Investigation of Chinese Herbal Decoctions with Enzymatic Hydrolysis and Sequential Fermentation as Potential Nutrient Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Herbal Materials
2.2. Preparation of Herbal Decoctions
2.3. Sample Preparation
2.4. Strains and Culture Conditions
2.5. In Vitro Antioxidative Assays
2.6. Analysis of Volatile Compounds
2.7. Examination of Total Flavonoids, Saponins, and Crude Polysaccharides
2.8. Measurement of Amino Acids
2.9. Untargeted Metabonomic Analysis
2.10. Animals and Treatments
2.11. Gut Microbiota Analysis
2.12. Statistical Analysis
3. Results
3.1. Determination of Functional Components
3.2. Determination of Amino Acids
3.3. Determination of Flavor Profiles and Herbal Compositions from Different Treatments
- •
- Volatile Gallery Plot and the Herbal Compositions of Samples with Enzymatic Hydrolysis
- •
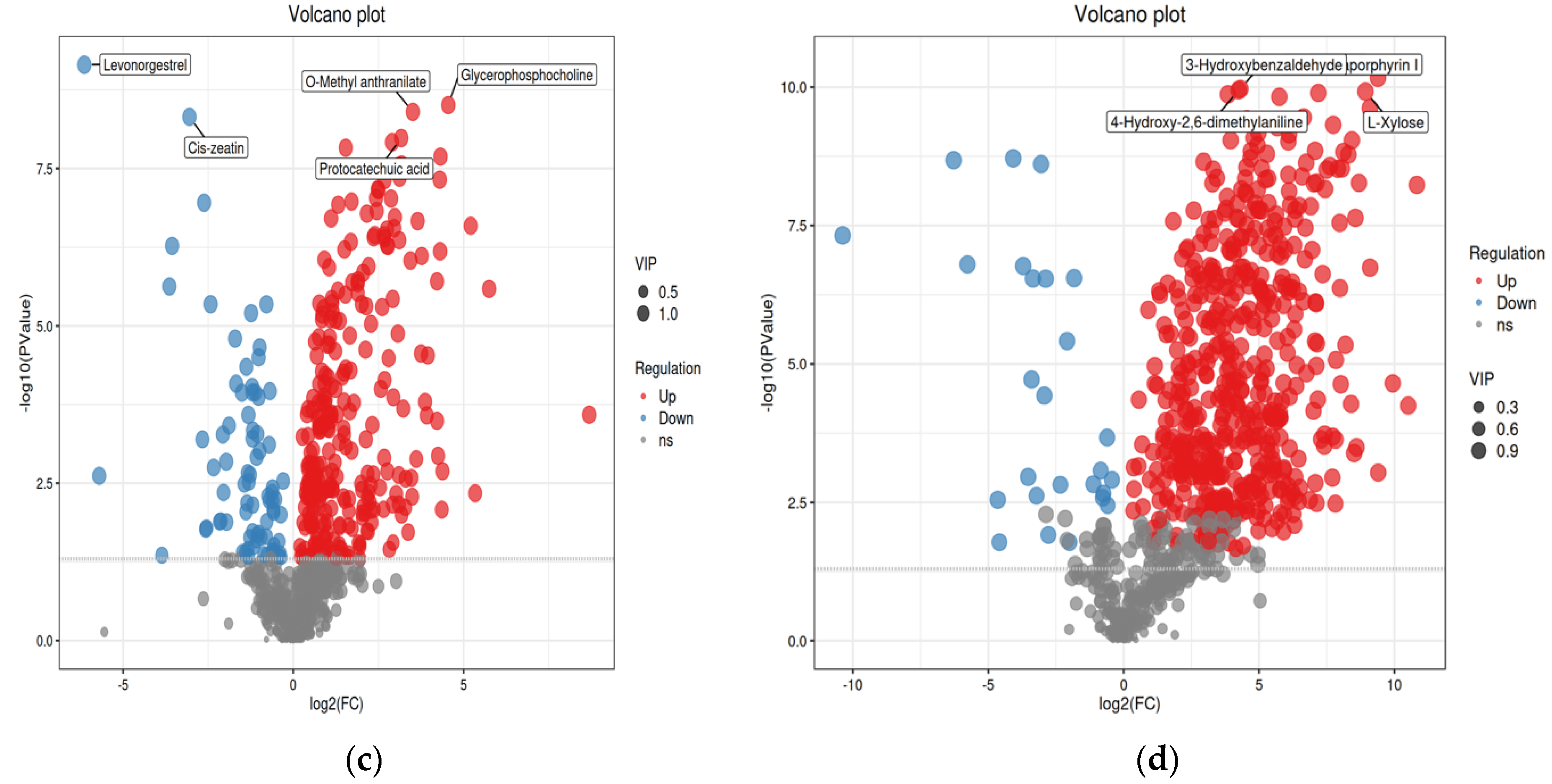
- Herbal compositions were identified by UHPLC-Q-TOF/MS. There were 115 compounds upregulated and 241 downregulated when comparing group #3 to group #2; 226 compounds were upregulated and 169 were downregulated when comparing group #4 to group #2. With different fermentation strategies in group #3 and group #4, 277 compounds were upregulated and 80 downregulated. These differential compounds were visualized in the volcano plots (p < 0.05, FC > 1.2 and VIP > 1, Figure 4).
- •
- Volatiles and Differential Compounds from Direct Plant Extraction and Probiotic Fermentation
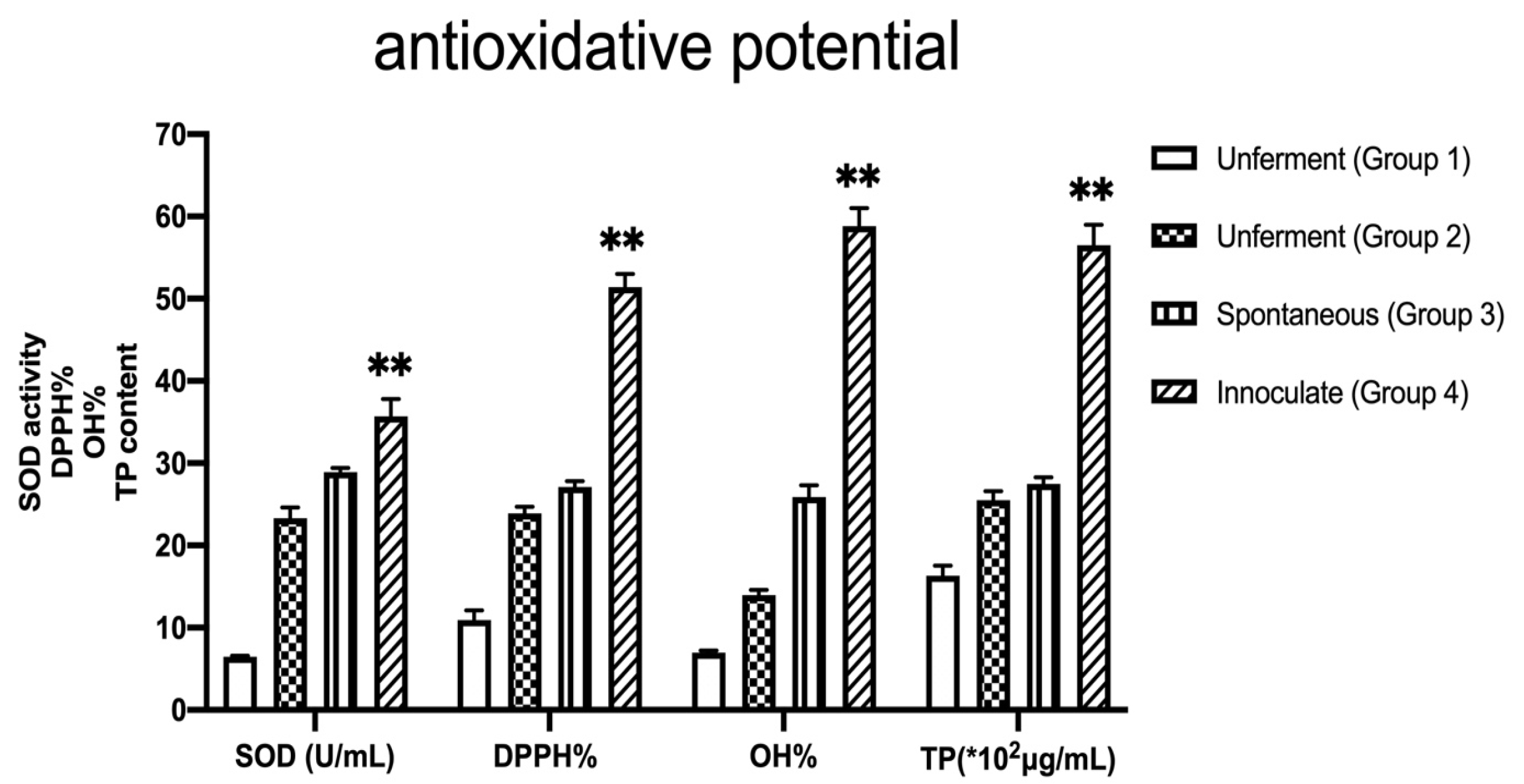
3.4. Antioxidant Capacities of Different Herbal Treatments
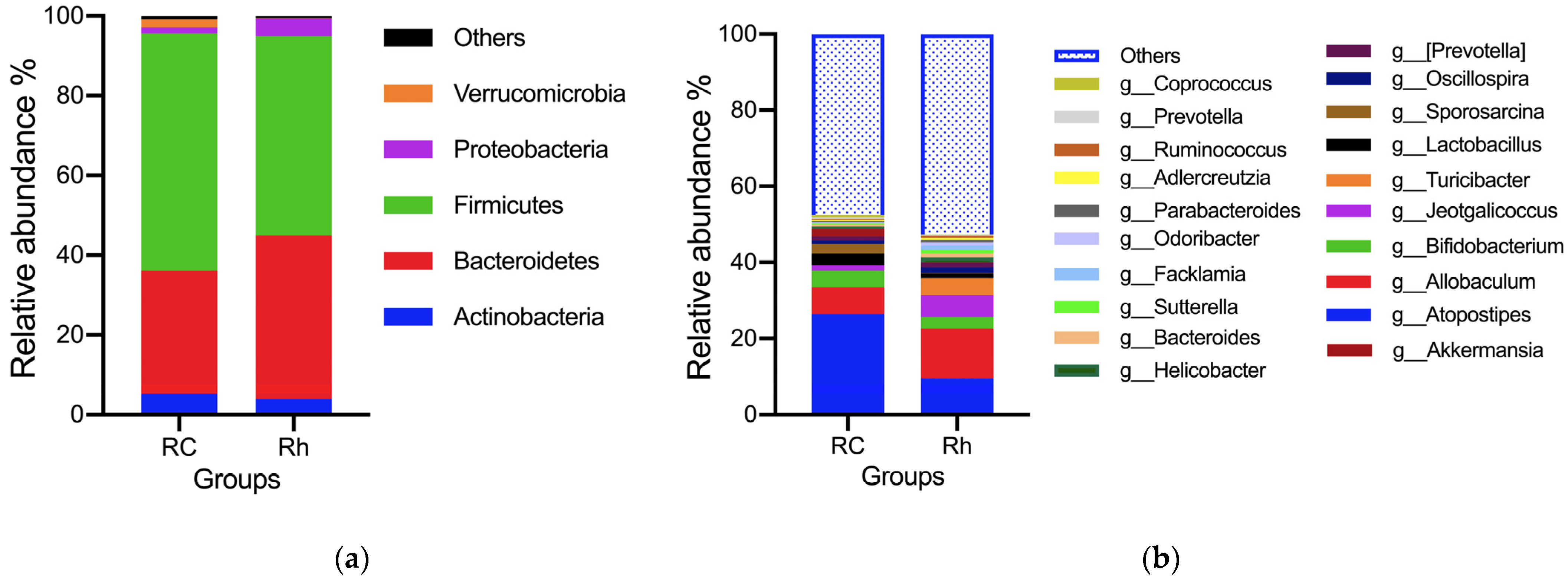
3.5. Gut Microbiota Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.-Y.; Zhang, H.; Chen, L.; Shan, L.-H.; Fan, G.-W.; Gao, X.-M. Liquorice, A Unique “Guide Drug” of Traditional Chinese Medicine: A Review of Its Role in Drug Interactions. J. Ethnopharmacol. 2013, 15, 781–790. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.-G.; Singer, A.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-X.; Xu, F.; Shang, M.-Y.; Wang, X.; Cai, S.-Q. The Relative Content and Distribution of Absorbed Volatile Organic Compounds in Rats Administered Asari Radix et Rhizoma Are Different between Powder- and Decoction-Treated Groups. Molecules 2020, 25, 4441. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef]
- Gong, J.; Yin, F.; Hou, Y.; Yin, Y. Review: Chinese herbs as alternatives to antibiotics in feed for swine and poultry production: Potential and challenges in application. Can. J. Anim. Sci. 2014, 94, 223–241. [Google Scholar] [CrossRef]
- Huang, C.; Li, Z.-X.; Wu, Y.; Huang, Z.-Y.; Hu, Y.; Gao, J. Treatment and bioresources utilization of traditional Chinese medicinal herb residues: Recent technological advances and industrial prospect. J. Environ. Manag. 2021, 299, 113607. [Google Scholar] [CrossRef]
- Fu, Y.-Q.; Wei, X.-Z.; Wang, Y.; Wang, H.; Lou, Z.-H. The acute toxicity appraises of the Asarum Heterotropoides fr. schmidt var. Mandshuricum (Maxim.) kitag, Asarum Sieboldii Miq. and Asarum Sieboldii Miq. var. seoulense nakai. Asia Pac. Tradit. Med. 2010, 6, 23–25. Available online: https://en.cnki.com.cn/Article_en/CJFDTOTAL-YTCT201012008.htm (accessed on 11 January 2023).
- Su, J.; Zhu, Q.; Zhao, Y.; Han, L.; Yin, Y.; Blachier, F.; Wang, Z.; Kong, X. Dietary Supplementation With Chinese Herbal Residues or Their Fermented Products Modifies the Colonic Microbiota, Bacterial Metabolites, and Expression of Genes Related to Colon Barrier Function in Weaned Piglets. Front. Microbiol. 2018, 9, 3181. [Google Scholar] [CrossRef]
- Lin, Z.; Ye, L.; Li, Z.; Huang, X.; Lu, Z.; Yang, Y.; Xing, H.; Bai, J.; Ying, Z. Chinese herb feed additives improved the growth performance, meat quality, and nutrient digestibility parameters of pigs. Anim. Model. Exp. Med. 2020, 3, 47–54. [Google Scholar] [CrossRef]
- Chen, G.; Li, Z.; Liu, S.; Tang, T.; Chen, Q.; Yan, Z.; Peng, J.; Yang, Z.; Zhang, G.; Liu, Y.; et al. Fermented Chinese Herbal Medicine Promoted Growth Performance, Intestinal Health, and Regulated Bacterial Microbiota of Weaned Piglets. Animals 2023, 13, 476. [Google Scholar] [CrossRef]
- Li, Y.; Sun, T.; Hong, Y.; Qiao, T.; Wang, Y.; Li, W.; Tang, S.; Yang, X.; Li, J.; Li, X.; et al. Mixture of Five Fermented Herbs (Zhihuasi Tk) Alters the Intestinal Microbiota and Promotes the Growth Performance in Piglets. Front. Microbiol. 2021, 12, 725196. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The Application of Fermentation Technology in Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Lu, C.-C.; Chang, C.-J.; Lin, C.-S.; Lai, H.-C.; Young, J.D. Immunomodulatory Properties of Plants and Mushrooms. Trends Pharmacol. Sci. 2017, 38, 967–981. [Google Scholar] [CrossRef]
- Maleš, I.; Pedisić, S.; Zorić, Z.; Elez-Garofulić, I.; Repajić, M.; You, L.; Vladimir-Knežević, S.; Butorac, D.; Dragović-Uzelac, V. The medicinal and aromatic plants as ingredients in functional beverage production. J. Funct. Foods 2022, 96, 105210. [Google Scholar] [CrossRef]
- Etheridge, C.J.; Derbyshire, E. Herbal infusions and health: A review of findings from human studies, mechanisms and future research directions. Food Sci. Nutr. 2020, 50, 969–985. [Google Scholar] [CrossRef]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef]
- Lin, B.; Guo, S.; Hong, X.; Jiang, X.; Li, H.; Li, J.; Guo, L.; Li, M.; Chen, J.; Huang, B.; et al. Profiling of the Compounds in the Chinese herbal Formula Li Chang Decoction by UPLC-QTOF/MS. Evid.-Based Complement. Altern. Med. 2022, 2022, 1–22. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Azmin, S.N.H.M.; Manan, Z.A.; Alwi, S.R.W.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal Processing and Extraction Technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Lu, J.; Jing, Y.; Li, M.; Cao, J.; Bian, B.; Hu, C. Seeing the unseen of Chinese herbal medicine processing (Paozhi): Advances in new perspectives. Chin. Med. 2018, 13, 4. [Google Scholar] [CrossRef]
- Sun, Y.-J.; Rukeya, J.; Tao, W.-Y.; Sun, P.-L.; Ye, X.-Q. Bioactive compounds and antioxidant activity of wolfberry infusion. Sci. Rep. 2017, 7, 40605. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, H.; Yin, H.; Zhang, Q. Characteristics of Inoculated Multi-Stage Apple Fermentation Over Spontaneous Fermentation for the Production of Traditional Functional Beverage Jiaosu: Antioxidant, Antibacterial Activities and Metabolic Changes. Curr. Res. Nutr. Food Sci. J. 2021, 9, 606–617. [Google Scholar] [CrossRef]
- Peng, L.-X.; Zou, L.; Tan, M.-L.; Deng, Y.-Y.; Yan, J.; Yan, Z.-Y.; Zhao, G. Free amino acids, fatty acids and phenolic compounds in Tartary buckwheat of different hull colour. Czech J. Food Sci. 2017, 35, 214–222. [Google Scholar] [CrossRef]
- Liu, R.-H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, C.; Bao, R.; Liu, M.; Lv, J.; Yang, Z.; Xu, W.; Liang, H.; Ji, C.; Li, S.; et al. Effects of flavourzyme addition on physicochemical properties, volatile compound components and microbial community succession of Suanzhayu. Int. J. Food Microbiol. 2020, 334, 108839. [Google Scholar] [CrossRef]
- FAO/WHO. Protein and Amino Acid Requirements in Human Nutrition. In Report of A Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series No. 935; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Lenis, N.P.; Van Diepen, H.T.M.; Bikker, P.; Jongbloed, A.W.; Van Der Meulen, J. Effect of the ratio between essential and nonessential amino acids in the diet on utilization of nitrogen and amino acids by growing pigs. J. Anim. Sci. 1999, 77, 1777–1787. [Google Scholar] [CrossRef]
- Park, M.K.; Kim, Y.-S. Distinctive Formation of Volatile Compounds in Fermented Rice Inoculated by Different Molds, Yeasts, and Lactic Acid Bacteria. Molecules 2019, 24, 2123. [Google Scholar] [CrossRef]
- Zeng, X.; Xia, W.; Jiang, Q.; Xu, Y.; Fan, J. Contribution of Mixed Starter Cultures to Flavor Profile of Suanyu—A Traditional Chinese Low-Salt Fermented Whole Fish. J. Food Process. Preserv. 2017, 41, e13131. [Google Scholar] [CrossRef]
- Zhang, L.; Mi, S.; Liu, R.-B.; Sang, Y.-X.; Wang, X.-H. Evaluation of Volatile Compounds during the Fermentation Process of Yogurts by Streptococcus thermophilus Based on Odor Activity Value and Heat Map Analysis. Int. J. Anal. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, W. Formation of Aldehyde and Ketone Compounds during Production and Storage of Milk Powder. Molecules 2012, 17, 9900–9911. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; DiBaise, J.K.; Ilhan, Z.E.; Crowell, M.D.; Rideout, J.R.; Caporaso, J.G.; Rittmann, B.E.; Krajmalnik-Brown, R. Gut microbial and short-chain fatty acid profiles in adults with chronic constipation before and after treatment with lubiprostone. Anaerobe 2015, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, L.; Guan, H.; Tong, S.; Liu, M.; Liu, C.; Zhang, Z.; Du, C.; Li, P. Effects of taurocholic acid on immunoregulation in mice. Int. Immunopharmacol. 2013, 15, 217–222. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, V.L.; Mateus, M.L.; Aschner, M.; Dos Santos, A.M. Assessment of Occupational Exposures to Multiple Metals with Urinary Porphyrin Profiles. J. Integr. OMICS 2018, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, P.W.M.; Melo, M.B.; Lima, R.D.F.; Gavioli, M.; Santiago, N.M.; Greco, L.; Jesus, I.C.; Nocchi, E.; Parreira, A.; Alves, M.N.; et al. Beneficial Effects of Angiotensin-(1–7) Against Deoxycorticosterone Acetate–Induced Diastolic Dysfunction Occur Independently of Changes in Blood Pressure. Hypertension 2015, 66, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Shivanandappa, T.; Zarei, M. Natural bioactive 4-Hydroxyisophthalic acid (4-HIPA) exhibited antiproliferative potential by upregulating apoptotic markers in in vitro and in vivo cancer models. Mol. Biol. Rep. 2020, 47, 5343–5353. [Google Scholar] [CrossRef]
- Li, S.; Li, S.-K.; Gan, R.-Y.; Song, F.-L.; Kuang, L.; Li, H.-B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop. Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Liao, W.; Shen, J.; Manickam, S.; Li, S.; Tao, Y.; Li, D.; Liu, D.; Han, Y. Investigation of blueberry juice fermentation by mixed probiotic strains: Regression modeling, machine learning optimization and comparison with fermentation by single strain in the phenolic and volatile profiles. Food Chem. 2023, 405, 134982. [Google Scholar] [CrossRef]
- Zheng, H.; Du, Q.; Yin, J.; Gao, Y. A narrative review on the main chemical constituents and bioactivity of Camellia nitidissima Chi. Longhua Chin. Med. 2022, 5, 1–17. [Google Scholar] [CrossRef]
- Son, C.-G.; Lee, S.-K.; Choi, I.-K.; Jang, E.-S.; Bang, K.-J. Herbal Transformation by Fermentation. J. Acupunct. Meridian Stud. 2020, 13, 167–168. [Google Scholar] [CrossRef]
- Pan, Y.; Li, H.; Chen, X.; Fan, Y.; Zhang, B.; Liu, R.; Deng, Z.-Y. Antioxidant interactions between hydrophilic and lipophilic phytochemicals are influenced by their ratios and total concentrations. Food Biosci. 2021, 45, 101465. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yamashita, T.; Osone, T.; Hosooka, T.; Shinohara, M.; Kitahama, S.; Sasaki, K.; Sasaki, D.; Yoneshiro, T.; Suzuki, T.; et al. Bacteroides spp. promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. Iscience 2021, 24, 103342. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Bradley, P.H.; Pollard, K.S. Proteobacteria explain significant functional variability in the human gut microbiome. Microbiome 2017, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Z.; Wang, B.; He, X.; Tang, J.; Peng, W.; Zhou, J.; Wang, Y. Flammulinavelutipes Polysaccharides Modulate Gut Microbiota and Alleviate Carbon Tetrachloride-Induced Hepatic Oxidative Injury in Mice. Front. Microbiol. 2022, 13, 847653. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef]
- Derrien, M.; Turroni, F.; Ventura, M.; van Sinderen, D. Insights into endogenous Bifidobacterium species in the human gut microbiota during adulthood. Trends Microbiol. 2022, 30, 940–947. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Sun, Y.; Ip, L.Y.T.; Wang, L.; Chan, F.K.L.; Miao, Y.; Ng, S.C. Prevotella species in the human gut is primarily comprised of Prevotella copri, Prevotella stercorea and related lineages. Sci. Rep. 2022, 12, 9055. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Usyk, M.; Vázquez-Baeza, Y.; Chen, G.-C.; Isasi, C.R.; Williams-Nguyen, J.S.; Hua, S.; McDonald, D.; Thyagarajan, B.; Daviglus, M.L.; et al. Microbial co-occurrence complicates associations of gut microbiome with US immigration, dietary intake and obesity. Genome Biol. 2021, 22, 336. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, Y.; Wang, J.; Li, P.; Duan, Y.; Dai, H.; An, Y.; Cheng, L.; Wang, T.; Wang, C.; et al. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomed. Pharmacother. 2020, 124, 109873. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Xu, H.; Liu, Y.; Qi, F.; Zhang, W.; Chen, H.; Wang, C.; Wang, Y.; Yang, W.; Li, S. Mutagenesis and redox partners analysis of the P450 fatty acid decarboxylase OleTJE. Sci. Rep. 2017, 7, 44258. [Google Scholar] [CrossRef]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Turicibacter Modifies Host Bile Acids and Lipids in A Strain-Specific Manner. BioRxiv 2022, 27, 497673. [Google Scholar] [CrossRef]
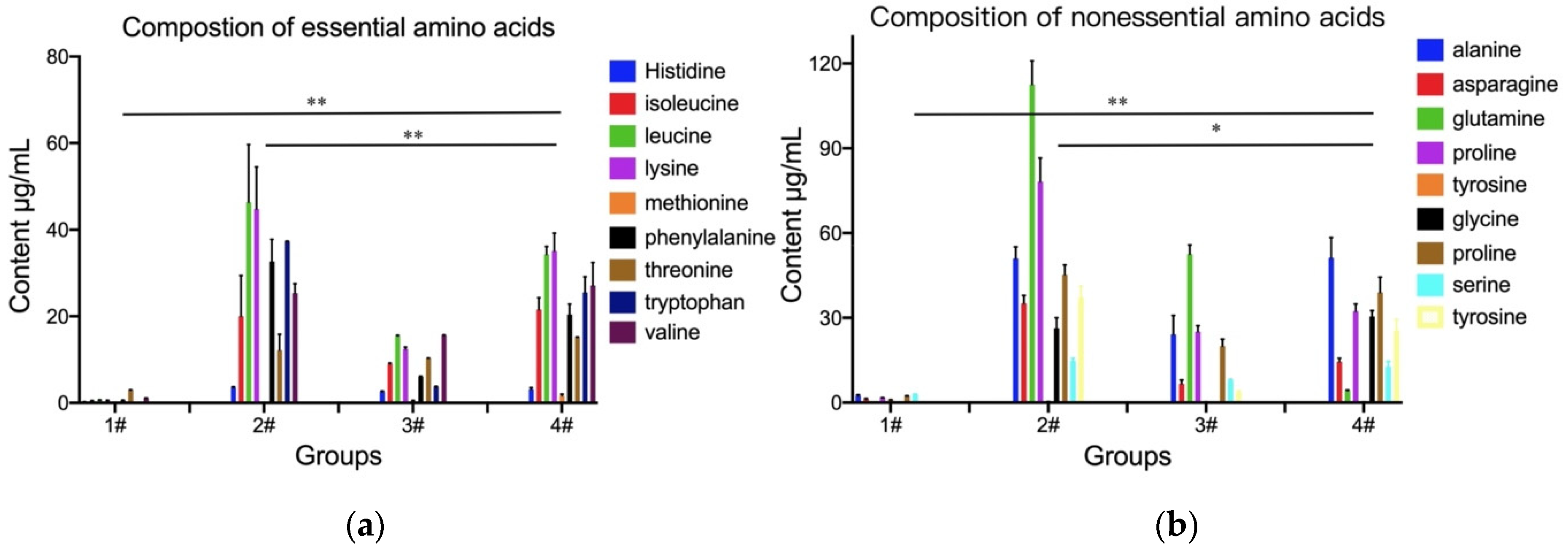
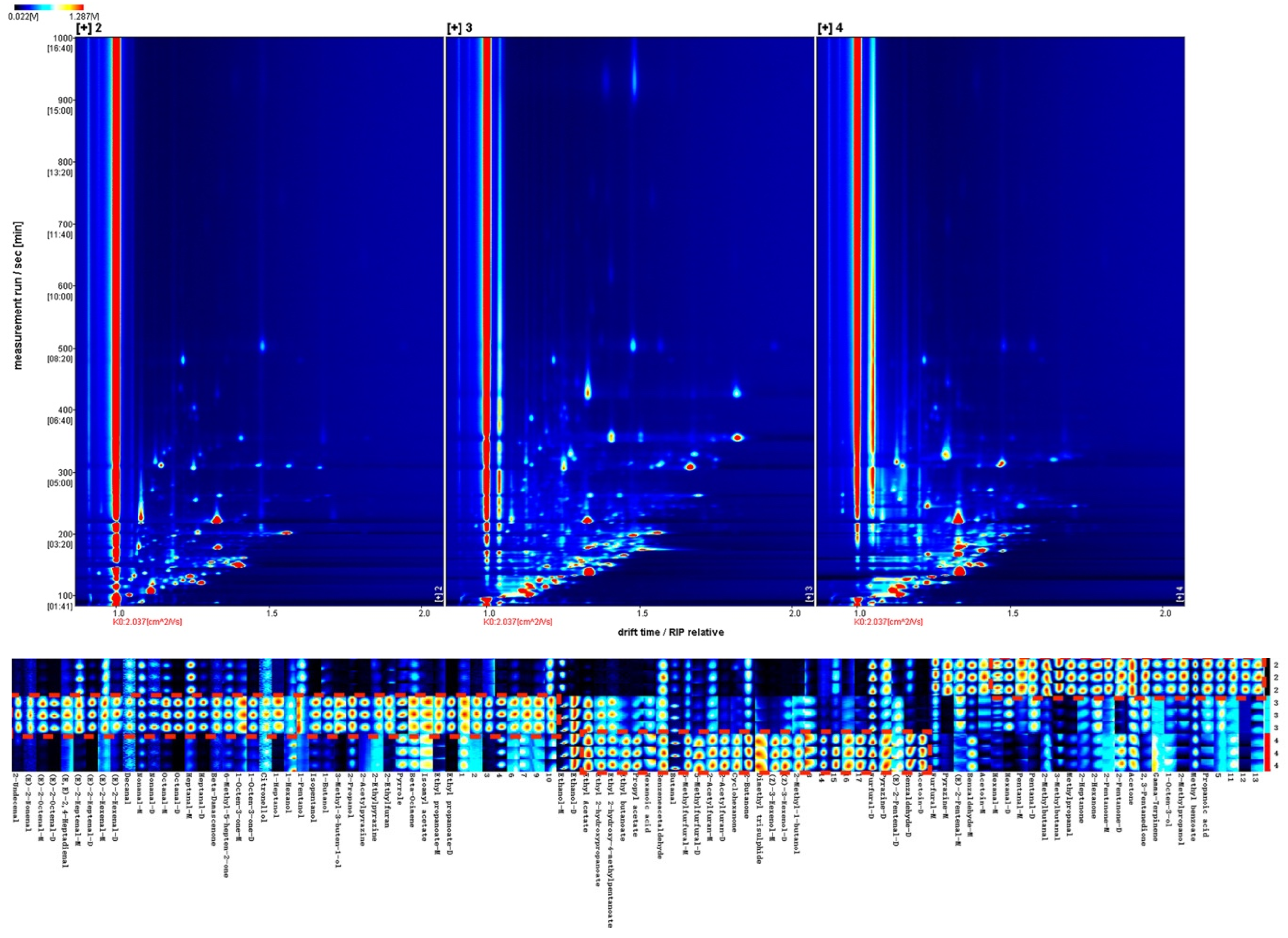
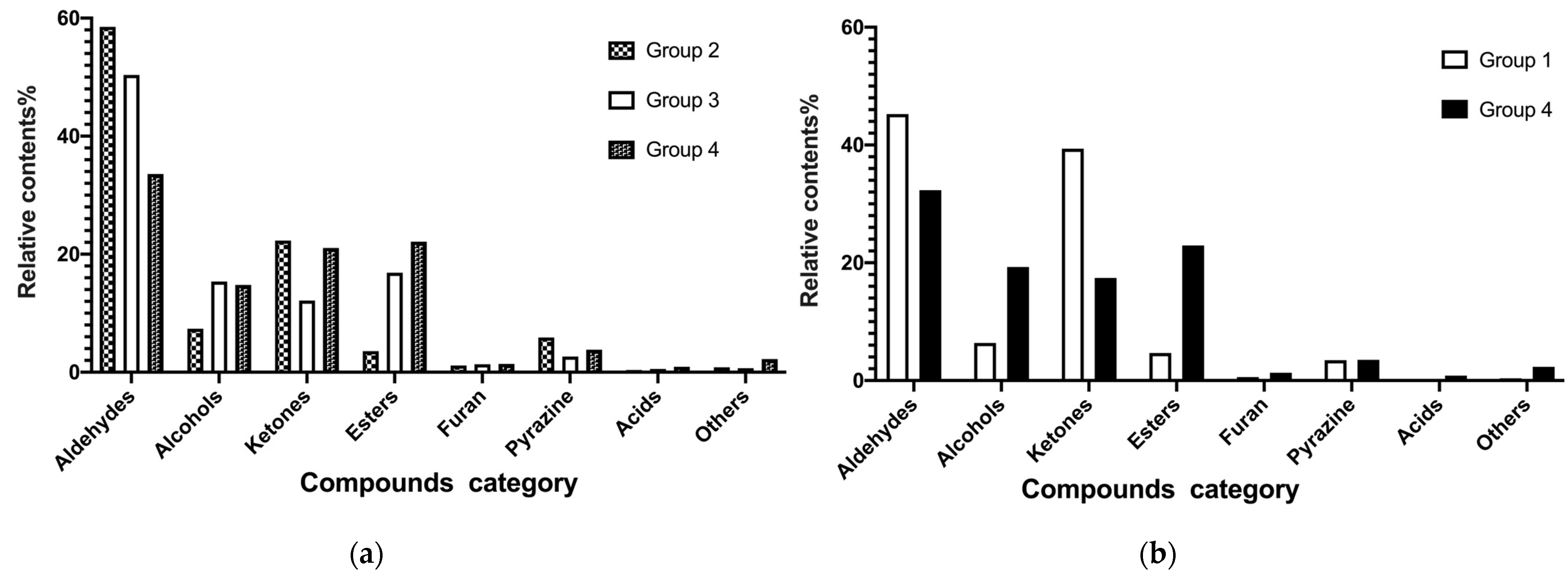
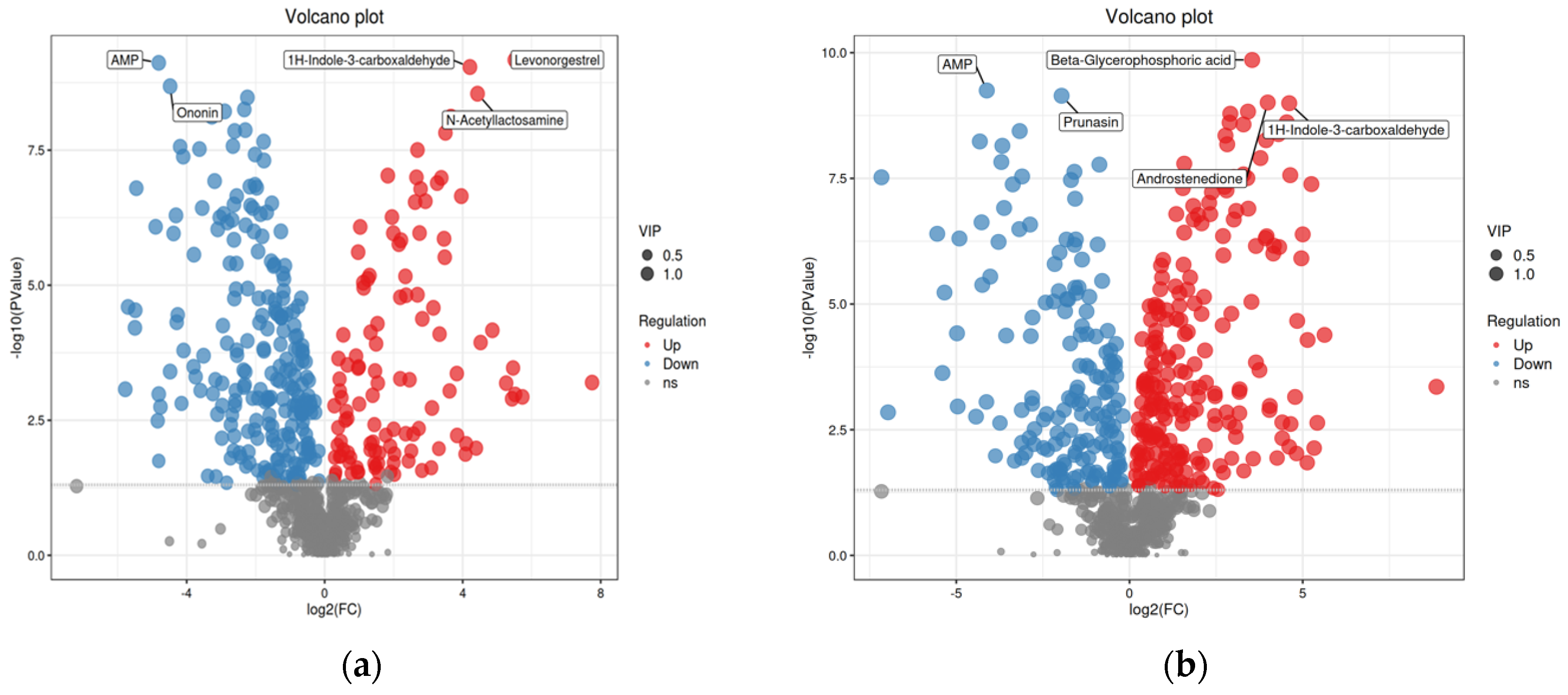

| Treatment (Group ID) | Extraction Procedure | Final Concentration |
|---|---|---|
| Direct extraction (DE, #1) | Ethanol maceration, then hot water decoction | 1 kg/L |
| Standard decoction (SD, #2) | Water maceration with enzymes, then hot water decoction | 1 kg/L |
| Spontaneous fermentation (SF, #3) | Water maceration with enzymes, then ferment with endogenous microorganisms, hot water decoction | 1 kg/L |
| Inoculated fermentation (IF, #4) | Water maceration with enzymes, hot water decoction, filtration to separate herbal residue, herbal solution ferment with probiotic inoculums | 1 kg/L |
| HS-GC-IMS Unit | Parameters |
|---|---|
| Analysis time (sample reading) | 20 min |
| Chromatographic Column Type | MXT-5, 15 m × 0.53 mm × 1 μm |
| Column temperature | 60 °C |
| Drift gas/carrier gas | N2 (99.999% purity) |
| IMS temperature | 45 °C |
| Sample injection volume | 200 μL |
| Incubation time | 15 min |
| Incubation temperature | 60 °C |
| Temperature of the injection syringe | 65 °C |
| Rotary incubation speed | 500 rpm |
| Sample ID | Flavonoids (g/kg) | Saponins Re (%) | Crude Polysaccharide (g/L) |
|---|---|---|---|
| #1 | 0.56 ± 0.12 | 5.50 ± 0.70 | 0.93 ± 0.10 |
| #2 | 4.27 ± 0.33 ** | 39.02 ± 2.19 ** | 6.54 ± 0.34 ** |
| #3 | 1.77 ± 0.18 ** | 1.61 ± 0.21 ** | 3.91 ± 0.11 ** |
| #4 | 4.07 ± 1.05 ** | 25.30 ± 2.26 ** | 7.50 ± 0.85 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Zhang, H.; Wang, Y. Investigation of Chinese Herbal Decoctions with Enzymatic Hydrolysis and Sequential Fermentation as Potential Nutrient Supplements. Appl. Sci. 2023, 13, 2154. https://doi.org/10.3390/app13042154
Han S, Zhang H, Wang Y. Investigation of Chinese Herbal Decoctions with Enzymatic Hydrolysis and Sequential Fermentation as Potential Nutrient Supplements. Applied Sciences. 2023; 13(4):2154. https://doi.org/10.3390/app13042154
Chicago/Turabian StyleHan, Shanshan, Hongyan Zhang, and Yongliang Wang. 2023. "Investigation of Chinese Herbal Decoctions with Enzymatic Hydrolysis and Sequential Fermentation as Potential Nutrient Supplements" Applied Sciences 13, no. 4: 2154. https://doi.org/10.3390/app13042154
APA StyleHan, S., Zhang, H., & Wang, Y. (2023). Investigation of Chinese Herbal Decoctions with Enzymatic Hydrolysis and Sequential Fermentation as Potential Nutrient Supplements. Applied Sciences, 13(4), 2154. https://doi.org/10.3390/app13042154






