Actin-Cytoskeleton Drives Caveolae Signaling to Mitochondria during Postconditioning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Electron Microscopy
2.3. Phalloidin Staining and F-Actin/G-Actin Levels
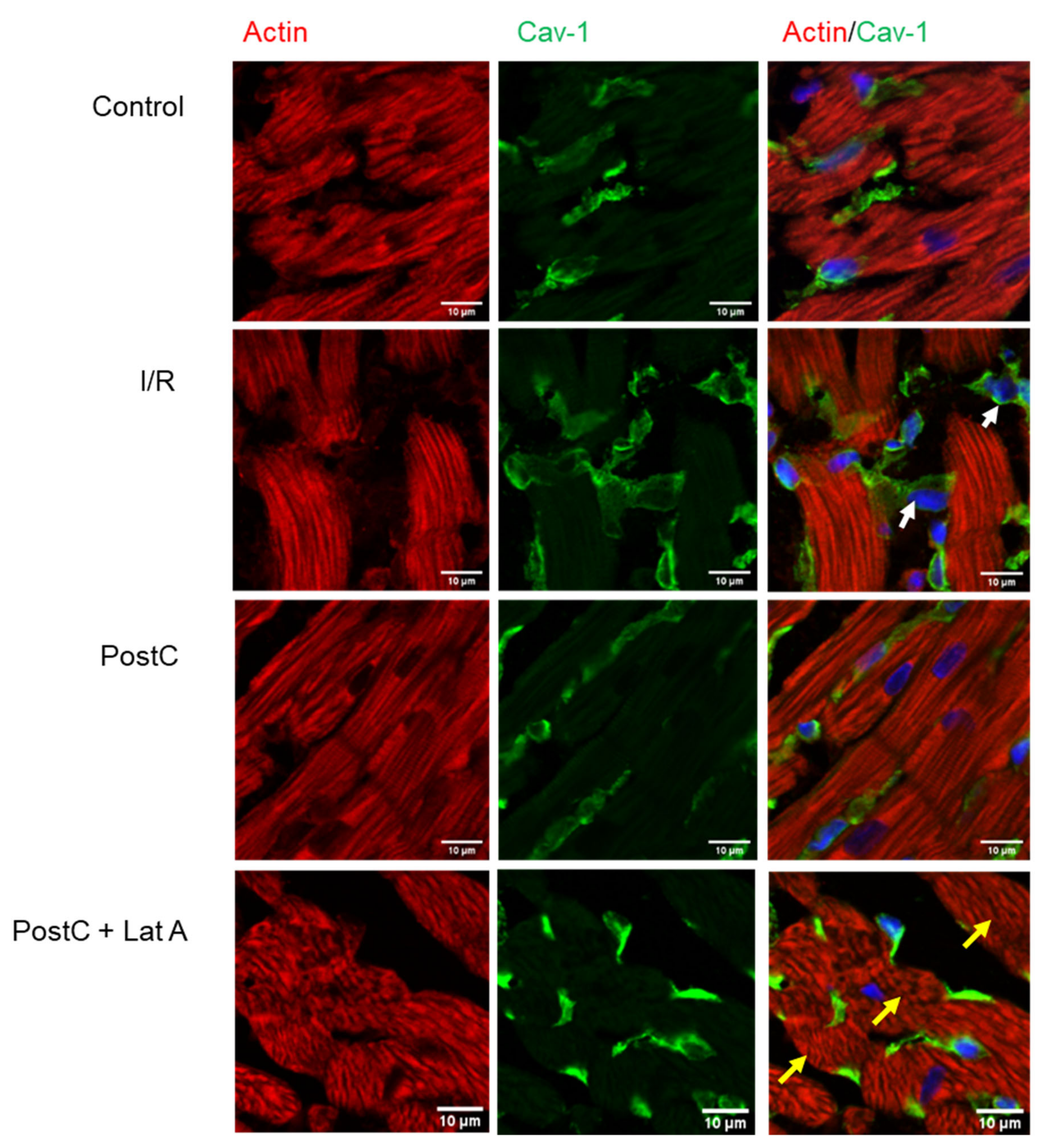
2.4. Immunofluorescence
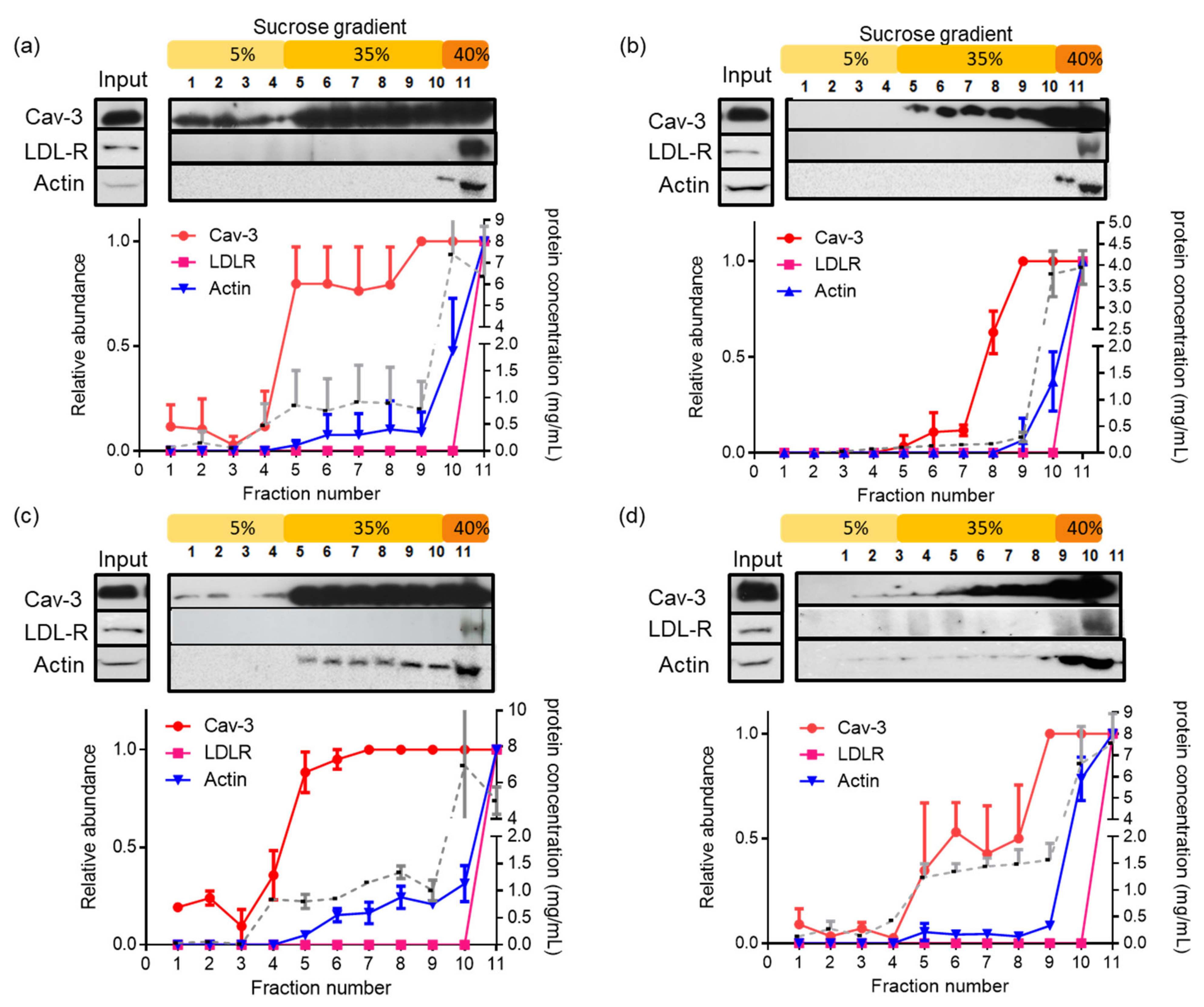
2.5. Caveolin-3 and Actin Segregation in Lipid Rafts
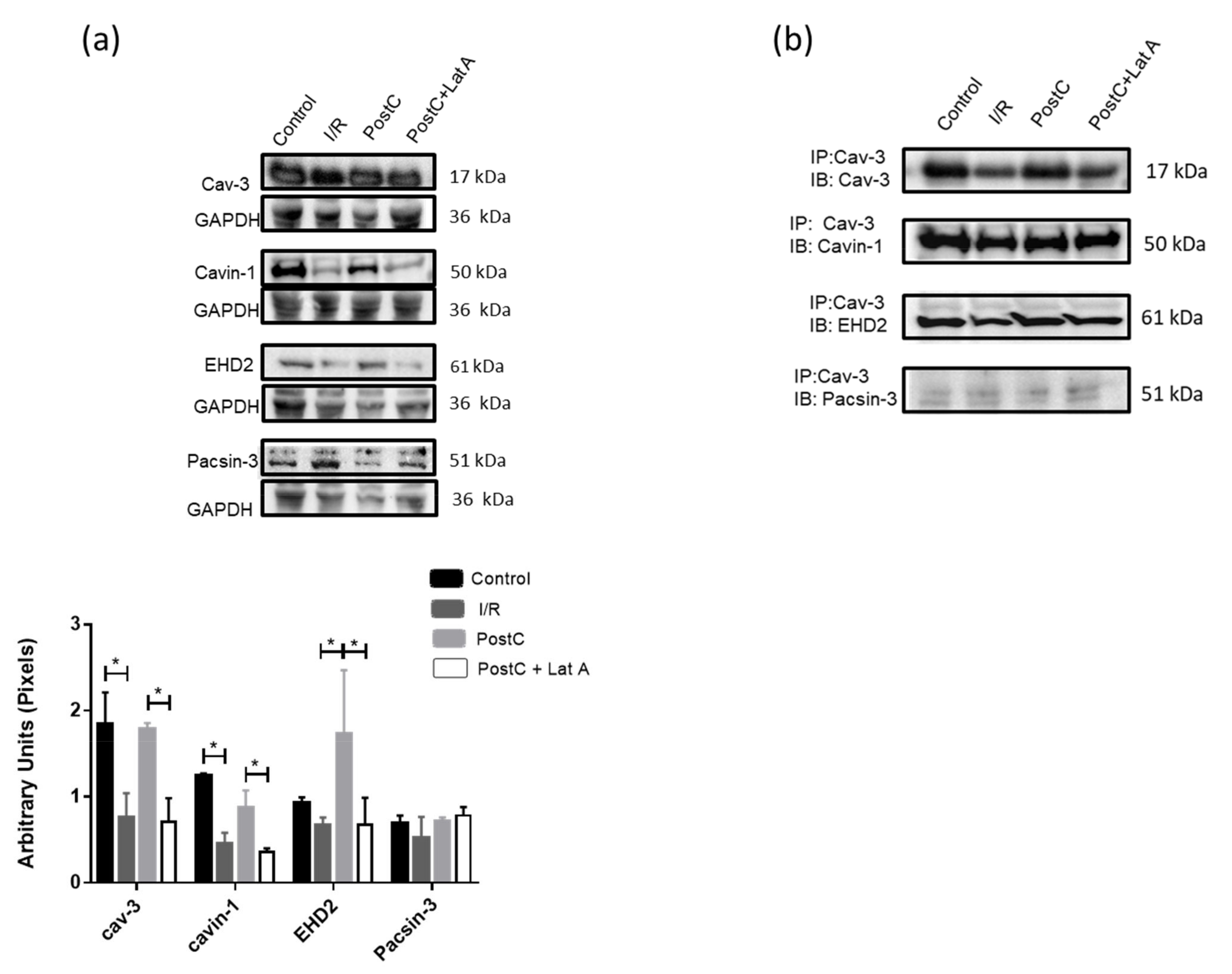
2.6. Western Blot Analysis and Co-Immunoprecipitation
2.7. Subsarcolemmal and Interfibrillar Mitochondrial Isolation
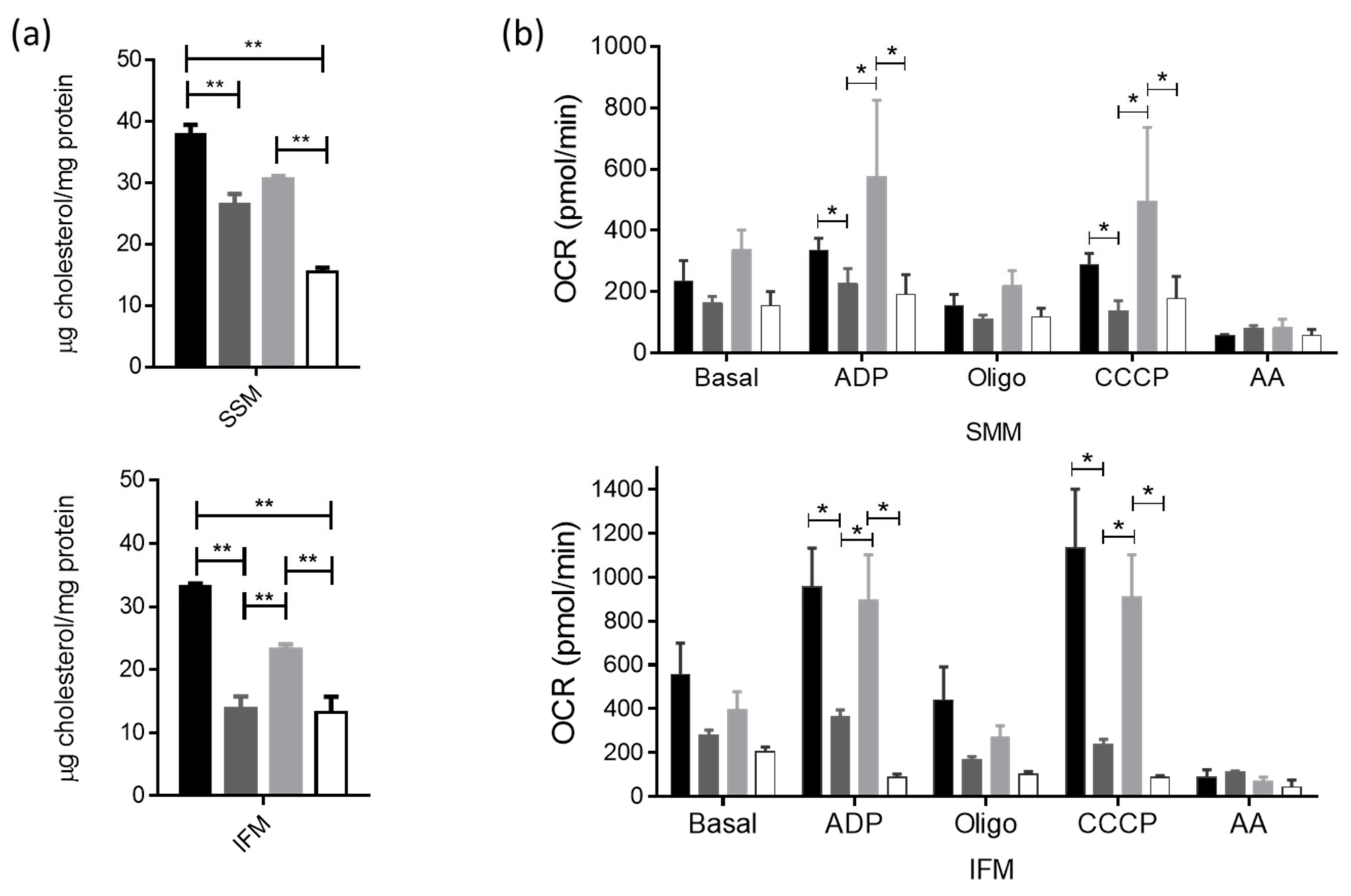
2.8. Cholesterol Measurement in Isolated Mitochondria
2.9. Mitochondrial Function
2.10. Statistical Analysis
3. Results
3.1. Changes in the F-Actin Microfilament Cytoskeleton Abolish the Cardioprotection and Reduce the Caveolae Content in PostC Hearts
3.2. Cav-1 and Cav-3 Co-Localization with Actin in Post-Conditioned Hearts Treated with Latrunculin A
3.3. Latrunculin A Reduces the Actin Association with Detergent-Insoluble Caveolae in PostC Hearts
3.4. Changes in Caveolar Proteins in PostC Hearts Treated with Latrunculin A
3.5. Actin-Cytoskeleton Disruption Reduces the Cholesterol Delivery towards the Mitochondria and Impacts Their Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaul, P.W.; Anderson, R.G. Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. 1998, 275, L843–L851. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Scherer, P.E.; Tang, Z.; Okamoto, T.; Li, S.; Chafel, M.; Chu, C.; Kohtz, D.S.; Lisanti, M.P. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 1996, 271, 15160–15165. [Google Scholar] [CrossRef] [PubMed]
- Feron, O.; Balligand, J.L. Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc. Res. 2006, 69, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, M.; van Deurs, B. Identification of filamin as a novel ligand for caveolin-1: Evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol. Biol. Cell 2000, 11, 325–337. [Google Scholar] [CrossRef]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef]
- Davidson, S.M.; Hausenloy, D.; Duchen, M.R.; Yellon, D.M. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int. J. Biochem. Cell Biol. 2006, 38, 414–419. [Google Scholar] [CrossRef]
- Tsibulnikov, S.Y.; Maslov, L.N.; Naryzhnaya, N.V.; Ma, H.; Lishmanov, Y.B.; Oeltgen, P.R.; Garlid, K. Role of protein kinase C, PI3 kinase, tyrosine kinases, NO-synthase, KATP channels and MPT pore in the signaling pathway of the cardioprotective effect of chronic continuous hypoxia. Gen. Physiol. Biophys. 2018, 37, 537–547. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Costa, A.D.; Costa, C.L.; Pierre, S.V.; Dos Santos, P.; Garlid, K.D. Conditioning the heart induces formation of signalosomes that interact with mitochondria to open mitoKATP channels. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H953–H961. [Google Scholar] [CrossRef]
- Hernández-Reséndiz, S.; Zazueta, C. PHO-ERK1/2 interaction with mitochondria regulates the permeability transition pore in cardioprotective signaling. Life Sci. 2014, 108, 13–21. [Google Scholar] [CrossRef]
- Lupi Herrera, E.; Gaspar, J.; González-Pacheco, H.; Martínez-Sánchez, C.; Pastelín-Hernández, G.; Luna-Ortiz, P.; Chávez-Cosio, E. Reperfusion and postconditioning in acute ST segment elevation myocardial infarction. A new paradigm for the treatment of acute myocardial infarction. From bench to bedside? Arch. Cardiol. Mex. 2006, 76 (Suppl. 4), S76–S101. [Google Scholar]
- Sun, J.; Nguyen, T.; Aponte, A.M.; Menazza, S.; Kohr, M.J.; Roth, D.M.; Patel, H.H.; Murphy, E.; Steenbergen, C. Ischaemic preconditioning preferentially increases protein S-nitrosylation in subsarcolemmal. Cardiovasc. Res. 2015, 106, 227–236. [Google Scholar] [CrossRef] [Green Version]
- García-Niño, W.R.; Correa, F.; Rodríguez-Barrena, J.I.; León-Contreras, J.C.; Buelna-Chontal, M.; Soria-Castro, E.; Hernández-Pando, R.; Pedraza-Chaverri, J.; Zazueta, C. Cardioprotective kinase signaling to subsarcolemmal and interfibrillar mitochondria is mediated by caveolar structures. Basic Res. Cardiol. 2017, 112, 15. [Google Scholar] [CrossRef]
- Revenu, C.; Athman, R.; Robine, S.; Louvard, D. The co-workers of actin filaments: From cell structures to signals. Nat. Rev. Mol. Cell Biol. 2004, 8, 635–646. [Google Scholar] [CrossRef]
- Jasper, J.R.; Post, S.R.; Desai, K.H.; Insel, P.A.; Bernstein, D. Colchicine and cytochalasin B enhance cyclic AMP accumulation via postreceptor actions. J. Pharmacol. Exp. Ther. 1995, 274, 937–942. [Google Scholar]
- Head, B.P.; Patel, H.H.; Roth, D.M.; Murray, F.; Swaney, J.S.; Niesman, I.R.; Farquhar, M.G.; Insel, P.A. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J. Biol. Chem. 2006, 281, 26391–26399. [Google Scholar] [CrossRef]
- Kostan, J.; Salzer, U.; Orlova, A.; Törö, I.; Hodnik, V.; Senju, Y.; Zou, J.; Schreiner, C.; Steiner, J.; Meriläinen, J.; et al. Direct interaction of actin filaments with F-BAR protein pacsin2. EMBO Rep. 2014, 15, 1154–1162. [Google Scholar] [CrossRef]
- Hernández-Reséndiz, S.; Roldán, F.J.; Correa, F.; Martínez-Abundis, E.; Osorio-Valencia, G.; Ruíz-de-Jesús, O.; Alexánderson-Rosas, E.; Vigueras, R.M.; Franco, M.; Zazueta, C. Postconditioning protects against reperfusion injury in hypertensive dilated cardiomyopathy by activating MEK/ERK1/2 signaling. J. Card. Fail. 2013, 19, 135–146. [Google Scholar] [CrossRef]
- Smyth, J.W.; Vogan, J.M.; Buch, P.J.; Zhang, S.; Fong, T.S.; Hong, T.; Shaw, R.M. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ. Res. 2012, 110, 978–989. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Fohn phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Rudel, L.L.; Morris, M.D. Determination of cholesterol using o-phthalaldehyde. J. Lipid Res. 1973, 14, 364–366. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Erdmann, K.S.; Roux, A.; Habermann, B.; Werner, H.; De Camilli, P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell 2005, 6, 791–804. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.A.; Zhu, M.; Kwon, S.W.; Liu, P.; Zhao, Y.; Anderson, R.G.W. Detergent-free caveolae proteome suggests an interaction with ER and mitochondria. Proteomics 2006, 6, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.Z.; McIntosh, V.J.; Abou-Samra, A.B.; Mohammad, R.M.; Lasley, R.D. Cholesterol Depletion Alters Cardiomyocyte Subcellular Signaling and Increases Contractility. PLoS ONE 2016, 11, e0154151. [Google Scholar] [CrossRef]
- Pelkmans, L.; Püntener, D.; Helenius, A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 2002, 296, 535–539. [Google Scholar] [CrossRef]
- Liu, L.; Pilch, P.F. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem. 2008, 283, 4314–4322. [Google Scholar] [CrossRef]
- Hernandez, V.J.; Weng, J.; Ly, P.; Pompey, S.; Dong, H.; Mishra, L.; Schwarz, M.; Anderson, R.G.W.; Michaely, P. Cavin-3 dictates the balance between ERK and Akt signaling. ELife 2013, 2, e00905. [Google Scholar] [CrossRef]
- Hubert, M.; Larsson, E.; Lundmark, R. Keeping in touch with the membrane; protein- and lipid-mediated confinement of caveolae to the cell surface. Biochem. Soc. Trans. 2020, 48, 155–163. [Google Scholar] [CrossRef]
- Parton, R.G.; Tillu, V.; McMahon, K.A.; Collins, B.M. Key phases in the formation of caveolae. Curr. Opin. Cell Biol. 2021, 71, 7–14. [Google Scholar] [CrossRef]
- Yeow, I.; Howard, G.; Chadwick, J.; Mendoza-Topaz, C.; Hansen, C.G.; Nichols, B.J.; Shvets, E. EHD proteins cooperate to generate caveolar clusters and to maintain caveolae during repeated mechanical stress. Curr. Biol. 2017, 27, 2951–2962. [Google Scholar] [CrossRef]
- Daumier, O.; Lundmark, R.; Vallis, Y.; Martens, S.; Butler, P.J.G.; McMahon, H.T. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 2007, 449, 923–927. [Google Scholar] [CrossRef]
- Seemann, E.; Sun, M.; Krueger, S.; Tröger, J.; Hou, W.; Haag, N.; Schüler, S.; Westermann, M.; Huebner, C.A.; Romeike, B.; et al. Deciphering caveolar functions by syndapin III KO-mediated impairment of caveolar invagination. Elife 2017, 6, e29854. [Google Scholar] [CrossRef]
- Qualmann, B.; Kelly, R.B. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J. Cell Biol. 2000, 148, 1047–1062. [Google Scholar] [CrossRef]
- Hansen, C.G.; Howard, G.; Nichols, B.J. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. J. Cell Sci. 2011, 124, 2777–2785. [Google Scholar] [CrossRef]
- Fridolfsson, H.N.; Roth, D.M.; Insel, P.A.; Patel, H.H. Regulation of intracellular signaling and function by caveolin. FASEB J. 2014, 28, 3823–3831. [Google Scholar] [CrossRef]
- Wang, J.W.; Xue, Z.Y.; Wu, A.S. Mechanistic insights into δ-opioid-induced cardioprotection: Involvement of caveolin translocation to the mitochondria. Life Sci. 2019, 247, 116942. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Grimm, M.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. Crosstalk between Mitochondria and Cytoskeleton in Cardiac Cells. Cells 2020, 9, 222. [Google Scholar] [CrossRef]
- Li, F.; Fan, X.; Zhang, Y.; Zhang, Y.; Ma, X.; Kou, J.; Yu, B. Inhibition of myosin IIA-actin interaction prevents ischemia/reperfusion induced cardiomyocytes apoptosis through modulating PINK1/Parkin pathway and mitochondrial fission. Int. J. Cardiol. 2018, 271, 211–218. [Google Scholar] [CrossRef]
- Boldogh, I.R.; Pon, L.A. Interactions of mitochondria with the actin cytoskeleton. Biochim. Biophys. Acta 2006, 1763, 450–462. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa, F.; Enríquez-Cortina, C.; Silva-Palacios, A.; Román-Anguiano, N.; Gil-Hernández, A.; Ostolga-Chavarría, M.; Soria-Castro, E.; Hernández-Rizo, S.; Heros, P.d.l.; Chávez-Canales, M.; et al. Actin-Cytoskeleton Drives Caveolae Signaling to Mitochondria during Postconditioning. Cells 2023, 12, 492. https://doi.org/10.3390/cells12030492
Correa F, Enríquez-Cortina C, Silva-Palacios A, Román-Anguiano N, Gil-Hernández A, Ostolga-Chavarría M, Soria-Castro E, Hernández-Rizo S, Heros Pdl, Chávez-Canales M, et al. Actin-Cytoskeleton Drives Caveolae Signaling to Mitochondria during Postconditioning. Cells. 2023; 12(3):492. https://doi.org/10.3390/cells12030492
Chicago/Turabian StyleCorrea, Francisco, Cristina Enríquez-Cortina, Alejandro Silva-Palacios, Nadia Román-Anguiano, Aurora Gil-Hernández, Marcos Ostolga-Chavarría, Elizabeth Soria-Castro, Sharik Hernández-Rizo, Paola de los Heros, María Chávez-Canales, and et al. 2023. "Actin-Cytoskeleton Drives Caveolae Signaling to Mitochondria during Postconditioning" Cells 12, no. 3: 492. https://doi.org/10.3390/cells12030492
APA StyleCorrea, F., Enríquez-Cortina, C., Silva-Palacios, A., Román-Anguiano, N., Gil-Hernández, A., Ostolga-Chavarría, M., Soria-Castro, E., Hernández-Rizo, S., Heros, P. d. l., Chávez-Canales, M., & Zazueta, C. (2023). Actin-Cytoskeleton Drives Caveolae Signaling to Mitochondria during Postconditioning. Cells, 12(3), 492. https://doi.org/10.3390/cells12030492






