Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture
Abstract
1. Introduction
2. Phosphorus and Phosphate Fertilizers
3. Phosphate-Solubilizing Microorganisms
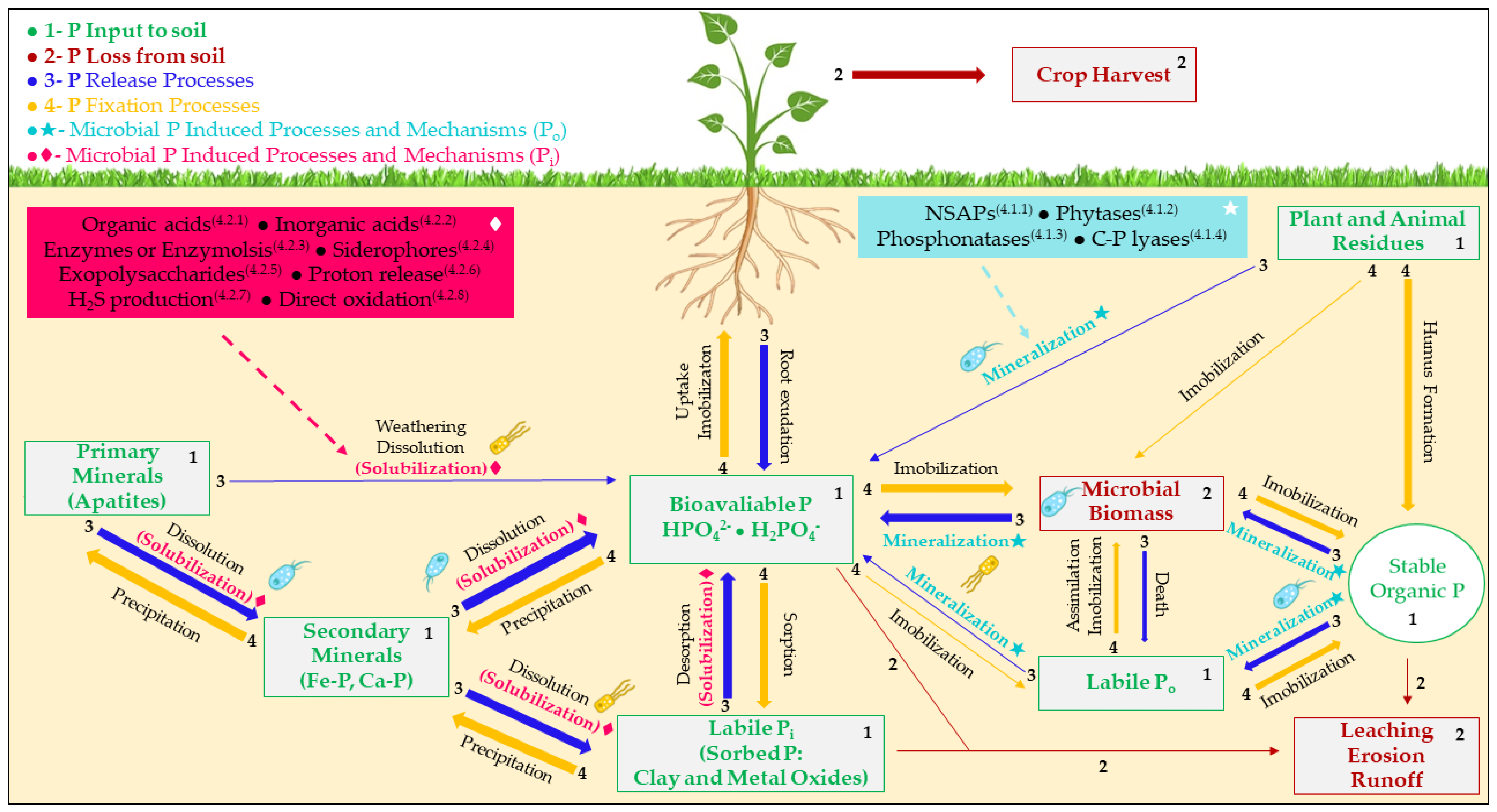
4. Phosphate Solubilization Mechanisms
4.1. Organic Phosphate
4.1.1. Non-Specific Acid Phosphatases (NSAPs)
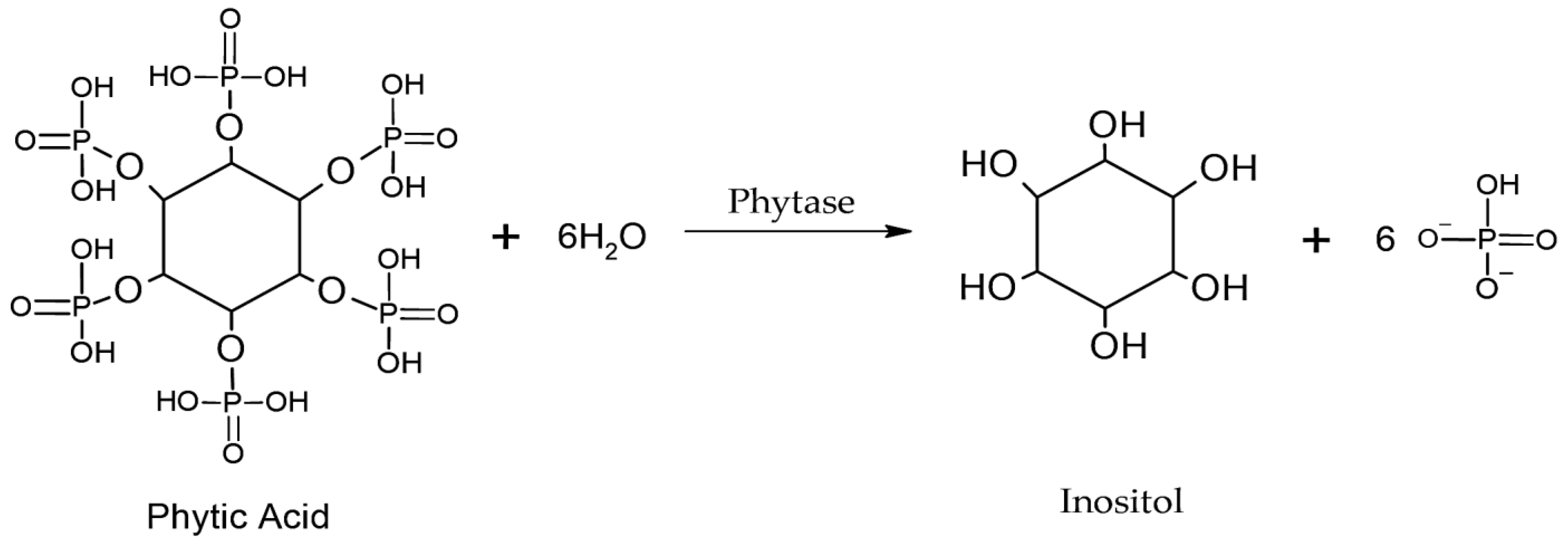
4.1.2. Phytases
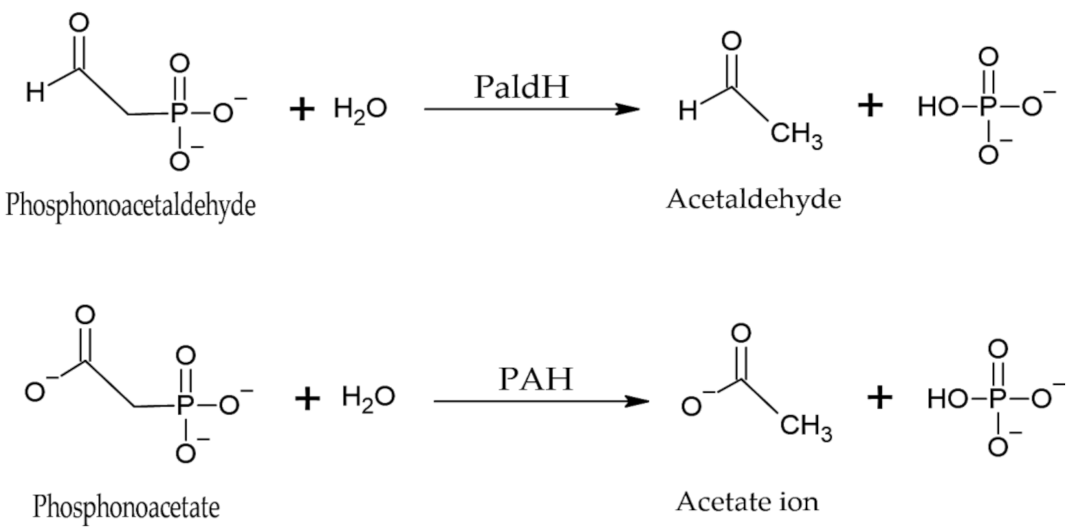
4.1.3. Phosphonatases
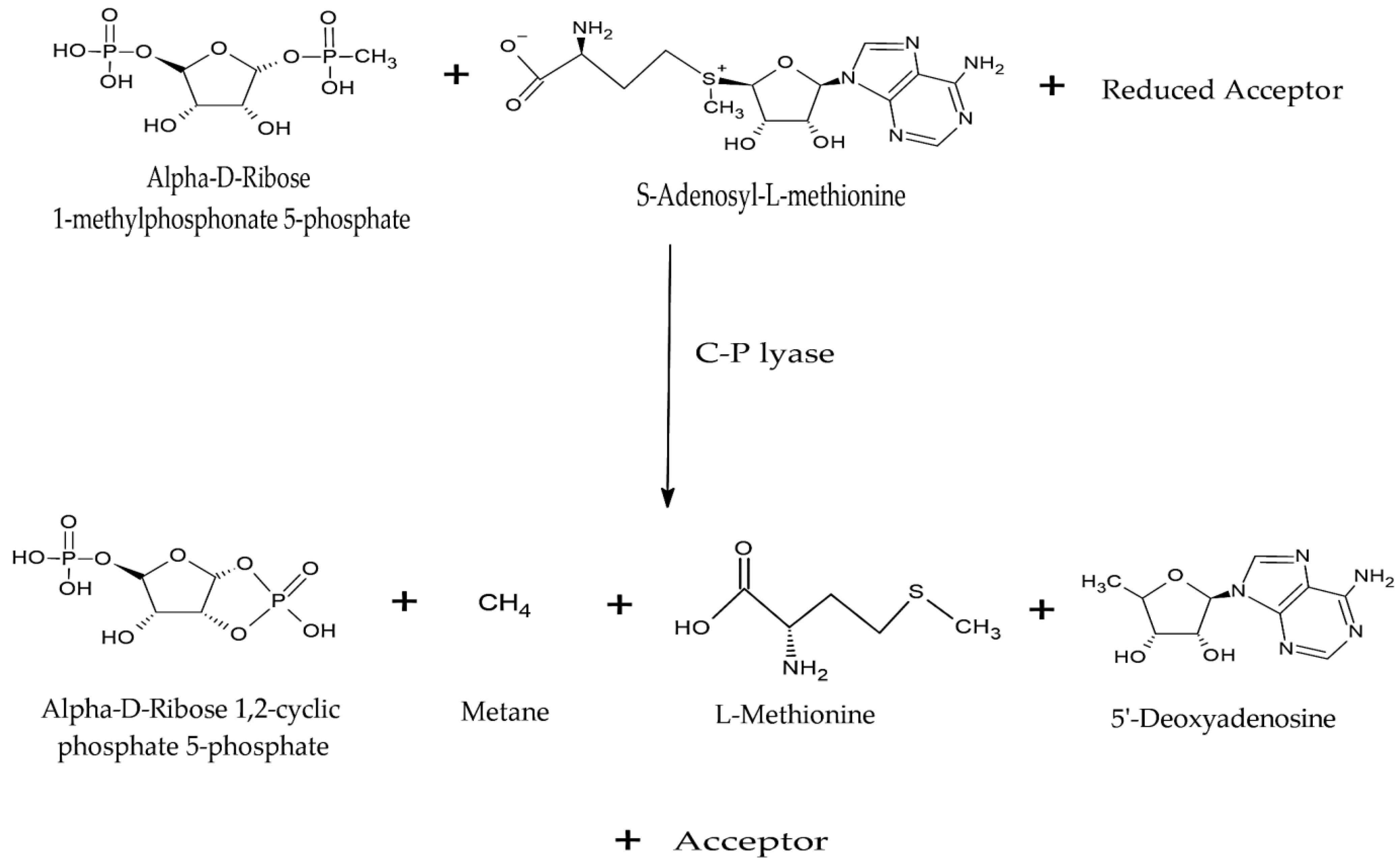
4.1.4. Carbon–Phosphorus Lyases
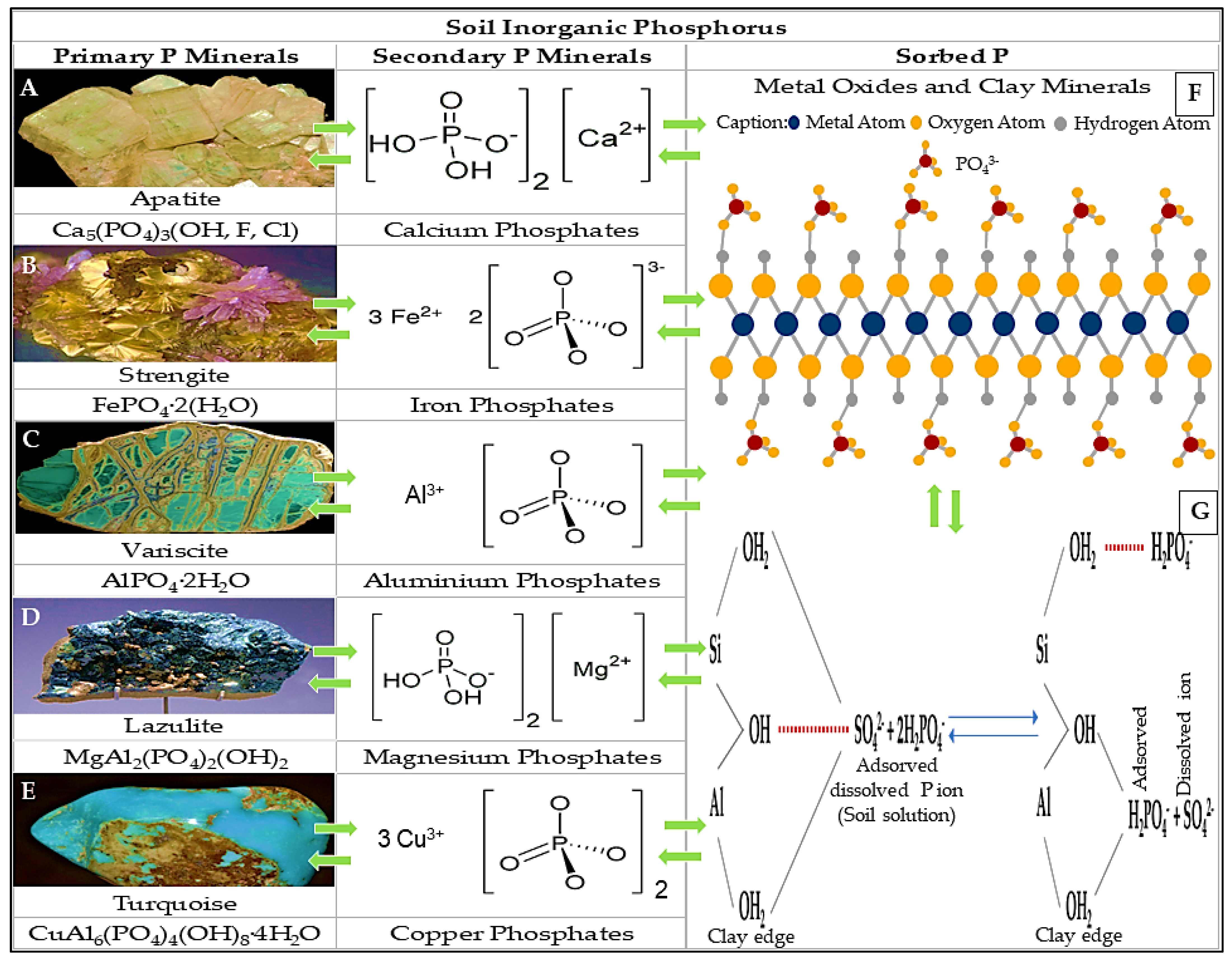
4.2. Inorganic Phosphate
4.2.1. Organic Acids
4.2.2. Inorganic Acids
4.2.3. Enzymes or Enzymolysis
4.2.4. Siderophores
4.2.5. Exopolysaccharides
4.2.6. Proton Release
4.2.7. H2S Production
4.2.8. Direct Oxidation of Glucose
5. Applications of Phosphate-Solubilizing Microorganisms as Plant Growth Promoters
6. Market and Agricultural Practices with Phosphate-Solubilizing Microorganisms
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kavanagh, P.H.; Vilela, B.; Haynie, H.J. Hindcasting global population densities reveal forces enabling the origin of agriculture. Nat. Hum. Behav. 2018, 2, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Ramankutty, N.; Evan, A.T.; Monfreda, C.; Foley, J.A. Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Glob. Biogeochem. Cycles 2008, 22, GB1003. [Google Scholar] [CrossRef]
- Ramankutty, N.; Mehrabi, Z.; Waha, K.; Jarvis, L.; Kremen, C.; Herrero, M.; Rieserberg, L. Trends in Global Agricultural Land Use: Implications for Environmental Health and Food Security. Annu. Rev. Plant Biol. 2018, 69, 789–815. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Hazafa, A.; Ashraf, I.; Alamri, S.; Siddiqui, M.H.; Ramzan, A.; Qamar, N.; Sher, F.; Naeem, M. Comparative Effect of Inoculation of Phosphorus-Solubilizing Bacteria and Phosphorus as Sustainable Fertilizer on Yield and Quality of Mung Bean (Vigna radiata L.). Plants 2021, 10, 2079. [Google Scholar] [CrossRef] [PubMed]
- Dokwal, D.; Romsdahl, T.B.; Kunz, D.A.; Alonso, A.P.; Dickstein, R. Phosphorus deprivation affects composition and spatial distribution of membrane lipids in legume nodules. Plant Physiol. 2021, 185, 1847–1859. [Google Scholar] [CrossRef]
- Elser, J.; Bennett, E. Phosphorus cycle: A broken biogeochemical cycle. Nature 2011, 478, 29–31. [Google Scholar] [CrossRef]
- Silva, F.B.V.; Nascimento, C.W.A.; Alvarez, A.M.; Araújo, P.R.M. Inputs of rare earth elements in Brazilian agricultural soils via P-containing fertilizers and soil correctives. J. Environ. Manag. 2019, 232, 90–96. [Google Scholar] [CrossRef]
- Blanco-Vargas, A.; Rodríguez-Gacha, L.M.; Sánchez-Castro, N.; Garzón-Jaramillo, R.; Pedroza-Camacho, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M. Phosphate-solubilizing Pseudomonas sp., and Serratia sp., co-culture for Allium cepa L. growth promotion. Heliyon 2020, 6, e05218. [Google Scholar] [CrossRef]
- Ajar, N.Y.; Priyanka, V.; Bhanumati, S.; Vinay, S.C.; Archna, S.; Anil, K.S. Plant Growth Promoting Bacteria: Biodiversity and Multifunctional Attributes for Sustainable Agriculture. Adv. Biotechnol. Microbiol. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Cardoso, I.M.; Kuyper, T. Mycorrhizas and tropical soil fertility. Agric. Ecosyst. Environ. 2006, 116, 72–84. [Google Scholar] [CrossRef]
- Doilom, M.; Guo, J.W.; Phookamsak, R.; Mortimer, P.E.; Karunarathna, S.C.; Dong, W.; Liao, C.F.; Yan, K.; Pem, D.; Suwannarach, N.; et al. Screening of Phosphate-Solubilizing Fungi From Air and Soil in Yunnan, China: Four Novel Species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Din, M.; Nelofer, R.; Salman, M.; Abdullah; Khan, F.H.; Khan, A.; Khan, M. Production of nitrogen fixing Azotobacter (SR-4) and phosphorus solubilizing Aspergillus niger and their evaluation on Lagenaria siceraria and Abelmoschus esculentus. Biotechnol. Rep. 2019, 22, e00323. [Google Scholar] [CrossRef]
- Wang, K.; Hou, J.; Zhang, S.; Hu, W.; Yi, G.; Chen, W.; Cheng, L.; Zhang, Q. Preparation of a new biochar-based microbial fertilizer: Nutrient release patterns and synergistic mechanisms to improve soil fertility. Sci. Total Environ. 2023, 860, 160478. [Google Scholar] [CrossRef]
- Day, A.D.; Ludeke, K.L. Plant Nutrients in Desert Environments. Adaptations of Desert Organisms; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. Plant Nutrients and Abiotic Stress Tolerance. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Bisson, C.; Adams, N.B.P.; Stevenson, B.; Brindley, A.A.; Polyviou, D.; Bibby, T.S.; Baker, P.J.; Hunter, C.N.; Hitchcock, A. The molecular basis of phosphite and hypophosphite recognition by ABC-transporters. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Xing, D.; Wu, Y. Effect of phosphorus deficiency on photosynthetic inorganic carbon assimilation of three climber plant species. Bot. Stud. 2014, 55, 60. [Google Scholar] [CrossRef]
- Tiziani, R.; Pii, Y.; Celletti, S.; Cesco, S.; Mimmo, T. Phosphorus deficiency changes carbon isotope fractionation and triggers exudate reacquisition in tomato plants. Sci. Rep. 2020, 10, 15970. [Google Scholar] [CrossRef]
- Meng, X.; Chen, W.W.; Wang, Y.Y.; Huang, Z.R.; Ye, X.; Chen, L.S. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef] [PubMed]
- Silva, U.C.; Cuadros-Orellana, S.; Silva, D.R.C.; Freitas-Júnior, L.F.; Fernandes, A.C.; Leite, L.R.; Oliveira, C.A.; dos Santos, V.L. Genomic and Phenotypic Insights Into the Potential of Rock Phosphate Solubilizing Bacteria to Promote Millet Growth in vivo. Front. Microbiol. 2021, 11, 574550. [Google Scholar] [CrossRef] [PubMed]
- McLaren, T.I.; Smernik, R.J.; McLaughlin, M.J.; Doolette, A.L.; Richardson, A.E.; Frossard, E. The chemical nature of soil organic phosphorus: A critical review and global compilation of quantitative data. Adv. Agron. 2019, 160, 51–124. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Binkley, D.; Doxtader, K.G. A new method for estimating gross phosphorus mineralization and immobilization rates in soils. Plant Soil 1992, 147, 243–250. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Guan, G.; Chen, S. Phosphate solubilizing bacteria stimulate wheat rhizosphere and endosphere biological nitrogen fixation by improving phosphorus content. PeerJ 2020, 4, e9062. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.S.; de Sousa, D.M.G.; Goedert, W.J.; de Oliveira, L.E.Z.; Pavinato, P.S.; Pinheiro, T.D. Distribution of Soil Phosphorus Fractions as a Function of Long-Term Soil Tillage and Phosphate Fertilization Management. Front. Earth Sci. 2020, 8, 350. [Google Scholar] [CrossRef]
- Sims, J.T.; Pierzynski, G.M. Chemistry of phosphorus in soil. In Chemical Processes in Soil; Tabatabai, M.A., Sparks, D.L., Eds.; SSSA: Madison, WI, USA, 2005; Volume 8, pp. 151–192. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef]
- Novais, R.F.; Smyth, T.J. Fósforo em Solo e Planta em Condições Tropicais; Universidade Federal de Viçosa: Viçosa, MG, Brazil, 1999. [Google Scholar]
- Paul, E.A. Soil Microbiology, Ecology, and Biochemistry, 3rd ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 389–432. [Google Scholar] [CrossRef]
- Devau, N.; Le Cadre, E.; Hinsinger, P.; Jaillard, B.; Gérard, F. Soil pH controls the environmental availability of phosphorus: Experimental and mechanistic modelling approaches. Appl. Geochem. 2009, 24, 2163–2174. [Google Scholar] [CrossRef]
- Foth, H.D. Fundamentals of Soil Science, 8th ed.; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Samreen, S.; Kausar, S. Phosphorus Fertilizer: The Original and Commercial Sources. In Phosphorus Recovery and Recycling; Zhang, T., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Bhattacharya, A. Changing Environmental Condition and Phosphorus-Use Efficiency in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 241–305. [Google Scholar] [CrossRef]
- Alabama Extension. Phosphorus Basics: Understanding Pathways of Soil Phosphorus Loss. Available online: https://www.aces.edu/blog/topics/crop-production/understanding-soil-phosphorus-loss/#:~:text=Water%20is%20the%20primary%20driver,soil%20is%20dissolved%20in%20water (accessed on 28 November 2022).
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 87, 85–91. [Google Scholar] [CrossRef]
- Etesami, H. Enhanced Phosphorus Fertilizer Use Efficiency with Microorganisms. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020; pp. 215–245. [Google Scholar] [CrossRef]
- Yu, X.; Keitel, C.; Dijkstra, F.A. Global analysis of phosphorus fertilizer use efficiency in cereal crops. Glob. Food Sec. 2021, 29, 100545. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Chen, X.; Yan, X.; Wang, M.; Cai, Y.; Weng, X.; Su, D. Long-term Excessive Phosphorus Fertilization Alters Soil Phosphorus Fractions in the Acidic Soil of Pomelo Orchards. Soil. Tillage Res. 2022, 215, 105214. [Google Scholar] [CrossRef]
- Beauregard, M.S.; Hamel, C.; Atul-Nayyar; St-Arnaud, M. Long-Term Phosphorus Fertilization Impacts Soil Fungal and Bacterial Diversity but not AM Fungal Community in Alfalfa. Microb. Ecol. 2010, 59, 379–389. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Phsophate Rock. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-phosphate.pdf (accessed on 24 January 2023).
- Abdelgalil, S.A.; Kaddah, M.M.Y.; Duab, M.E.A. A sustainable and effective bioprocessing approach for improvement of acid phosphatase production and rock phosphate solubilization by Bacillus haynesii strain ACP1. Sci. Rep. 2022, 12. [Google Scholar] [CrossRef]
- International Fertilizer Association. Fertilizer Consumption—Historical Trends by Country or Region. Available online: https://www.ifastat.org/databases/graph/1_1 (accessed on 24 January 2023).
- Roy-Bolduc, A.; Hijri, M. The Use of Mycorrhizae to Enhance Phosphorus Uptake: A Way Out the Phosphorus Crisis. J. Biofertil. Biopestic. 2011, 2, 1000104. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Bouwman, A.F.; Giller, K.E.; van Ittersum, M.K. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. USA 2012, 109, 6348–6353. [Google Scholar] [CrossRef] [PubMed]
- Daneshgarm, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Wijaya, B.A.; Hidayat, W.; Riniarti, M.; Prasetia, H.; Niswati, A.; Hasanudin, U.; Banuwa, I.S.; Kim, S.; Lee, S.; Yoo, J. Meranti (Shorea sp.) Biochar Application Method on the Growth of Sengon (Falcataria moluccana) as a Solution of Phosphorus Crisis. Energies 2022, 15, 2110. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Q.; Li, J.; Poon, C.S.; Cheeseman, C.R.; Donatello, S.; Tsang, D.C.W. Feasibility of wet extraction of phosphorus from incinerated sewage sludge ash (ISSA) for phosphate fertilizer production: A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 939–971. [Google Scholar] [CrossRef]
- Ibáñez, A.; Diez-Galán, A.; Cobos, R.; Calvo-Peña, C.; Barreiro, C.; Medina-Turienzo, J.; Coque, J.J.R. Using Rhizosphere Phosphate Solubilizing Bacteria to Improve Barley (Hordeum vulgare) Plant Productivity. Microorganisms 2021, 9, 1619. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Bouwman, A.F.; Beusen, A.H.W. Phosphorus demand for the 1970–2100 period: A scenario analysis of resource depletion. Glob. Environ. Chang. 2010, 20, 428–439. [Google Scholar] [CrossRef]
- Van Kauwenbergh, S.J. World Phosphate Rock Reserves and Resources; International Fertilizer Development Center (IFDC): Alabama, USA, 2010. [Google Scholar]
- Olagunju, K.O.; Feng, S.; Patton, M. Dynamic relationships among phosphate rock, fertilizers and agricultural commodity markets: Evidence from a vector error correction model and Directed Acyclic Graphs. Resour. Policy 2021, 74, 102301. [Google Scholar] [CrossRef]
- Neset, T.S.S.; Cordell, D. Global phosphorus scarcity: Identifying synergies for a sustainable future. J. Sci. Food Agric. 2011, 92, 2–6. [Google Scholar] [CrossRef]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Dai, M.; Diao, X.; Dai, S.; Fang, T.; Dong, X. Input of Cd from agriculture phosphate fertilizer application in China during 2006–2016. Sci. Total Environ. 2019, 698, 134–149. [Google Scholar] [CrossRef]
- Vieira da Silva, F.B.; Araújo, N.C.W.; Muniz, A.P.R. Environmental risk of trace elements in P-containing fertilizers marketed in Brazil. J. Soil Sci. Plant Nutr. 2017, 17, 635–647. [Google Scholar] [CrossRef]
- Campos, V. Arsenic in groundwater affected by phosphate fertilizers at São Paulo, Brazil. Environ. Geol. 2002, 42, 83–87. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Shrivastava, M.; Srivastava, P.C.; D’Souza, S.F. Phosphate-Solubilizing Microbes: Diversity and Phosphates Solubilization Mechanism. In Rhizospheric Microbes in Soil; Meena, V., Ed.; Springer: Singapore, 2018; pp. 137–165. [Google Scholar] [CrossRef]
- Djuuna, I.A.F.; Prabawardani, S.; Massora, M. Population Distribution of Phosphate-solubilizing Microorganisms in Agricultural Soil. Microbes Environ. 2022, 37, ME21041. [Google Scholar] [CrossRef]
- Behera, B.C.; Singdevsachan, S.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Diversity, mechanism and biotechnology of phosphate solubilizing microorganism in mangrove—A review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, G.; Declerck, S. Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 2018, 12, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Dastager, S.G.; Deepa, C.K.; Pandey, A. Isolation and characterization of novel plant growth promoting Micrococcus sp. NII-0909 and its interaction with cowpea. Plant Physiol. Biochem. 2010, 48, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Franco-Correa, M.; Quintana, A.; Duque, C.; Suarez, C.; Rodríguez, M.X.; Barea, J.M. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl. Soil Ecol. 2010, 45, 209–217. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Lee, J.S.; Lee, K.C.; Saravanan, V.S.; Santhanakrishnan, P. Microbacterium azadirachtae sp. nov., a plant-growth-promoting actinobacterium isolated from the rhizoplane of neem seedlings. Int. J. Syst. Evol. Microbiol. 2010, 60, 1687–1692. [Google Scholar] [CrossRef]
- Saif, S.; Khan, M.S.; Zaidi, A.; Ahmad, E. Role of Phosphate-Solubilizing Actinomycetes in Plant Growth Promotion: Current Perspective. In Phosphate Solubilizing Microorganisms; Khan, M., Zaidi, A., Musarrat, J., Eds.; Springer: Cham, Switzerland, 2014; pp. 137–156. [Google Scholar] [CrossRef]
- McGill, W.B.; Cole, C.V. Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 1981, 26, 267–268. [Google Scholar] [CrossRef]
- Schnepf, A.; Jones, D.; Roose, T. Modelling nutrient uptake by individual hyphae of arbuscular mycorrhizal fungi: Temporal and spatial scales for an experimental design. Bull. Math. Biol. 2011, 73, 2175–2200. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Bünemann, E.K. Assessment of gross and net mineralization rates of soil organic phosphorus—A review. Soil Biol. Biochem. 2015, 89, 82–98. [Google Scholar] [CrossRef]
- Tate, K.R. The biological transformation of P in soil. Plant Soil 1984, 76, 245–256. [Google Scholar] [CrossRef]
- Tamburini, F.; Pfahler, V.; Bunemann, E.K.; Guelland, K.; Bernasconi, S.M.; Frossard, E. Oxygen isotopes unravel the role of microorganisms in phosphate cycling in soils. Environ. Sci. Technol. 2012, 46, 5956–5962. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Nygren, C.M.R.; Rosling, A. Localisation of phosphomonoesterase activity in ectomycorrhizal fungi grown on different phosphorus sources. Mycorrhiza 2009, 19, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.U.; Chandra, S.B. A comparative analysis of three classes of bacterial non-specific Acid phosphatases and archaeal phosphoesterases: Evolutionary perspective. Acta Inform. Med. 2012, 20, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Rejsek, K.; Vranova, V.; Formanek, P. Determination of the Proportion of Total Soil Extracellular Acid Phosphomonoesterase (E.C. 3.1.3.2) Activity Represented by Roots in the Soil of Different Forest Ecosystems. Sci. World J. 2012, 2012, 250805. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Pan, F.; Ma, J.; Yang, Z.; Ling, T.; Song, Z. Alkaline Phosphomonoesterase-Harboring Microorganisms Mediate Soil Phosphorus Transformation With Stand Age in Chinese Pinus massoniana Plantations. Front. Microbiol. 2020, 11, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gaiero, J.R.; Bent, E.; Fraser, T.D. Validating novel oligonucleotide primers targeting three classes of bacterial non-specific acid phosphatase genes in grassland soils. Plant Soil 2018, 427, 39–51. [Google Scholar] [CrossRef]
- Neal, A.L.; Blackwell, M.; Akkari, E. Phylogenetic distribution, biogeography and the effects of land management upon bacterial non-specific Acid phosphatase Gene diversity and abundance. Plant Soil 2018, 427, 175–189. [Google Scholar] [CrossRef]
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Appl. Soil Ecol. 2017, 111, 48–56. [Google Scholar] [CrossRef]
- Singh, B.; Satyanarayana, T. Microbial phytases in phosphorus acquisition and plant growth promotion. Physiol. Mol. Biol. Plants 2011, 17, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Riaz, A.; Chaudhary, A.N.; Hayat, R.; Hussain, Q.; Tahir, M.I.; Imran, M. Microbial phytase activity and their role in organic P mineralization. Arch. Agron. Soil Sci. 2014, 61, 751–766. [Google Scholar] [CrossRef]
- Kour, D.; Kaur, T.; Yadav, N.; Rastegari, A.A.; Singh, B.; Kumar, V.; Yadav, A.N. Chapter 10—Phytases from microbes in phosphorus acquisition for plant growth promotion and soil health. In New and Future Developments in Microbial Biotechnology and Bioengineering; Rastegari, A.A., Yadav, A.N., Yadav, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–176. [Google Scholar] [CrossRef]
- Sadaf, N.; Muhammad, Z.H.; Naeem, I.; Muyassar, H.A.; Aishah, A. Harnessing the Phytase Production Potential of Soil-Borne Fungi from Wastewater Irrigated Fields Based on Eco-Cultural Optimization under Shake Flask Method. Agriculture 2022, 12, 103. [Google Scholar] [CrossRef]
- Wang, X.X.; Hoffland, E.; Feng, G.; Kuyper, T.W. Phosphate Uptake from Phytate Due to Hyphae-Mediated Phytase Activity by Arbuscular Mycorrhizal Maize. Front. Plant Sci. 2017, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- Kamat, S.S.; Raushel, F.M. The enzymatic conversion of phosphonates to phosphate by bacteria. Curr. Opin. Chem. Biol. 2013, 17, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P. Phosphonates: Their Natural Occurrence and Physiological Role. In Contemporary Topics about Phosphorus in Biology and Materials; Churchill, D.G., Sikirić, M.D., Čolović, B., Milhofer, H.F., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Horsak, R.D.; Bedient, P.B.; Hamilton, M.C.; Thomas, F.B. Pesticides. Environ. Forensics 1964, 143–165. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Hagner, M.; Mikola, J.; Saloniemi, I. Effects of a glyphosate-based herbicide on soil animal trophic groups and associated ecosystem functioning in a northern agricultural field. Sci. Rep. 2019, 9, 8540. [Google Scholar] [CrossRef]
- Helander, M.; Pauna, A.; Saikkonen, K. Glyphosate residues in soil affect crop plant germination and growth. Sci. Rep. 2019, 9, 19653. [Google Scholar] [CrossRef]
- Kryuchkova, Y.V.; Burygin, G.L.; Gogoleva, N.E.; Gogolev, Y.V.; Chernyshova, M.P.; Makarov, O.E.; Turkovskaya, O.V. Isolation and characterization of a glyphosate-degrading rhizosphere strain, Enterobacter cloacae K7. Microbiol. Res. 2014, 169, 99–105. [Google Scholar] [CrossRef]
- Chávez-Ortiz, P.; Tapia-Torres, Y.; Larsen, J.; García-Oliva, F. Glyphosate-based herbicides alter soil carbon and phosphorus dynamics and microbial activity. Appl. Soil Ecol. 2022, 169, 104256. [Google Scholar] [CrossRef]
- Stosiek, N.; Talma, M.; Klimek-Ochab, M. Carbon-Phosphorus Lyase—The State of the Art. Appl. Biochem. Biotechnol. 2020, 190, 1525–1552. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, P.; Van, L.B.; Kjeldgaard, M.; Russo, C.J.; Passmore, L.A.; Hove-Jensen, B.; Jochimsen, B.; Brodersen, D.E. Structural insights into the bacterial carbon-phosphorus lyase machinery. Nature 2015, 525, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Chiu, J.F. The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front. Microbiol. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Manav, M.C.; Sofos, N.; Hove-Jensen, B.; Brodersen, D.E. The Abc of Phosphonate Breakdown: A Mechanism for Bacterial Survival. BioEssays 2018, 40, 800091. [Google Scholar] [CrossRef]
- Smeck, N.E. Phosphorus dynamics in soils and landscapes. Geoderma 1985, 36, 185–199. [Google Scholar] [CrossRef]
- Kome, G.; Enang, R.; Tabi, F.; Yerima, B. Influence of Clay Minerals on Some Soil Fertility Attributes: A Review. Open J. Soil Sci. 2019, 9, 155–188. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 13th ed.; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus Transformation in Soils Following Co-Application of Charcoal and Wood Ash. Agronomy 2021, 11, 2010. [Google Scholar] [CrossRef]
- Marra, L.M.; de Oliveira-Longatti, S.M.; Soares, C.R.F.S.; Olivares, F.L.; Moreira, F.M.S. The Amount of Phosphate Solubilization Depends on the Strain, C-Source, Organic Acids and Type of Phosphate. Geomicrobiol. J. 2019, 36, 232–242. [Google Scholar] [CrossRef]
- Duebel, A.; Gransee, A.; Merbach, W. Transformation of organic rhizodeposits by rhizoplane bacteria and its influence on the availability of tertiary calcium phosphate. J. Plant Nutr. Soil Sci. 2000, 163, 387–392. [Google Scholar] [CrossRef]
- Satyaprakash, M.; Nikitha, T.; Reddi, E.U.B.; Sadhana, B.; Vani, S.S. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2133–2144. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Patel, H. Role of microbes in phosphorus availability and acquisition by plants. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1344–1347. [Google Scholar] [CrossRef]
- Mander, C.; Wakelin, S.; Young, S.; Condron, L.; O’Callaghan, M. Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol. Biochem. 2012, 44, 93–101. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef]
- Kishore, N.; Pindi, P.K.; Reddy, S.R. Phosphate-solubilizing microorganisms: A critical review. In Plant Biology and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K., Eds.; Springer: New Delhi, India, 2015; pp. 307–333. [Google Scholar] [CrossRef]
- Prabhu, N.; Borkar, S.; Garg, S. Phosphate solubilization by microorganisms. Adv. Biol. Res. 2019, 161–176. [Google Scholar] [CrossRef]
- Mendes, G.O.; Murta, H.M.; Valadares, R.V.; Silveira, W.B.; Silva, I.R.; Costa, M.D. Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization. Miner. Eng. 2020, 155, 106458. [Google Scholar] [CrossRef]
- Patel, D.K.; Archana, G.; Kumar, G.N. Variation in the Nature of Organic Acid Secretion and Mineral Phosphate Solubilization by Citrobacter sp. DHRSS in the Presence of Different Sugars. Curr Microbiol. 2008, 56, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; McDonald, G.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Siddique, R.; Gul, A.; Ozturk, M.; Altay, V. Phosphate Solubilizing Bacteria for Soil Sustainability. In Handbook of Assisted and Amendment-Enhanced Sustainable Remediation Technology; Prasad, M.N., Ed.; Willey: Telangana, India, 2021; pp. 425–432. [Google Scholar] [CrossRef]
- Cantin, P.; Karam, A.; Guay, R. Solubilization of Phosphorus from Apatite by Sulfuric Acid Produced from the Microbiological Oxidation of Sulfur. In Effect of Mineral-Organic-Microorganism Interactions on Soil and Freshwater Environments; Berthelin, J., Huang, P.M., Bollag, J.M., Andreux, F., Eds.; Springer: Boston, MA, USA, 1999; pp. 247–252. [Google Scholar] [CrossRef]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid. Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar] [CrossRef]
- Verma, V.; Joshi, K.; Mazumdar, B. Study of Siderophore Formation in Nodule-Forming Bacterial Species. Res. J. Chem. Sci. 2012, 2, 26–29. [Google Scholar]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; Umar, S.; Lee, J. Sorghum-Phosphate Solubilizers Interactions: Crop Nutrition, Biotic Stress Alleviation, and Yield Optimization. Front. Plant Sci. 2021, 12, 746780. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.R.; McArdle, J.V.; Raymond, K.N. Siderophore electrochemistry: Relation to intracellular iron release mechanism. Proc. Natl. Acad. Sci. USA 1978, 75, 3551–3554. [Google Scholar] [CrossRef]
- Jyothi, V.; Sowmya, H.V.; Thippeswamy, B. Siderophore production by phosphate solubilizing fungi from rhizospheric soil of medicinal plants. Int. J. Biol. Biotechnol. 2020, 17, 599–606. [Google Scholar]
- Cui, K.; Xu, T.; Chen, J.; Yang, H.; Liu, X.; Zhuo, R.; Peng, Y.; Tang, W.; Wang, R.; Chen, L. Siderophores, a potential phosphate solubilizer from the endophyte Streptomyces sp. CoT10, improved phosphorus mobilization for host plant growth and rhizosphere modulation. J. Clean. Prod. 2022, 367, 133110. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, W.; Ge, Y. Exopolysaccharide: A novel important factor in the microbial dissolution of tricalcium phosphate. World J. Microbiol. Biotechnol. 2007, 24, 1059–1065. [Google Scholar] [CrossRef]
- Prabhu, N.; Borkar, S.; Garg, S. Phosphate Solubilization Mechanisms in Alkaliphilic Bacterium Bacillus marisflavi FA7. Curr. Sci. 2018, 114, 845–853. [Google Scholar] [CrossRef]
- Kailasam, S.; Arumugam, S.; Balaji, K.; Kanth, S.V. Adsorption of chromium by exopolysaccharides extracted from lignolytic phosphate solubilizing bacteria. Int. J. Biol. Macromol. 2022, 206, 788–798. [Google Scholar] [CrossRef]
- Ochoa-Loza, F.J.; Artiola, J.F.; Maier, R.M. Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. J. Environ. Qual. 2001, 30, 479–485. [Google Scholar] [CrossRef]
- Mohite, B.V.; Koli, S.H.; Narkhede, C.P. Prospective of Microbial Exopolysaccharide for Heavy Metal Exclusion. Appl. Biochem. Biotechnol. 2017, 183, 582–600. [Google Scholar] [CrossRef]
- Gaind, S. Phosphate dissolving fungi: Mechanism and application in alleviation of salt stress in wheat. Microbiol. Res. 2016, 193, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.D.M.; Gomes, I.C.P.; Nietsche, S.; Xavier, A.A.; Gomes, W.S.; dos Santos Neto, J.A.; Pereira, M.C.T. Phosphate solubilization by endophytic bacteria isolated from banana trees. An. Acad. Bras. Cienc. 2017, 89, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Arcand, M.M.; Schneider, K.D. Plant- and microbial-based mechanisms to improve the agronomic effectiveness of phosphate rock: A review. An. Acad. Bras. Cienc. 2006, 78, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Öğüt, M.; Er, F.; Neumann, G. Increased proton extrusion of wheat roots by inoculation with phosphorus solubilising microorganims. Plant Soil 2011, 339, 285–297. [Google Scholar] [CrossRef]
- Habte, M.; Osorio, N.W. Effect of Nitrogen Form on the Effectiveness of a Phosphate-Solubilizing Fungus to Dissolve Rock Phosphate. J Biofertil. Biopestic. 2012, 3, 127. [Google Scholar] [CrossRef]
- Florentino, A.P.; Weijma, J.; Stams, A.J.; Sanchez-Andrea, I. Ecophysiology and application of acidophilic sulfur-reducing microorganisms. In Biotechnology of Extremophiles: Grand Challenges in Biology and Biotechnology; Rampelotto, P., Ed.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 141–175. [Google Scholar] [CrossRef]
- Sperber, J. Release of Phosphate from Soil Minerals by Hydrogen Sulphide. Nature 1958, 181, 934. [Google Scholar] [CrossRef]
- Wilfert, P.; Meerdink, J.; Degaga, B.; Temmink, H.; Korving, L.; Witkamp, G.J.; van Loosdrecht, M.C.M. Sulfide induced phosphate release from iron phosphates and its potential for phosphate recovery. Water Res. 2020, 171, 115389. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Wei, Y. Isolation and screening of multifunctional phosphate solubilizing bacteria and its growth-promoting effect on Chinese fir seedlings. Sci Rep. 2021, 11, 9081. [Google Scholar] [CrossRef]
- Zutter, N.; Maarten, A.; Pieter, V.; Verwaeren, J.; Leen, D.G.; Kris, A. Innovative Rhizosphere-Based Enrichment under P-Limitation Selects for Bacterial Isolates with High-Performance P-Solubilizing Traits. Microbiol. Spectr. 2022, 10, e02052-22. [Google Scholar] [CrossRef]
- Singh, T.B.; Sahai, V.; Ali, A.; Prasad, M.; Yadav, A.; Shrivastav, P.; Goyal, D.; Dantu, P.K. Screening and evaluation of PGPR strains having multiple PGP traits from the hilly terrain. J. Appl. Biol. Biotechnol. 2020, 8, 38–44. [Google Scholar] [CrossRef]
- Song, O.R.; Lee, S.J.; Lee, Y.S.; Lee, S.C.; Kim, K.K.; Choi, Y.L. Solubilization of insoluble inorganic phosphate by Burkholderia cepacia DA23 isolated from cultivated soil. Braz. J. Microbiol. 2008, 39, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Sashidhar, B.; Podile, A.R. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J. Appl. Microbiol. 2010, 109, 1–12. [Google Scholar] [CrossRef]
- Mei, C.; Chretien, R.L.; Amaradasa, B.S.; He, Y.; Turner, A.; Lowman, S. Characterization of Phosphate Solubilizing Bacterial Endophytes and Plant Growth Promotion In Vitro and in Greenhouse. Microorganisms 2021, 9, 1935. [Google Scholar] [CrossRef] [PubMed]
- Rasul, M.; Yasmin, S.; Suleman, M.; Zaheer, A.; Reitz, T.; Tarkka, M.T.; Islam, E.; Sajjad, M.M. Glucose dehydrogenase gene containing phosphobacteria for biofortification of Phosphorus with growth promotion of rice. Microbiol. Res. 2019, 223–225, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bharwad, K.; Rajkumar, S. Modulation of PQQ-dependent glucose dehydrogenase (mGDH and sGDH) activity by succinate in phosphate solubilizing plant growth promoting Acinetobacter sp. SK2. 3 Biotech 2020, 10, 5. [Google Scholar] [CrossRef]
- Iyer, B.; Rajkumar, S. Succinate irrepressible periplasmic glucose dehydrogenase of Rhizobium sp. Td3 and SN1 contributes to its phosphate solubilization ability. Arch. Microbiol. 2019, 201, 649–659. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, P.; Li, H.B. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane: A comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 2020, 20, 220. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions Between Plants and Non-Symbiotic Growth Promoting Bacteria Under Edaphic Stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Nadal, M.C.; Ferreira, G.M.R.; Andrade, G.V.S.; Buttrós, V.H.; Rodrigues, F.A.; Silva, C.M.; Martins, A.D.; Rufato, L.; Luz, J.M.Q.; Dória, J.; et al. Endophytic Bacteria Can Replace the Need for Synthetic Auxin during In Vitro Rooting of Pyrus communis. Agronomy 2022, 12, 1226. [Google Scholar] [CrossRef]
- Marulanda, A.; Barea, J.M.; Azcón, R. Stimulation of Plant Growth and Drought Tolerance by Native Microorganisms (AM Fungi and Bacteria) from Dry Environments: Mechanisms Related to Bacterial. J. Plant Growth Regul. 2009, 28, 115–124. [Google Scholar] [CrossRef]
- Zaheer, A.; Mirza, B.S.; Mclean, J.E.; Yasmin, S.; Shah, T.M.; Malik, K.A.; Mirza, M.S. Association of plant growth-promoting Serratia spp. with the root nodules of chickpea. Res. Microbiol. 2016, 167, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The Ecological Role of Volatile and Soluble Secondary Metabolites Produced by Soil Bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Moura, G.G.D.; Barros, A.V.; Machado, F.; Martins, A.D.; Silva, C.M.; Durango, L.G.C.; Forim, M.; Alves, E.; Pasqual, M.; Dória, J. Endophytic bacteria from strawberry plants control gray mold in fruits via production of antifungal compounds against Botrytis cinerea L. Microbiol. Res. 2021, 251, 126793. [Google Scholar] [CrossRef]
- Rani, M.; Weadge, J.T.; Jabaji, S. Isolation and Characterization of Biosurfactant-Producing Bacteria From Oil Well Batteries With Antimicrobial Activities Against Food-Borne and Plant Pathogens. Front. Microbiol. 2020, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Buttrós, V.H.; Araújo, N.A.F.; D’Ávila, V.A.; Pereira, M.M.A.; Melo, D.S.; Pasqual, M.; Dória, J. A Little Helper: Beneficial Bacteria with Growth-Promoting Mechanisms Can Reduce Asian Soybean Rust Severity in a Cell-Free Formulation. Agronomy 2022, 12, 2635. [Google Scholar] [CrossRef]
- Araújo, R.C.; Ribeiro, M.S.; Rodrigues, F.A.; Silva, B.S.; Dória, J.; Pasqual, M. Association of growth-promoting bacteria and hydroponic system aiming at reducing the time of production of banana seedlings. Arch. Agron. Soil Sci. 2022, 1–14. [Google Scholar] [CrossRef]
- Silva, L.I.; Oliveira, I.P.; Jesus, E.C.; Pereira, M.C.; Pasqual, M.; Araújo, R.C.; Dória, J. Fertilizer of the Future: Beneficial Bacteria Promote Strawberry Growth and Yield and May Reduce the Need for Chemical Fertilizer. Agronomy 2022, 12, 2465. [Google Scholar] [CrossRef]
- Moura, G.G.D.; Barros, A.V.; Machado, F.; Silva, C.M.; Lienke, C.; Petters-Vandresen, D.A.L.; Alves, E.; Schwan, R.F.; Pasqual, M.; Dória, J. The Friend Within: Endophytic Bacteria as a Tool for Sustainability in Strawberry Crops. Microorganisms 2022, 10, 2341. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Zhang, Q.; Li, S.; Sun, Y.; Lu, W.; Ma, C. Response of alfalfa growth to arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria under different phosphorus application levels. AMB Express 2020, 10, 200. [Google Scholar] [CrossRef]
- Liu, F.P.; Liu, H.Q.; Zhou, H.L.; Dong, Z.G.; Bai, X.H.; Bai, P.; Qiao, J.J. Isolation and characterization of phosphate-solubilizing bacteria from betel nut (Areca catechu) and their effects on plant growth and phosphorus mobilization in tropical soils. Biol. Fertil. Soils 2014, 50, 927–937. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Gonzalez-Mendoza, D. Isolation and Identification of Phosphate Solubilizing Bacillus spp. from Tamarix ramosissima Rhizosphere and Their Effect on Growth of Phaseolus vulgaris Under Salinity Stress. Geomicrobiol. J. 2020, 37, 901–908. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, I.; Hilger, T.H.; Nadeem, S.M.; Akhtar, M.F.; Jamil, M. Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. PeerJ 2018, 6, e5122. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, X.; Chen, C.; Zheng, X.; Han, H.; Li, C.; Li, J. Enrichment of phosphate solubilizing bacteria during late developmental stages of eggplant (Solanum melongena L.). FEMS Microbiol. Ecol. 2019, 95, fiz023. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef]
- Shen, M.; Li, J.; Dong, Y.; Liu, H.; Peng, J.; Hu, Y.; Sun, Y. Profiling of Plant Growth-Promoting Metabolites by Phosphate-Solubilizing Bacteria in Maize Rhizosphere. Plants 2021, 10, 1071. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Fu, J.; Hu, S.; Qu, J. Degradation of acetochlor and beneficial effect of phosphate-solubilizing Bacillus sp. ACD-9 on maize seedlings. 3 Biotech 2020, 10, 67. [Google Scholar] [CrossRef]
- Batista, B.D.; Lacava, P.T.; Ferrari, A.; Teixeira-Silva, N.S.; Bonatelli, M.L.; Tsui, S.; Quecine, M.C. Screening of tropically derived, multi-trait plant growth- promoting rhizobacteria and evaluation of corn and soybean colonization ability. Microbiol. Res. 2018, 206, 33–42. [Google Scholar] [CrossRef]
- Mosela, M.; Andrade, G.; Massucato, L.R. Bacillus velezensisstrain Ag75 as a new multifunctional agent for biocontrol, phosphate solubilization and growth promotion in maize and soybean crops. Sci. Rep. 2022, 12, 15284. [Google Scholar] [CrossRef]
- Lucero, C.T.; Lorda, G.S.; Anzuay, M.S.; Ludueña, L.M.; Taurian, T. Peanut Endophytic Phosphate Solubilizing Bacteria Increase Growth and P Content of Soybean and Maize Plants. Curr. Microbiol. 2021, 78, 1961–1972. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, X.R.; Wu, X.Q.; Li, G.E.; Wang, Z.; Li, D.W. The phosphate-solubilizing ability of Penicillium guanacastense and its effects on the growth of Pinus massoniana in phosphate-limiting conditions. Biol. Open. 2019, 8, bio046797. [Google Scholar] [CrossRef]
- Biswas, J.K.; Banerjee, A.; Rai, M.; Naidu, R.; Biswas, B.; Vithanage, M.; Meers, E. Potential application of selected metal resistant phosphate solubilizing bacteria isolated from the gut of earthworm (Metaphire posthuma) in plant growth promotion. Geoderma 2018, 330, 117–124. [Google Scholar] [CrossRef]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B. Potential PGPR Properties of Cellulolytic, Nitrogen-Fixing, Phosphate-Solubilizing Bacteria in Rehabilitated Tropical Forest Soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, S.; Wang, T.; Chi, X.; Qi, P.; Chen, G. Interaction Between Halotolerant Phosphate-Solubilizing Bacteria (Providencia rettgeri Strain TPM23) and Rock Phosphate Improves Soil Biochemical Properties and Peanut Growth in Saline Soil. Front. Microbiol. 2021, 12, 777351. [Google Scholar] [CrossRef] [PubMed]
- Yañez-Ocampo, G. Isolated Phosphate-Solubilizing Soil Bacteria Promotes In vitro Growth of Solanum tuberosum L. Pol. J. Microbiol. 2020, 69, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 Isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Valetti, L.; Iriarte, L.; Fabra, A. Growth promotion of rapeseed (Brassica napus) associated with the inoculation of phosphate solubilizing bacteria. Appl. Soil Ecol. 2018, 132, 1–10. [Google Scholar] [CrossRef]
- Adhikari, A.; Lee, K.E.; Khan, M.A.; Kang, S.M.; Adhikari, B.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.J. Effect of Silicate and Phosphate Solubilizing Rhizobacterium Enterobacter ludwigii GAK2 on Oryza sativa L. under Cadmium Stress. J. Microbiol. Biotechnol. 2020, 30, 118–126. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, F.; Ma, L. Phosphate-solubilizing Bacteria from Safflower Rhizosphere and their Effect on Seedling Growth. Open Life Sci. 2019, 10, 246–254. [Google Scholar] [CrossRef]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- He, D.; Wan, W. Phosphate-Solubilizing Bacterium Acinetobacter pittii gp-1 Affects Rhizosphere Bacterial Community to Alleviate Soil Phosphorus Limitation for Growth of Soybean (Glycine max). Front. Microbiol. 2021, 12, 737116. [Google Scholar] [CrossRef]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Kuyper, T.W.; Boonlue, S. Interaction between Phosphate Solubilizing Bacteria and Arbuscular Mycorrhizal Fungi on Growth Promotion and Tuber Inulin Content of Helianthus tuberosus L. Sci. Rep. 2020, 10, 4916. [Google Scholar] [CrossRef] [PubMed]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Ekprasert, J.; Cooper, J. Combination of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria on growth and production of Helianthus tuberosus under field condition. Sci. Rep. 2021, 11, 6501. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, J.; Chen, Y.; Zhang, L.; Zhang, Y.; Wang, S.; Liang, J. Effects of Phosphate Solubilizing Bacteria on the Growth, Photosynthesis, and Nutrient Uptake of Camellia oleifera Abel. Forests 2019, 10, 348. [Google Scholar] [CrossRef]
- Tchakounté, G.V.T.; Berger, B.; Patz, S.; Becker, M.; Fankem, H.; Taffouo, V.D.; Ruppel, S. Selected Rhizosphere Bacteria Help Tomato Plants Cope with Combined Phosphorus and Salt Stresses. Microorganisms 2020, 8, 844. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.J.; Nam, I.H. Effect of Treating Acid Sulfate Soils with Phosphate Solubilizing Bacteria on Germination and Growth of Tomato (Lycopersicon esculentum L.). Int. J. Environ. Res. Public Health 2021, 18, 8919. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Compant, S.; Ballhausen, M.B.; Ruppel, S.; Franken, P. The interaction between Rhizoglomus irregulare and hyphae attached phosphate solubilizing bacteria increases plant biomass of Solanum lycopersicum. Microbiol. Res. 2020, 240, 126556. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Aeron, A.; Shaw, N.; Singh, A.; Bajpai, V.K.; Pant, S.; Dubey, R.C. Seed bio-priming with tri-species consortia of phosphate solubilizing rhizobacteria (PSR) and its effect on plant growth promotion. Heliyon 2020, 6, e05701. [Google Scholar] [CrossRef]
- Yahya, M.; Islam, E.; Rasul, M.; Farooq, I.; Mahreen, N.; Tawab, A.; Irfan, M.; Rajput, L.; Amin, I.; Yasmin, S. Differential Root Exudation and Architecture for Improved Growth of Wheat Mediated by Phosphate Solubilizing Bacteria. Front. Microbiol. 2021, 12, 744094. [Google Scholar] [CrossRef]
- Tahir, M.; Khalid, U.; Ijaz, M.; Shah, G.M.; Naeem, M.A.; Shahid, M. Combined application of bio-organic phosphate and phosphorus solubilizing bacteria (Bacillus strain MWT 14) improve the performance of bread wheat with low fertilizer input under an arid climate. Braz. J. Microbiol. 2018, 49, 15–24. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The Screening of Potassium- and Phosphate-Solubilizing Actinobacteria and the Assessment of Their Ability to Promote Wheat Growth Parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef]
- Prakash, J.; Arora, N.K. Phosphate-solubilizing Bacillus sp. enhances growth, phosphorus uptake and oil yield of Mentha arvensis L. 3 Biotech 2019, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.K.; Guaraha, A.K. Global biofertilizer market: Emerging trends and opportunities. In Developments in Applied Microbiology and Biotechnology, Trends of Applied Microbiology for Sustainable Economy; Soni, R., Suyal, D.P., Yadav, A.N., Goel, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 689–697. [Google Scholar] [CrossRef]
- Pirttilä, A.M.; Mohammad Parast Tabas, H.; Baruah, N.; Koskimäki, J.J. Biofertilizers and Biocontrol Agents for Agriculture: How to Identify and Develop New Potent Microbial Strains and Traits. Microorganisms 2021, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Mącik, M.; Agata, G.; Magdalena, F. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 162, pp. 31–87. [Google Scholar] [CrossRef]
- Jokkaew, S.; Jantharadej, K.; Pokhum, C.; Chawengkijwanich, C.; Suwannasilp, B.B. Free and Encapsulated Phosphate-Solubilizing Bacteria for the Enhanced Dissolution of Swine Wastewater-Derived Struvite—An Attractive Approach for Green Phosphorus Fertilizer. Sustainability 2022, 14, 12627. [Google Scholar] [CrossRef]
- Cheng, Y.; Wan, W. Alkaline phosphomonoesterase-harboring bacteria facilitate phosphorus availability during winter composting with different animal manures. J. Clean. Prod. 2022, 376, 134299. [Google Scholar] [CrossRef]



| Microorganism/Consortia | Crop | Mechanism of Action | Highlights | Reference |
|---|---|---|---|---|
| Glomus mosseae +/or Bacillus megaterium | Alfalfa (Medicago sativa) | Increased the mycorrhizae infection rate, shoot biomass, chlorophyll content in leaves, and soluble sugar content | AMF and PSB significantly promoted the nutritious quality of alfalfa under different phosphorus application conditions | Liu et al. [161] |
| Advenella mimigardefordensis, Bacillus cereus, Bacillus megaterium, and Burkholderia fungorum | Barley (Hordeum vulgare) | Improved levels of assimilated phosphate, dry weight of ears, and total starch accumulated on ears | The use of PSB is a promising strategy to take advantage of non-accessible soil P reserves | Ibáñez et al. [52] |
| Acinetobacter pittii +/or Escherichia coli +/or Enterobacter cloacae | Betel nut (Areca catechu) | The strains significantly improved plant height, shoot and root dry weight, and nutrient uptake. Moreover, the co-inoculation enhanced the solubilization of tricalcium and aluminum phosphate. | The strains can be potentially applied as inoculants in tropical and aluminum-rich soils | Liu et al. [162] |
| Azotobacter sp. SR-4 +/or Aspergillus niger | Calabash (Lagenaria siceraria) and Okra (Abelmoschus esculentus) | Increased plant height, leaf length/width, fruit size, and the number of fruits per plant. Consortium shows better results | Selected strains may replace costly and the environment-toxic chemical fertilizers | Din et al. [13] |
| Pseudomonas donghuensis JLP2, Pseudomonas grimontii JRP22, Pantoea roadsii HRP2, Enterobacter hormaechei SSP2, Paraburkholderia caffeinilytica JRP13, Novosphingobium barchaimii JRP23 and Ochrobactrum pseudogrignonense JRP24 | Chinese fir (Cunninghamia lanceolata) | Improved plant height, stem diameter, biomass, and nutrient content. Also enhanced soil nutrient content and enzyme activity | PSB could be used as biological agents instead of chemical fertilizers for agroforestry production | Chen et al. [140] |
| Bacillus megaterium +/or Bacillus cereus | Common bean (Phaseolus vulgaris) | Single and dual inoculation increases root length, plant height, root and shoot dry weight, P content in plants and photosynthetic pigments even in salt stress conditions | Decreased the harmful effects of salinity and improve plant growth in stress conditions | Abdelmoteleb et al. [163] |
| Bacillus subtilis Q3 and Paenibacillus sp. Q6 | Cotton (Gossypium sp.) | Increased root length, shoot and root fresh and dry weight, and root/shoot ratio | Selected strains are potential candidates for promoting cotton growth under alkaline conditions | Ahmad et al. [164] |
| Enterobacter sp. | Eggplant (Solanum melongena) | Eggplant recruited Enterobacter PSBs during fruiting stages | The rhizosphere bacterial community was susceptible to farming strategies and was largely shaped during the plant development stages | Li et al. [165] |
| Achromobacter, Agrobacterium, Bacillus, Burkholderia, Erwinia, Flavobacterium, Micrococcus, Pseudomonas, and Rhizobia | Maize (Zea mays) | Improved growth, its P concentration, and uptake | PSB inoculation may nullify the negative effects of liming (such as decreased maize growth and P uptake, and increased post-harvest soil salinity and calcification) on plant growth and P availability | Adnan et al. [166] |
| Citrobacter amalonaticus M16 +/or Bacillus safensis M44 | Maize (Zea mays) | Increased the length of the root and sprout, also the underground and aboveground biomass. Enhanced plant amino acids, metabolites, and other molecules | This study supplies a theoretical basis for the application of PSB in sustainable agriculture | Shen et al. [167] |
| Bacillus sp. ACD-9 | Maize (Zea mays) | Improve growth (9%) and phosphorus uptake (15%) and decrease the accumulation (70%) and toxic effects of herbicide acetochlor | The strain may be useful in the degradation of acetochlor in soil and the promotion of the growth and phosphorus uptake of maize | Li et al. [168] |
| Bacillus sp. RZ2MS9 and Burkholderia ambifaria RZ2MS16 | Maize (Zea mays) and Soybean (Glycine max) | Increases in root and shoots dry weight of both plants when compared to non-inoculated control | The PSB isolated of guarana (Paullinia cupana) a tropical plant shows the ability to endophytically colonize plants of agricultural interest | Batista et al. [169] |
| Bacillus velezensis Ag75 | Maize (Zea mays) and Soybean (Glycine max) | Increased maize and soybean yield by 18% and 27%, respectively, while also being a biocontrol agent. | The bacterium has multifunctional traits for promoting plant growth and makes it possible to reduce the demand for phosphate fertilization | Mosela et al. [170] |
| Enterobacter sp J49 and Serratia sp. S119 | Maize (Zea mays), Soybean (Glycine max) and Peanut (Arachis hypogaea) | Promote plant growth and P tissue uptake and increased the phosphate-solubilizing ability of the rhizosphere. Root exudates of the plants showed to produce changes in the pectinase and cellulase activities of the strains | The strains analyzed constitute potential sources for the formulation of biofertilizers for application in agricultural soils with low P content | Lucero et al. [171] |
| Penicillium guanacastense JP-NJ2 | Masson pine (Pinus massoniana) | Extracellular metabolites and fungal suspension from the strain promoted the shoot lengths by 60% and 98%, respectively, while root crown diameters increased by 28% and 47% | The strain might be used to improve soil fertility in nurseries and forestry practice | Qiao et al. [172] |
| Bacillus megaterium UFMG50, Klebsiella variicola UFMG51, Pantoea ananatis UFMG54, Microbacterium sp. UFMG61, Pseudomonas sp. UFMG81 and Ochrobactrum pseudogrignonense CNPMS2088 | Millet (Pennisetum glaucum) | Increased P both in soil and in the plant. Organic acids and the production of phytohormones are among the mechanisms of plant growth | RP and the isolates described here are used as adjuvants to a P-fertilization strategy in tropical soils. | Silva et al. [21] |
| Pseudomonas spp. | Mung bean (Vigna radiata) | Increased seed yield, 1000-grain weight, biological yield, shoot and root P concentration, and uptake | PSB inoculation with less P fertilization | Bilal et al. [4] |
| Bacillus megaterium MF 589715, Staphylococcus haemolyticus MF 589716, and Bacillus licheniformis MF 589720 | Mung bean (Vigna radiata) | Isolated PSBs from earthworm gut is capable of plant growth promotion and metal resistance | Integrated use of earthworms and associated bacteria as the powerful biofertilizer in the sustainable crop production | Biswas et al. [173] |
| Burkholderia cepacia strains 5.5, 2EJ5 and ATCC 35254, Burkholderia uboniae, Gluconacetobacter diazotrophicus PAl 5 | Mung bean (Vigna radiata) | The PSB improved root and shoot lengths, and seedling vigor | Bacterial strains could potentially be included in bio-fertilizer formulations for crop growth on acid soils | Tang et al. [174] |
| Pseudomonas sp. +/or Serratia sp. | Onion (Allium cepa) | Consortium increases the seeds germination rates (90% of evaluated) and plant’s total dry weight | Consortium application twice a week for two months favored onion total dry weight increase in comparison with controls | Blanco-Vargas et al. [8] |
| Providencia rettgeri TPM23 | Peanut (Arachis hypogaea) | Combined application of RP and PSB increased plant length, biomass, and uptake of NPK. Decrease of soil Na+, Cl-, and pH. Also increased soil beneficial enzymes and microbial diversity | The combination of PSB and RP might be a low-cost and environmentally safe strategy to remediate the problem of low nutrient availability in saline soils. | Jiang et al. [175] |
| Bacillus pumilus | Potato (Solanum tuberosum) | In vitro increased root (68%) and stems (79%) length. Also, duplicate the fresh weight of plants | Growth promotion under in vitro conditions is a step forward in the use of innocuous bacterial strain biofertilizer | Yañez-Ocampo et al. [176] |
| Bacillus licheniformis QA1 and Enterobacter asburiae QF11 | Quinoa (Chenopodium quinoa) | The strains significantly improve germination rate and seedling height and weight. Also reduces Na+ uptake under saline conditions | Isolation of potential biofertilizers PSB strains from the rhizosphere of quinoa from Moroccan soil | Mahdi et al. [177] |
| Bacillus sp. LTAD-52, LRCP-2, LRCP-3, LRCP-4, Serratia sp. LRCP-29, Pantoea sp. LRCP-17 and Arthrobacter sp. LRCP-11 | Rapeseed (Brassica napus) | Increased significantly plant growth and crop yield (from 21% to 40%), reaching values like or even higher than the fertilized control | Extend the knowledge of the diversity of bacteria associated with rapeseed plants. Contributes to the development of biotechnological strategies | Valetti et al. [178] |
| Enterobacter ludwigii GAK2 | Rice (Oryza sativa) | Enhanced plant fresh, shoot and root weight, plant height, and chlorophyll content | The strain solubilizes the silicate and phosphate in the soil and thereby promotes the growth of plants in cadmium-contaminated soil | Adhikari et al. [179] |
| Acinetobacter sp. RC04 + Sinorhizobium sp. RC02 | Safflower (Carthamus tinctorious) | Improved seed germination and, when co-inoculated, improved seedling growth | Reveal the potential of Acinetobacter sp. and Sinorhizobium sp. as biofertilizer agents. | Zhang et al. [180] |
| Trichoderma spp. | Soybean (Glycine max) | Increased soybean growth from 2% to 41% as well as in the efficiency of P uptake-up to 141% | Reveal the potential of Trichoderma spp. from the Amazon biome as a promising biofertilizer agent. | Bononi et al. [181] |
| Acinetobacter pittii | Soybean (Glycine max) | Promoted plant growth. Increased activities of phosphatase, phytase, and indole acetic acid | A. pittii promotes inorganic and organic P use and increases the function of P-cycling-related enzymes of the rhizosphere bacterial community. | He and Wan [182] |
| Klebsiella variicola + Rhizophagus intraradices | Sunchoke (Helianthus tuberosus) | Increased plant growth and tuber inulin content | Dual inoculation may be a promising strategy to both reduce expensive synthetic fertilizers and enhance insulin production | Nacoon et al. [183] |
| Klebsiella variicola +/or Rhizophagus intraradices | Sunchoke (Helianthus tuberosus) | In 2016 (year) the consortium improved the growth and production of the plant more than the inoculation of AMF or PSB alone. In 2017 showed that the inoculation of AMF alone played a more significant role in enhancing plant growth and production | Different years of sunchoke plantation could result in distinct levels of plant response and PSB and AMF status in soil | Nacoon et al. [184] |
| Bacillus aryabhattai JX285 +/or Pseudomonas auricularis HN038 | Tea-Oil Camellia (Camellia oleifera) | Improved plant growth, photosynthetic ability, the N and P content of the leaves, and the available N, P, and K content of rhizosphere soil | The inoculation effect of mixed PSB strains was better than that of single strains | Wu et al. [185] |
| Arthrobacter sp. and Bacillus sp. | Tomato (Solanum lycopersicum) | Enhanced plant growth in P-deficient and salt-affected soils by 47–115%. The PGPB effect was increased in higher salt stress conditions | Selected bacteria solubilize phosphate in the presence of high salt concentrations, promoting plant growth even under combined P and salt stresses | Tchakounté et al. [186] |
| Methylobacterium sp. PS and Caballeronia sp. EK | Tomato (Solanum lycopersicum) | In acid sulfate soils treated with each bacterial strain led to 38% to 60% increased germination (52 days), a 2–3-fold increased number of leaves (52 days), and 19–45% increased soil tATP levels (50 days) | Strains of PSB described have the potential for use as biofertilizers that promote vegetation growth in acid sulfate soils | Kim et al. [187] |
| Pseudomonas fluorescens PSB1 and PSB11, P. koreensis PSB18 +/or Rhizoglomus irregulare (One PSB + AMF consortium) | Tomato (Solanum lycopersicum) | PSB and AMF increased the plant biomass. Also, PSB increased hyphal length and colonization | Plants inoculated with the combination of fungus and bacteria had significantly higher plant biomass compared to single inoculations | Sharma et al. [188] |
| Burkholderia gladioli +/or Pseudomonas sp. +/or Bacillus subtilis | Tomato (Solanum lycopersicum) and Fenugreek (Trigonella foenum-graecum) | Seed germination, plant height, and weight significantly increased | Reveals the strains and consortium ability to solubilize insoluble inorganic and organic P into absorbable form for plant | Kumar et al. [189] |
| Paenibacillus beijingensis BJ-18 + Paenibacillus sp. B1 | Wheat (Triticum aestivum) | Increase plant biomass, improve plant nutrition and rhizosphere soil physicochemical properties | PSB and diazotrophic bacteria can improve the sustainability of agriculture. | Li et al. [25] |
|
Consortium 1 (Enterobacter spp. ZW9, ZW32, and Ochrobactrum sp. SSR). Consortium 2 (Pantoea sp. S1, Enterobacter sp. D1, and Ochrobactrum sp. SSR). Consortium 3 (Ochrobactrum sp. SSR, Pseudomonas sp. TJA, and Bacillus sp. TAYB) | Wheat (Triticum aestivum) | Alleviation of P stress through induced sequential production of root exudates, modification of root architecture, and mitigation of oxidative damage by induced activities of antioxidant enzymes | P-solubilizing bacteria employed beneficial impact on morpho-physiological attributes of inoculated plants | Yahya et al. [190] |
| Bacillus sp. MWT-14 | Wheat (Triticum aestivum) | Increased number of productive tillers, 1000-grain weight, grains per spike | Combined use of Bio-organic P and PSB can increase the soil fertility, crop growth, and productivity of wheat | Tahir et al. [191] |
| Streptomyces alboviridis P18, Streptomyces griseorubens BC3, Streptomyces griseorubens BC10, and Nocardiopsis alba BC11 | Wheat (Triticum aestivum) | Improved root length (2–24%), root volume (42–72%), root dry weight (47–162%), shoot length (9–24%) and shoot dry weight (3–66%) | Significant ability to solubilize mica and RPs under the in vitro condition. BC10 and BC11 are promising candidates for the implementation of efficient biofertilization | Boubekri et al. [192] |
| Bacillus sp. | Wild mint (Mentha arvensis) | Increased in the plant growth parameters, oil yield, and P uptake | PS Bacillus enhanced the menthol content of M. arvensis | Prakash and Arora, [193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.I.d.; Pereira, M.C.; Carvalho, A.M.X.d.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture 2023, 13, 462. https://doi.org/10.3390/agriculture13020462
Silva LId, Pereira MC, Carvalho AMXd, Buttrós VH, Pasqual M, Dória J. Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture. 2023; 13(2):462. https://doi.org/10.3390/agriculture13020462
Chicago/Turabian StyleSilva, Leandro Israel da, Marlon Correa Pereira, André Mundstock Xavier de Carvalho, Victor Hugo Buttrós, Moacir Pasqual, and Joyce Dória. 2023. "Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture" Agriculture 13, no. 2: 462. https://doi.org/10.3390/agriculture13020462
APA StyleSilva, L. I. d., Pereira, M. C., Carvalho, A. M. X. d., Buttrós, V. H., Pasqual, M., & Dória, J. (2023). Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture, 13(2), 462. https://doi.org/10.3390/agriculture13020462






