SiC Foams for the Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SiC Foam Fabrication
2.3. SiC Foam Characterization
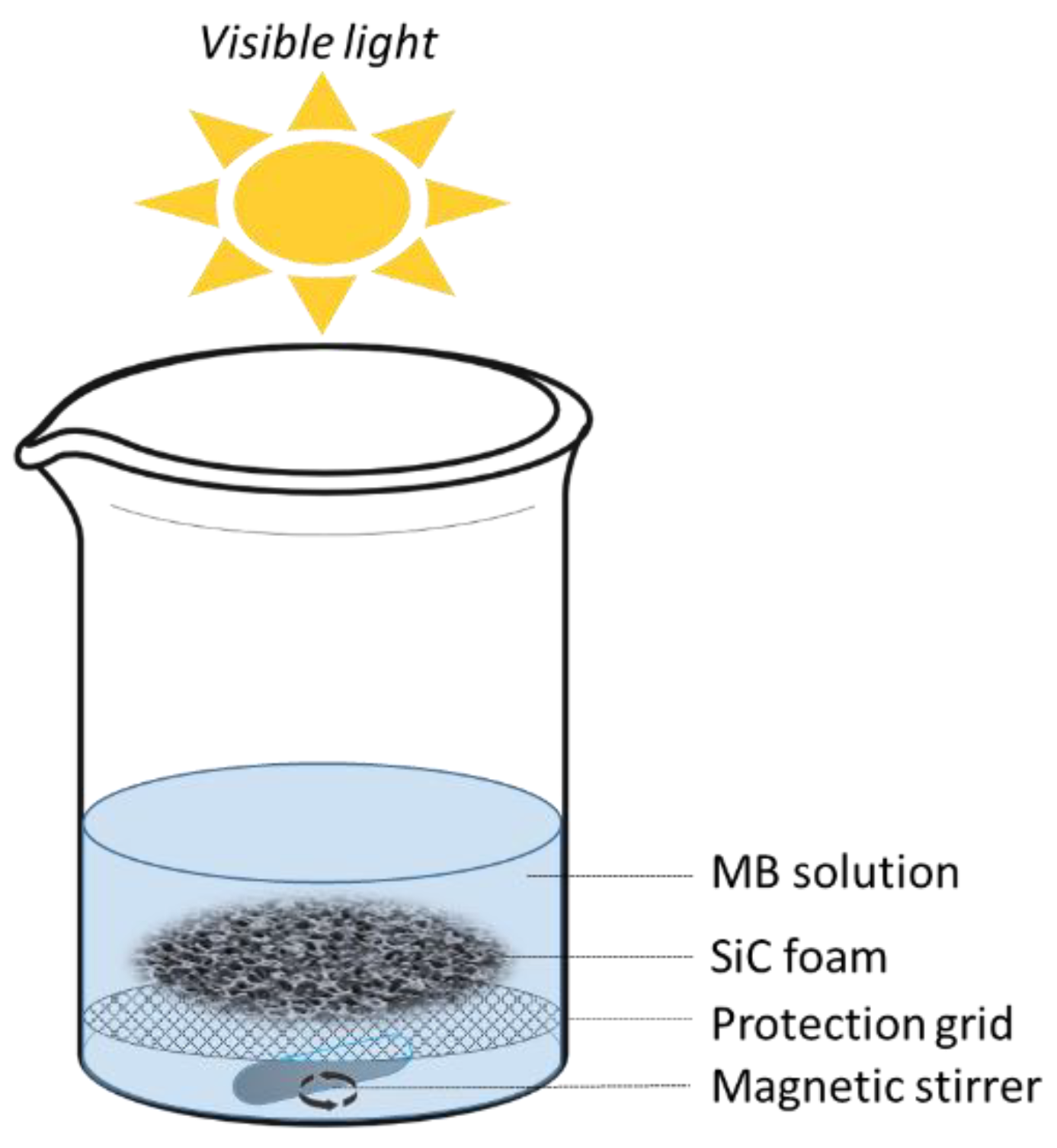
2.4. Black Experiments and Photocatalytic Degradation under Visible Light Irradiation
3. Results
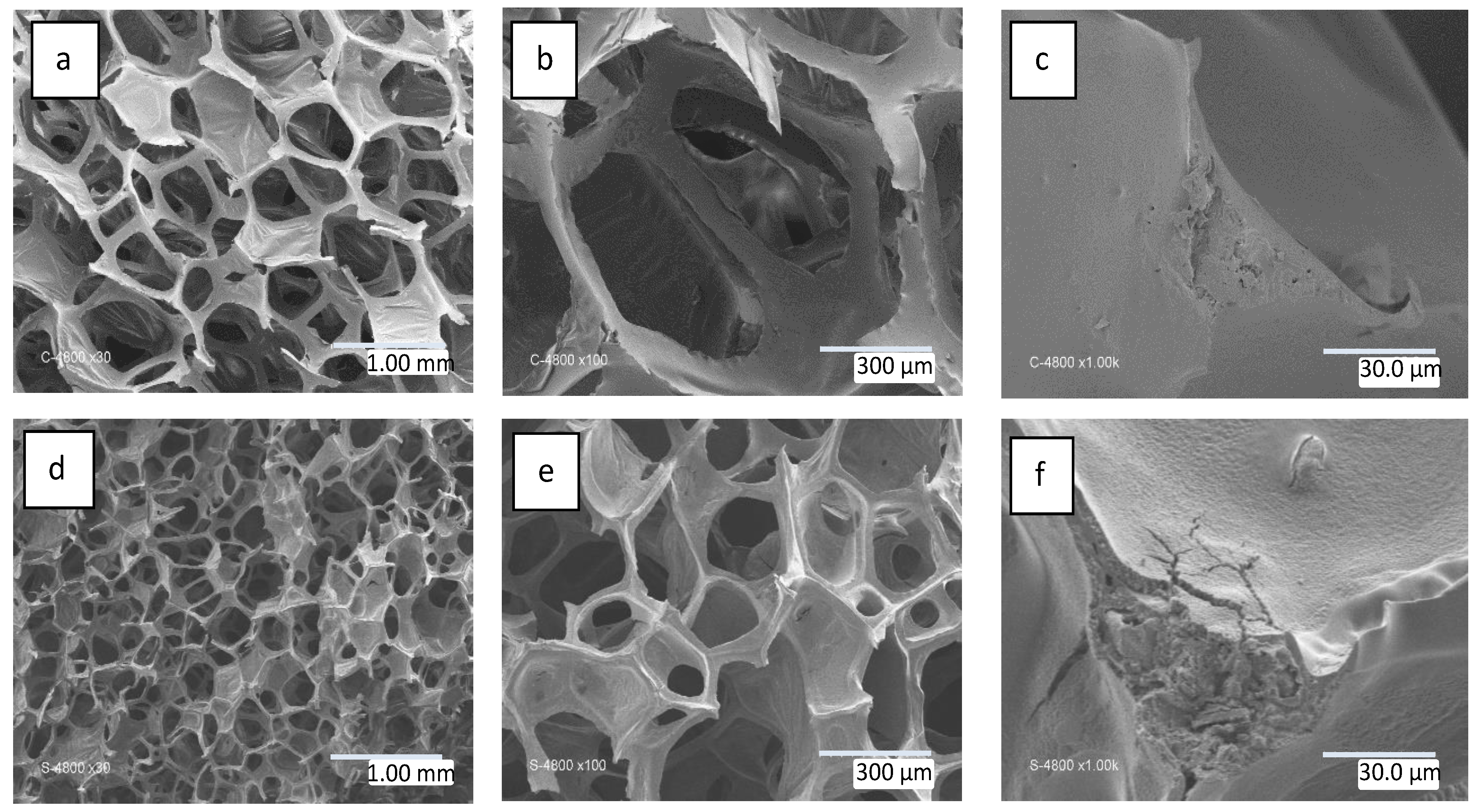
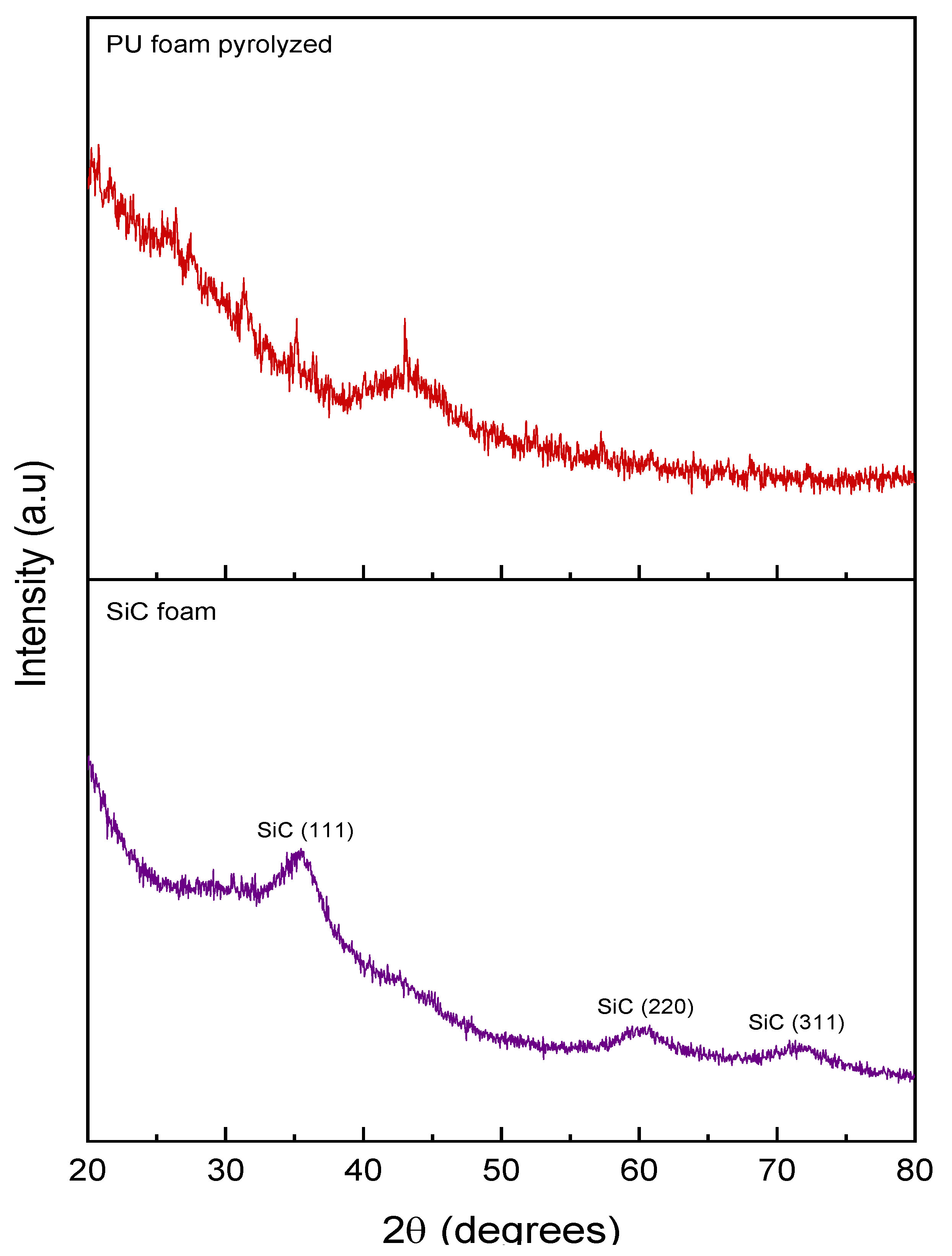
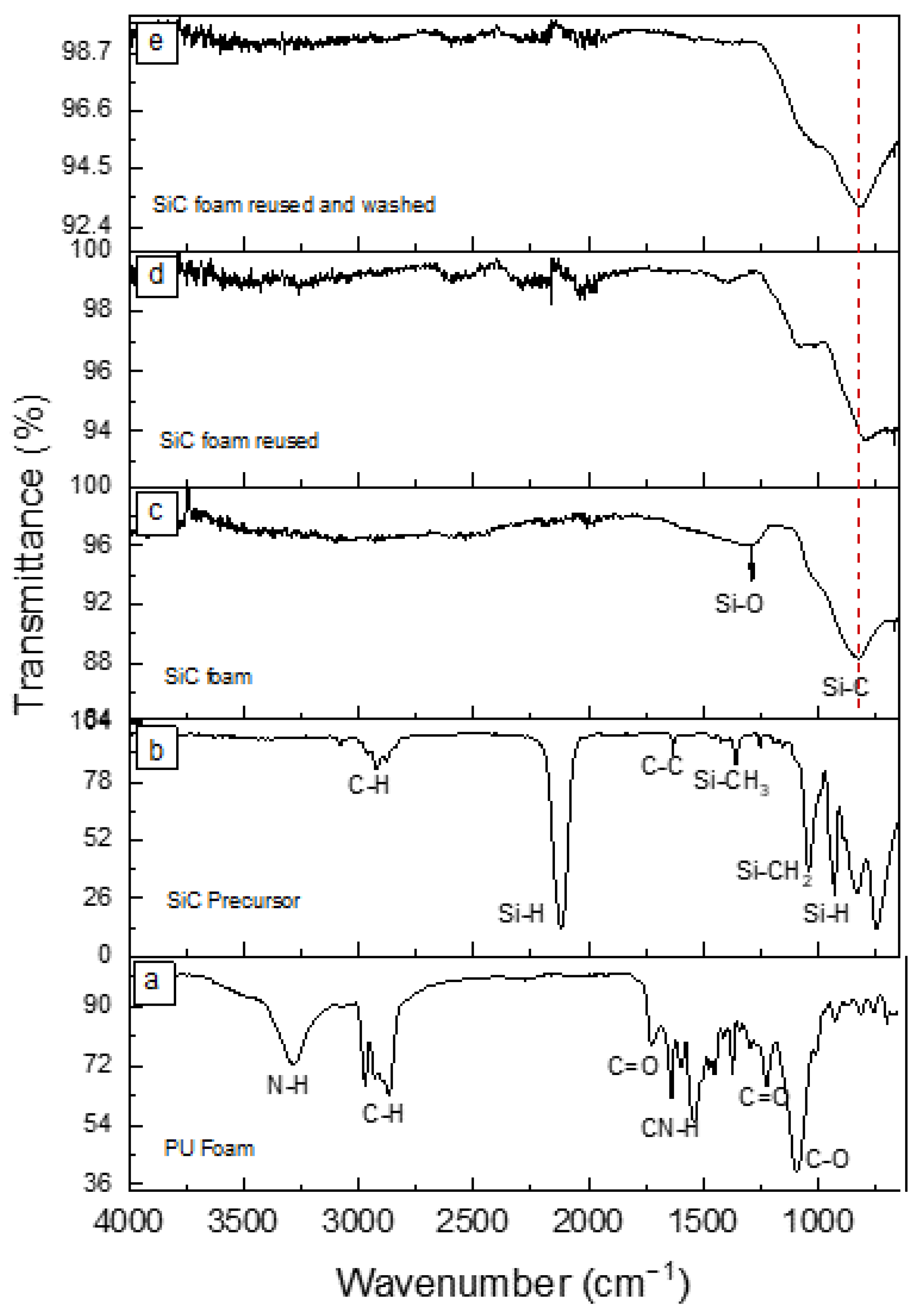
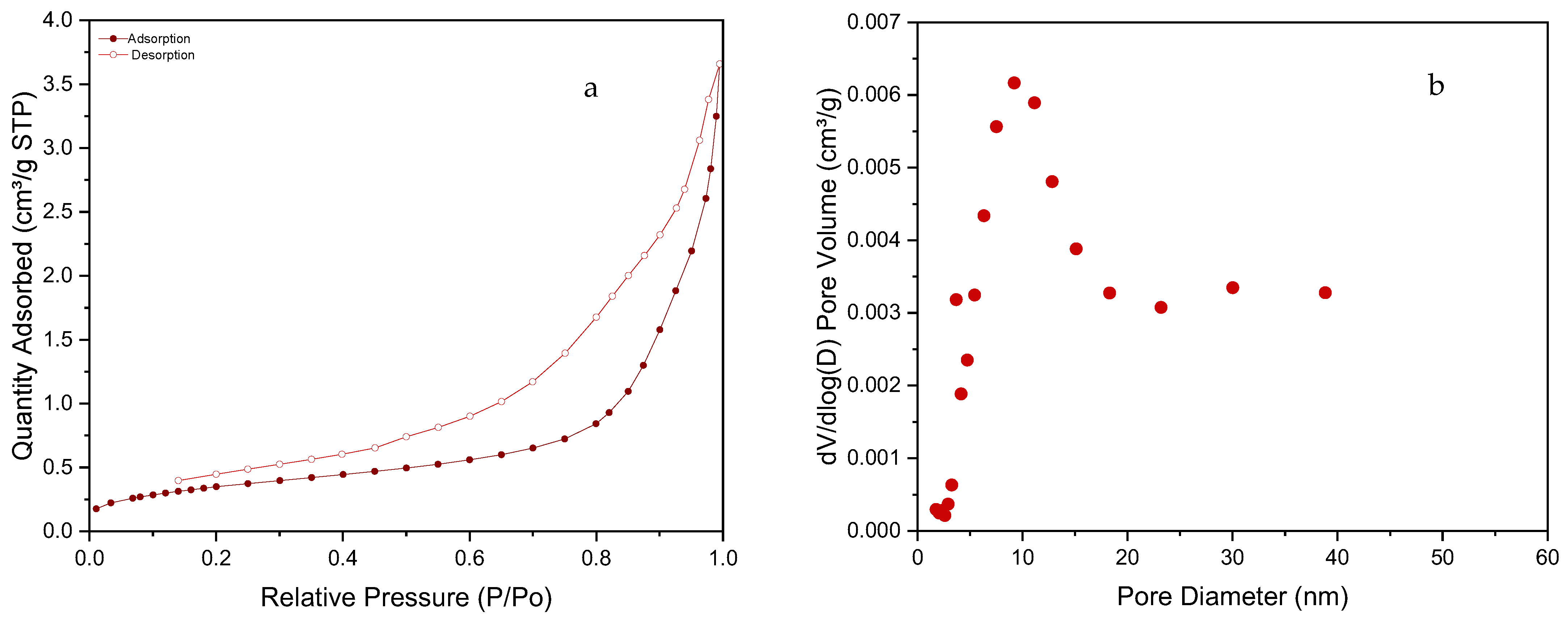
3.1. SiC Foam Characterization
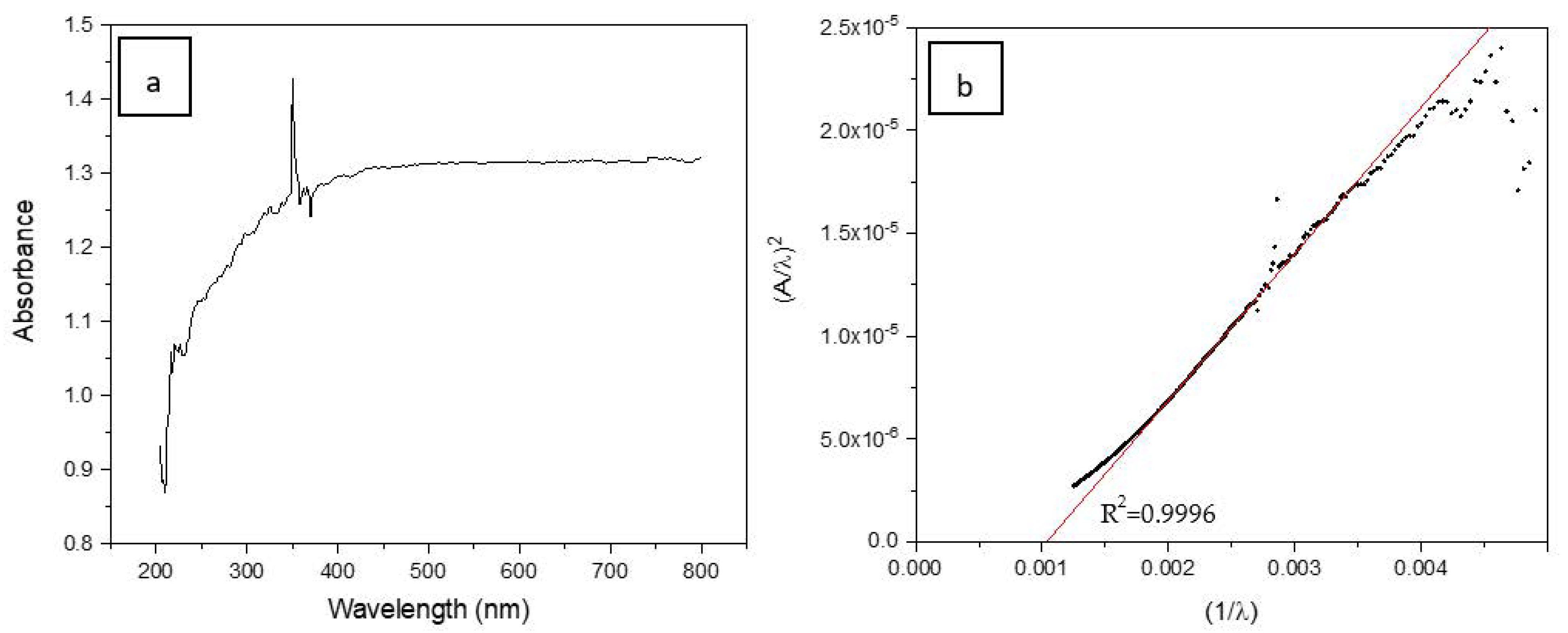
3.2. Bandgap Calculations of the Prepared SiC Foam
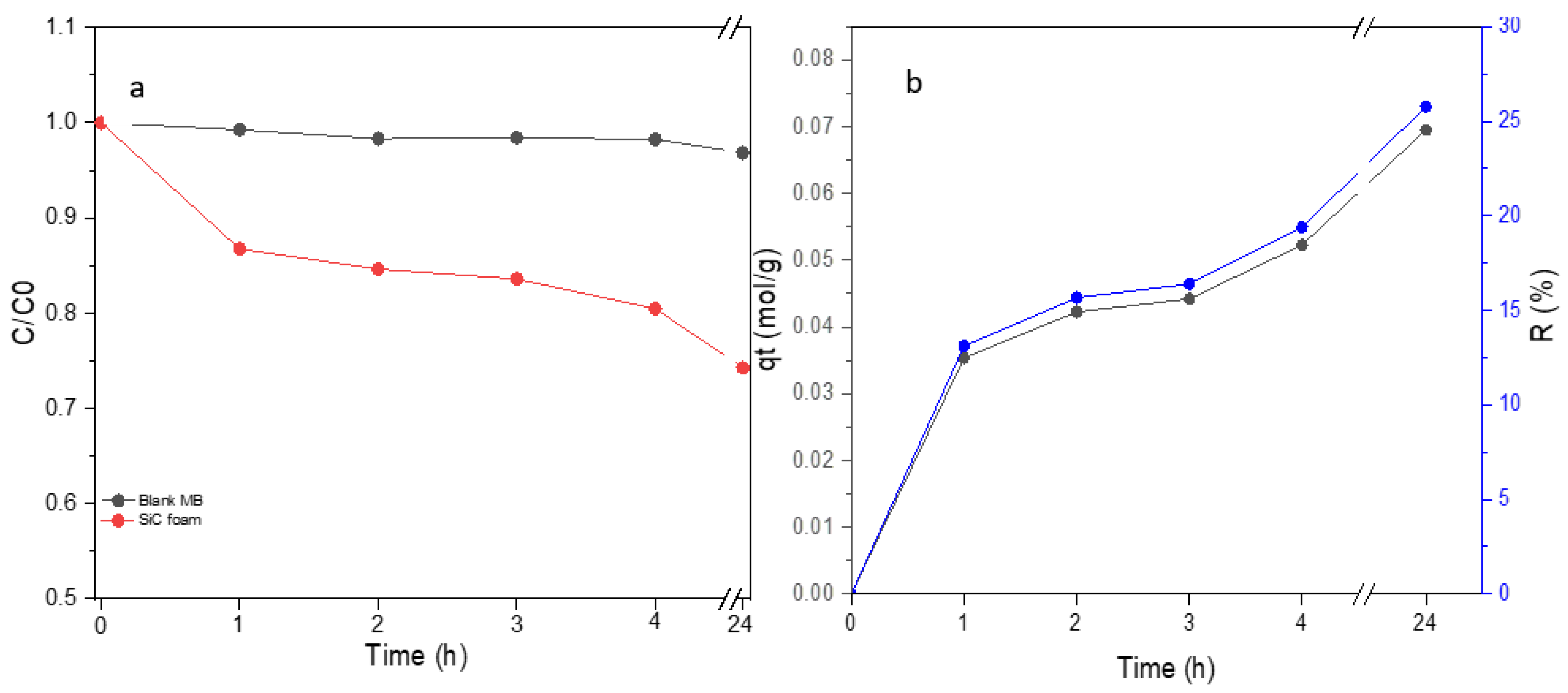
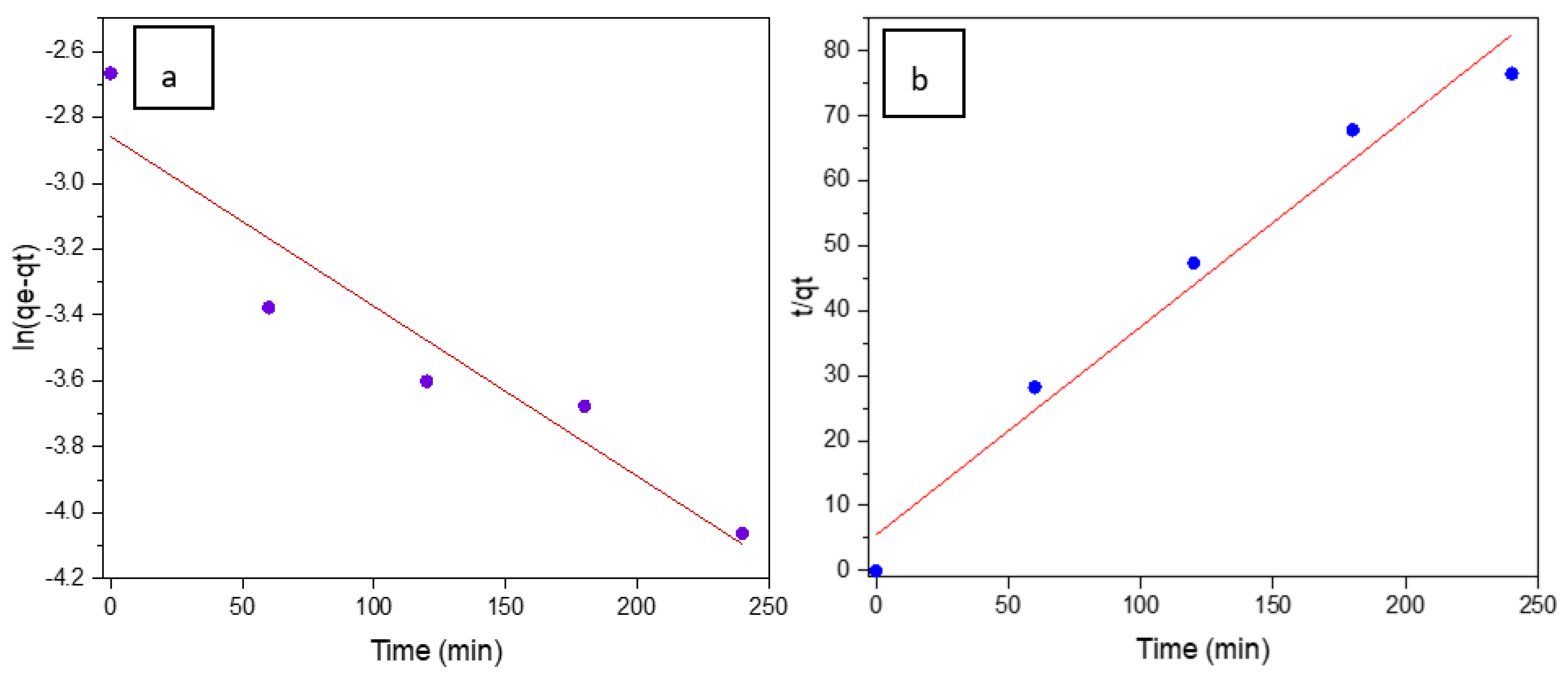
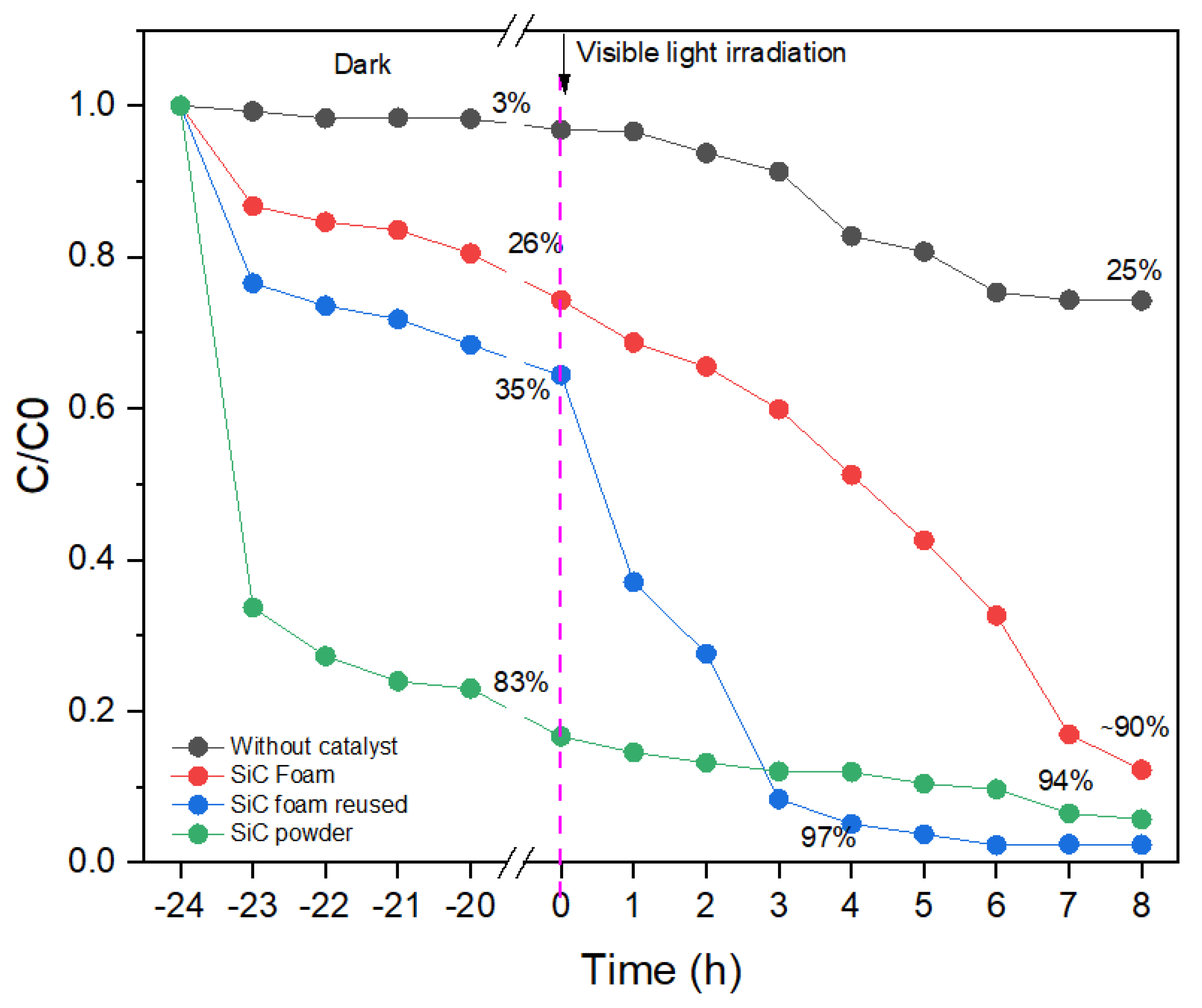
3.3. Adsorption Capacity and Photocatalytic Performance of SiC Foams under Visible Light Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q. Pollution and Treatment of Dye Waste-Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052001. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Sonawane, R.S.; Kale, B.B.; Dongare, M.K. Preparation and Photo-Catalytic Activity of Fe-TiO2 Thin Films Prepared by Sol-Gel Dip Coating. Mater. Chem. Phys. 2004, 85, 52–57. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO2 Based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Kouamé, N.A.; Robert, D.; Keller, V.; Keller, N.; Pham, C.; Nguyen, P. Preliminary Study of the Use of β-SiC Foam as a Photocatalytic Support for Water Treatment. Catal. Today 2011, 161, 3–7. [Google Scholar] [CrossRef]
- Hariganesh, S.; Vadivel, S.; Maruthamani, D.; Rangabhashiyam, S. Disinfection By-Products in Drinking Water: Detection and Treatment Methods. In Disinfection By-products in Drinking Water; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–304. ISBN 9780081029770. [Google Scholar]
- Abebe, B.; Murthy, H.C.A.; Amare, E. Summary on Adsorption and Photocatalysis for Pollutant Remediation: Mini Review. J. Encapsulation Adsorpt. Sci. 2018, 08, 225–255. [Google Scholar] [CrossRef]
- Aziz, N.A.A.; Palaniandy, P.; Aziz, H.A.; Dahlan, I. Review of the Mechanism and Operational Factors Influencing the Degradation Process of Contaminants in Heterogenous Photocatalysis. J. Chem. Res. 2016, 40, 704–712. [Google Scholar] [CrossRef]
- Xu, C.; Rangaiah, G.P.; Zhao, X.S. Photocatalytic Degradation of Methylene Blue by Titanium Dioxide: Experimental and Modeling Study. Ind. Eng. Chem. Res. 2014, 53, 14641–14649. [Google Scholar] [CrossRef]
- Chang, X.; Li, Z.; Zhai, X.; Sun, S.; Gu, D.; Dong, L.; Yin, Y.; Zhu, Y. Efficient Synthesis of Sunlight-Driven ZnO-Based Heterogeneous Photocatalysts. Mater. Des. 2016, 98, 324–332. [Google Scholar] [CrossRef]
- Wang, H.; Sun, F.; Zhang, Y.; Li, L.; Chen, H.; Wu, Q.; Yu, J.C. Photochemical Growth of Nanoporous SnO2 at the Air-Water Interface and Its High Photocatalytic Activity. J. Mater. Chem. 2010, 20, 5641–5645. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A Review on Exploration of Fe2O3 Photocatalyst towards Degradation of Dyes and Organic Contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Belessiotis, G.V.; Arfanis, M.K.; Athanasekou, C.; Philippopoulos, A.I.; Mitsopoulou, C.A.; Romanos, G.E.; Falaras, P. Surfactant Effects on the Synthesis of Redox Bifunctional V2O5 Photocatalysts. Materials 2020, 13, 4665. [Google Scholar] [CrossRef]
- Guo, X.-N.; Tong, X.-L.; Guo, X.-Y. Application of Silicon Carbide in Photocatalysis. In Novel Carbon Materials and Composites; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 73–97. [Google Scholar]
- Ibrahim, I.; Belessiotis, G.V.; Elseman, A.M.; Mohamed, M.M.; Ren, Y.; Salama, T.M.; Mohamed, M.B.I. Magnetic TiO2/CoFe2O4 Photocatalysts for Degradation of Organic Dyes and Pharmaceuticals without Oxidants. Nanomaterials 2022, 12, 3290. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Kaltzoglou, A.; Athanasekou, C.; Katsaros, F.; Devlin, E.; Kontos, A.G.; Ioannidis, N.; Perraki, M.; Tsakiridis, P.; Sygellou, L.; et al. Magnetically Separable TiO2/CoFe2O4/Ag Nanocomposites for the Photocatalytic Reduction of Hexavalent Chromium Pollutant under UV and Artificial Solar Light. Chem. Eng. J. 2020, 381, 122730. [Google Scholar] [CrossRef]
- Hao, Q.; Niu, X.; Nie, C.; Hao, S.; Zou, W.; Ge, J.; Chen, D.; Yao, W. A Highly Efficient G-C3N4/SiO2 Heterojunction: The Role of SiO2 in the Enhancement of Visible Light Photocatalytic Activity. Phys. Chem. Chem. Phys. 2016, 18, 31410–31418. [Google Scholar] [CrossRef]
- Sun, S.; Ding, H.; Mei, L.; Chen, Y.; Hao, Q.; Chen, W.; Xu, Z.; Chen, D. Construction of SiO2-TiO2/g-C3N4 Composite Photocatalyst for Hydrogen Production and Pollutant Degradation: Insight into the Effect of SiO2. Chinese Chem. Lett. 2020, 31, 2287–2294. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Shen, K.; Lu, W.; Wang, J.; Chen, F. Defect Engineering in G-C3N4 Quantum-Dot-Modified TiO2 Nanofiber: Uncovering Novel Mechanisms for the Degradation of Tetracycline in Coexistence with Cu2+. Adv. Fiber Mater. 2022, 1–15. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Antoniadou, M.; Kaltzoglou, A.; Sakellis, E.; Katsaros, F.; Sygellou, L.; Arfanis, M.K.; Salama, T.M.; Falaras, P. Silver Decorated TiO2/g-C3N4 Bifunctional Nanocomposites for Photocatalytic Elimination of Water Pollutants under UV and Artificial Solar Light. Results Eng. 2022, 14, 100470. [Google Scholar] [CrossRef]
- Jana, P.; Bruzzoniti, M.C.; Appendini, M.; Rivoira, L.; Del Bubba, M.; Rossini, D.; Ciofi, L.; Sorarù, G.D. Processing of Polymer-Derived Silicon Carbide Foams and Their Adsorption Capacity for Non-Steroidal Anti-Inflammatory Drugs. Ceram. Int. 2016, 42, 18937–18943. [Google Scholar] [CrossRef]
- Kouamé, A.N.; Masson, R.; Robert, D.; Keller, N.; Keller, V. β-SiC Foams as a Promising Structured Photocatalytic Support for Water and Air Detoxification. Catal. Today 2013, 209, 13–20. [Google Scholar] [CrossRef]
- Adhikari, S.; Eswar, N.K.R.; Sangita, S.; Sarkar, D.; Madras, G. Investigation of Nano Ag-Decorated SiC Particles for Photoelectrocatalytic Dye Degradation and Bacterial Inactivation. J. Photochem. Photobiol. A Chem. 2018, 357, 118–131. [Google Scholar] [CrossRef]
- van Dorp, D.H.; Hijnen, N.; Di Vece, M.; Kelly, J.J. SiC: A Photocathode for Water Splitting and Hydrogen Storage. Angew. Chemie Int. Ed. 2009, 48, 6085–6088. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, X.; Jin, G.; Guo, X. Visible-Light-Enhanced Photocatalytic Sonogashira Reaction over Silicon Carbide Supported Pd Nanoparticles. Catal. Commun. 2017, 98, 81–84. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Xin, L.; Wang, M. Hierarchical 3C-SiC Nanowires as Stable Photocatalyst for Organic Dye Degradation under Visible Light Irradiation. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2014, 179, 6–11. [Google Scholar] [CrossRef]
- Haibo, O.; Jianfeng, H.; Xierong, Z.; Liyun, C.; Cuiyan, L.; Xinbo, X.; Jie, F. Visible-Light Photocatalytic Activity of SiC Hollow Spheres Prepared by a Vapor-Solid Reaction of Carbon Spheres and Silicon Monoxide. Ceram. Int. 2014, 40, 2619–2625. [Google Scholar] [CrossRef]
- Nardin, T.; Gouze, B.; Cambedouzou, J.; Diat, O. Soft Templated Mesoporous SiC from Polycarbosilane Grafted onto Triblock Copolymers. Mater. Lett. 2016, 185, 424–427. [Google Scholar] [CrossRef]
- Souri, D.; Tahan, Z.E. A New Method for the Determination of Optical Band Gap and the Nature of Optical Transitions in Semiconductors. Appl. Phys. B Lasers Opt. 2015, 119, 273–279. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Mahmood Mat Yunus, W.; Navasery, M.; Bahmanrokh, G.; Erfani, M.; Zare, M.R.; Gharibshahi, E. Photocatalytic Degradation of Methylene Blue under Visible Light Using PVP-Capped ZnS and CdS Nanoparticles. Sol. Energy 2013, 97, 147–154. [Google Scholar] [CrossRef]
- Iram, M.; Guo, C.; Guan, Y.; Ishfaq, A.; Liu, H. Adsorption and Magnetic Removal of Neutral Red Dye from Aqueous Solution Using Fe3O4 Hollow Nanospheres. J. Hazard. Mater. 2010, 181, 1039–1050. [Google Scholar] [CrossRef]
- Andronic, L.; Isac, L.; Cazan, C.; Enesca, A. Simultaneous Adsorption and Photocatalysis Processes Based on Ternary TiO2–CuxS–Fly Ash Hetero-Structures. Appl. Sci. 2020, 10, 8070. [Google Scholar] [CrossRef]
- Baldez, E.E.; Robaina, N.F.; Cassella, R.J. Employment of Polyurethane Foam for the Adsorption of Methylene Blue in Aqueous Medium. J. Hazard. Mater. 2008, 159, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Gouze, B.; Cervantes-Diaz, K.B.; Nardin, T.; Diat, O.; Cambedouzou, J. Highly Crystalline Silicon Carbide of Controlled Mesoporosity. Mater. Chem. Phys. 2020, 250, 123208. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Roh, J.S. Analysis of Activation Process of Carbon Black Based on Structural Parameters Obtained by XRD Analysis. Crystals 2021, 11, 153. [Google Scholar] [CrossRef]
- Wang, Q.; Yokoji, M.; Nagasawa, H.; Yu, L.; Kanezashi, M.; Tsuru, T. Microstructure Evolution and Enhanced Permeation of SiC Membranes Derived from Allylhydridopolycarbosilane. J. Memb. Sci. 2020, 612, 118392. [Google Scholar] [CrossRef]
- Pradhan, A.C.; Paul, A.; Rao, G.R. Sol-Gel- Cum -Hydrothermal Synthesis of Mesoporous Co-Fe @ Al2O3—MCM-41 for Methylene Blue Remediation. J. Chem. Sci. 2017, 129, 381–395. [Google Scholar] [CrossRef]
- Romolini, G.; Ricciarelli, M.G.D.; Zampini, L.T.G. Photocatalytic Activity of Silica and Silica—Silver Nanocolloids Based on Photo—Induced Formation of Reactive Oxygen Species. Photochem. Photobiol. Sci. 2021, 20, 1161–1172. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ghobadi, N. Band Gap Determination Using Absorption Spectrum Fitting Procedure. Int. Nano Lett. 2013, 3, 2–5. [Google Scholar] [CrossRef]
- Souri, D.; Shomalian, K. Band Gap Determination by Absorption Spectrum Fitting Method (ASF) and Structural Properties of Different Compositions of (60−x) V2O5-40TeO2-xSb2O3 Glasses. J. Non. Cryst. Solids 2009, 355, 1597–1601. [Google Scholar] [CrossRef]
- Low, J.; Kreider, M.; Pulsifer, D.; Jones, A.; Gilani, T. Band Gap Energy in Silicon. Am. J. Undergrad. Res. 2008, 7, 27–32. [Google Scholar] [CrossRef]
- Vadivelan, V.; Vasanth Kumar, K. Equilibrium, Kinetics, Mechanism, and Process Design for the Sorption of Methylene Blue onto Rice Husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N. Differences between Chemical Reaction Kinetics and Adsorption Kinetics: Fundamentals and Discussion. J. Tech. Educ. Sci. 2022, 70B, 33–47. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, X.; Tang, Y.; Su, K.; Kong, J. Hierarchically Porous Silicon-Carbon-Nitrogen Hybrid Materials towards Highly Efficient and Selective Adsorption of Organic Dyes. Sci. Rep. 2015, 5, 7910. [Google Scholar] [CrossRef]
- Fazal, T.; Razzaq, A.; Javed, F.; Hafeez, A.; Rashid, N.; Amjad, U.S.; Ur Rehman, M.S.; Faisal, A.; Rehman, F. Integrating Adsorption and Photocatalysis: A Cost Effective Strategy for Textile Wastewater Treatment Using Hybrid Biochar-TiO2 Composite. J. Hazard. Mater. 2020, 390, 121623. [Google Scholar] [CrossRef] [PubMed]
- Bruzzoniti, M.C.; Appendini, M.; Onida, B.; Castiglioni, M.; Del Bubba, M.; Vanzetti, L.; Jana, P.; Sorarù, G.D.; Rivoira, L. Regenerable, Innovative Porous Silicon-Based Polymer-Derived Ceramics for Removal of Methylene Blue and Rhodamine B from Textile and Environmental Waters. Environ. Sci. Pollut. Res. 2018, 25, 10619–10629. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, J.; Chen, Y.; Liu, X.; Zhao, H.; Yao, Y.; Cao, H. Promising Application of SiC without Co-Catalyst in Photocatalysis and Ozone Integrated Process for Aqueous Organics Degradation. Catal. Today 2018, 315, 223–229. [Google Scholar] [CrossRef]
- Yang, J.; Peng, Y.; Yang, B. Enhanced photocatalytic activity of SiC modified by BiVO4 under visible light irradiation. J. Dispers. Sci. Technol. 2019, 40, 408–414. [Google Scholar] [CrossRef]
- Hao, D.; Liu, Y.; Gao, S.; Arandiyan, H.; Bai, X.; Kong, Q.; Wei, W.; Shen, P.K.; Ni, B.-J. Emerging artificial nitrogen cycle processes through novel electrochemical and photochemical synthesis. Mater. Today 2021, 46, 212–233. [Google Scholar] [CrossRef]
- Florea, I.; Ersen, O.; Hirlimann, C.; Roiban, L.; Deneuve, A.; Houlle, M.; Janowska, I.; Nguyen, P.; Pham, C.; Pham-Huu, C. Analytical electron tomography mapping of the SiC pore oxidation at the nanoscale. Nanoscale 2010, 2, 2668–2678. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.J.; Vaughan, B.; Yeomans, J.A.; Smith, P.A.; Burnage, S.T. Surface preparation of silicon carbide for improved adhesive bond strength in armour applications. J. Eur. Ceram. Soc. 2013, 33, 2925–2934. [Google Scholar] [CrossRef] [Green Version]
| Photocatalyst [ref] | Bandgap (eV) | Efficiency for MB Abatement |
|---|---|---|
| SiC nanowires [28] | - | 96% after 360 min |
| SiC hollow spheres [29] | 2.15 | 98% after 300 min |
| PVP-capped ZnS [32] | 4.07 | 81% after 360 min |
| Er2O3-coated silicon nanowires [52] | - | 98% after 120 min |
| SiC foams (this work) | 1.29 | 88% after 480 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes-Diaz, K.B.; Drobek, M.; Julbe, A.; Cambedouzou, J. SiC Foams for the Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation. Materials 2023, 16, 1328. https://doi.org/10.3390/ma16041328
Cervantes-Diaz KB, Drobek M, Julbe A, Cambedouzou J. SiC Foams for the Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation. Materials. 2023; 16(4):1328. https://doi.org/10.3390/ma16041328
Chicago/Turabian StyleCervantes-Diaz, Karla Begonia, Martin Drobek, Anne Julbe, and Julien Cambedouzou. 2023. "SiC Foams for the Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation" Materials 16, no. 4: 1328. https://doi.org/10.3390/ma16041328
APA StyleCervantes-Diaz, K. B., Drobek, M., Julbe, A., & Cambedouzou, J. (2023). SiC Foams for the Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation. Materials, 16(4), 1328. https://doi.org/10.3390/ma16041328