Hot Isostatic Pressing Control of Tungsten-Based Composites
Abstract
:1. Introduction
2. Results and Discussion
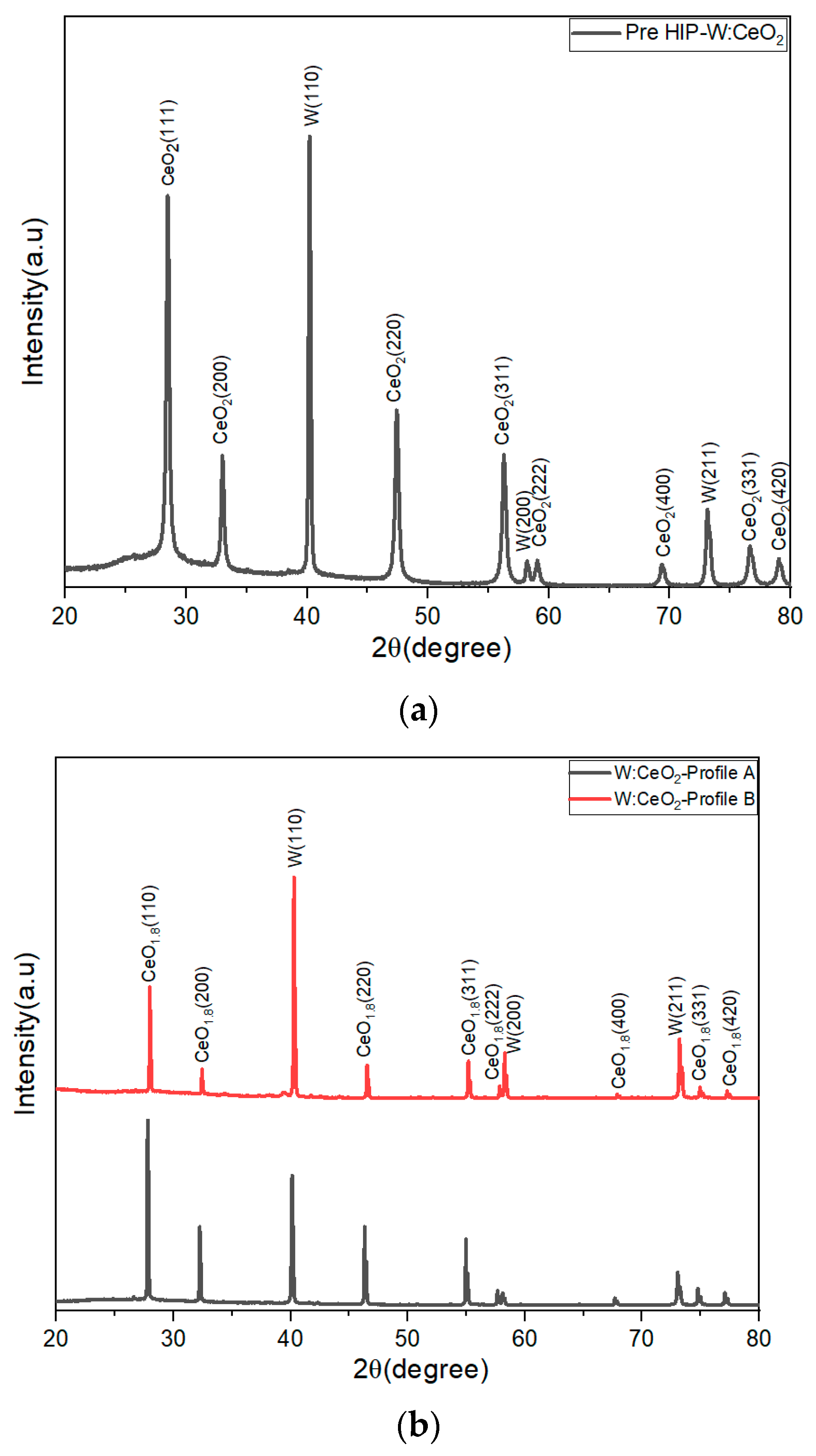
2.1. XRD
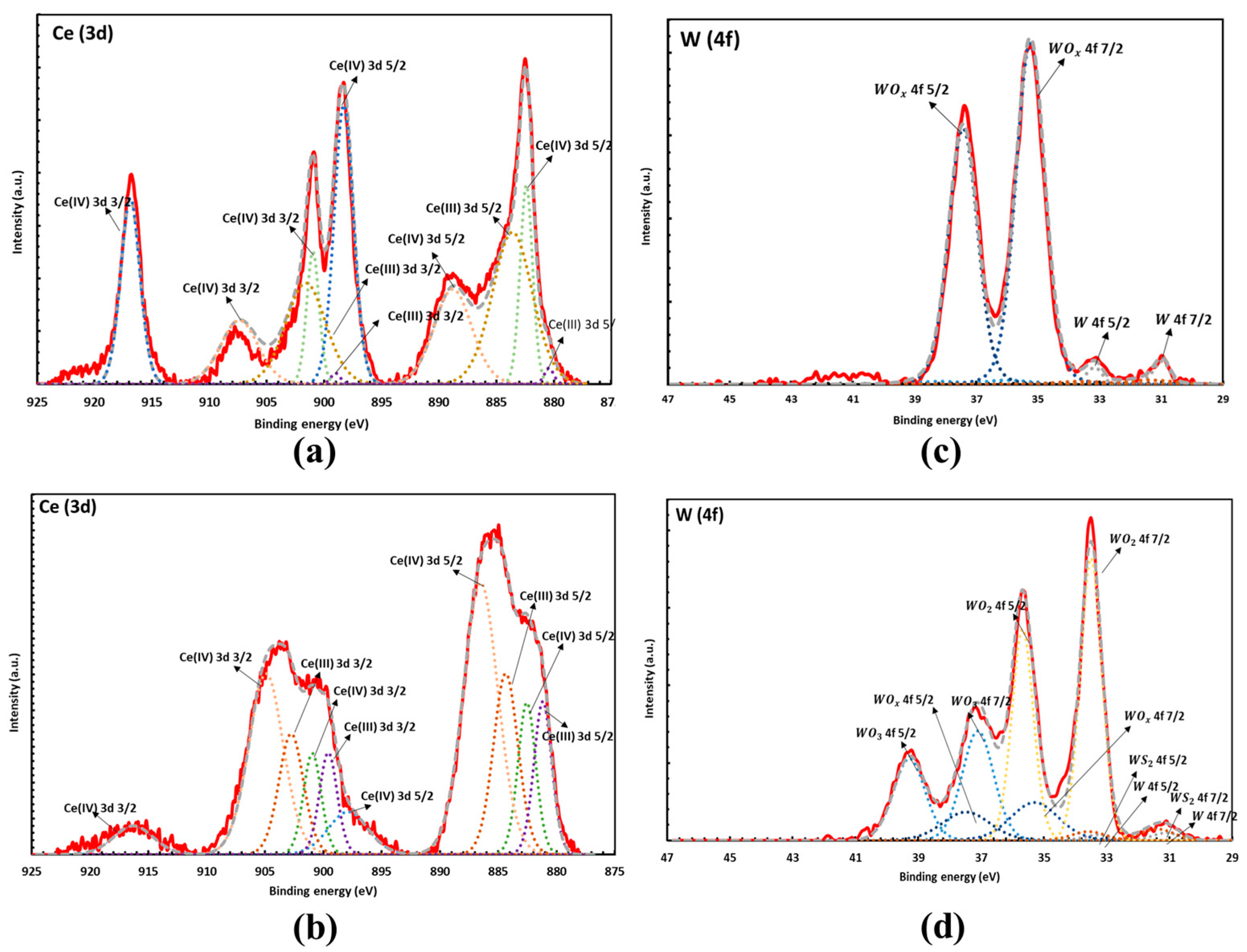
2.2. XPS
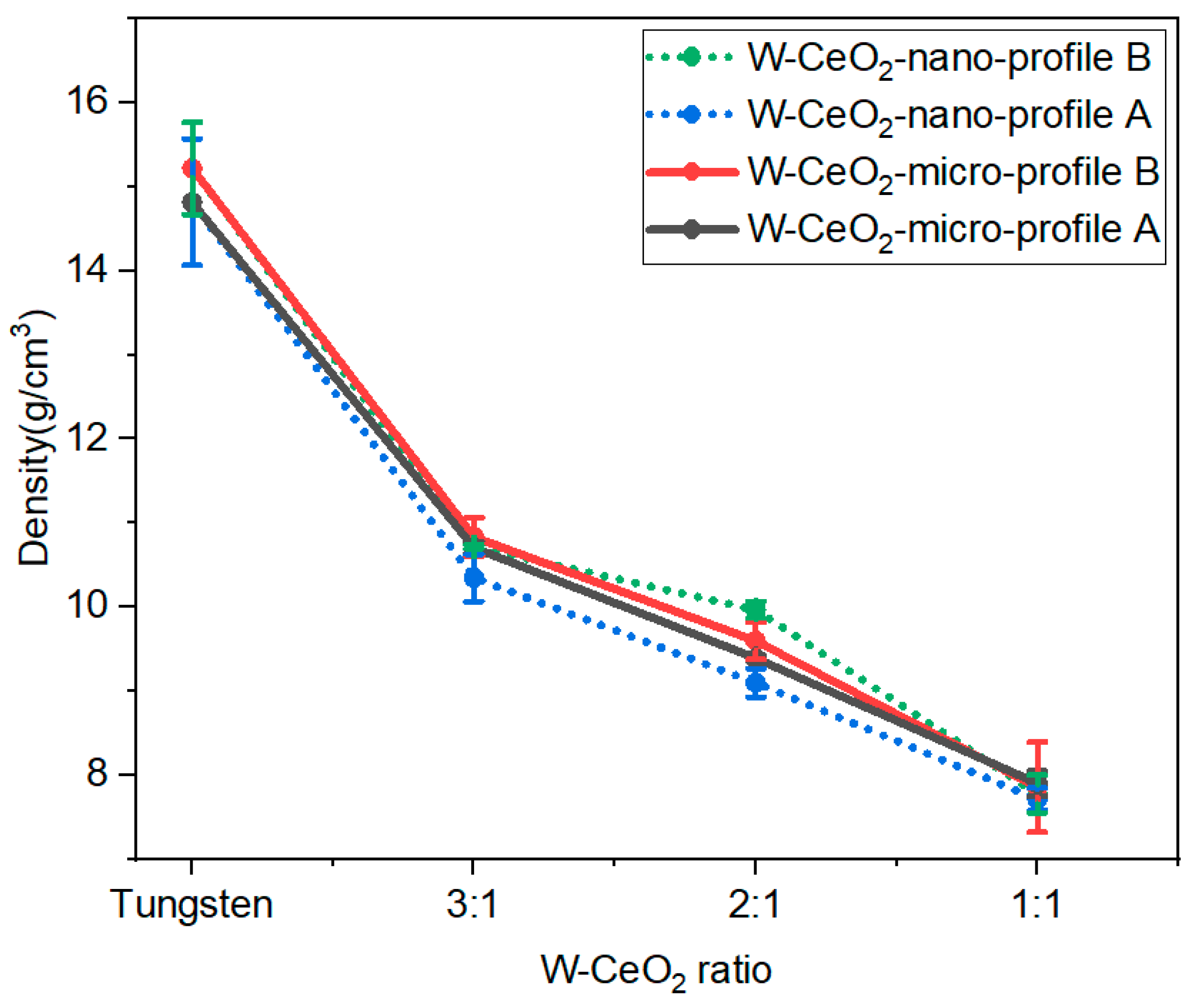
2.3. Density
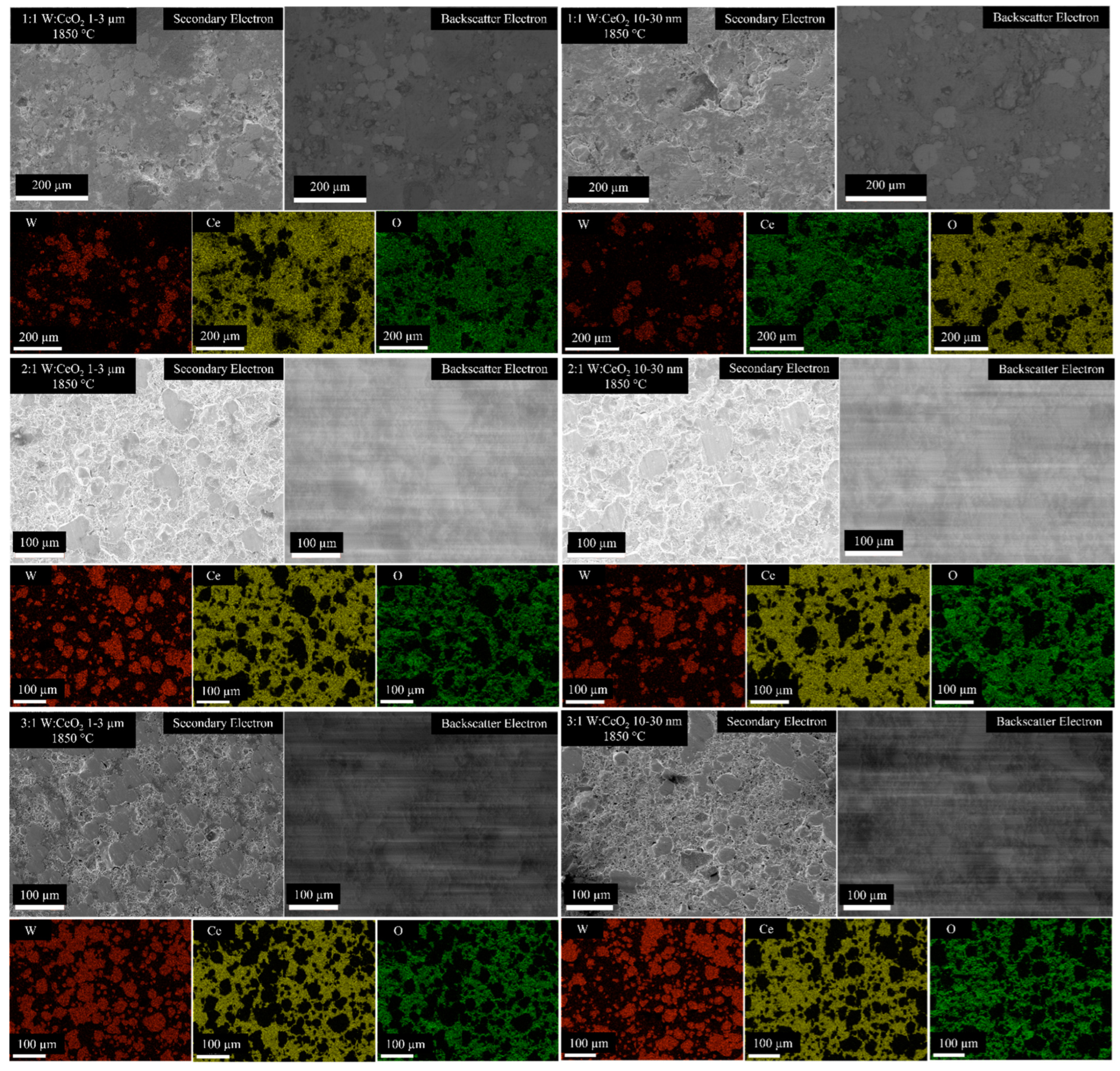
2.4. Microstructure
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. HIP Processing
4.3. Characterization Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, G.; Zhou, Y.; Wang, Y.; Lei, T. Throat materials for solid rocket motors. J. Solid Rocket. Technol. 1998, 21, 51–55. [Google Scholar]
- Lassner, E.; Schubert, W.-D. Tungsten: Properties, Chemistry, Technology of the Elements, Alloys, and Chemical Compounds; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Yi, S.; Wang, C. Tungsten: Sources, Metallurgy, Properties, and Applications; Plenum Press: New York, NY, USA, 1979. [Google Scholar]
- Maloof, S. Development of Ultra-High Temperature Tungsten-Base Composites for Rocket Nozzle Applications. In Proceedings of the 15th Annual Meeting of the American Rocket Society; 1960; pp. 1560–1573. [Google Scholar]
- Schade, P. 100 years of doped tungsten wire. Int. J. Refract. Met. Hard Mater. 2010, 28, 648–660. [Google Scholar] [CrossRef]
- Zan, X.; Gu, M.; Wang, K.; Luo, L.; Zhu, X.; Wu, Y. Recrystallization kinetics of 50% hot-rolled 2% Y2O3 dispersed tungsten. Fusion Eng. Des. 2019, 144, 1–5. [Google Scholar] [CrossRef]
- Wang, K.; Ren, D.; Zan, X.; Luo, L.; Pantleon, W.; Wu, Y. Evolution of microstructure and texture in a warm-rolled yttria dispersion-strengthened tungsten plate during annealing in the temperature range between 1200 °C and 1350 °C. J. Alloys Compd. 2021, 883, 160767. [Google Scholar] [CrossRef]
- Mabuchi, M.; Okamoto, K.; Saito, N.; Asahina, T.; Igarashi, T. Deformation behavior and strengthening mechanisms at intermediate temperatures in W-La2O3. Mater. Sci. Eng. A 1997, 237, 241–249. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, M.; Zuo, T. Morphological evolution of second-phase particles during thermomechanical processing of W-La2O3 alloy. Scr. Mater. 2000, 43, 291–297. [Google Scholar] [CrossRef]
- Rieth, M.; Dafferner, B. Limitations of W and W–1% La2O3 for use as structural materials. J. Nucl. Mater. 2005, 342, 20–25. [Google Scholar] [CrossRef]
- Song, G.; Wang, Y.; Zhou, Y. Elevated temperature ablation resistance and thermophysical properties of tungsten matrix composites reinforced with ZrC particles. J. Mater. Sci. 2001, 36, 4625–4631. [Google Scholar] [CrossRef]
- Lee, D.; Umer, M.; Ryu, H.; Hong, S. Elevated temperature ablation resistance of HfC particle-reinforced tungsten composites. Int. J. Refract. Met. Hard Mater. 2014, 43, 89–93. [Google Scholar] [CrossRef]
- El-Genk, M.; Tournier, J.-M. A review of refractory metal alloys and mechanically alloyed-oxide dispersion strengthened steels for space nuclear power systems. J. Nucl. Mater. 2005, 340, 93–112. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, Z.; Liu, Y. Accelerated sintering of high-performance oxide dispersion strengthened alloy at low temperature. Acta Mater. 2021, 220, 117309. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, R.; Fang, Q.; Zhang, T.; Jiang, Y.; Wang, X.; Liu, C. Microstructure and mechanical properties of nano-size zirconium carbide dispersion strengthened tungsten alloys fabricated by spark plasma sintering method. Plasma Sci. Technol. 2015, 17, 1066. [Google Scholar] [CrossRef] [Green Version]
- Lang, E.; Schamis, H.; Madden, N.; Smith, C.; Kolasinski, R.; Krogstad, J.; Allain, J. Recrystallization suppression through dispersion-strengthening of tungsten. J. Nucl. Mater. 2021, 545, 152613. [Google Scholar] [CrossRef]
- Swinkels, F.; Ashby, M. A second report on sintering diagrams. Acta Metall. 1981, 29, 259–281. [Google Scholar] [CrossRef]
- Johnson, D. Particle drag retardation of surface smoothing and sintering. J. Mater. Sci. 1976, 11, 2312–2318. [Google Scholar] [CrossRef]
- Kuczynski, G.; Lavendel, H. Effect of Dispersed Oxide Particles upon Sintering of Metallic Compacts; University of Notre Dame: Notre Dame, IN, USA, 1969. [Google Scholar]
- Tardiff, G. Sintering Behavior of Submicron Tungsten—Thoria Powder Blends; University of California: Livermore, CA, USA, 1969. [Google Scholar]
- Haerdtle, S.; Schmidberger, R. Tungsten and molybdenum with oxide dispersion, production and properties. In Proceedings of the 12th International Plansee Seminar’89 V. 1. Refractory Metals and Related Topics, Superconductors; 1989. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:20075165 (accessed on 1 January 2023).
- Chen, L. Dilatometric analysis of sintering of tungsten and tungsten with ceria and hafnia dispersions. Int. J. Refract. Met. Hard Mater. 1993, 12, 41–51. [Google Scholar] [CrossRef]
- Chen, L.-C.; Bewlay, B. Microstructural Evolution and Densification Kinetics During Sintering of Oxide-Dispersed Tungsten Alloys. MRS Online Proc. Libr. 1993, 322, 483–489. [Google Scholar]
- Truffault, L.; Ta, M.-T.; Devers, T.; Konstantinov, K.; Harel, V.; Simmonard, C.; Andreazza, C.; Nevirkovets, I.; Pineau, A.; Veron, O.; et al. Application of nanostructured Ca doped CeO2 for ultraviolet filtration. Mater. Res. Bull. 2010, 45, 527–535. [Google Scholar] [CrossRef]
- Carvalho, J.; Rocha, L.; Renzetti, R.; Procopio, A.; Mastelaro, V.; Simões, A.; Ponce, M.; Macchi, C.; Somoza, A.; Aldao, C.; et al. High-performance CeO2: Co nanostructures for the elimination of accidental poisoning caused by CO intoxication. Open Ceram. 2022, 12, 100298. [Google Scholar] [CrossRef]
- Burroughs, P.; Hamnett, A.; Orchard, A.; Thornton, G. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium. J. Chem. Soc. Dalton Trans. 1976, 9, 1686–1698. [Google Scholar] [CrossRef]
- Zhou, Y. The Influence of Redox Reaction of the Sintering of Cerium Oxide. J. Mater. Synth. Process. 1998, 6, 411–414. [Google Scholar] [CrossRef]
- da Fonseca, D.; Monteiro, W. The Microstructure and Properties of Copper with Ceria Nanoparticles Addition. J. Mater. Sci. Chem. Eng. 2019, 7, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Inaba, H.; Nakajima, T.; Tagawa, H. Sintering behaviors of ceria and gadolinia-doped ceria. Solid State Ion. 1998, 106, 263–268. [Google Scholar] [CrossRef]
- Umer, M.; Lee, D.; Ryu, H.; Hong, S. High temperature thermo-mechanical properties of HfC reinforced tungsten matrix composites. Compos. Res. 2015, 28, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

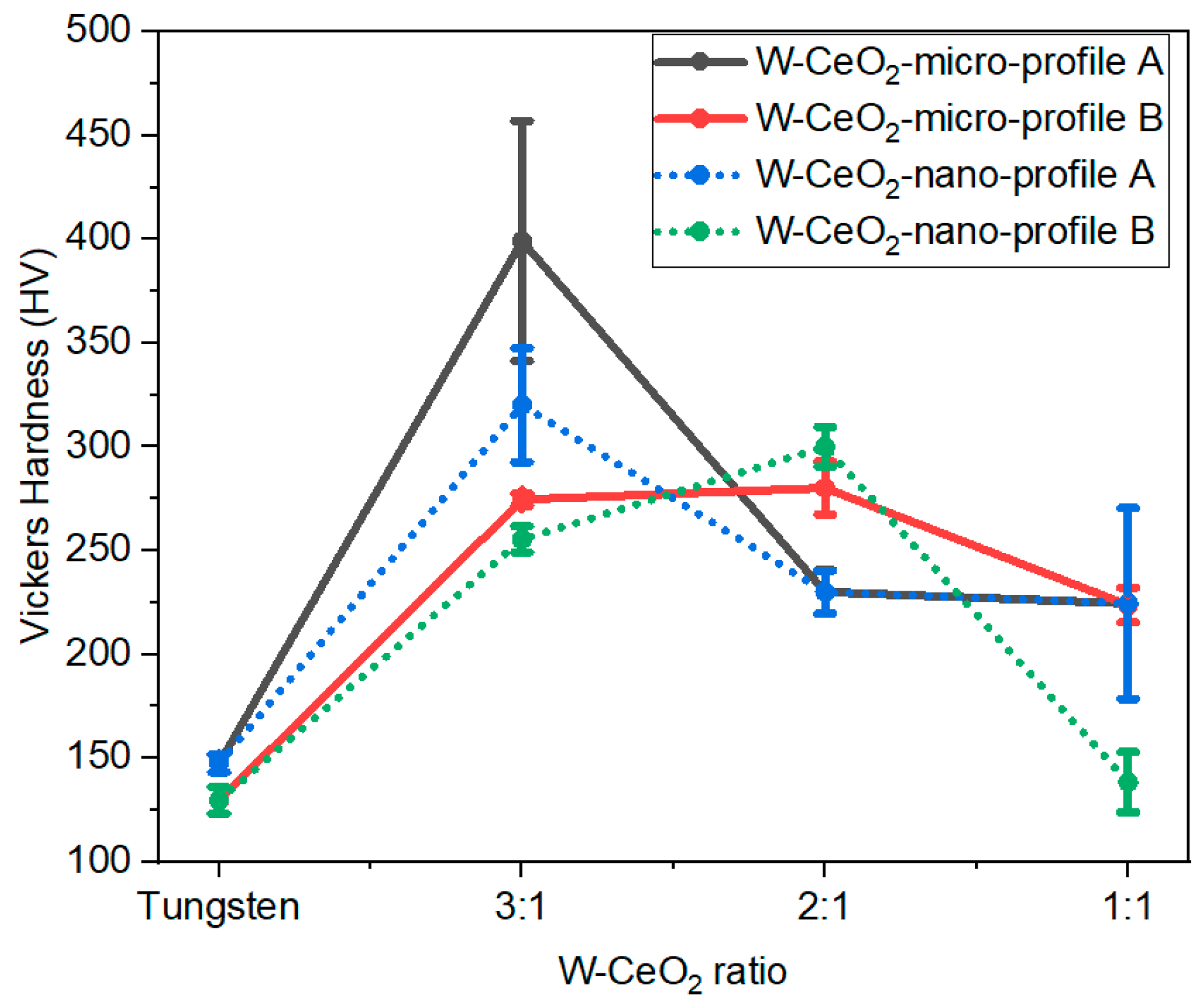
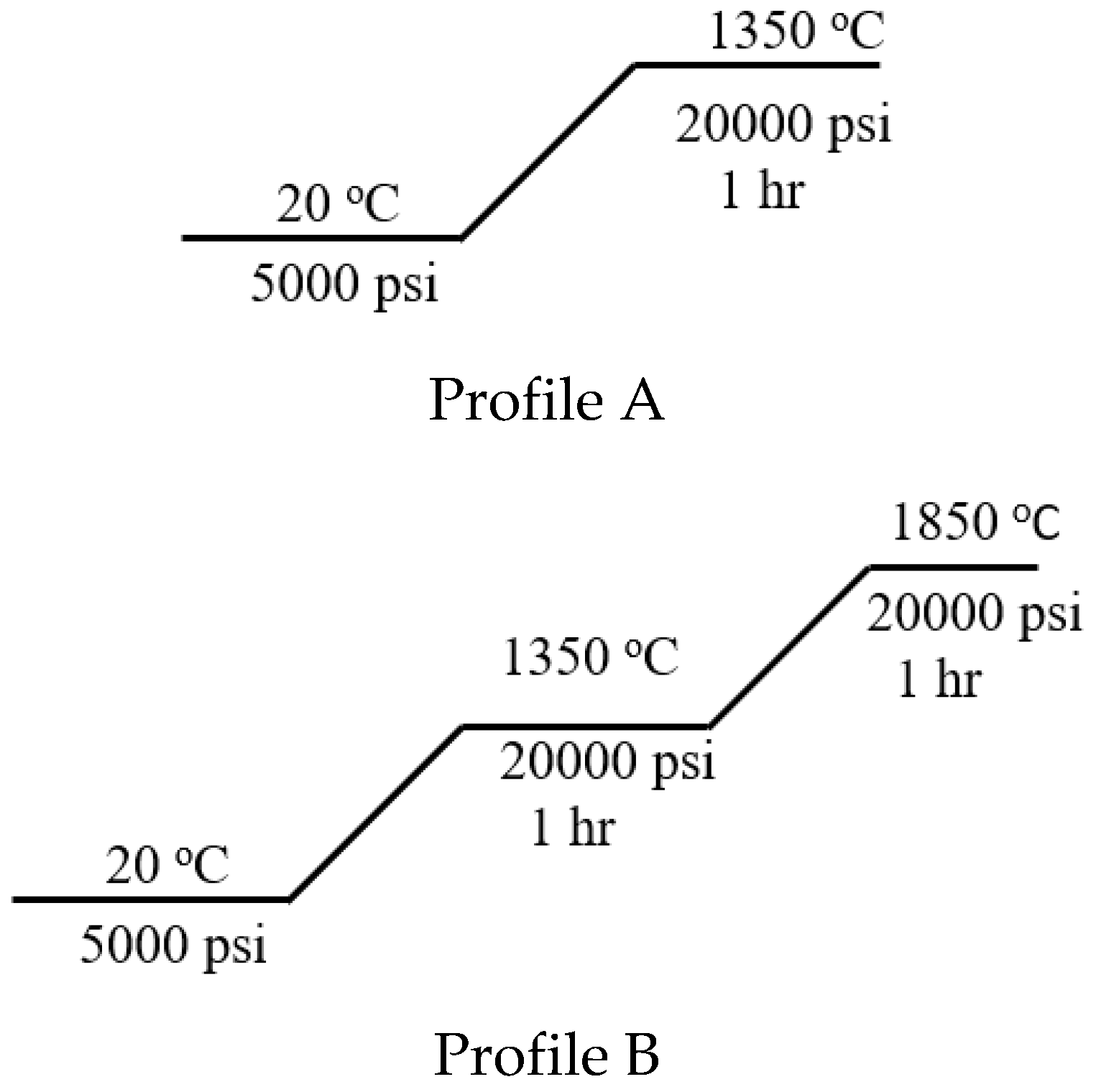
| Sample Name | Maximum HIP Temperature | CeO2 Powder Size | Density [g/cm3] | Relative Density | W Particle Size [µm] | Vickers Hardness [HV] |
|---|---|---|---|---|---|---|
| 1:1 1–3 µm 1850 °C | 1850 °C | 1–3 µm | 8.617 ± 0.5374 | 0.64 | 7.6 | 223.7 ± 8.4 |
| 1:1 10–30 nm 1350 °C | 1350 °C | 10–30 nm | 7.714 ± 0.1322 | 0.58 | 2.3 | 224.6 ± 46.0 |
| 1:1 1–3 µm 1350 °C | 1350 °C | 1–3 µm | 7.902 ± 0.1513 | 0.59 | 5.4 | 155.1 ± 17.5 |
| 1:1 10–30 nm 1850 °C | 1850 °C | 10–30 nm | 8.091 ± 0.2227 | 0.61 | 3.7 | 138.42 ± 14.4 |
| 2:1 1–3 µm 1350 °C | 1350 °C | 1–3 µm | 9.392 ± 0.0368 | 0.62 | 3.9 | 230.1 ± 10.6 |
| 2:1 1–3 µm 1850 °C | 1850 °C | 1–3 µm | 9.598 ± 0.2199 | 0.63 | 4.2 | 280.2 ± 12.8 |
| 2:1 10–30 nm 1350 °C | 1350 °C | 10–30 nm | 9.100 ± 0.1697 | 0.60 | 2.4 | 230.0 ± 10.3 |
| 2:1 10–30 nm 1850 °C | 1850 °C | 10–30 nm | 9.966 ± 0.1004 | 0.66 | 3.7 | 300.0 ± 9.5 |
| 3:1 1–3 µm 1350 °C | 1350 °C | 1–3 µm | 10.708 ± 0.0750 | 0.66 | 5.9 | 399.2 ± 57.9 |
| 3:1 1–3 µm 1850 °C | 1850 °C | 1–3 µm | 10.833 ± 0.2312 | 0.66 | 4.4 | 274.6 ± 3.0 |
| 3:1 10–30 nm 1350 °C | 1350 °C | 10–30 nm | 10.348 ± 0.2814 | 0.63 | 6.3 | 320.2 ± 27.4 |
| 3:1 10–30 nm 1850 °C | 1850 °C | 10–30 nm | 10.718 ± 0.0198 | 0.66 | 7.6 | 255.4 ± 6.2 |
| W | 1350 °C. | N/A | 14.81 ± 0.7531 | 0.76 | N/A | 147.5 ± 4.3 |
| W | 1850 °C | N/A | 15.19 ± 0.5494 | 0.79 | N/A | 129.7 ± 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoell, R.; Reyes, A.; Suman, G.; Lam, M.N.; Hamil, J.; Rosenberg, S.G.; Treadwell, L.; Hattar, K.; Lang, E. Hot Isostatic Pressing Control of Tungsten-Based Composites. Inorganics 2023, 11, 82. https://doi.org/10.3390/inorganics11020082
Schoell R, Reyes A, Suman G, Lam MN, Hamil J, Rosenberg SG, Treadwell L, Hattar K, Lang E. Hot Isostatic Pressing Control of Tungsten-Based Composites. Inorganics. 2023; 11(2):82. https://doi.org/10.3390/inorganics11020082
Chicago/Turabian StyleSchoell, Ryan, Aspen Reyes, Guddi Suman, Mila Nhu Lam, Justin Hamil, Samantha G. Rosenberg, LaRico Treadwell, Khalid Hattar, and Eric Lang. 2023. "Hot Isostatic Pressing Control of Tungsten-Based Composites" Inorganics 11, no. 2: 82. https://doi.org/10.3390/inorganics11020082
APA StyleSchoell, R., Reyes, A., Suman, G., Lam, M. N., Hamil, J., Rosenberg, S. G., Treadwell, L., Hattar, K., & Lang, E. (2023). Hot Isostatic Pressing Control of Tungsten-Based Composites. Inorganics, 11(2), 82. https://doi.org/10.3390/inorganics11020082






