Vitreous Substitutes from Bench to the Operating Room in a Translational Approach: Review and Future Endeavors in Vitreoretinal Surgery
Abstract
:1. Introduction
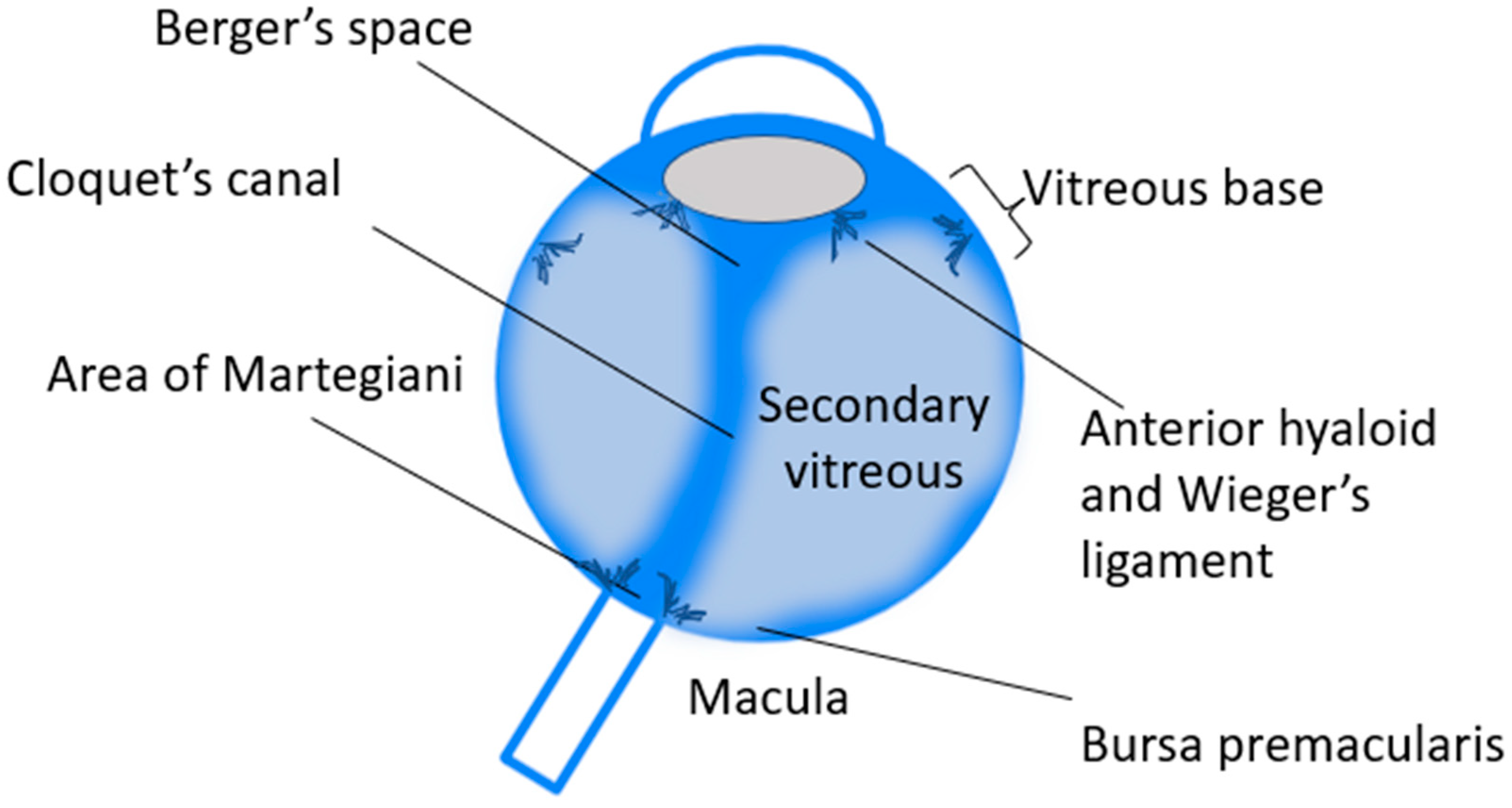
2. The Anatomy of the Vitreous Body: General Principles
- Viscosity: 300–2000 cP;
- pH: 7.40–7.52;
- Density: 1.0053–1.008 g/cm3;
- Intraocular pressure (IOP) maintenance to less than 20 mmHg;
- Osmolality: 304 mOsm;
- Hydrophilic and insoluble in water;
- Easy to remove after surgery, if required;
- Provide support to intraocular tissues and maintains its proper position;
- Allow proper diffusion of ions, electrolytes, and other molecules (oxygen, lactic acid, and ascorbic acid);
- Easy to inject through a small syringe during vitrectomy surgery;
- Inert;
- Biocompatible;
- Transparent;
- Non-absorbable if required to be kept indefinitely or for a very long time.
3. Physical Properties of the Currently Available Vitreous Substitutes and Clinical Correlates
- Specific Gravity and Density
- b.
- Buoyancy
- c.
- Interfacial Tension
- d.
- Viscosity
4. Chemical Properties of the Currently Available Tamponades and Clinical Correlates
- Densiron 68. Perfluorohexyloctane, 30.5% SFA F6H8 and 69.5% SiO 5000 cSt are combined to create this compound (Fluoron Co, Ulm, Germany). By adding SiO, F6H8’s viscosity rises from 2.5 to over 1400 mPa·s, decreasing its tendency to disperse, which is thought to be the root of the issues associated with long-term usage of F6H8. It has a refractive index of 1.387 and a specific gravity of 1.06 g/mL [99,100].
- Oxane HD. A blend of 88.1% Oxane 5700, a 5000 mPa SiO, and 11.9% RMN3, a partly fluorinated olefin, makes up this compound (Bausch & Lomb, Toulouse, France). Its specific gravity is only about 1.02–1.04 g/mL. It is the least heavy HSiO of the three compounds and has the highest viscosity (3800 mPa·s) [101,102,103].
- HWS 46-3000. The most recent HSiO to reach the market is known as HWS 46-3000, and it is composed of 55% perfluorobutylhexane (F4H6), a semifluorinated alkane with viscosity of 1.28 mPa, and 45% ultrapurified SiO 100,000 (viscosity 97,100 mPa·s and specific gravity 0.977 g/cm3) [97]. The resultant solution is homogenous and stable in the presence of aqueous humor, air, or PFCLs, with a specific gravity of 1.105 g/mL and a viscosity of 3109 mPa·s. Of the three HSiOs, it is the heaviest and most viscous [97,98]. In a pilot trial, HWS 46-3000 was used as a long-term tamponade (1–3 months), and although it is a compound with increased viscosity, which could make this compound difficult to handle, a good success and low complication rates could be observed [98].
5. Classification of the Vitreous Substitutes: General Principles, Past and Future Attempts and Clinical Correlates
- Gases, such as air and expansile gases;
- Liquids, such as BSS, PFCL, SFAs and SiO;
- Gases
- AirThe air in the vitreous cavity is inert and colorless. Ohm utilized it for the first time to treat RD in 1911 [59,112]. Air has the advantage of being always available at no cost, but it needs to be purified via a filter before being injected into the vitreous cavity to avoid contamination [113]. Since air remains in the vitreous chamber only for a few days before being replaced by aqueous humor, it has the clinical advantage of a rapid visual rehabilitation and for this purpose has been largely utilized in the vitreoretinal surgery for RDs and macular holes [114,115,116,117,118]. However, this could be considered a disadvantage as a vitreous replacement due to its short time effect, especially in case of PVR or complex RDs. In these cases, a clear superiority of longer lasting tamponade effect has been demonstrated [119,120,121,122]. Another negative aspect of air is its low refractive index, which results in total light reflection and hence poor optical performance [123]. Air is generally used as a tamponade for RDs with upper retinal breaks [66,124,125], but it has also been recently shown to have a role in inferior retinal breaks [118].
- Expansile GasesIntraocular gas tamponades have been an integral aspect of vitreoretinal surgery since the early 1970s, when E.W. Norton reported the use of SF6 as a vitreous substitute [126]. Today, SF6, C2F6, and C3F8 are being used to treat a variety of vitreoretinal disorders. These gases are heavier than air, colorless, odorless, harmless and have different lasting times in the eye (Table 1). According to Kontos et al. the mean duration for 30% SF6 in the fluid-filled vitreous chamber is equal to 18.0 days with a standard deviation of ±2.6 days. The mean duration for 20% C2F6 is 34.5 days with a standard deviation of ±3.3 days. Finally, the mean duration for 15% C3F8 is 67.7 days with a standard deviation of ±5.5 days [75]. This is clinically important because the longer the tamponading agent persists into the eye, the longer the retina is supported, the displacement of the proinflammatory aqueous humor is carried out and the passage of fluid via the retinal breaks is blocked [1]. Within approximately a couple of days, pure SF6 increases to twice its initial volume, whereas C2F6 grows to roughly three times and C3F8 to about four times their respective initial volumes [127]. The knowledge of this effect is also important in surgical practice when the surgeon is confronted with subretinal fluid or choroidal effusion. The ensuing underfilling of the vitreous chamber at the end of the operation can be overcome by a slightly higher gas concentration titrated on the specific gas’s expandability [74,128,129,130]. Furthermore, it has been shown that quick variations in ambient air pressure cause significant changes in intraocular pressure and therefore patients are recommended to postpone flying travel and stay away from high altitudes for approximately 2 weeks, 4 weeks, and 6 weeks after receiving SF6, C2F6, and C3F8, respectively [76,131]. Extreme caution is recommended in these cases of expansile gas tamponade as the risk of post-operative ocular hypertension could lead to dreadful consequences such as central retinal artery occlusion, among others [132,133,134]. Finally, thanks to the buoyancy that characterizes gases, the surgeon can keep on treating the patient for an optimal subretinal fluid expression, as happens in the steamroller maneuver [135], for displacing the subretinal fluid from the macula (face-down positioning) [136] and for allowing an optimal retinochoroidal contact to develop into a firm adhesion. In fact, chorioretinal adhesions caused by cryopexy or laser retinopexy have been described to take between 2 and 4 weeks to reach the maximum strength of adhesion, and in order to occur, a retinochoroidal contact needs to be established and maintained [137,138,139].
- Liquids
- Perfluorocarbon Liquid (PFCL)Various types of PFCLs have been used in vitreoretinal surgery, such as perfluorooctane (PFO), perfluoroperhydrophenanthrene (Vitreon), perfluorodecalin (PFD), and perfluorotributylamide (PFTB) [9,140]. The PFCLs are a class of fluorochemicals in which all the hydrogen atoms have been replaced by fluorine, and have been artificially produced to exhibit high specific gravities between 1.76 and 2.03, [140] with low surface tension and low viscosity as defining characteristics [141]. Such a high specific gravity is useful in flattening the retina up from the posterior pole, at the same time expressing the subretinal fluid out of the macula through the retinal breaks and at the same time anteriorizing the peripheral vitreous for an easier removal [142,143]. One of the main disadvantages of PFCLs is that they are approved only for intra-operative use only, since many animal studies have indicated that leaving PFCL in the vitreous cavity as a post-operative tamponade agent causes retinal toxicity [144,145,146]. It is not completely clear where PFCL’s toxicity stems from, but it has been shown that it could be a combination of induced inflammation, impurities and chemical toxicity and mechanical trauma, not only in the long term but also acutely [147,148,149,150]. Some of these impurities have been identified as molecules with nitrogen bonds, as well as compounds containing hydrogen and fluoride [151,152]. Nevertheless, there are studies reporting on the use of PFCLs as short-, medium-, and long-term vitreous substitute to exploit their high specific gravity in a way similar to HSiO [153,154,155,156,157].
- Semifluorinated Alkanes-(SFAs)SFAs were introduced in the early 2000s as a novel family of chemicals with excellent characteristics for application in vitreoretinal surgery [99]. They have an index of refraction of 1.3, which is similar to the aqueous humor (1.336) [158,159], they are soluble in PFCL, hydrocarbons, SiOs and contain perfluorocarbon and hydrocarbon side chains. SFAs have a specific gravity of 1.35 g/mL, are inert, and are heavier than the aqueous humor, which is the main characteristic for which they were combined with SiO [99,160]. SFAs have been used alone in the past as a temporary vitreous substitute for maximum of two to three months length of use due to their instability and inflammatory side effect [47,161,162,163]. SFAs today are used only in combination with SiO to produce HSiO and obtain a lower retinal tamponading effect [100,164].
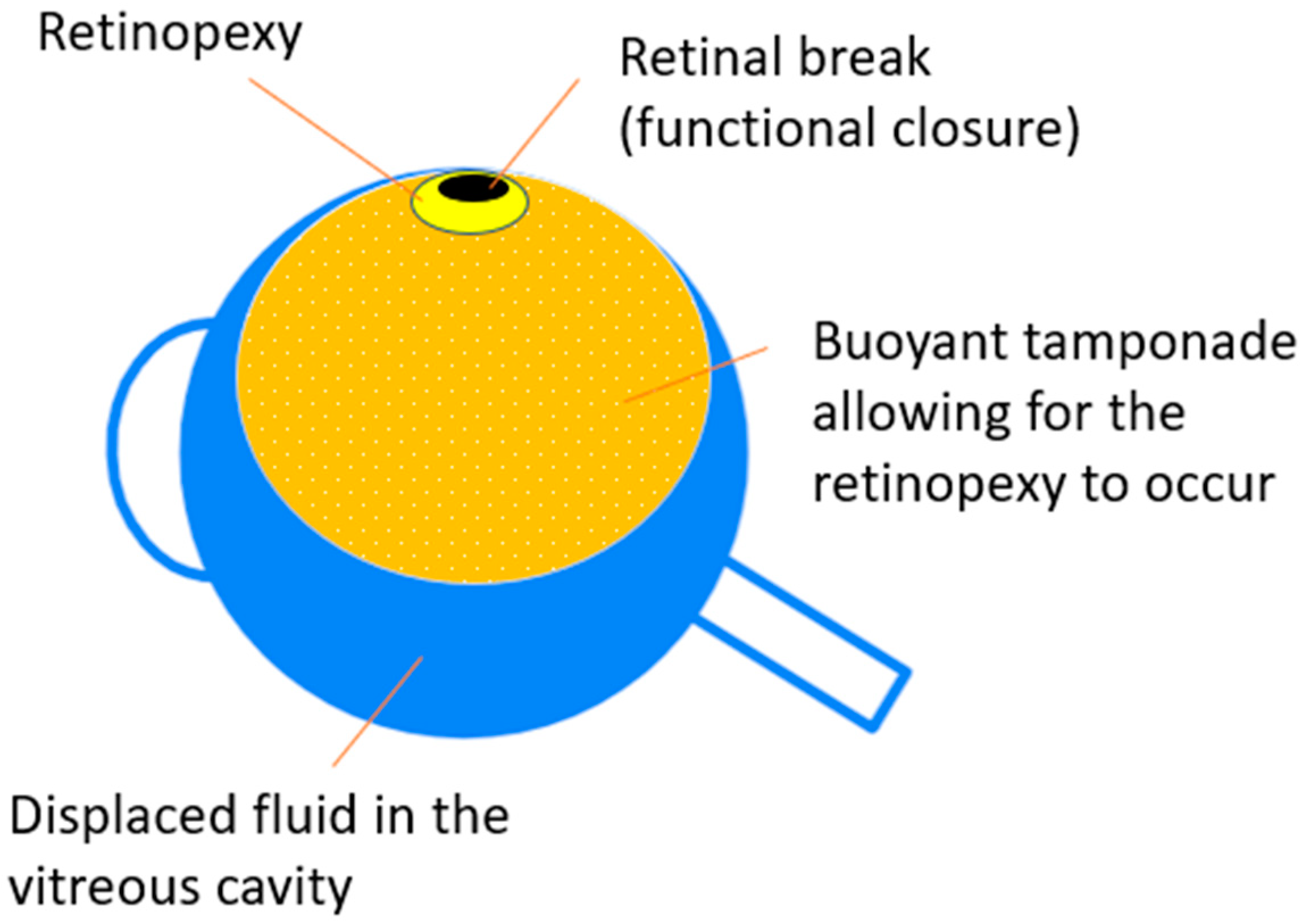
- Silicone Oil (SiO)SiOs have been used as an intraocular tamponade due to its physical qualities of transparency, chemical inertness, high surface tension, and higher interfacial tension than aqueous humor since Cibis et al. introduced it in 1962 [165]. These characteristics made SiO the only material approved by the Food and Drug Administration (FDA, USA) for long-term vitreous replacement [84,86]. Moreover, SiO has a higher refractive index (1.405) as compared to the vitreous (1.336), in contrast to air and gases (about 1.0003) and the understanding of this concept is of clinical importance in the visual rehabilitation of the patient (Figure 5) [166,167].Similarly to what happens with a gas tamponade, SiO is capable of blocking intravitreal fluid from migrating into the subretinal space so that, if retinopexy has been correctly applied, chorioretinal adhesion and scarring can occur [84,86]. Once the retinochoroidal adhesion has developed and there is no longer any retinal traction, SiO can be removed [168].In contrast to gases, SiOs have a lower buoyancy, thus, the SiO bubble’s resting position cannot produce a high pressure against the retinal tear, and the sealing occurs almost exclusively as a result of the strong interfacial tension of the bubble over the break [86].The main clinical difference that sets SiO apart from gases is its ability to persist for a long time into the vitreous chamber, until surgical removal is performed [84]. This feature is crucial when contrasting the tendency of the retina to contract, shorten, and detach, a phenomenon produced by PVR or proliferative diabetic retinopathy (PDR) with residual tractions after surgery [84,169,170]. Moreover, the persistence of SiO in the vitreous chamber allows the eye to contrast the tendency to hypotony and eventually phthisis [171], thus allowing a retinal support when the retinal breaks but could not be adequately treated [172] or when the patient cannot posture properly after the surgery [84].Even though SiO has proved to be safe and effective in the above-mentioned circumstances, it is today demonstrated to have a low grade toxicity and side effects, especially in the long run [173,174]. The main mechanism causing SiO complications has been shown to be emulsification, a process that produces emulsified SiO droplets, which detach from the main SiO bubble and generate ocular inflammation, keratopathy, late-onset glaucoma, retinal toxicity, and optic neuropathy as well as loss of the tamponading capability.
- Heavy Silicone Oil (HSiO)After the first description of SFAs by Meinert and Roy in 2000 [99], the HSiO was created through a combination of unstable, proinflammatory SFAs with high viscosity HSiO to overcome the resting position of the buoyant or ‘’light’’ SiO. Clinically, the HSiOs are mainly utilized as an endotamponade agent for complex RDs, particularly those with inferior breaks and inferior PVR [175]. HSiO has the advantage of tamponading the retina inferiorly without the need of posturing the patient, displacing the ‘’PVR soup’’ from the bottom of the eye and therefore preventing inferior redetachments [102,104,176]. Nevertheless, HSiOs, containing the instable SFA molecules, have the disadvantages of producing inflammation and emulsion [104,177].
- Polymers
6. Limitations of the Current Vitreous Substitutes: Mimicking the Vitreous Body
- a.
- The Physiology of Human Vitreous: What should we mimic?
- b.
- Aging changes of the vitreous body
- c.
- The Vitreous Substitutes in Current Use: Advantages and Disadvantages
7. Vitreous Substitutes as Drug Delivery Agents
8. The Ideal Vitreous Substitute
9. Conclusions and Future Perspectives
10. Methods of Literature Search
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, S.G.; Flynn, H.W.; Lee, W.-H.; Ssemanda, E.; Ervin, A.-M. Tamponade in Surgery for Retinal Detachment Associated with Proliferative Vitreoretinopathy. Cochrane Database Syst. Rev. 2009, CD006126. [Google Scholar] [CrossRef]
- Haave, H.; Petrovski, B.É.; Zając, M.; Lumi, X.; Melekidou, W.; Lytvynchuk, L.; Ruban, A.; Znaor, L.; Nawrocki, J.; Nawrocka, Z.A.; et al. Outcomes from the Retrospective Multicenter Cross-Sectional Study on Lamellar Macular Hole Surgery. Clin. Ophthalmol. 2022, 16, 1847–1860. [Google Scholar] [CrossRef]
- Stene-Johansen, I.; Bragadóttir, R.; Petrovski, B.É.; Petrovski, G. Macular Hole Surgery Using Gas Tamponade—An Outcome from the Oslo Retrospective Cross-Sectional Study. J. Clin. Med. 2019, 8, 704. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.-C.; Hsieh, Y.-T.; Yang, C.-M. Silicone Oil Removal after Extended Tamponade in Proliferative Diabetic Retinopathy—A Long Range of Follow-Up. Eye 2020, 34, 2307–2314. [Google Scholar] [CrossRef]
- Jun, S.Y.; Hwang, D.D.-J. Effect of Vitrectomy with Silicone Oil Tamponade and Internal Limiting Membrane Peeling on Eyes with Proliferative Diabetic Retinopathy. Sci. Rep. 2022, 12, 8076. [Google Scholar] [CrossRef] [PubMed]
- Deobhakta, A.; Rosen, R. Retinal Tamponades: Current Uses and Future Technologies. Curr. Ophthalmol. Rep. 2020, 8, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Tripathy, K. Agents for Vitreous Tamponade; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Williamson, T.H. Principles of Internal Tamponade. In Vitreoretinal Surgery; Williamson, T.H., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 93–133. ISBN 978-3-030-68769-4. [Google Scholar]
- Foster, W.J. Vitreous Substitutes. Expert Rev. Ophthalmol. 2008, 3, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J. The Physical and Surgical Aspects of Silicone Oil in the Vitreous Cavity. Graefe’s Arch. Clin. Exp. Ophthalmol. 1987, 225, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.J.; Chou, T. Physical Mechanisms of Gas and Perfluoron Retinopexy and Sub-Retinal Fluid Displacement. Phys. Med. Biol. 2004, 49, 2989–2997. [Google Scholar] [CrossRef]
- Su, X. A Novel Thermogel Which Encourages Vitreous Regeneration in Retinal Detachments. Available online: https://bioengineeringcommunity.nature.com/posts/48592-a-novel-thermogel-which-encourages-vitreous-regeneration-in-retinal-detachments (accessed on 17 December 2022).
- Ophthalmic Measurements. Available online: https://www.aao.org/young-ophthalmologists/yo-info/article/ophthalmic-measurements (accessed on 12 December 2021).
- Le Goff, M.M.; Bishop, P.N. Adult Vitreous Structure and Postnatal Changes. Eye 2008, 22, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Ishibashi, T. Hyalocytes: Essential Cells of the Vitreous Cavity in Vitreoretinal Pathophysiology? Retina 2011, 31, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ankamah, E.; Sebag, J.; Ng, E.; Nolan, J.M. Vitreous Antioxidants, Degeneration, and Vitreo-Retinopathy: Exploring the Links. Antioxidants 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.N. Embryology of the Vitreous. Congenital and Developmental Abnormalities. In The Vitreous and Vitreoretinal Interface; Schepens, C.L., Neetens, A., Eds.; Springer: New York, NY, USA, 1987; pp. 11–35. ISBN 978-1-4757-1901-7. [Google Scholar]
- Lee, B.; Litt, M.; Buchsbaum, G. Rheology of the Vitreous Body. Part I: Viscoelasticity of Human Vitreous. Biorheology 1992, 29, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-Y.; Fu, Y.; Hui, Y.-N. Vitreous Substitutes: Challenges and Directions. Int. J. Ophthalmol. 2015, 8, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, Q.; Jiang, Z.; Lin, J.; Liu, Y.; Chen, J.; Zhou, L.; Li, H.; Yang, Q.; Wang, T. Biocompatibility and Retinal Support of a Foldable Capsular Vitreous Body Injected with Saline or Silicone Oil Implanted in Rabbit Eyes. Clin. Exp. Ophthalmol. 2012, 40, e67–e75. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Shimizu, K. Posterior Precortical Vitreous Pocket. Arch. Ophthalmol. 1990, 108, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Itakura, H.; Kishi, S.; Li, D.; Akiyama, H. Observation of Posterior Precortical Vitreous Pocket Using Swept-Source Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3102–3107. [Google Scholar] [CrossRef]
- Apple, D.J.; Rabb, M.F. Ocular Pathology: Clinical Applications and Self-Assessment; Vanderbilt University: Nashville, TN, USA, 1998; Available online: https://catalog.library.vanderbilt.edu/discovery/fulldisplay/alma991033533179703276/01VAN_INST:vanui (accessed on 7 November 2022).
- Kagemann, L.; Wollstein, G.; Ishikawa, H.; Gabriele, M.L.; Srinivasan, V.J.; Wojtkowski, M.; Duker, J.S.; Fujimoto, J.G.; Schuman, J.S. Persistence of Cloquet’s Canal in Normal Healthy Eyes. Am. J. Ophthalmol. 2006, 142, 862–864. [Google Scholar] [CrossRef]
- Lamb, T.D.; Collin, S.P.; Pugh, E.N. Evolution of the Vertebrate Eye: Opsins, Photoreceptors, Retina and Eye Cup. Nat. Rev. Neurosci. 2007, 8, 960–976. [Google Scholar] [CrossRef]
- Silva, A.F.; Alves, M.A.; Oliveira, M.S.N. Rheological Behaviour of Vitreous Humour. Rheol. Acta 2017, 56, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Kokavec, J.; Min, S.H.; Tan, M.H.; Gilhotra, J.S.; Newland, H.S.; Durkin, S.R.; Grigg, J.; Casson, R.J. Biochemical Analysis of the Living Human Vitreous. Clin. Exp. Ophthalmol. 2016, 44, 597–609. [Google Scholar] [CrossRef]
- Asencio-Duran, M.; Vallejo-Garcia, J.L.; Pastora-Salvador, N.; Fonseca-Sandomingo, A.; Romano, M.R. Vitreous Diagnosis in Neoplastic Diseases. Mediat. Inflamm. 2012, 2012, 930704. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, D.A.; Skandalis, S.S.; Noulas, A.V.; Papageorgakopoulou, N.; Theocharis, A.D.; Karamanos, N.K. Hyaluronan and Chondroitin Sulfate Proteoglycans in the Supramolecular Organization of the Mammalian Vitreous Body. Connect. Tissue Res. 2008, 49, 124–128. [Google Scholar] [CrossRef]
- Murthy, K.R.; Goel, R.; Subbannayya, Y.; Jacob, H.K.; Murthy, P.R.; Manda, S.S.; Patil, A.H.; Sharma, R.; Sahasrabuddhe, N.A.; Parashar, A.; et al. Proteomic Analysis of Human Vitreous Humor. Clin. Proteom. 2014, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.N.; Crossman, M.V.; McLeod, D.; Ayad, S. Extraction and Characterization of the Tissue Forms of Collagen Types II and IX from Bovine Vitreous. Biochem. J. 1994, 299 Pt 2, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Bos, K.J.; Holmes, D.F.; Kadler, K.E.; McLeod, D.; Morris, N.P.; Bishop, P.N. Axial Structure of the Heterotypic Collagen Fibrils of Vitreous Humour and Cartilage. J. Mol. Biol. 2001, 306, 1011–1022. [Google Scholar] [CrossRef]
- Ponsioen, T.L.; Hooymans, J.M.M.; Los, L.I. Remodelling of the Human Vitreous and Vitreoretinal Interface—A Dynamic Process. Prog. Retin. Eye Res. 2010, 29, 580–595. [Google Scholar] [CrossRef]
- Migacz, J.V.; Otero-Marquez, O.; Zhou, R.; Rickford, K.; Murillo, B.; Zhou, D.B.; Castanos, M.V.; Sredar, N.; Dubra, A.; Rosen, R.B.; et al. Imaging of Vitreous Cortex Hyalocyte Dynamics Using Non-Confocal Quadrant-Detection Adaptive Optics Scanning Light Ophthalmoscopy in Human Subjects. Biomed. Opt. Express 2022, 13, 1755–1773. [Google Scholar] [CrossRef]
- Boneva, S.K.; Wolf, J.; Rosmus, D.-D.; Schlecht, A.; Prinz, G.; Laich, Y.; Boeck, M.; Zhang, P.; Hilgendorf, I.; Stahl, A.; et al. Transcriptional Profiling Uncovers Human Hyalocytes as a Unique Innate Immune Cell Population. Front. Immunol. 2020, 11, 567274. [Google Scholar] [CrossRef]
- Noda, Y.; Hata, Y.; Hisatomi, T.; Nakamura, Y.; Hirayama, K.; Miura, M.; Nakao, S.; Fujisawa, K.; Sakamoto, T.; Ishibashi, T. Functional Properties of Hyalocytes under PDGF-Rich Conditions. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2107–2114. [Google Scholar] [CrossRef] [Green Version]
- Salu, P.; Claeskens, W.; De Wilde, A.; Hijmans, W.; Wisse, E. Light and Electron Microscopic Studies of the Rat Hyalocyte after Perfusion Fixation. Ophthalmic Res. 1985, 17, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.S.; Hageman, G.S. In Situ Characterization of the Human Hyalocyte. Arch. Ophthalmol. 1994, 112, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Pederson, J.E. Chapter 113—Fluid Physiology of the Subretinal Space. In Retina, 4th ed.; Ryan, S.J., Hinton, D.R., Schachat, A.P., Wilkinson, C.P., Eds.; Mosby: Edinburgh, UK, 2006; pp. 1909–1920. ISBN 978-0-323-02598-0. [Google Scholar]
- Semeraro, F.; Morescalchi, F.; Russo, A.; Romano, M.R.; Costagliola, C. Tamponade or Filling Effect: Changes of Forces in Myopic Eyes. BioMed Res. Int. 2014, 2014, 618382. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. The Silicone Study: A Small Piece of the PVR Puzzle Is Put Into Place. Arch. Ophthalmol. 1997, 115, 407–408. [Google Scholar] [CrossRef]
- Stern, W.H.; Lean, J.S. Intraocular Silicone Oil Versus Gas in the Management of PVR: A Multicenter Clinical Study. In Proliferative Vitreoretinopathy (PVR); Freeman, H.M., Tolentino, F.I., Eds.; Springer: New York, NY, USA, 1988; pp. 88–96. ISBN 978-1-4612-3910-9. [Google Scholar]
- Wetterqvist, C.; Wong, D.; Williams, R.; Stappler, T.; Herbert, E.; Freeburn, S. Tamponade Efficiency of Perfluorohexyloctane and Silicone Oil Solutions in a Model Eye Chamber. Br. J. Ophthalmol. 2004, 88, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Wong, D. The Concept of Heavy Tamponades—Chances and Limitations. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Chan, Y.K.; Bek, T.; Wilson, I.; Stefánsson, E. Intraocular Currents, Bernoulli’s Principle and Non-Drainage Scleral Buckling for Rhegmatogenous Retinal Detachment. Eye 2018, 32, 213–221. [Google Scholar] [CrossRef]
- Schetz, J.A.; Fuhs, A.E. Fundamentals of Fluid Mechanics; John Wiley & Sons: Hoboken, NJ, USA, 1999; ISBN 978-0-471-34856-6. [Google Scholar]
- Gabel, V.P.; Kampik, A.; Gabel, C.; Spiegel, D. Silicone Oil with High Specific Gravity for Intraocular Use. Br. J. Ophthalmol. 1987, 71, 262–267. [Google Scholar] [CrossRef]
- Sunderland, D.K.; Sapra, A. Physiology, Aqueous Humor Circulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Su, X.; Vesco, C.; Fleming, J.; Choh, V. Density of Ocular Components of the Bovine Eye. Optom. Vis. Sci. 2009, 86, 1187–1195. [Google Scholar] [CrossRef]
- Galili, I. Weight versus Gravitational Force: Historical and Educational Perspectives. Int. J. Sci. Educ. 2001, 23, 1073–1093. [Google Scholar] [CrossRef]
- Interrante, C.G.; Heymann, F.J. Standardization of Technical Terminology: Principles and Practices: A Symposium Sponsored by ASTM, Committee on Terminology, Toronto, Canada, 23 June 1982; ASTM International: West Conshohocken, PA, USA, 1983; ISBN 978-0-8031-1183-7. [Google Scholar]
- Archimedes’ Principle. Available online: https://en.wikipedia.org/wiki/Archimedes%27_principle (accessed on 17 December 2022).
- Fundamentals of Surface Tension. Available online: http://web.mit.edu/nnf/education/wettability/index1.html (accessed on 17 December 2022).
- Saab, M.; Javidi, S.; Dirani, A.; Cordahi, G. Displacement of Retained Subretinal Perfluorocarbon Liquid Through Therapeutic Retinal Detachment Induced by Balanced Salt Solution Injection. Int. Med. Case Rep. J. 2020, 13, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Mirshahi, A.; Ghasemi, F.; Zarei, M.; Karkhaneh, R.; Ahmadraji, A.; Polkinghorne, P.J. Removal of Subfoveal Perfluorocarbon Liquid: Report of 3 Cases. J. Curr. Ophthalmol. 2017, 29, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Padhy, S.K.; Takkar, B.; Kelgaonkar, A.; Sahu, S. Retinal Pigment Epithelial Step Sign: Optical Coherence Tomography Clue for Diagnosing Retained Subretinal Perfluorocarbon Liquid. BMJ Case Rep. 2021, 14, e244464. [Google Scholar] [CrossRef] [PubMed]
- JaypeeDigital | Interfacial Tension Agents, Exchanges and Subretinal Fluid Drainage. Available online: https://www.jaypeedigital.com/book/9789351520979/chapter/ch15 (accessed on 17 December 2022).
- Elert, G. Viscosity. The Physics Hypertextbook. 2021. Available online: https://physics.info/viscosity/ (accessed on 17 December 2022).
- Kleinberg, T.T.; Tzekov, R.T.; Stein, L.; Ravi, N.; Kaushal, S. Vitreous Substitutes: A Comprehensive Review. Surv. Ophthalmol. 2011, 56, 300–323. [Google Scholar] [CrossRef]
- Yadav, I.; Purohit, S.D.; Singh, H.; Bhushan, S.; Yadav, M.K.; Velpandian, T.; Chawla, R.; Hazra, S.; Mishra, N.C. Vitreous Substitutes: An Overview of the Properties, Importance, and Development. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1156–1176. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B.; Papakostas, T.D.; Vavvas, D.G. Complications of Emulsified Silicone Oil after Retinal Detachment Repair. Semin. Ophthalmol. 2014, 29, 312–318. [Google Scholar] [CrossRef]
- Chen, Y.; Ip, Y.L.; Zhou, L.; Li, P.Y.; Chan, Y.M.; Lam, W.C.; Li, K.K.W.; Steel, D.H.; Chan, Y.K. What Is the Cause of Toxicity of Silicone Oil? Materials 2021, 15, 269. [Google Scholar] [CrossRef]
- Merrill, D.L.; Fleming, T.C.; Girard, L.J. The Effects of Physiologic Balanced Salt Solutions and Normal Saline on Intraocular and Extraocular Tissues. Am. J. Ophthalmol. 1960, 49, 895–898. [Google Scholar] [CrossRef]
- Balanced Salt Solution | Encyclopedia.Com. Available online: https://www.encyclopedia.com/caregiving/dictionaries-thesauruses-pictures-and-press-releases/balanced-salt-solution (accessed on 30 August 2022).
- Matsuda, M.; Kinoshita, S.; Ohashi, Y.; Shimomura, Y.; Ohguro, N.; Okamoto, H.; Omoto, T.; Hosotani, H.; Yoshida, H. Comparison of the Effects of Intraocular Irrigating Solutions on the Corneal Endothelium in Intraocular Lens Implantation. Br. J. Ophthalmol. 1991, 75, 476–479. [Google Scholar] [CrossRef]
- Zhou, C.; Qiu, Q.; Zheng, Z. Air versus Gas Tamponade in Rhegmatogenous Retinal Detachment with Inferior Breaks after 23-Gauge Pars Plana Vitrectomy: A Prospective, Randomized Comparative Interventional Study. Retina 2015, 35, 886–891. [Google Scholar] [CrossRef]
- Sabates, W.I.; Abrams, G.W.; Swanson, D.E.; Norton, E.W.D. The Use of Intraocular Gases: The Results of Sulfur Hexafluoride Gas in Retinal Detachment Surgery. Ophthalmology 1981, 88, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Mouries, O.; Bonnet, M. [C2F6 in the treatment of retinal detachment associated to PVR: Therapeutic trial]. J. Fr. Ophtalmol. 1992, 15, 601–604. [Google Scholar] [PubMed]
- Sigler, E.J.; Randolph, J.C.; Charles, S.; Calzada, J.I. Intravitreal Fluorinated Gas Preference and Occurrence of Rare Ischemic Postoperative Complications after Pars Plana Vitrectomy: A Survey of the American Society of Retina Specialists. J. Ophthalmol. 2012, 2012, 230596. [Google Scholar] [CrossRef]
- Fleury, J.; Bonnet, M. [C3F8 in the treatment of retinal detachment associated with vitreoretinal proliferation]. J. Fr. Ophtalmol. 1989, 12, 89–94. [Google Scholar] [PubMed]
- Cekic, O.; Ohji, M. Intraocular Gas Tamponades. Semin. Ophthalmol. 2000, 15, 3–14. [Google Scholar] [CrossRef]
- Crittenden, J.J.; de Juan, E., Jr.; Tiedeman, J. Expansion of Long-Acting Gas Bubbles for Intraocular Use: Principles and Practice. Arch. Ophthalmol. 1985, 103, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, M.; Shariati, M.M.; Bolouki, A.; Zamani, G. Gas Expansion Three Days after Pars Plana Vitrectomy with Sulfur Hexafluoride 20% Tamponade Following Carbon Monoxide Toxicity and Oxygen Therapy. Case Rep. Ophthalmol. Med. 2022, 2022, 5035361. [Google Scholar] [CrossRef]
- Barak, Y.; Heroman, J.W.; Schaal, S. Use of 25% Sulfur Hexafluoride Gas Mixture May Minimize Short-Term Postoperative Hypotony in Sutureless 25-Gauge Pars Plana Vitrectomy Surgery. Clin. Ophthalmol. 2013, 7, 423–426. [Google Scholar] [CrossRef]
- Kontos, A.; Tee, J.; Stuart, A.; Shalchi, Z.; Williamson, T.H. Duration of Intraocular Gases Following Vitreoretinal Surgery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 231–236. [Google Scholar] [CrossRef]
- Gandorfer, A.; Kampik, A. Expansion intraokularer Gase infolge Reduktion des Atmosphärendruckes Kasuistik und Literaturübersicht. Ophthalmologe 2000, 97, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Herbert, L.; Rüschen, H.; Cooling, R.J. Lesson of the Week: Nitrous Oxide Anaesthesia in the Presence of Intraocular Gas Can Cause Irreversible Blindness. BMJ 2002, 325, 532. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.Y.; Wong, D. Chapter 104—Special Adjuncts to Treatment. In Retina, 5th ed.; Ryan, S.J., Sadda, S.R., Hinton, D.R., Schachat, A.P., Sadda, S.R., Wilkinson, C.P., Wiedemann, P., Schachat, A.P., Eds.; W.B. Saunders: London, UK, 2013; pp. 1735–1783. ISBN 978-1-4557-0737-9. [Google Scholar]
- Mendichi, R.; Schieroni, A.G.; Piovani, D.; Allegrini, D.; Ferrara, M.; Romano, M.R. Comparative Study of Chemical Composition, Molecular and Rheological Properties of Silicone Oil Medical Devices. Transl. Vis. Sci. Technol. 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Barca, F.; Caporossi, T.; Rizzo, S. Silicone Oil: Different Physical Proprieties and Clinical Applications. BioMed Res. Int. 2014, 2014, 502143. [Google Scholar] [CrossRef]
- Mark, J.E.; Schaefer, D.W.; Lin, G. Types of Polysiloxanes. In The Polysiloxanes; Mark, J.E., Schaefer, D.W., Lin, G., Eds.; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-518173-9. [Google Scholar]
- Steel, D.H.W.; Wong, D.; Sakamoto, T. Silicone Oils Compared and Found Wanting. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 11–12. [Google Scholar] [CrossRef]
- Schatz, M.F.; Howden, K. Purification of Silicone Oils for Fluid Experiments. Exp. Fluids 1995, 19, 359–361. [Google Scholar] [CrossRef]
- Giordano, G.G.; Refojo, M.F. Silicone Oils as Vitreous Substitutes. Prog. Polym. Sci. 1998, 23, 509–532. [Google Scholar] [CrossRef]
- Setiadji, S.; Sumiyanto, E.; Fitrilawati; Syakir, N.; Noviyanti, A.R.; Rahayu, I.; Risdiana, R. Synthesis of Polydimethylsiloxane and Its Monomer from Hydrolysis of Dichlorodimethylsilane. Key Eng. Mater. 2020, 860, 234–238. [Google Scholar] [CrossRef]
- Chen, Y.; Kearns, V.R.; Zhou, L.; Sandinha, T.; Lam, W.C.; Steel, D.H.; Chan, Y.K. Silicone Oil in Vitreoretinal Surgery: Indications, Complications, New Developments and Alternative Long-Term Tamponade Agents. Acta Ophthalmol. 2021, 99, 240–250. [Google Scholar] [CrossRef]
- Chmielowiec, A.; Woś, W.; Gumieniak, J. Viscosity Approximation of PDMS Using Weibull Function. Materials 2021, 14, 6060. [Google Scholar] [CrossRef]
- Kartasasmita, A.; Kusdiono, W.; Virgana, R.; Boesorie, S. In Vivo Emulsification Analysis of 1000 Cs and 5000 Cs Silicone Oil after Rhegmatogenous Retinal Detachment Vitrectomy Surgery. Open J. Ophthalmol. 2017, 7, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Ratanapakorn, T.; Thongmee, W.; Meethongkam, K.; Sinawat, S.; Sanguansak, T.; Bhoomibunchoo, C.; Laovirojjanakul, W.; Yospaiboon, Y. Emulsification of Different Viscosity Silicone Oil in Complicated Retinal Detachment Surgery: A Randomized Double-Blinded Clinical Trial. Clin. Ophthalmol. 2020, 14, 359–367. [Google Scholar] [CrossRef]
- Caramoy, A.; Schröder, S.; Fauser, S.; Kirchhof, B. In Vitro Emulsification Assessment of New Silicone Oils. Br. J. Ophthalmol. 2010, 94, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Chen, K.-H.; Hsu, W.-M.; Li, Y.-S. Cytotoxicity of Silicone Oil on Cultivated Human Corneal Endothelium. Eye 2008, 22, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.X.; Sawant, R.; Jonas, A.; Lochhead, J. The Incidence of Silicone Oil-Related Visual Loss Following the Removal of Heavy Silicone Oil. Eye 2019, 33, 1969–1970. [Google Scholar] [CrossRef]
- Mazzeo, T.J.M.M.; Jacob, G.A.V.; Horizonte, P.H.; Leber, H.M.; Gomes, A.M.V. Intraocular Silicone Oil Brain Migration Associated with Severe Subacute Headaches: A Case Report. Int. J. Retin. Vitr. 2021, 7, 10. [Google Scholar] [CrossRef]
- Horozoglu, F.; Sener, H.; Polat, O.A.; Sever, O.; Potoglu, B.; Celik, E.; Turkoglu, E.B.; Evereklioglu, C. Evaluation of Long-Term Outcomes Associated with Extended Heavy-Silicone Oil Use for the Treatment of Inferior Retinal Detachment. Sci. Rep. 2022, 12, 11636. [Google Scholar] [CrossRef] [PubMed]
- Heavy Silicone Oil: A “Novel” Intraocular Tamponade Agent. Available online: https://oce-ovid-com.ezproxy.uio.no/article/00006324-201106000-00017/PDF (accessed on 18 December 2022).
- Wong, D.; Van Meurs, J.C.; Stappler, T.; Groenewald, C.; Pearce, I.A.; McGalliard, J.N.; Manousakis, E.; Herbert, E.N. A Pilot Study on the Use of a Perfluorohexyloctane/Silicone Oil Solution as a Heavier than Water Internal Tamponade Agent. Br. J. Ophthalmol. 2005, 89, 662–665. [Google Scholar] [CrossRef]
- Genovesi–Ebert, F.; Andrea, V.; Cresti, F.; Bartolo, E.D.; Miniaci, S.; Palla, M.; Belting, C.; Rizzo, S. A New Heavier–Than–Water Silicone Oil (HWS 46–300) as Prolonged Internal Tamponade Agent in Complicated Vitreo–Retinal Surgery: A Pilot Study. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4218. [Google Scholar]
- Rizzo, S.; Genovesi-Ebert, F.; Vento, A.; Cresti, F.; Di Bartolo, E.; Belting, C. A New Heavy Silicone Oil (HWS 46-3000) Used as a Prolonged Internal Tamponade Agent in Complicated Vitreoretinal Surgery: A Pilot Study. Retina 2007, 27, 613–620. [Google Scholar] [CrossRef]
- Meinert, H.; Roy, T. Semifluorinated Alkanes—A New Class of Compounds with Outstanding Properties for Use in Ophthalmology. Eur. J. Ophthalmol. 2000, 10, 189–197. [Google Scholar] [CrossRef]
- Caporossi, T.; Franco, F.; Finocchio, L.; Barca, F.; Giansanti, F.; Tartaro, R.; Virgili, G.; Rizzo, S. Densiron 68 Heavy Silicone Oil in the Management of Inferior Retinal Detachment Recurrence: Analysis on Functional and Anatomical Outcomes and Complications. Int. J. Ophthalmol. 2019, 12, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Gora, F.; Lohmann, C.P.; Sachs, H.; Gabel, V.P. Heavy Silicone Oil (Oxane Hd) as Long–Term Tamponade for Complicated Retinal Detachment. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2047. [Google Scholar]
- Wolf, S.; Schön, V.; Meier, P.; Wiedemann, P. Silicone Oil-RMN3 Mixture (“Heavy Silicone Oil”) as Internal Tamponade for Complicated Retinal Detachment. Retina 2003, 23, 335–342. [Google Scholar] [CrossRef]
- Rizzo, S.; Genovesi-Ebert, F.; Belting, C.; Vento, A.; Cresti, F. A Pilot Study on the Use of Silicone Oil-RMN3 as Heavier-than-Water Endotamponade Agent. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, J.; Magalhães, O.; Lucatto, L.F.A.; Navarro, R.M.; Moraes, N.S.; Farah, M.E.; Maia, A.; Maia, M. Heavy Silicone Oil as a Long-Term Endotamponade Agent for Complicated Retinal Detachments. BioMed Res. Int. 2014, 2014, 136031. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Kirchhof, B.; Schrage, N.; Ocklenburg, C.; Hilgers, R.-D.; HSO Study Group. Heavy Silicone Oil versus Standard Silicone Oil as Vitreous Tamponade in Inferior PVR (HSO Study): Design Issues and Implications. Acta Ophthalmol. Scand. 2007, 85, 623–630. [Google Scholar] [CrossRef]
- Russo, A.; Morescalchi, F.; Donati, S.; Gambicorti, E.; Azzolini, C.; Costagliola, C.; Semeraro, F. Heavy and Standard Silicone Oil: Intraocular Inflammation. Int. Ophthalmol. 2018, 38, 855–867. [Google Scholar] [CrossRef]
- Moussa, G.; Tadros, M.; Ch’ng, S.W.; Sharma, A.; Lett, K.S.; Mitra, A.; Tyagi, A.K.; Andreatta, W. Outcomes of Heavy Silicone Oil (Densiron) Compared to Silicone Oil in Primary Rhegmatogenous Retinal Detachment: A Multivariable Regression Model. Int. J. Retin. Vitr. 2022, 8, 61. [Google Scholar] [CrossRef]
- Retinal Physician—Heavy Oils in Vitreoretinal Surgery. Available online: https://www.retinalphysician.com/issues/2018/july-august-2018/heavy-oils-in-vitreoretinal-surgery (accessed on 18 December 2022).
- Auriol, S.; Pagot-Mathis, V.; Mahieu, L.; Lemoine, C.; Mathis, A. Efficacy and Safety of Heavy Silicone Oil Densiron 68 in the Treatment of Complicated Retinal Detachment with Large Inferior Retinectomy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1383–1389. [Google Scholar] [CrossRef]
- Kurt, R.A.; Kapran, Z. Heavy Silicone Oil as an Endotamponade in Recurrent or Complicated Retinal Detachment and Macular Hole. Turk. J. Ophthalmol. 2022, 52, 119–124. [Google Scholar] [CrossRef]
- Alovisi, C.; Panico, C.; de Sanctis, U.; Eandi, C.M. Vitreous Substitutes: Old and New Materials in Vitreoretinal Surgery. J. Ophthalmol. 2017, 2017, 3172138. [Google Scholar] [CrossRef] [PubMed]
- Ohm, J. Über die Behandlung der Netzhautablösung durch operative Entleerung der subretinalen Flüssigkeit und Einspritzung von Luft in den Glaskörper. Albrecht Graefes Arch. Ophthalmol. 1911, 79, 442–450. [Google Scholar] [CrossRef]
- Techniques for In-Office Vitreoretinal Procedures. Available online: https://retinatoday.com/articles/2020-apr/techniques-for-in-office-vitreoretinal-procedures (accessed on 18 December 2022).
- Mateo-Montoya, A.; de Smet, M.D. Air as Tamponade for Retinal Detachments. Eur. J. Ophthalmol. 2014, 24, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, C.; Eckert, T.; Eckardt, U.; Porkert, U.; Gesser, C. Macular Hole Surgery with Air Tamponade and Optical Coherence Tomography-Based Duration of Face-down Positioning. Retina 2008, 28, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Forsaa, V.A.; Krohn, J. Air Tamponade Combined with Nonsupine Positioning in Macular Hole Surgery for Pseudophakic Eyes. Retina 2017, 37, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Law, J.; Cherney, E.; Recchia, F.; Kim, S.J. Air vs Gas Tamponade During Macular Hole Repair Surgery. J. Vitr. Dis. 2020, 4, 360–363. [Google Scholar] [CrossRef]
- Shen, P.; Kong, X.; Zhou, L.; Su, P.; Lu, X.; He, M. Air Tamponade for Rhegmatogenous Retinal Detachment With Inferior Breaks After 25-Gauge Pars Plana Vitrectomy: Technique and Outcome. Front. Med. 2022, 9, 724234. [Google Scholar] [CrossRef]
- Govers, B.M.; Lamers, M.P.M.; Klevering, B.J.; Keijser, S. Air versus Fluorinated Gas Tamponades in Pars Plana Vitrectomy Treatment for Primary Rhegmatogenous Retinal Detachment. Acta Ophthalmol. 2022, 100, e1600–e1605. [Google Scholar] [CrossRef]
- Vitrectomy with Silicone Oil or Sulfur Hexafluoride Gas in Eyes with Severe Proliferative Vitreoretinopathy: Results of a Randomized Clinical Trial. Arch. Ophthalmol. 1992, 110, 770–779. [CrossRef]
- Haller, J.A.; Campochiaro, P.A. Oil and Gas on Troubled Waters. The Proliferative Vitreoretinopathy Studies. Arch. Ophthalmol. 1992, 110, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nishi, K.; Nishitsuka, K. Selection Criteria for Air Tamponade During Vitrectomy for Rhegmatogenous Retinal Detachment. Clin. Ophthalmol. 2022, 16, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Caprani, S.M.; Airaghi, G.; Vinciguerra, R.; Bartalena, L.; Testa, F.; Mariotti, C.; Porta, G.; Simonelli, F.; Azzolini, C. Vitreous Substitutes: The Present and the Future. BioMed Res. Int. 2014, 2014, 351804. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.S.; Oberstein, S.Y.L.; Mura, M.; Bijl, H.M. Air versus Gas Tamponade in Retinal Detachment Surgery. Br. J. Ophthalmol. 2013, 97, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, V.; Boixadera, A.; Verdugo, A.; García-Arumí, J. Pars Plana Vitrectomy Alone for the Management of Inferior Breaks in Pseudophakic Retinal Detachment without Facedown Position. Ophthalmology 2005, 112, 1222–1226. [Google Scholar] [CrossRef]
- Norton, E.W. Intraocular Gas in the Management of Selected Retinal Detachments. Trans. Am. Acad. Ophthalmol. Otolaryngol. Am. Acad. Ophthalmol. Otolaryngol. 1973, 77, OP85–OP98. [Google Scholar]
- Lincoff, A.; Haft, D.; Liggett, P.; Reifer, C. Intravitreal Expansion of Perfluorocarbon Bubbles. Arch. Ophthalmol. 1980, 98, 1646. [Google Scholar] [CrossRef]
- Choi, M.; Hong, S.; Yun, C.; Kim, S.-W. Objective Analysis of Perfluoropropane Tamponade Area after Pars Plana Vitrectomy Using Ultra-Widefield Fundus Stereographic Projection Images. Sci. Rep. 2020, 10, 18268. [Google Scholar] [CrossRef]
- Ocular Bubbly: A Vitreoretinal Update on the Art of Gases. Available online: https://www.eyenews.uk.com/education/trainees/post/ocular-bubbly-a-vitreoretinal-update-on-the-art-of-gases (accessed on 7 November 2022).
- Management of Hemorrhagic Choroidal Detachment. Available online: https://retinatoday.com/articles/2012-sept/management-of-hemorrhagic-choroidal-detachment (accessed on 7 November 2022).
- Aronowitz, J.D.; Brubaker, R.F. Effect of Intraocular Gas on Intraocular Pressure. Arch. Ophthalmol. 1976, 94, 1191–1196. [Google Scholar] [CrossRef]
- Kanclerz, P.; Grzybowski, A. Complications Associated with the Use of Expandable Gases in Vitrectomy. J. Ophthalmol. 2018, 2018, e8606494. [Google Scholar] [CrossRef]
- Abrams, G.W.; Swanson, D.E.; Sabates, W.I.; Goldman, A.I. The Results of Sulfur Hexafluoride Gas in Vitreous Surgery. Am. J. Ophthalmol. 1982, 94, 165–171. [Google Scholar] [CrossRef]
- Kornmann, H.L.; Gedde, S.J. Glaucoma Management after Vitreoretinal Surgeries. Curr. Opin. Ophthalmol. 2016, 27, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Tornambe, P.E.; Hilton, G.F. The Steamroller Maneuver and Proliferative Vitreoretinopathy. Arch. Ophthalmol. 1992, 110, 15. [Google Scholar] [CrossRef]
- Casswell, E.J.; Yorston, D.; Lee, E.; Heeren, T.F.C.; Harris, N.; Zvobgo, T.M.; Tarafdar, S.; Xing, W.; Bourmpaki, E.; Bunce, C.; et al. Effect of Face-Down Positioning vs Support-the-Break Positioning After Macula-Involving Retinal Detachment Repair: The PostRD Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 634–642. [Google Scholar] [CrossRef]
- Cheung, N.; Wong, D. Graefe’s Archive for Clinical and Experimental Ophthalmology—Instant Glue for Retinal Detachment Surgery? Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1695–1696. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.H.; Marmor, M.F. Rapid Enhancement of Retinal Adhesion by Laser Photocoagulation. Ophthalmology 1988, 95, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Negi, A.; Kawano, S.; Honda, Y. Photothermal, Cryogenic, and Diathermic Effects of Retinal Adhesive Force in Vivo. Retina 1991, 11, 441–444. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, K.; Su, L.; Xia, X.; Xu, X. Perfluorocarbon Liquid: Its Application in Vitreoretinal Surgery and Related Ocular Inflammation. BioMed Res. Int. 2014, 2014, 250323. [Google Scholar] [CrossRef]
- Banks, R.E.; Smart, B.E.; Tatlow, J.C. Organofluorine Chemistry: Principles and Commercial Applications; Springer Science & Business Media: Dordrecht, Germany, 2013; ISBN 978-1-4899-1202-2. [Google Scholar]
- Imamura, Y.; Minami, M.; Ueki, M.; Satoh, B.; Ikeda, T. Use of Perfluorocarbon Liquid during Vitrectomy for Severe Proliferative Diabetic Retinopathy. Br. J. Ophthalmol. 2003, 87, 563–566. [Google Scholar] [CrossRef]
- Chang, S.; Ozmert, E.; Zimmerman, N.J. Intraoperative Perfluorocarbon Liquids in the Management of Proliferative Vitreoretinopathy. Am. J. Ophthalmol. 1988, 106, 668–674. [Google Scholar] [CrossRef]
- Stolba, U.; Krepler, K.; Velikay, M.; Binder, S. Anterior Segment Changes in Rabbits after Experimental Aqueous Replacement with Various Amounts of Different Perfluorocarbon Liquids. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 501–507. [Google Scholar] [CrossRef]
- Nabih, M.; Peyman, G.A.; Clark, L.C.; Hoffman, R.E.; Miceli, M.; Abou-Steit, M.; Tawakol, M.; Liu, K.R. Experimental Evaluation of Perfluorophenanthrene as a High Specific Gravity Vitreous Substitute: A Preliminary Report. Ophthalmic Surg. Lasers Imaging Retin. 1989, 20, 286–293. [Google Scholar] [CrossRef]
- Moreira, H.; de Queiroz, J.M.; Liggett, P.E.; McDonnell, P.J. Corneal Toxicity Study of Two Perfluorocarbon Liquids in Rabbit Eyes. Cornea 1992, 11, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.K.; Kalaiselvan, V.; Andrés-Iglesias, C.; Shukla, S.; Saxena, R.; Pastor, J.C. Acute Intraocular Toxicity Caused by Perfluorocarbon Liquids: Safety Control Systems of Medical Devices. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.S.; Casas, D.R. Inflammation Induced by Perfluorocarbon Liquid: Intra- and Postoperative Use. BioMed Res. Int. 2014, 2014, 907816. [Google Scholar] [CrossRef]
- Januschowski, K.; Irigoyen, C.; Pastor, J.C.; Srivastava, G.K.; Romano, M.R.; Heimann, H.; Stalmans, P.; Van Keer, K.; Boden, K.; Szurman, P.; et al. Retinal Toxicity of Medical Devices Used during Vitreoretinal Surgery: A Critical Overview. Ophthalmologica 2018, 240, 236–243. [Google Scholar] [CrossRef]
- Pastor, J.-C.; Andres_iglesias, C.; Srivastava, G.K.; Fernandez-Bueno, I.; Dueñas-Laita, A.; Rull, F.; Medina, J.; García-Serna, J.; Garcia-Layana, A.; Coco-Martin, R. Clinical Characteristics of Proven Acute Intraocular Toxicity by Perfluorocarbon Liquids (PFCL) Used in Retinal Surgery. Investig. Ophthalmol. Vis. Sci. 2019, 60, 6597. [Google Scholar]
- Using Perfluorocarbon Liquids as Short-Term Postoperative Intravitreal Tamponade. Available online: https://retinatoday.com/articles/2009-mar/0309RT_F08_Global_Wu-php (accessed on 26 August 2022).
- Peyman, G.A.; Schulman, J.A.; Sullivan, B. Perfluorocarbon Liquids in Ophthalmology. Surv. Ophthalmol. 1995, 39, 375–395. [Google Scholar] [CrossRef]
- Bhurayanontachai, P.; Seepongphun, U. Outcomes of a Postoperative Perfluorocarbon Liquid Tamponade for Complex Retinal Detachments: 12 Years of Experience in Southern Thailand. BMC Ophthalmol. 2020, 20, 358. [Google Scholar] [CrossRef]
- Sheridan, A.M.; Essex, R.W.; Yeoh, J.; Allen, P.; Campbell, W.G.; Edwards, T.L. Is Post-Operative Perfluorocarbon Liquid Tamponade for Macula-on Giant Retinal Tear Safer than Silicone Oil? Eye 2019, 33, 689–691. [Google Scholar] [CrossRef]
- Sirimaharaj, M.; Balachandran, C.; Chan, W.C.; Hunyor, A.P.; Chang, A.A.; Gregory-Roberts, J.; Hunyor, A.B.; Playfair, T.J. Vitrectomy with Short Term Postoperative Tamponade Using Perfluorocarbon Liquid for Giant Retinal Tears. Br. J. Ophthalmol. 2005, 89, 1176–1179. [Google Scholar] [CrossRef]
- Randolph, J.C.; Diaz, R.I.; Sigler, E.J.; Calzada, J.I.; Charles, S. 25-Gauge Pars Plana Vitrectomy with Medium-Term Postoperative Perfluoro-n-Octane for the Repair of Giant Retinal Tears. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Eiger-Moscovich, M.; Gershoni, A.; Axer-Siegel, R.; Weinberger, D.; Ehrlich, R. Short-Term Vitreoretinal Tamponade with Heavy Liquid Following Surgery for Giant Retinal Tear. Curr. Eye Res. 2017, 42, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, A.M.; Krause, A.C.; Goldstein, H.H.; Berman, M.B. A Chemical Study of the Normal Aqueous Humor. Exp. Biol. Med. 1927, 24, 451–452. [Google Scholar] [CrossRef]
- Dong, J.; Yao, J.; Chang, S.; Kanclerz, P.; Khoramnia, R.; Wang, X. Comparison of Mean Corneal Power of Annular Rings and Zones Using Swept-Source Optical Coherence Tomography. Diagnostics 2022, 12, 754. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Lois, N. Perfluorocarbons and Semifluorinated Alkanes. Semin. Ophthalmol. 2000, 15, 25–35. [Google Scholar] [CrossRef]
- Tognetto, D.; Lepori, L.; Lapasin, R.; Minutola, D.; Sanguinetti, G.; Michelone, L.; Fanni, D.; Ravalico, G. A New Heavy Internal Tamponade in Vitreoretinal Surgery: An in Vitro Study. Eye 2008, 22, 1082–1088. [Google Scholar] [CrossRef]
- De Molfetta, V.; Bottoni, F.; Arpa, P.; Vinciguerra, P.; Zenoni, S. The Effect of Simultaneous Internal Tamponade on Fluid Compartmentalization and Its Relationship to Cell Proliferation. Retina 1992, 12, S40–S45. [Google Scholar] [CrossRef]
- Nakamura, K.; Refojo, M.F.; Crabtree, D.V.; Pastor, J.; Leong, F.L. Ocular Toxicity of Low-Molecular-Weight Components of Silicone and Fluorosilicone Oils. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3007–3020. [Google Scholar]
- Kirchhof, B.; Wong, D.; Van Meurs, J.; Hilgers, R.D.; Macek, M.; Lois, N.; Schrage, N.F. Use of Perfluorohexyloctane as a Long-Term Internal Tamponade Agent in Complicated Retinal Detachment Surgery. Am. J. Ophthalmol. 2002, 133, 95–101. [Google Scholar] [CrossRef]
- Cibis, P.A.; Becker, B.; Okun, E.; Canaan, S. The Use of Liquid Silicone in Retinal Detachment Surgery. Arch. Ophthalmol. 1962, 68, 590–599. [Google Scholar] [CrossRef]
- Smith, R.C.; Smith, G.T.; Wong, D. Refractive Changes in Silicone Filled Eyes. Eye 1990, 4, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Stefánsson, E.; Anderson, M.M.; Landers, M.B.; Tiedeman, J.S.; McCuen, B.W. Refractive Changes from Use of Silicone Oil in Vitreous Surgery. Retina 1988, 8, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Mondelo-García, C.; Bandín-Vilar, E.; García-Quintanilla, L.; Castro-Balado, A.; del Amo, E.M.; Gil-Martínez, M.; Blanco-Teijeiro, M.J.; González-Barcia, M.; Zarra-Ferro, I.; Fernández-Ferreiro, A.; et al. Current Situation and Challenges in Vitreous Substitutes. Macromol. Biosci. 2021, 21, e2100066. [Google Scholar] [CrossRef]
- Brourman, N.D.; Blumenkranz, M.S.; Cox, M.S.; Trese, M.T. Silicone Oil for the Treatment of Severe Proliferative Diabetic Retinopathy. Ophthalmology 1989, 96, 759–764. [Google Scholar] [CrossRef]
- Abrams, G.W.; Azen, S.P.; McCuen, B.W.; Flynn, H.W.; Lai, M.Y.; Ryan, S.J. Vitrectomy with Silicone Oil or Long-Acting Gas in Eyes with Severe Proliferative Vitreoretinopathy: Results of Additional and Long-Term Follow-up. Arch. Ophthalmol. 1997, 115, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.C.; Lai, M.Y.; Lean, J.S.; Linton, K.L.; Trese, M.; Abrams, G.; Ryan, S.J.; Azen, S.P. Postoperative Intraocular Pressure Abnormalities in the Silicone Study. Ophthalmology 1993, 100, 1629–1635. [Google Scholar] [CrossRef]
- Scholda, C.; Wirtitsch, M.; Biowski, R.; Stur, M. Primary Silicone Oil Tamponade without Retinopexy in Highly Myopic Eyes with Central Macular Hole Detachments. Retina 2005, 25, 141–146. [Google Scholar] [CrossRef]
- Abu-Yaghi, N.E.; Abu Gharbieh, Y.A.; Al-Amer, A.M.; AlRyalat, S.A.S.; Nawaiseh, M.B.; Darweesh, M.J.; Alkukhun, L.R.; Abed, A.M.; Saleh, O.A.; Ababneh, O.H. Characteristics, Fates and Complications of Long-Term Silicone Oil Tamponade after Pars Plana Vitrectomy. BMC Ophthalmol. 2020, 20, 336. [Google Scholar] [CrossRef]
- Federman, J.L.; Schubert, H.D. Complications Associated with the Use of Silicone Oil in 150 Eyes after Retina-Vitreous Surgery. Ophthalmology 1988, 95, 870–876. [Google Scholar] [CrossRef]
- Sandner, D.; Herbrig, E.; Engelmann, K. High-Density Silicone Oil (Densiron) as a Primary Intraocular Tamponade: 12-Month Follow Up. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1097–1105. [Google Scholar] [CrossRef]
- Boscia, F.; Furino, C.; Recchimurzo, N.; Besozzi, G.; Sborgia, G.; Sborgia, C. Oxane HD vs Silicone Oil and Scleral Buckle in Retinal Detachment with Proliferative Vitreoretinopathy and Inferior Retinal Breaks. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Morescalchi, F.; Costagliola, C.; Duse, S.; Gambicorti, E.; Parolini, B.; Arcidiacono, B.; Romano, M.R.; Semeraro, F. Heavy Silicone Oil and Intraocular Inflammation. BioMed Res. Int. 2014, 2014, 574825. [Google Scholar] [CrossRef]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Su, X.; Loh, X.J. Polymeric Hydrogels as a Vitreous Replacement Strategy in the Eye. Biomaterials 2021, 268, 120547. [Google Scholar] [CrossRef] [PubMed]
- Oelker, A.M.; Grinstaff, M.W. Ophthalmic Adhesives: A Materials Chemistry Perspective. J. Mater. Chem. 2008, 18, 2521–2536. [Google Scholar] [CrossRef]
- Schulz, A.; Szurman, P. Vitreous Substitutes as Drug Release Systems. Transl. Vis. Sci. Technol. 2022, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.T.; Wang, A.; Nguyen, A.B.; Iyer, J.; Tran, S.D. Recent Advances in Hydrogels: Ophthalmic Applications in Cell Delivery, Vitreous Substitutes, and Ocular Adhesives. Biomedicines 2021, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Schneider, N.; Hohenadl, C.; Hurst, J.; Schatz, A.; Januschowski, K.; Spitzer, M.S. Efficacy of Two Different Thiol-Modified Crosslinked Hyaluronate Formulations as Vitreous Replacement Compared to Silicone Oil in a Model of Retinal Detachment. PLoS ONE 2017, 12, e0172895. [Google Scholar] [CrossRef]
- Schulz, A.; Rickmann, A.; Wahl, S.; Germann, A.; Stanzel, B.V.; Januschowski, K.; Szurman, P. Alginate- and Hyaluronic Acid–Based Hydrogels as Vitreous Substitutes: An In Vitro Evaluation. Transl. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef]
- Schramm, C.; Spitzer, M.S.; Henke-Fahle, S.; Steinmetz, G.; Januschowski, K.; Heiduschka, P.; Geis-Gerstorfer, J.; Biedermann, T.; Bartz-Schmidt, K.U.; Szurman, P. The Cross-Linked Biopolymer Hyaluronic Acid as an Artificial Vitreous Substitute. Investig. Ophthalmol. Vis. Sci. 2012, 53, 613–621. [Google Scholar] [CrossRef]
- Barth, H.; Crafoord, S.; Andréasson, S.; Ghosh, F. A Cross-Linked Hyaluronic Acid Hydrogel (Healaflow®) as a Novel Vitreous Substitute. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 697–703. [Google Scholar] [CrossRef]
- Su, W.-Y.; Chen, Y.-C.; Lin, F.-H. Injectable Oxidized Hyaluronic Acid/Adipic Acid Dihydrazide Hydrogel for Nucleus Pulposus Regeneration. Acta Biomater. 2010, 6, 3044–3055. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Tanaka, M.; Miyata, T. Evaluation of Collagen Gel and Hyaluronic Acid as Vitreous Substitutes. Ophthalmic Res. 1997, 29, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chi, J.; Jiang, Z.; Hu, H.; Yang, C.; Liu, W.; Han, B. A Self-Healing and Injectable Hydrogel Based on Water-Soluble Chitosan and Hyaluronic Acid for Vitreous Substitute. Carbohydr. Polym. 2021, 256, 117519. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Peng, Y.; Yang, C.; Liu, W.; Han, B. The Feasibility Study of an in Situ Marine Polysaccharide-Based Hydrogel as the Vitreous Substitute. J. Biomed. Mater. Res. Part A 2018, 106, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.; Purohit, S.D.; Singh, H.; Das, N.; Roy, P.; Mishra, N.C. A Highly Transparent Tri-Polymer Complex in Situ Hydrogel of HA, Collagen and Four-Arm-PEG as Potential Vitreous Substitute. Biomed. Mater. 2021, 16, 065018. [Google Scholar] [CrossRef]
- Santhanam, S.; Liang, J.; Struckhoff, J.; Hamilton, P.D.; Ravi, N. Biomimetic Hydrogel with Tunable Mechanical Properties for Vitreous Substitutes. Acta Biomater. 2016, 43, 327–337. [Google Scholar] [CrossRef]
- Suri, S.; Banerjee, R. In Vitro Evaluation of in Situ Gels as Short Term Vitreous Substitutes. J. Biomed. Mater. Res. Part A 2006, 79, 650–664. [Google Scholar] [CrossRef]
- Yang, H.; Wang, R.; Gu, Q.; Zhang, X. Feasibility Study of Chitosan as Intravitreous Tamponade Material. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1097–1105. [Google Scholar] [CrossRef]
- Cavalieri, F.; Miano, F.; D’Antona, P.; Paradossi, G. Study of Gelling Behavior of Poly(Vinyl Alcohol)-Methacrylate for Potential Utilizations in Tissue Replacement and Drug Delivery. Biomacromolecules 2004, 5, 2439–2446. [Google Scholar] [CrossRef]
- Maruoka, S.; Matsuura, T.; Kawasaki, K.; Okamoto, M.; Yoshiaki, H.; Kodama, M.; Sugiyama, M.; Annaka, M. Biocompatibility of Polyvinylalcohol Gel as a Vitreous Substitute. Curr. Eye Res. 2006, 31, 599–606. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Aggravi, M.; Donati, A.; Lamponi, S.; Magnani, A. PVA/STMP Based Hydrogels as Potential Substitutes of Human Vitreous. J. Mater. Sci. Mater. Med. 2010, 21, 2491–2500. [Google Scholar] [CrossRef] [PubMed]
- Lamponi, S.; Leone, G.; Consumi, M.; Greco, G.; Magnani, A. In Vitro Biocompatibility of New PVA-Based Hydrogels as Vitreous Body Substitutes. J. Biomater. Sci. Polym. Ed. 2012, 23, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, H.; Liu, Y.; Huang, Z.; Sun, X.; Zhou, L.; Lu, X.; Gao, Q. A Novel Vitreous Substitute of Using a Foldable Capsular Vitreous Body Injected with Polyvinylalcohol Hydrogel. Sci. Rep. 2013, 3, srep01838. [Google Scholar] [CrossRef] [PubMed]
- Morandim-Giannetti, A.D.A.; Silva, R.C.; Magalhães, O.; Schor, P.; Bersanetti, P.A. Conditions for Obtaining Polyvinyl Alcohol/Trisodium Trimetaphosphate Hydrogels as Vitreous Humor Substitute. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1386–1395. [Google Scholar] [CrossRef]
- Chen, H.; Feng, S.; Liu, Y.; Huang, Z.; Sun, X.; Zhou, L.; Lu, X.; Gao, Q. Functional Evaluation of a Novel Vitreous Substitute Using Polyethylene Glycol Sols Injected into a Foldable Capsular Vitreous Body. J. Biomed. Mater. Res. Part A 2013, 101, 2538–2547. [Google Scholar] [CrossRef]
- Davis, J.T.; Hamilton, P.D.; Ravi, N. Poly(Acrylamide Co-Acrylic Acid) for Use as an in Situ Gelling Vitreous Substitute. J. Bioact. Compat. Polym. 2017, 32, 528–541. [Google Scholar] [CrossRef]
- Liang, J.; Struckhoff, J.J.; Du, H.; Hamilton, P.D.; Ravi, N. Synthesis and Characterization of in Situ Forming Anionic Hydrogel as Vitreous Substitutes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 977–988. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Cui, C.; Yang, J.; Liu, W. Antifouling Super Water Absorbent Supramolecular Polymer Hydrogel as an Artificial Vitreous Body. Adv. Sci. 2018, 5, 1800711. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.Q.; Liow, S.S.; Ye, E.; Lakshminarayanan, R.; Loh, X.J. Biodegradable Thermogelling Polymers: Working Towards Clinical Applications. Adv. Healthc. Mater. 2014, 3, 977–988. [Google Scholar] [CrossRef]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In Situ Gelling Biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Ko, D.Y.; Park, M.H.; Joo, M.K.; Jeong, B. Temperature-Responsive Compounds as in Situ Gelling Biomedical Materials. Chem. Soc. Rev. 2012, 41, 4860–4883. [Google Scholar] [CrossRef]
- Liu, Z.; Liow, S.S.; Lai, S.L.; Alli-Shaik, A.; Holder, G.E.; Parikh, B.H.; Krishnakumar, S.; Li, Z.; Tan, M.J.; Gunaratne, J.; et al. Retinal-Detachment Repair and Vitreous-like-Body Reformation via a Thermogelling Polymer Endotamponade. Nat. Biomed. Eng. 2019, 3, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.S.; Yoeruek, E.; Kaczmarek, R.T.; Sierra, A.; Aisenbrey, S.; Grisanti, S.; Bartz-Schmidt, K.U.; Szurman, P. Sodium Hyaluronate Gels as a Drug-Release System for Corticosteroids: Release Kinetics and Antiproliferative Potential for Glaucoma Surgery. Acta Ophthalmol. 2008, 86, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ma, S.; Wu, M.; Cui, H.; Wu, R.; Chen, S.; Xu, C.; Lu, X.; Feng, S. Self-Assembling Hydrogel Loaded with 5-FU PLGA Microspheres as a Novel Vitreous Substitute for Proliferative Vitreoretinopathy. J. Biomed. Mater. Res. Part A 2020, 108, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.K.; Jiang, P.; Torres-Flores, T.C.; Jacobs, K.M.; Chandler, H.L.; Swindle-Reilly, K.E. A Hydrogel Vitreous Substitute That Releases Antioxidant. Macromol. Biosci. 2020, 20, e1900305. [Google Scholar] [CrossRef]
- Tram, N.K.; McLean, R.M.; Swindle-Reilly, K.E. Glutathione Improves the Antioxidant Activity of Vitamin C in Human Lens and Retinal Epithelial Cells: Implications for Vitreous Substitutes. Curr. Eye Res. 2021, 46, 470–481. [Google Scholar] [CrossRef]
- Thakur, S.S.; Shenoy, S.K.; Suk, J.S.; Hanes, J.S.; Rupenthal, I.D. Validation of Hyaluronic Acid-Agar-Based Hydrogels as Vitreous Humor Mimetics for in Vitro Drug and Particle Migration Evaluations. Eur. J. Pharm. Biopharm. 2020, 148, 118–125. [Google Scholar] [CrossRef]
- Lovett, M.L.; Wang, X.; Yucel, T.; York, L.; Keirstead, M.; Haggerty, L.; Kaplan, D.L. Silk Hydrogels for Sustained Ocular Delivery of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) Therapeutics. Eur. J. Pharm. Biopharm. 2015, 95, 271–278. [Google Scholar] [CrossRef]
- Xue, K.; Zhao, X.; Zhang, Z.; Qiu, B.; Tan, Q.S.W.; Ong, K.H.; Liu, Z.; Parikh, B.H.; Barathi, V.A.; Yu, W.; et al. Sustained Delivery of Anti-VEGFs from Thermogel Depots Inhibits Angiogenesis without the Need for Multiple Injections. Biomater. Sci. 2019, 7, 4603–4614. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for Protein Delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Cui, H. Peptide-Based Supramolecular Hydrogels for Delivery of Biologics. Bioeng. Transl. Med. 2016, 1, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lin, R.; Patel, K.; Cheetham, A.G.; Kan, C.; Cui, H. Spatiotemporal Control of the Creation and Immolation of Peptide Assemblies. Coord. Chem. Rev. 2016, 320–321, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Lin, R.; Zhang, P.; Fern, J.; Cheetham, A.G.; Patel, K.; Schulman, R.; Kan, C.; Cui, H. Electrostatic-Driven Lamination and Untwisting of β-Sheet Assemblies. ACS Nano 2016, 10, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.A.E.; Zhang, S. Designer Self-Assembling Peptide Nanofiber Biological Materials. Chem. Soc. Rev. 2010, 39, 2780–2790. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, F.; Xu, H.; Yaseen, M.; Shan, H.; Hauser, C.A.E.; Zhang, S.; Lu, J.R. Molecular Self-Assembly and Applications of Designer Peptide Amphiphiles. Chem. Soc. Rev. 2010, 39, 3480–3498. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum Hydrogels with Tunable Physical and Mechanical Properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef]
- Januschowski, K.; Schnichels, S.; Hurst, J.; Hohenadl, C.; Reither, C.; Rickmann, A.; Pohl, L.; Bartz-Schmidt, K.-U.; Spitzer, M.S. Ex Vivo Biophysical Characterization of a Hydrogel-Based Artificial Vitreous Substitute. PLoS ONE 2019, 14, e0209217. [Google Scholar] [CrossRef]
- Raia, N.R.; Jia, D.; Ghezzi, C.E.; Muthukumar, M.; Kaplan, D.L. Characterization of Silk-Hyaluronic Acid Composite Hydrogels towards Vitreous Humor Substitutes. Biomaterials 2020, 233, 119729. [Google Scholar] [CrossRef]
- Uesugi, K.; Sakaguchi, H.; Hayashida, Y.; Hayashi, R.; Baba, K.; Suganuma, Y.; Yokoi, H.; Tsujikawa, M.; Nishida, K. A Self-Assembling Peptide Gel as a Vitreous Substitute: A Rabbit Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.D.; Crafoord, S.; Andréasson, S.; Arnér, K.M.; O’Shea, T.M.; Langer, R.; Ghosh, F.K. Evaluation of Viscoelastic Poly(Ethylene Glycol) Sols as Vitreous Substitutes in an Experimental Vitrectomy Model in Rabbits. Acta Biomater. 2011, 7, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, Y.; Iwasaki, T.; Ishikawa, T.; Yamakawa, N.; Suzuki, H.; Usui, M. Application of Thermo-Setting Gel as Artificial Vitreous. Jpn. J. Ophthalmol. 2005, 49, 491–496. [Google Scholar] [CrossRef]
- Annaka, M.; Mortensen, K.; Vigild, M.E.; Matsuura, T.; Tsuji, S.; Ueda, T.; Tsujinaka, H. Design of an Injectable in Situ Gelation Biomaterials for Vitreous Substitute. Biomacromolecules 2011, 12, 4011–4021. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Yang, X.; Tong, X.; Huang, Y. A Method to Accelerate the Gelation of Disulfide-Crosslinked Hydrogels. Chin. J. Polym. Sci. 2015, 33, 118–127. [Google Scholar] [CrossRef]
- Hayashi, K.; Okamoto, F.; Hoshi, S.; Katashima, T.; Zujur, D.C.; Li, X.; Shibayama, M.; Gilbert, E.P.; Chung, U.I.; Ohba, S.; et al. Fast-Forming Hydrogel with Ultralow Polymeric Content as an Artificial Vitreous Body. Nat. Biomed. Eng. 2017, 1, 0044. Available online: https://www.nature.com/articles/s41551-017-0044 (accessed on 2 September 2022). [CrossRef]
- Chang, J.; Tao, Y.; Wang, B.; Guo, B.; Xu, H.; Jiang, Y.; Huang, Y. An in Situ-Forming Zwitterionic Hydrogel as Vitreous Substitute. J. Mater. Chem. B 2015, 3, 1097–1105. [Google Scholar] [CrossRef]
- Morozova, S.; Hamilton, P.; Ravi, N.; Muthukumar, M. Development of a Vitreous Substitute: Incorporating Charges and Fibrous Structures in Synthetic Hydrogel Materials. Macromolecules 2016, 49, 4619–4626. [Google Scholar] [CrossRef]
- Liang, H.; Labbé, A.; Le Mouhaër, J.; Plisson, C.; Baudouin, C. A New Viscous Cysteamine Eye Drops Treatment for Ophthalmic Cystinosis: An Open-Label Randomized Comparative Phase III Pivotal Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2275–2283. [Google Scholar] [CrossRef]
- Xue, K.; Liu, Z.; Jiang, L.; Kai, D.; Li, Z.; Su, X.; Loh, X.J. A New Highly Transparent Injectable PHA-Based Thermogelling Vitreous Substitute. Biomater. Sci. 2020, 8, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Laradji, A.; Shui, Y.-B.; Karakocak, B.B.; Evans, L.; Hamilton, P.; Ravi, N. Bioinspired Thermosensitive Hydrogel as a Vitreous Substitute: Synthesis, Properties, and Progress of Animal Studies. Materials 2020, 13, 1337. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J. Structure, Function, and Age-Related Changes of the Human Vitreous. In The Vitreous and Vitreoretinal Interface; Schepens, C.L., Neetens, A., Eds.; Springer: New York, NY, USA, 1987; pp. 37–57. ISBN 978-1-4757-1901-7. [Google Scholar]
- Nickerson, C.S.; Park, J.; Kornfield, J.A.; Karageozian, H. Rheological Properties of the Vitreous and the Role of Hyaluronic Acid. J. Biomech. 2008, 41, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Landwehr, G.; Kilp, H.; Neubauer, H. The Mechanical Properties of the Vitreous of Pig and Human Donor Eyes. Ophthalmic Res. 1982, 14, 335–343. [Google Scholar] [CrossRef]
- Boettner, E.A.; Wolter, J.R. Transmission of the Ocular Media. Investig. Ophthalmol. Vis. Sci. 1962, 1, 776–783. [Google Scholar]
- Balazs, E.A. Fine Structure and Function of Ocular Tissues. The Vitreous. Int. Ophthalmol. Clin. 1973, 13, 169–187. [Google Scholar]
- Coleman, D.J. Unified Model for Accommodative Mechanism. Am. J. Ophthalmol. 1970, 69, 1063–1079. [Google Scholar] [CrossRef]
- Käsdorf, B.T.; Arends, F.; Lieleg, O. Diffusion Regulation in the Vitreous Humor. Biophys. J. 2015, 109, 2171–2181. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. The Blood-Ocular Barriers: Past, Present, and Future. Doc. Ophthalmol. 1997, 93, 149–157. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-Mediated Angiogenesis in Vascular Disease: Uncovering RHAMM and CD44 Receptor Signaling Pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.; Patrick, R.S. Constituent Monosaccharides and Hexosamine Concentration of Normal Human Vitreous Humour. Exp. Eye Res. 1967, 6, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Shui, Y.-B.; Holekamp, N.M.; Kramer, B.C.; Crowley, J.R.; Wilkins, M.A.; Chu, F.; Malone, P.E.; Mangers, S.J.; Hou, J.H.; Siegfried, C.J.; et al. The Gel State of the Vitreous and Ascorbate-Dependent Oxygen Consumption: Relationship to the Etiology of Nuclear Cataracts. Arch. Ophthalmol. 2009, 127, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Spitzer, M.S.; Januschowski, K. [Aging and age-related changes of the vitreous body]. Ophthalmologe 2015, 7, 552–558. [Google Scholar] [CrossRef]
- Sebag, J. Ageing of the Vitreous. Eye 1987, 1 Pt 2, 254–262. [Google Scholar] [CrossRef]
- Meral, I.; Bilgili, Y. Diffusion Changes in the Vitreous Humor of the Eye during Aging. AJNR Am. J. Neuroradiol. 2011, 32, 1563–1566. [Google Scholar] [CrossRef] [Green Version]
- Ponsioen, T.L.; van Deemter, M.; Bank, R.A.; Snabel, J.M.; Zijlstra, G.S.; van der Worp, R.J.; Hooymans, J.M.M.; Los, L.I. Mature Enzymatic Collagen Cross-Links, Hydroxylysylpyridinoline and Lysylpyridinoline, in the Aging Human Vitreous. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Lumi, X.; Hawlina, M.; Glavač, D.; Facskó, A.; Moe, M.C.; Kaarniranta, K.; Petrovski, G. Ageing of the Vitreous: From Acute Onset Floaters and Flashes to Retinal Detachment. Ageing Res. Rev. 2015, 21, 71–77. [Google Scholar] [CrossRef]
- de Smet, M.D.; Gad Elkareem, A.M.; Zwinderman, A.H. The Vitreous, the Retinal Interface in Ocular Health and Disease. Ophthalmologica 2013, 230, 165–178. [Google Scholar] [CrossRef]
- Itakura, H.; Kishi, S.; Kotajima, N.; Murakami, M. Decreased Vitreal Hyaluronan Levels with Aging. Ophthalmologica 2009, 223, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Thomas, A.; Gilbert, S.J.; Duance, V.C. Elevated Levels of Proteolytic Enzymes in the Aging Human Vitreous. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3299–3304. [Google Scholar]
- Bishop, P.N.; Holmes, D.F.; Kadler, K.E.; McLeod, D.; Bos, K.J. Age-Related Changes on the Surface of Vitreous Collagen Fibrils. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Los, L.I.; van der Worp, R.J.; van Luyn, M.J.A.; Hooymans, J.M.M. Age-Related Liquefaction of the Human Vitreous Body: LM and TEM Evaluation of the Role of Proteoglycans and Collagen. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2828–2833. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M.; Harocopos, G.J.; Shui, Y.-B.; Beebe, D.C. Myopia and Axial Length Contribute to Vitreous Liquefaction and Nuclear Cataract. Arch. Ophthalmol. 2008, 126, 744. [Google Scholar] [CrossRef]
- Neal, R.E.; Bettelheim, F.A.; Lin, C.; Winn, K.C.; Garland, D.L.; Zigler, J.S. Alterations in Human Vitreous Humour Following Cataract Extraction. Exp. Eye Res. 2005, 80, 337–347. [Google Scholar] [CrossRef]
- Framme, C.; Wolf, S. Retinal Complications after Damaging the Vitreolenticular Barrier. Ophthalmologica 2012, 227, 20–33. [Google Scholar] [CrossRef]
- Sebag, J. Vitreous and Vision Degrading Myodesopsia. Prog. Retin. Eye Res. 2020, 79, 100847. [Google Scholar] [CrossRef]
- Su, X.; Tan, M.J.; Li, Z.; Wong, M.; Rajamani, L.; Lingam, G.; Loh, X.J. Recent Progress in Using Biomaterials as Vitreous Substitutes. Biomacromolecules 2015, 16, 3093–3102. [Google Scholar] [CrossRef]
- Dogramaci, M. The Effect of the Anterior Ocular Structures on the Fluid Dynamics in Eyes with Gas Tamponades. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1324. [Google Scholar] [CrossRef]
- How Should Patients with a Gas Bubble Position Their Head and What Precautions Should They Follow? Available online: https://www.healio.com/news/ophthalmology/20200930/how-should-patients-with-a-gas-bubble-position-their-head-and-what-precautions-should-they-follow (accessed on 1 September 2022).
- Mertens, S.; Bednarz, J.; Engelmann, K. Evidence of Toxic Side Effects of Perfluorohexyloctane after Vitreoretinal Surgery as Well as in Previously Established in Vitro Models with Ocular Cell Types. Graefes Arch. Clin. Exp. Ophthalmol. 2002, 240, 989–995. [Google Scholar] [CrossRef]
- Migliavacca, L.; Bottoni, F.; Miglior, S. Experimental Short-Term Tolerance to Perfluorodecalin in the Rabbit Eye: A Histopathological Study. Curr. Eye Res. 1998, 17, 828–835. [Google Scholar] [CrossRef]
- Kociok, N.; Gavranic, C.; Kirchhof, B.; Joussen, A.M. Influence on Membrane-Mediated Cell Activation by Vesicles of Silicone Oil or Perfluorohexyloctane. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 345–358. [Google Scholar] [CrossRef]
- Bhende, P.S.; Biswas, J.; Gopal, L. Silicone Oil Complications. Ophthalmology 2004, 111, 2144–2145. [Google Scholar] [CrossRef]
- Rizzo, S.; Barca, F. Vitreous Substitute and Tamponade Substances for Microincision Vitreoretinal Surgery. Dev. Ophthalmol. 2014, 54, 92–101. [Google Scholar] [CrossRef]
- Romano, M.R.; Xu, X.; Li, K.K.W. Vitreous Substitutes: From Tamponade Effect to Intraocular Inflammation. BioMed Res. Int. 2014, 2014, 159832. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular Drug Delivery Systems: An Overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- García-Quintanilla, L.; Luaces-Rodríguez, A.; Gil-Martínez, M.; Mondelo-García, C.; Maroñas, O.; Mangas-Sanjuan, V.; González-Barcia, M.; Zarra-Ferro, I.; Aguiar, P.; Otero-Espinar, F.J.; et al. Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration. Pharmaceutics 2019, 11, 365. [Google Scholar] [CrossRef]
- Colthurst, M.J.; Williams, R.L.; Hiscott, P.S.; Grierson, I. Biomaterials Used in the Posterior Segment of the Eye. Biomaterials 2000, 21, 649–665. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Scholda, C.; Egger, S.; Lakits, A.; Walch, K.; Von Eckardstein, E.; Biowski, R. Retinal Detachment after Silicone Oil Removal. Acta Ophthalmol. Scand. 2000, 78, 182–186. [Google Scholar] [CrossRef]
- Kawaguchi, M. Silicone Oil Emulsions Stabilized by Polymers and Solid Particles. Adv. Colloid Interface Sci. 2016, 233, 186–199. [Google Scholar] [CrossRef]
- Zauberman, H.; Admoni, M.M.; Treister, G.; Nesher, R. [Age-related macular degeneration after retinal detachment surgery]. J. Fr. Ophtalmol. 1989, 12, 701–707. [Google Scholar]
- Paulk, P.; Crosson, J.; Swain, T.A.; Eloubeidi, D. Macula-Off Rhegmatogenous Retinal Detachment (RRD) Outcomes in Age-Related Macular Degeneration (AMD) versus Non-AMD Eyes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3740. [Google Scholar]
- Phelps, C.D.; Burton, T.C. Glaucoma and Retinal Detachment. Arch. Ophthalmol. 1977, 95, 418–422. [Google Scholar] [CrossRef]


| Type of Gas | Molecular Weight (g/mol) | Maximal Expansion (h) | Duration in the Vitreous Chamber | Non-Expansile Concentration | Capacity to Expand (Times) |
|---|---|---|---|---|---|
| Air | 29 | No expansion | 5–7 days | No expansion | 0 |
| SF6 | 146 | 24–48 | 1–2 weeks | 20% | 2 |
| C2F6 | 138 | 36–60 | 4–5 weeks | 16% | 3 |
| C3F8 | 188 | 72–96 | 6–8 weeks | 12% | 4 |
| (a) | |||||
|---|---|---|---|---|---|
| Hydrogels | Polymer Content [%] | Refractive Index | Light Transmittance [%] | In Vivo Studies | Ref. |
| Gellan and hyaluronic acid | 1 | - | 85–95 | Not conducted | [192] |
| Methacrylated gellan gum | 1 | - | - | Not conducted | [224] |
| Hyaluronic acid | 1 | 1.34 | - | rabbits | [184] |
| 3 | 1.34 | - | rabbits | [185] | |
| 1–2.2 | 1.32–1.34 | - | rabbits | [182] | |
| 1 | 1.32–1.33 | - | rabbits | [225] | |
| 1 | 1.34 | 75–91 | Not conducted | [226] | |
| Peptide gel | 0.10 | 1.33 | 96.7 | rabbits | [227] |
| Synthetic polymers | |||||
| Polyvinyl alcohol methacrylate | 9 | - | - | Not conducted | [194] |
| Polyvinyl alcohol | 7 | - | - | macaques | [195] |
| 4 | - | 85 | Not conducted | [196] | |
| 5 | - | - | Not conducted | [197] | |
| 1–7 | 1.34 | 93 | rabbits | [198] | |
| 4 | 1.34 | - | Not conducted | [199] | |
| Poly (ethylene glycol) | 5 | 1.34 | - | rabbits | [228] |
| Acrylic acid and acrylamide | 1.25–1.75 | - | - | Not conducted | [201] |
| Poly N-acryloy| glycinamide-polycarboxybetaine acrylamide | 1.60 | 1.34 | 93.2 | rabbits | [203] |
| Smart hydrogels | |||||
| WTG-127 | - | - | 89.3 | rabbits | [229] |
| Poly (ethylene glycol) | 25 | 1.35 | >90 | Not conducted | [230] |
| 10 | 1.33 | - | rabbits | [231] | |
| 0.4–0.7 | - | - | rabbits | [232] | |
| Sulfobetaine methacrylamide and acryloyl cystamine monomers | 5 | - | >90 | rabbits | [233] |
| Gellan and poly (methacrylamide-co-methacrylate) | 0.65–1.29 | 1.34–1.34 | 87.6–94 | rabbits | [191] |
| (b) | |||||
| Hydrogels | Polymer Content [%] | Refractive Index | Light Transmittance [%] | In Vivo Studies | Ref. |
| Smart hydrogels | |||||
| Methacrylic acid, methylacrylamide, and bismethacryloyloystamine | 1–1.4 | Not conducted | [234] | ||
| Polymethacrylamide and poly-methacrylate | 0.9–1.8 | 1.33–1.33 | >95 | Not conducted | [235] |
| Hydroxypropyl chitosan and alginate dialdehyde | 1–3 | 1.33 | >80 | rabbits | [189] |
| poly(ε-caprolactone)-based (PCL) thermogel | 3–12 | 1.34–1.34 | rabbits | [207] | |
| Polyhydroxyalkanoate (PHA) thermogel | 2–20 | <90 | rabbits | [236] | |
| Poly (ethylene glycol) methacrylate and poly (ethylene glycol) diacrylate | 0.75–5.7 | 1.34–1.34 | >90 | Not conducted | [210] |
| Gellan and poly(methacrylamide-co-methacrylate-co- bis (methacryloy))lorstamine) | 1.34–1.34 | >83 | rabbits | [237] | |
| Vitreous Substitute | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| BSS | Transparent, physiologic refractive index, non-toxic, such as aqueous humor and vitreous | Low interfacial tension, density nearly equal to 1 | [7,265] |
| Air | Chemically inert, non-toxic, inexpensive, colorless, low density, high interfacial tension, no need for a second operation for removal | Very short duration inside the vitreous cavity, low refractive index (1.0008) | [7,114] |
| Gas (SF6, C2F6, C3F8) | Colorless, odorless, non-toxic, high interfacial tension and buoyancy | Short duration inside the vitreous cavity, induction of cataract development, high IOP, need for positioning | [7,266,267] |
| PFCL | Transparent, high density, moderate interfacial tension | Only approved for intra-operative use due to high reactivity | [268,269] |
| SFAs | Inert, colorless, physiologic refractive index | Low specific gravity; cataract; emulsification. Epiretinal membrane development | [99,164,270] |
| Silicone Oil | Moderate interfacial tension, Transparent, inert | Cataract, glaucoma, corneal toxicity, silicone retinopathy risk; second surgery for its removal | [86,271] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Confalonieri, F.; Josifovska, N.; Boix-Lemonche, G.; Stene-Johansen, I.; Bragadottir, R.; Lumi, X.; Petrovski, G. Vitreous Substitutes from Bench to the Operating Room in a Translational Approach: Review and Future Endeavors in Vitreoretinal Surgery. Int. J. Mol. Sci. 2023, 24, 3342. https://doi.org/10.3390/ijms24043342
Confalonieri F, Josifovska N, Boix-Lemonche G, Stene-Johansen I, Bragadottir R, Lumi X, Petrovski G. Vitreous Substitutes from Bench to the Operating Room in a Translational Approach: Review and Future Endeavors in Vitreoretinal Surgery. International Journal of Molecular Sciences. 2023; 24(4):3342. https://doi.org/10.3390/ijms24043342
Chicago/Turabian StyleConfalonieri, Filippo, Natasha Josifovska, Gerard Boix-Lemonche, Ingar Stene-Johansen, Ragnheidur Bragadottir, Xhevat Lumi, and Goran Petrovski. 2023. "Vitreous Substitutes from Bench to the Operating Room in a Translational Approach: Review and Future Endeavors in Vitreoretinal Surgery" International Journal of Molecular Sciences 24, no. 4: 3342. https://doi.org/10.3390/ijms24043342






