- Alimohamadi, Y.; Tola, H.H.; Abbasi-Ghahramanloo, A.; Janani, M.; Sepandi, M. Case fatality rate of COVID-19: A systematic re-view and meta-analysis. J. Prev. Med. Hyg. 2021, 62, E311–E320. [Google Scholar]
- Lim, Z.J.; Subramaniam, A.; Reddy, M.P.; Blecher, G.; Kadam, U.; Afroz, A.; Billah, B.; Ashwin, S.; Kubicki, M.; Bilotta, F.; et al. Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 54–66. [Google Scholar] [CrossRef]
- Dhillon, R.A.; Qamar, M.A.; Gilani, J.A.; Irfan, O.; Waqar, U.; Sajid, M.I.; Mahmood, S.F. The mystery of COVID-19 reinfections: A global systematic review and meta-analysis. Ann. Med. Surg. 2021, 72, 103130. [Google Scholar] [CrossRef]
- Malhotra, S.; Mani, K.; Lodha, R.; Bakhshi, S.; Mathur, V.P.; Gupta, P.; Kedia, S.; Sankar, M.J.; Kumar, P.; Kumar, A.; et al. COVID-19 infection, and reinfection, and vaccine effectiveness against symptomatic infec-tion among health care workers in the setting of omicron variant transmission in New Delhi, India. Lancet Reg. Health 2022, 3, 100023. [Google Scholar]
| Variables | Non-Vaccinated (n = 2070) | Fully Vaccinated (n = 114) | p-Value |
|---|---|---|---|
| Age (years), median [IQR] | 68 [59–75] | 66 [56–72] | 0.057 |
| Male gender, n (%) | 1077 (52) | 51 (45) | 0.129 |
| ICU admission and management | |||
| Fever (yes), n (%) | 736 (36) | 34 (30) | 0.212 |
| Dyspnea (yes), n (%) | 1489 (72) | 84 (84) | 0.685 |
| ARDS (yes), n (%) | 938 (41) | 43 (38) | 0.558 |
| Invasive mechanical ventilation (yes), n (%) | 1157 (56) | 60 (53) | 0.495 |
| Non-invasive ventilation (yes), n (%) | 1277 (62) | 64 (56) | 0.236 |
| High flow nasal cannula oxygen therapy (yes), n (%) | 993 (48) | 50 (44) | 0.392 |
| GCS, median [IQR] | 15 [7–15] | 15 [12–15] | 0.010 |
| SOFA, median [IQR] | 7 [4–12] | 8 [4–12] | 0.202 |
| Corticosteroid usage, n (%) | 1788 (86) | 87 (76) | 0.003 |
| Received remdesivir (yes), n (%) | 828 (40) | 42 (37) | 0.503 |
| Associated medical conditions | |||
| Ischemic heart disease (yes), n (%) | 526 (25) | 41 (36) | 0.012 |
| Autoimmune disease (yes), n (%) | 49 (2) | 6 (5) | 0.055 |
| Dialysis patient (yes), n (%) | 16 (1) | 6 (5) | <0.001 |
| COPD (yes), n (%) | 111 (5) | 5 (4) | 0.651 |
| Past or current cancer (yes), n (%) | 136 (7) | 12 (11) | 0.102 |
| Chronic kidney disease 1 (yes), n (%) | 244 (12) | 24 (21) | 0.003 |
| Diabetes (yes), n (%) | 640 (31) | 44 (39) | 0.085 |
| Heart failure (yes), n (%) | 354 (17) | 21 (18) | 0.716 |
| Arterial hypertension (yes), n (%) | 1328 (64) | 78 (68) | 0.354 |
| Number of comorbidities, median [IQR] | 2 [1–2] | 2 [1–3] | 0.016 |
| Comorbidities, n (%) | <0.001 | ||
| - No comorbidity (yes), n (%) | 455 (22) | 25 (22) | |
| - 1 comorbidity (yes), n (%) | 564 (27) | 21 (18) | |
| - 2 comorbidities (yes), n (%) | 563 (27) | 27 (24) | |
| - 3 comorbidities (yes), n (%) | 295 (14) | 19 (17) | |
| - 4 comorbidities (yes), n (%) | 145 (7) | 9 (8) | |
| - 5 comorbidities (yes), n (%) | 39 (2) | 9 (8) | |
| - 6 comorbidities (yes), n (%) | 9 (0.4) | 4 (4) | |
| COVID-19 period, n (%) | <0.001 | ||
| - A (01–02/21) | 136 (7) | 4 (4) | 0.240 |
| - B (03–06/21) | 1169 (57) | 17 (15) | <0.001 |
| - C (07–12/21) | 752 (36) | 90 (79) | <0.001 |
| - D (01–03/22) | 13 (0.6) | 3 (3) | 0.047 |
| Outcomes | |||
| ICU Death, n (%) | 1387 (67) | 66 (58) | 0.045 |
| - No comorbidities | 206 (10) | 9 (8) | - |
| - With comorbidities | 1181 (57) | 57 (50) | - |
| ICU LOS (days), median [IQR] | 7 [4–12] | 8 [5–16] | 0.057 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Study endpoint | ||||
| Vaccinated (yes) | 0.68 (0.46–0.99) | 0.046 | 0.54 (0.31–0.93) | 0.027 |
| Patients’ baseline characteristics | ||||
| Age (year) | 1 (0.99–1) | 0.777 | ||
| Gender (male) | 1.18 (0.98–1.4) | 0.076 | ||
| COVID-19 period | ||||
| - A (01–02/21) | comparator | |||
| - B (03–06/21) | 1.02 (0.7–1.5) | 0.900 | 1.2 (0.7–2) | 0.485 |
| - C (07–12/21) | 0.90 (0.6–1.3) | 0.489 | 1.3 (0.8–2.3) | 0.262 |
| - D (01–03/22) | 0.03 (0.01–0.25) | 0.001 | 0.1 (0.01–1.6) | 0.107 |
| Comorbidities | ||||
| AHT (yes) | 2 (1.7–2.4) | <0.001 | 1.2 (0.91–1.6) | 0.205 |
| IHD (yes) | 2.1 (1.7–2.6) | <0.001 | 1.9 (1.4–2.6) | <0.001 |
| CHF (yes) | 1.8 (1.4–2.3) | <0.001 | 1 (0.7–1.5) | 0.857 |
| Diabetes (yes) | 1.7 (1.4–2) | <0.001 | 1.3 (0.99–1.7) | 0.056 |
| Autoimmunity (yes) | 1.13 (0.63–2) | 0.684 | ||
| CKD (yes) | 2.7 (1.9–3.7) | <0.001 | 2.2 (1.5–3.3) | <0.001 |
| Chronic dialysis (yes) | 2.3 (0.8–6.7) | 0.137 | ||
| COPD (yes) | 1.6 (1.05–2.5) | 0.030 | 1.6 (0.91–2.7) | 0.104 |
| Past or current neoplasia (yes) | 2.1 (1.4–3.1) | 0.001 | 1.6 (0.92–2.7) | 0.101 |
| ICU admission and evolution | ||||
| Fever (yes) | 1.1 (0.9–1.3) | 0.356 | ||
| Dyspnea (yes) | 0.96 (0.8–1.2) | 0.955 | ||
| SOFA score (each score point) | 1.08 (1.06–1.1) | <0.001 | 1.03 (1.01–1.06) | 0.003 |
| GCS (each score point) | 0.8 (0.77–0.82) | <0.001 | 0.9 (0.85–0.92) | <0.001 |
| Need for non-invasive ventilation (yes) | 2.5 (2–2.9) | <0.001 | 2 (1.6–2.7) | <0.001 |
| Need for invasive mechanical ventilation (yes) | 22 (17–28) | <0.001 | 14 (11–18) | <0.001 |
| Corticosteroid usage | 2.2 (1.8–2.8) | <0.001 | 0.9 (0.6–1.2) | 0.475 |
| Received remdesivir | 1.2 (0.98–1.4) | 0.076 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valeanu, L.; Andrei, S.; Morosanu, B.; Longrois, D.; Bubenek-Turconi, S.-I.; COVATI-RO Collaborative. The COVID-19 Vaccination Coverage in ICU Patients with Severe COVID-19 Infection in a Country with Low Vaccination Coverage—A National Retrospective Analysis. J. Clin. Med. 2023, 12, 1749. https://doi.org/10.3390/jcm12051749
Valeanu L, Andrei S, Morosanu B, Longrois D, Bubenek-Turconi S-I, COVATI-RO Collaborative. The COVID-19 Vaccination Coverage in ICU Patients with Severe COVID-19 Infection in a Country with Low Vaccination Coverage—A National Retrospective Analysis. Journal of Clinical Medicine. 2023; 12(5):1749. https://doi.org/10.3390/jcm12051749
Chicago/Turabian StyleValeanu, Liana, Stefan Andrei, Bianca Morosanu, Dan Longrois, Serban-Ion Bubenek-Turconi, and COVATI-RO Collaborative. 2023. "The COVID-19 Vaccination Coverage in ICU Patients with Severe COVID-19 Infection in a Country with Low Vaccination Coverage—A National Retrospective Analysis" Journal of Clinical Medicine 12, no. 5: 1749. https://doi.org/10.3390/jcm12051749
APA StyleValeanu, L., Andrei, S., Morosanu, B., Longrois, D., Bubenek-Turconi, S.-I., & COVATI-RO Collaborative. (2023). The COVID-19 Vaccination Coverage in ICU Patients with Severe COVID-19 Infection in a Country with Low Vaccination Coverage—A National Retrospective Analysis. Journal of Clinical Medicine, 12(5), 1749. https://doi.org/10.3390/jcm12051749






