The Role of Tryptophan Metabolism in Alzheimer’s Disease
Abstract
1. Introduction
2. Tryptophan Metabolism
2.1. Serotonergic Pathway
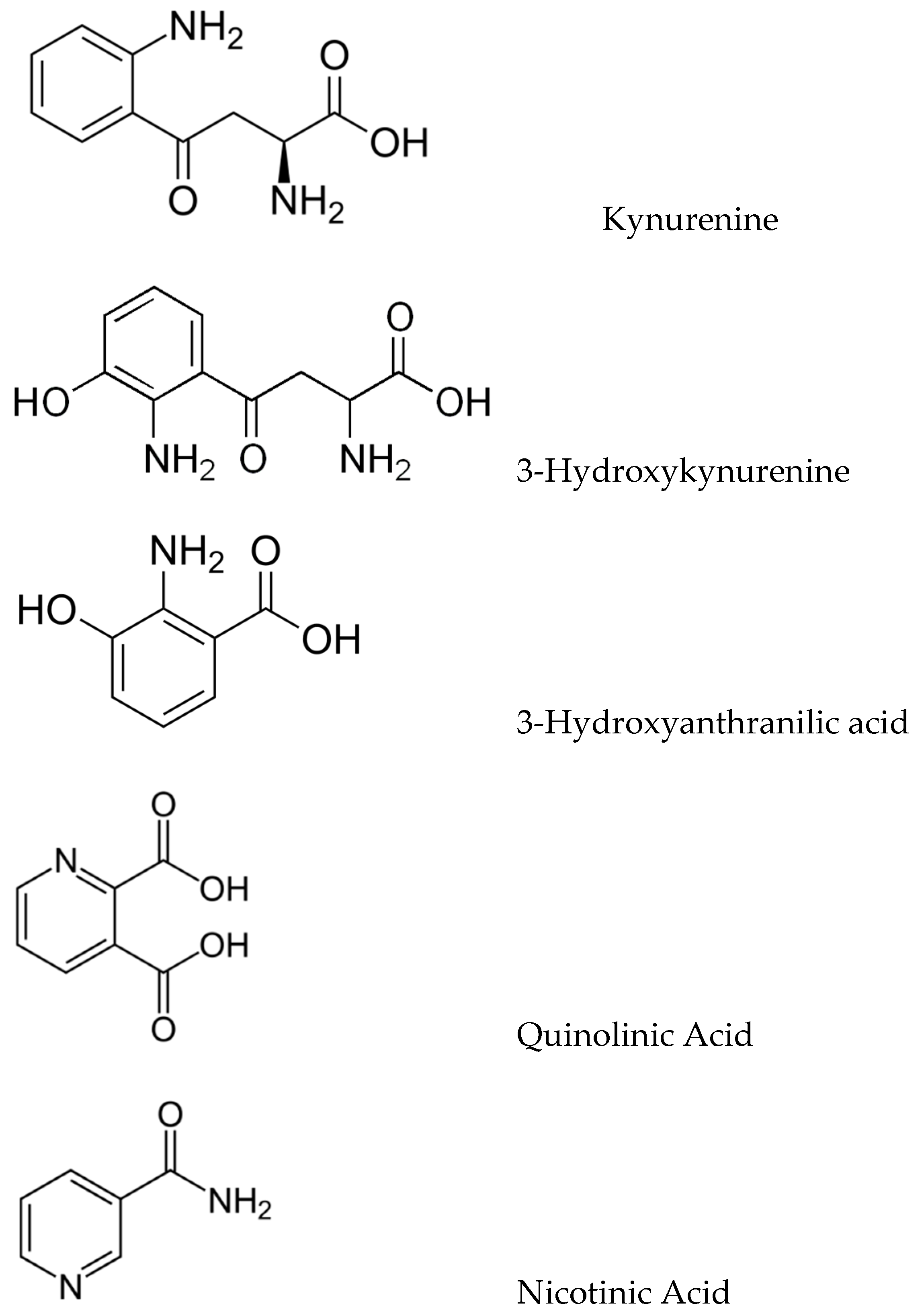
2.2. Kynurenic Pathway
3. Tryptophan and Alzheimer’s Disease Pathogenesis
3.1. Trp and Proteopathy in AD
3.2. Trp and Sleep Disorders in AD
3.3. Trp and Kynurenic Neurotoxicity in AD
3.4. Trp and Neuroinflammation in AD
3.5. Trp and Innate Autoimmunity in AD
4. Conclusions
Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Hogan, D.B.; Smith, E.E.; Frolkis, A.; Cohen, A.; Kirk, A.; Pearson, D.; Pringsheim, T.; et al. The Prevalence and Incidence of Dementia Due to Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. 2016, 43, S51–S82. [Google Scholar] [CrossRef] [PubMed]
- Guehne, U.; Riedel-Heller, S.; Angermeyer, M.C. Mortality in Dementia. Neuroepidemiology 2005, 25, 153–162. [Google Scholar] [CrossRef]
- Villarejo, A.; Benito-León, J.; Trincado, R.; Posada, I.J.; Puertas-Martín, V.; Boix, R.; Medrano, M.J.; Bermejo-Pareja, F. Dementia-Associated Mortality at Thirteen Years in the NEDICES Cohort Study. J. Alzheimers Dis. 2011, 26, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, H.; Saido, T.C. Amyloid-β in Brain Aging and Alzheimer’s Disease. In Aging Mechanisms II: Longevity, Metabolism, and Brain Aging; Mori, N., Ed.; Springer Nature: Singapore, 2022; pp. 335–354. [Google Scholar] [CrossRef]
- Brion, J.-P. Neurofibrillary Tangles and Alzheimer’s Disease. Eur. Neurol. 1998, 40, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Tapiola, T.; Overmyer, M.; Lehtovirta, M.; Helisalmi, S.; Ramberg, J.; Alafuzoff, I.; Riekkinen, P.S.; Soininen, H. The Level of Cerebrospinal Fluid Tau Correlates with Neurofibrillary Tangles in Alzheimer’s Disease. NeuroReport 1997, 8, 3961–3963. [Google Scholar] [PubMed]
- Fathi, M.; Vakili, K.; Yaghoobpoor, S.; Tavasol, A.; Jazi, K.; Hajibeygi, R.; Shool, S.; Sodeifian, F.; Klegeris, A.; McElhinney, A.; et al. Dynamic changes in metabolites of the kynurenine pathway in Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A systematic Review and meta-analysis. Front. Immunol. 2022, 13, 997240. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; Giacovazzo, G.; Decandia, D.; Coccurello, R. Alzheimer’s disease and depression in the elderly: A trajectory linking gut microbiota and serotonin signaling. Front. Psych. 2022, 13, 1010169. [Google Scholar] [CrossRef]
- Elsworthy, R.J.; Aldred, S. Depression in Alzheimer’s Disease: An Alternative Role for Selective Serotonin Reuptake Inhibitors? J. Alz. Dis. 2019, 69, 651–661. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Routy, J.P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Trp. Res. 2016, 9, 67–77. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Farber, N.B.; Olney, J.W. NMDA receptor function, memory, and brain aging. Dialogues Clin. Neurosci. 2000, 2, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Donev, R.; Kolev, M.; Millet, B.; Thome, J. Neuronal Death in Alzheimer’s Disease and Therapeutic Opportunities. J. Cell. Mol. Med. 2009, 13, 4329–4348. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, S.F. Alpha-Secretase in Alzheimer’s Disease: Molecular Identity, Regulation and Therapeutic Potential. J. Neurochem. 2011, 116, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alvarez, A.; Chávez-Ángel, E.; Nelson, R. Understanding the Molecular Basis of 5-HT4 Receptor Partial Agonists through 3D-QSAR Studies. Internat. J. Mol. Sci. 2021, 22, 3602. [Google Scholar] [CrossRef]
- Howell, E.H.; Cameron, S.J. Neprilysin inhibition: A brief review of past pharmacological strategies for heart failure treatment and future directions. Cardiol. J. 2016, 23, 591–598. [Google Scholar] [CrossRef]
- Humpel, C. Intranasal neprilysin rapidly eliminates amyloid-beta plaques, but causes plaque compensations: The explanation why the amyloid-beta cascade may fail? Neural Regen. Res. 2022, 17, 1881–1884. [Google Scholar] [CrossRef]
- Sutphin, G.L. Systemic Elevation of 3-Hydroxyanthranilic Acid (3HAA) to Extend Lifespan and Delay Alzheimer’s Pathology. Innov. Aging. 2018, 2 (Suppl. 1), 74. [Google Scholar] [CrossRef]
- Owens, J.; Adolescent Sleep Working Group; Committee on Adolescence; Au, R.; Carskadon, M.; Millman, R.; Wolfson, A.; Braverman, P.K.; Adelman, W.P.; Breuner, C.C.; et al. Insufficient Sleep in Adolescents and Young Adults: An Update on Causes and Consequences. Pediatrics 2014, 134, e921–e932. [Google Scholar] [CrossRef]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2019, 7, 1. [Google Scholar] [CrossRef]
- Products—Data Briefs—Number 127—August 2013. Available online: https://www.cdc.gov/nchs/products/databriefs/db127.htm (accessed on 9 December 2022).
- Sabia, S.; Fayosse, A.; Dumurgier, J.; van Hees, V.T.; Paquet, C.; Sommerlad, A.; Kivimäki, M.; Dugravot, A.; Singh-Manoux, A. Association of Sleep Duration in Middle and Old Age with Incidence of Dementia. Nat. Commun. 2021, 12, 2289. [Google Scholar] [CrossRef]
- Porter, V.R.; Buxton, W.G.; Avidan, A.Y. Sleep, Cognition and Dementia. Curr. Psychiatry Rep. 2015, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, A.; Pak, V.M. The effects of time-restricted eating on sleep, cognitive decline, and Alzheimer’s disease. Exp. Gerontol. 2023, 171, 112033. [Google Scholar] [CrossRef] [PubMed]
- Panossian, L.A.; Avidan, A.Y. Review of sleep disorders. Med. Clin. North Am. 2009, 93, 407–409. [Google Scholar] [CrossRef]
- Brown, B.M.; Rainey-Smith, S.R.; Villemagne, V.L.; Weinborn, M.; Bucks, R.S.; Sohrabi, H.R.; Laws, S.M.; Taddei, K.; Macaulay, S.L.; Ames, D.; et al. The Relationship between Sleep Quality and Brain Amyloid Burden. Sleep 2016, 39, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Kojori, E.; Wang, G.-J.; Wiers, C.E.; Demiral, S.B.; Guo, M.; Kim, S.W.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. β-Amyloid Accumulation in the Human Brain after One Night of Sleep Deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef]
- Holth, J.K.; Fritschi, S.K.; Wang, C.; Pedersen, N.P.; Cirrito, J.R.; Mahan, T.E.; Finn, M.B.; Manis, M.; Geerling, J.C.; Fuller, P.M.; et al. The Sleep-Wake Cycle Regulates Brain Interstitial Fluid Tau in Mice and CSF Tau in Humans. Science 2019, 363, 880–884. [Google Scholar] [CrossRef]
- Blattner, M.S.; Panigrahi, S.K.; Toedebusch, C.D.; Hicks, T.J.; McLeland, J.S.; Banks, I.R.; Schaibley, C.; Ovod, V.; Mawuenyega, K.G.; Bateman, R.J.; et al. Increased Cerebrospinal Fluid Amyloid-β During Sleep Deprivation in Healthy Middle-Aged Adults Is Not Due to Stress or Circadian Disruption. J. Alzheimers Dis. 2020, 75, 471–482. [Google Scholar] [CrossRef]
- Van Leeuwen, W.M.A.; Lehto, M.; Karisola, P.; Lindholm, H.; Luukkonen, R.; Sallinen, M.; Härmä, M.; Porkka-Heiskanen, T.; Alenius, H. Sleep Restriction Increases the Risk of Developing Cardiovascular Diseases by Augmenting Proinflammatory Responses through IL-17 and CRP. PLoS ONE 2009, 4, e4589. [Google Scholar] [CrossRef]
- Wu, H.; Dunnett, S.; Ho, Y.-S.; Chang, R.C.-C. The Role of Sleep Deprivation and Circadian Rhythm Disruption as Risk Factors of Alzheimer’s Disease. Front. Neuroendocrinol. 2019, 54, 100764. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Ameliorates Anxiety-like Behaviors Induced by Sleep Deprivation in Mice: Role of Oxidative Stress, Neuroinflammation, Autophagy and Apoptosis. Brain Res. Bull. 2021, 174, 161–172. [Google Scholar] [CrossRef]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Trp. Res. 2018, 11, 1178646918802282. [Google Scholar] [CrossRef]
- Silber, B.Y.; Schmitt, J.A.J. Effects of Tryptophan Loading on Human Cognition, Mood, and Sleep. Neurosci. Biobehav. Rev. 2010, 34, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. IJTR 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Nicholas, D.A.; Torrez, T.W.; Kim, N.-S.; Firek, A.F.; Langridge, W.H.R. The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines 2015, 3, 703–729. [Google Scholar] [CrossRef]
- Kwidzinski, E.; Bechmann, I. IDO Expression in the Brain: A Double-Edged Sword. J. Mol. Med. 2007, 85, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Alavi Naini, S.M.; Soussi-Yanicostas, N. Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies? Oxid. Med. Cell Longev. 2015, 2015, e151979. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Brew, B.J.; Noonan, C.E.; Takikawa, O.; Cullen, K.M. Indoleamine 2,3 Dioxygenase and Quinolinic Acid Immunoreactivity in Alzheimer’s Disease Hippocampus. Neuropathol. Appl. Neurobiol. 2005, 31, 395–404. [Google Scholar] [CrossRef]
- Thevandavakkam, M.A.; Schwarcz, R.; Muchowski, P.J.; Giorgini, F. Targeting Kynurenine 3-Monooxygenase (KMO): Implications for Therapy in Huntington’s Disease. CNS Neurol. Disord. Drug Targets CNS Neurol. Disord. 2010, 9, 791–800. [Google Scholar] [CrossRef]
- Yu, D.; Tao, B.-B.; Yang, Y.-Y.; Du, L.-S.; Yang, S.-S.; He, X.-J.; Zhu, Y.-W.; Yan, J.-K.; Yang, Q. The IDO Inhibitor Coptisine Ameliorates Cognitive Impairment in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2015, 43, 291–302. [Google Scholar] [CrossRef]
- Zwilling, D.; Huang, S.-Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.-Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.W.; et al. Kynurenine 3-Monooxygenase Inhibition in Blood Ameliorates Neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef]
- Gupta, M.; Lee, H.J.; Barden, C.J.; Weaver, D.F. The Blood–Brain Barrier (BBB) Score. J. Med. Chem. 2019, 62, 9824–9836. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.S.; Iradukunda, E.C.; Hughes, T.; Bowen, J.P. Modulation of Enzyme Activity in the Kynurenine Pathway by Kynurenine Monooxygenase Inhibition. Front. Mol. Biosci. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Stafford, P.M.; Stover, K.R.; Mohan, D.C.; Gupta, M.; Keske, E.C.; Schiavini, P.; Villar, L.; Wu, F.; Kreft, A.; et al. A Series of 2-((1-Phenyl-1H-imidazol-5-yl)methyl)-1H-indoles as Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitors. ChemMedChem 2021, 16, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.D.; Güner, O.F.; Iradukunda, E.C.; Phillips, R.S.; Bowen, J.P. The Kynurenine Pathway and Kynurenine 3-Monooxygenase Inhibitors. Molecules 2022, 27, 273. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-Inflammatory Stimuli (LPS, IFNγ+TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Boche, D.; Perry, V.H.; Nicoll, J.a.R. Review: Activation Patterns of Microglia and Their Identification in the Human Brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 Potently Enhances Murine Macrophage Mannose Receptor Activity: A Marker of Alternative Immunologic Macrophage Activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 Microglia: The Good, the Bad, and the Inflamed. J. Neuroinflam. 2014, 11, 98. [Google Scholar] [CrossRef]
- Cunningham, C.; Wilcockson, D.C.; Campion, S.; Lunnon, K.; Perry, V.H. Central and Systemic Endotoxin Challenges Exacerbate the Local Inflammatory Response and Increase Neuronal Death during Chronic Neurodegeneration. J. Neurosci. 2005, 25, 9275–9284. [Google Scholar] [CrossRef]
- Sheffield, L.G.; Marquis, J.G.; Berman, N.E.J. Regional Distribution of Cortical Microglia Parallels That of Neurofibrillary Tangles in Alzheimer’s Disease. Neurosci. Lett. 2000, 285, 165–168. [Google Scholar] [CrossRef]
- Koenigsknecht-Talboo, J.; Landreth, G.E. Microglial Phagocytosis Induced by Fibrillar β-Amyloid and IgGs Are Differentially Regulated by Proinflammatory Cytokines. J. Neurosci. 2005, 25, 8240–8249. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.F. β-Amyloid is an Immunopeptide and Alzheimer’s is an Autoimmune Disease. Curr. Alzheimer Res. 2021, 18, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.F. Alzheimer’s disease as an innate autoimmune disease (AD2): A new molecular paradigm. Alzheimers Dement. 2022. early view. [Google Scholar] [CrossRef]
- Meier-Stephenson, F.S.; Meier-Stephenson, V.C.; Carter, M.D.; Meek, A.R.; Wang, Y.; Pan, L.; Chen, Q.; Jacobo, S.; Wu, F.; Lu, E.; et al. Alzheimer’s disease as an autoimmune disorder of innate immunity endogenously modulated by tryptophan metabolites. Alzheimers Dement. 2022, 8, e12283. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savonije, K.; Weaver, D.F. The Role of Tryptophan Metabolism in Alzheimer’s Disease. Brain Sci. 2023, 13, 292. https://doi.org/10.3390/brainsci13020292
Savonije K, Weaver DF. The Role of Tryptophan Metabolism in Alzheimer’s Disease. Brain Sciences. 2023; 13(2):292. https://doi.org/10.3390/brainsci13020292
Chicago/Turabian StyleSavonije, Karl, and Donald F. Weaver. 2023. "The Role of Tryptophan Metabolism in Alzheimer’s Disease" Brain Sciences 13, no. 2: 292. https://doi.org/10.3390/brainsci13020292
APA StyleSavonije, K., & Weaver, D. F. (2023). The Role of Tryptophan Metabolism in Alzheimer’s Disease. Brain Sciences, 13(2), 292. https://doi.org/10.3390/brainsci13020292





