Prevalence, Virulence, and Antibiotics Gene Profiles in Lactococcus garvieae Isolated from Cows with Clinical Mastitis in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
2.2. Dairy Farm Information and Clinical Mastitis Sample Collection
2.3. Bacterial Culture of L. garvieae
2.4. 16 S rDNA Sequencing Identification and Biochemical Testing
2.5. Post-Treatment Milk Sample Collection for SCC and Bacteriological Cure Evaluation
2.6. Growth Curve of Lactococcus garvieae
2.7. Antimicrobial Resistance Determination
2.8. Virulence Gene Detection
2.9. Cell Cultures
2.10. Cytotoxic Lactate Dehydrogenase (LDH) Release Assay
2.11. Adhesion Assay
2.12. Morphology of Lactococcus garvieae on MAC-T
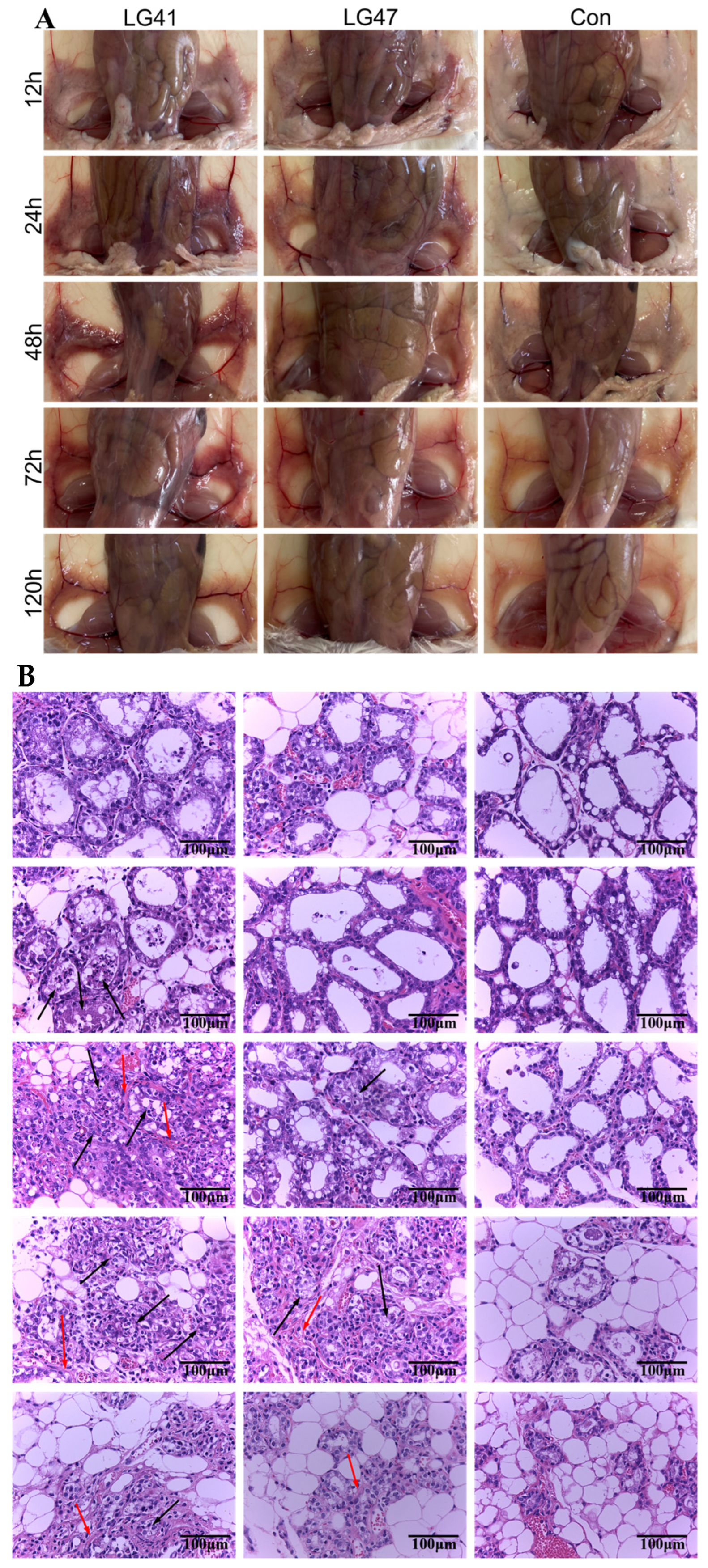
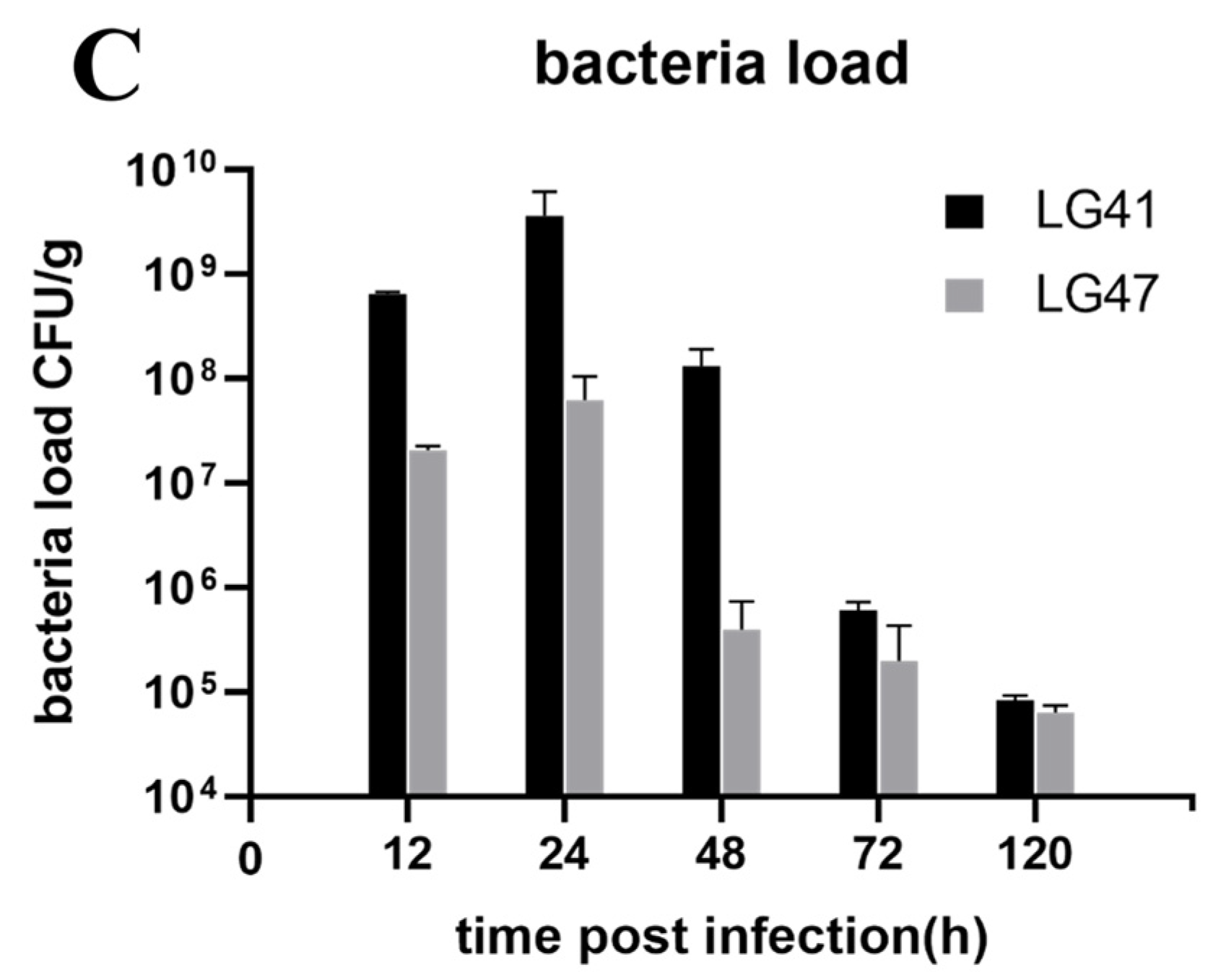
2.13. Lactococcus garvieae Experimental Infection in a Mouse Mammary Gland
2.14. Statistical Analyses
3. Results
3.1. Morphological Characteristics of Lactococcus garvieae
3.2. Identification of Suspected Isolates by 16S rDNA Sequence
3.3. Biochemical Testing of Lactococcus garvieae
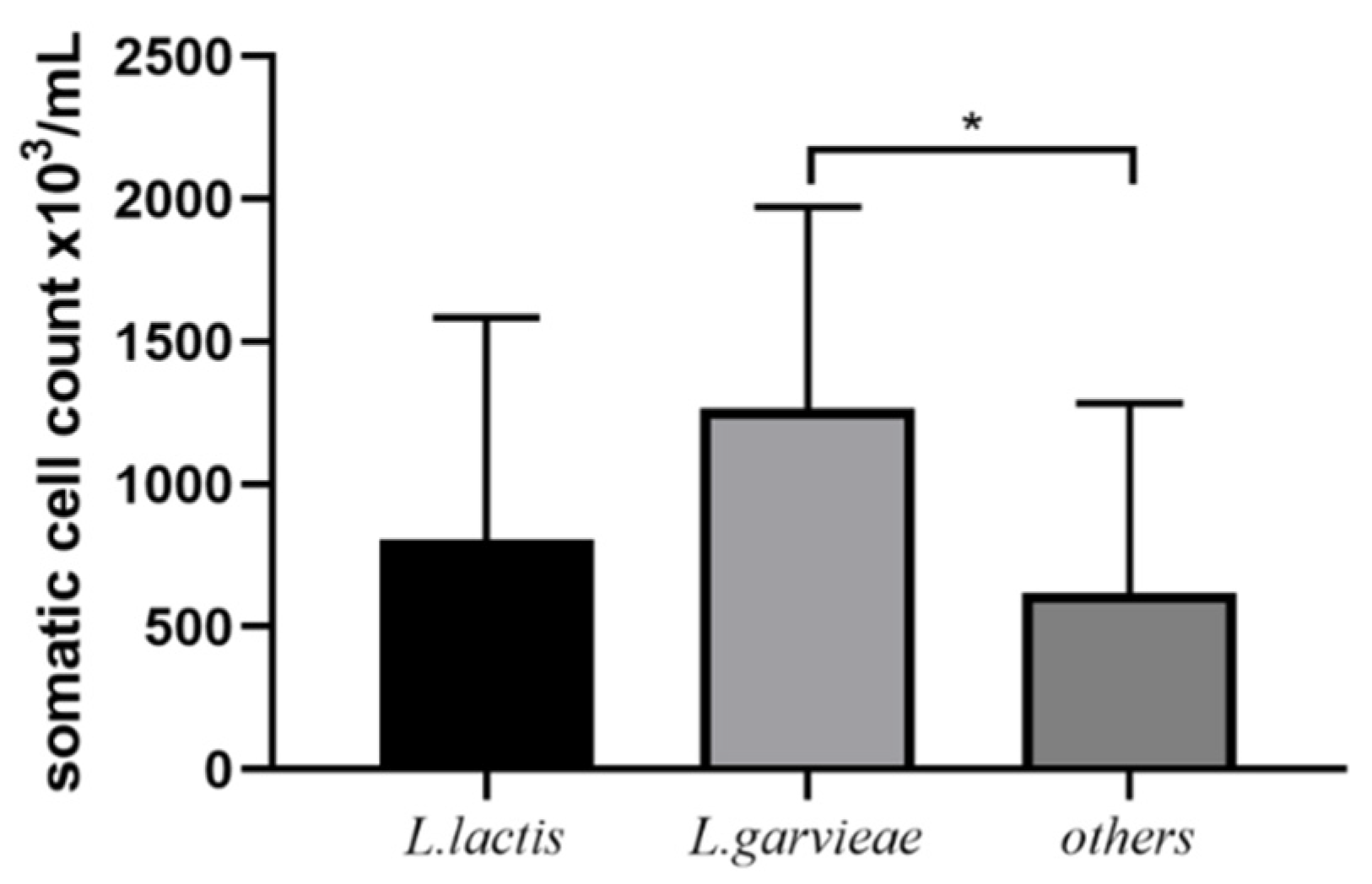
3.4. Post-Treatment SCC and Bacteriological Cure
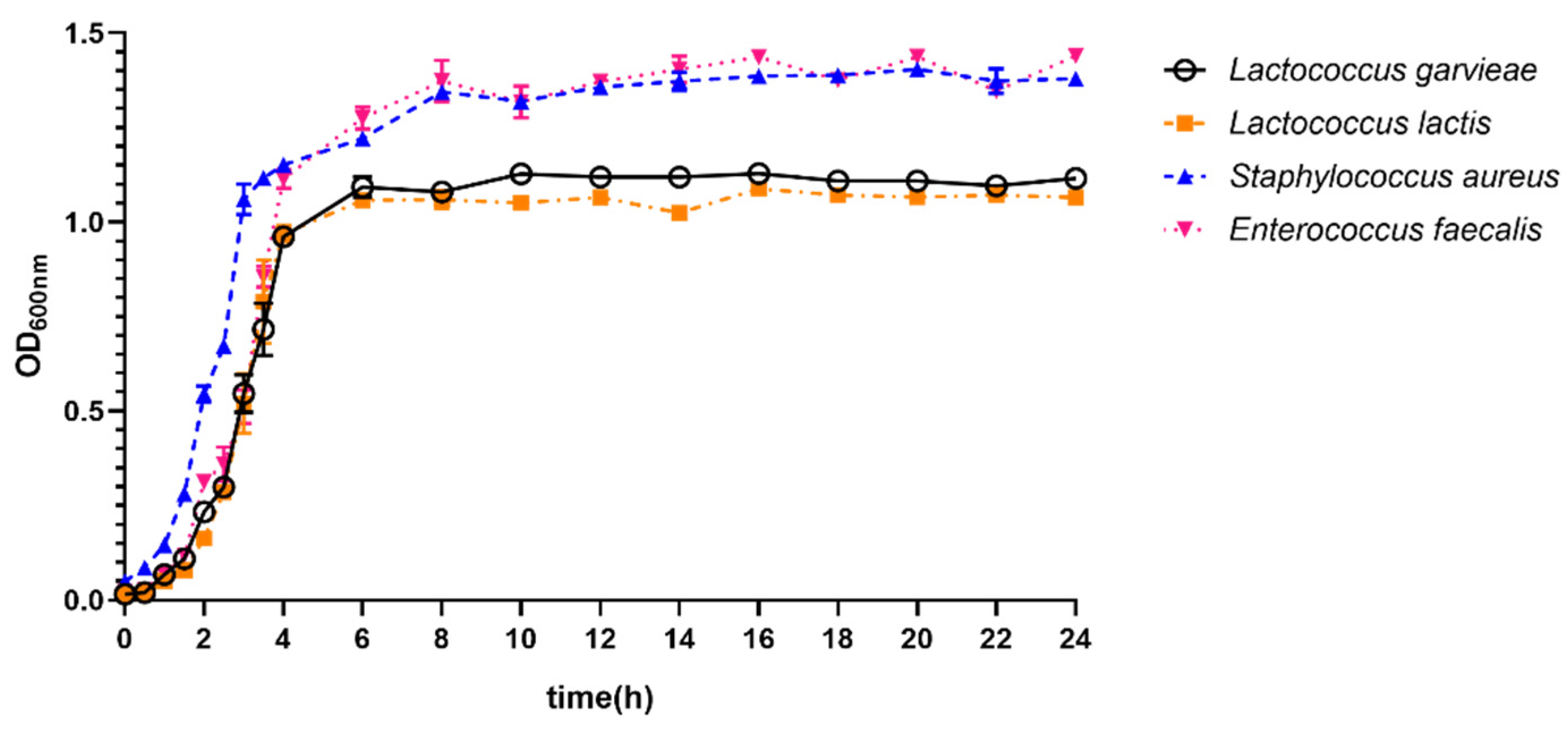
3.5. Growth Ability of Lactococcus garvieae
3.6. Antimicrobial Resistance Profiles of Lactococcus garvieae
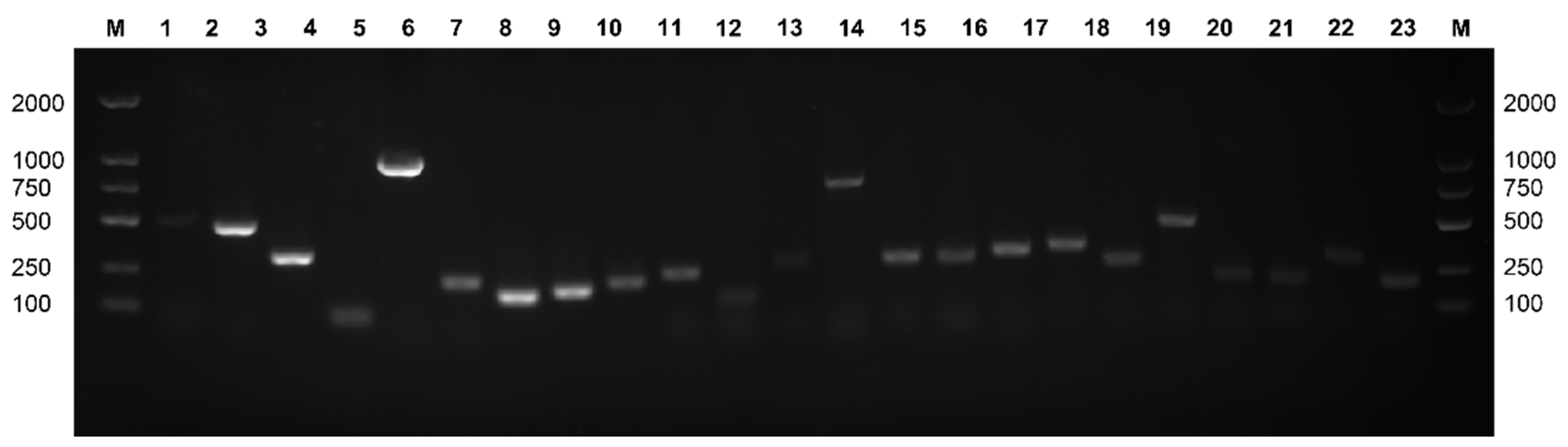
3.7. Detection of Virulence Genes in Lactococcus garvieae
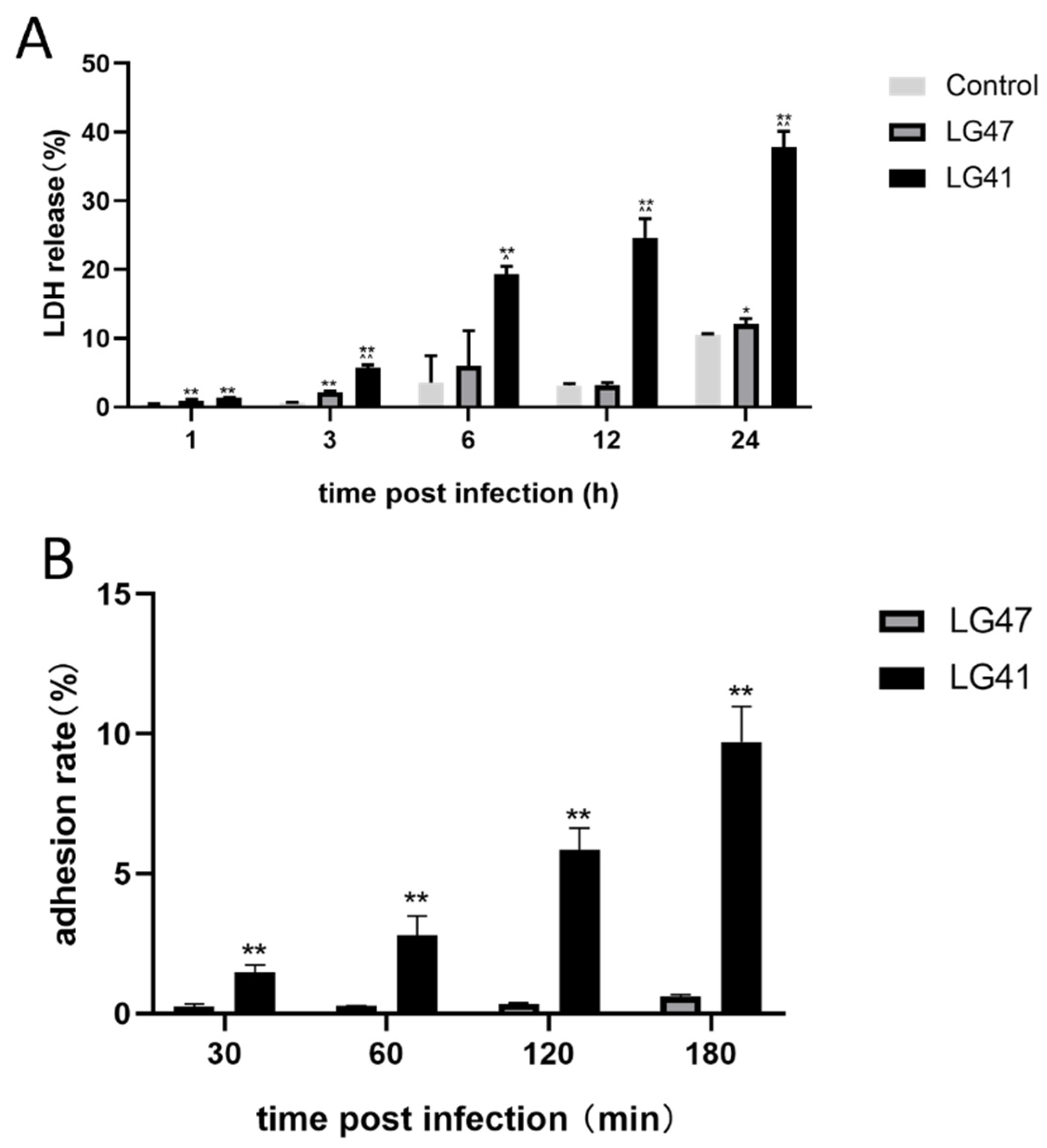
3.8. Pathogenic Effects of Lactococcus garvieae on MAC-T
3.9. Morphology of Lactococcus garvieae on MAC-T
3.10. Inflammation of Murine Mammary Gland Infected by Lactococcus garvieae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruegg, P.L. What Is Success? A Narrative Review of Research Evaluating Outcomes of Antibiotics Used for Treatment of Clinical Mastitis. Front. Vet. Sci. 2021, 8, 639641. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Qiu, Y.; Liu, G.; Li, X.; Cheng, J.; Liu, K.; Qu, W.; Zhu, C.; Kastelic, J.P.; Han, B.; et al. Characterization of Streptococcus lutetiensis isolated from clinical mastitis of dairy cows. J. Dairy Sci. 2021, 104, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.A.; Hommez, J.; Laevens, H.; Pot, B.; Vandamme, P.; Haesebrouck, F. Identification of aesculin-hydrolyzing streptococci, lactococci, aerococci and enterococci from subclinical intramammary infections in dairy cows. Vet. Microbiol. 1999, 70, 87–94. [Google Scholar] [CrossRef]
- Goyache, J.; Vela, A.I.; Gibello, A.; Blanco, M.M.; Briones, V.; González, S.; Téllez, S.; Ballesteros, C.; Domínguez, L.; Fernández-Garayzábal, J.F. Lactococcus lactis subsp. lactis infection in waterfowl: First confirmation in animals. Emerg. Infect. Dis. 2001, 7, 884–886. [Google Scholar] [CrossRef]
- Werner, B.; Moroni, P.; Gioia, G.; Lavin-Alconero, L.; Yousaf, A.; Charter, M.E.; Carter, B.M.; Bennett, J.; Nydam, D.V.; Welcome, F.; et al. Short communication: Genotypic and phenotypic identification of environmental streptococci and association of Lactococcus lactis ssp. lactis with intramammary infections among different dairy farms. J. Dairy Sci. 2014, 97, 6964–6969. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K. Transfer of Streptococcus lactis and related streptococci to the genus Lactococcus gen. nov. Syst. Appl. Microbiol. 1985, 6, 183–195. [Google Scholar] [CrossRef]
- Meyburgh, C.M.; Bragg, R.R.; Boucher, C.E. Lactococcus garvieae: An emerging bacterial pathogen of fish. Dis. Aquat. Org. 2017, 123, 67–79. [Google Scholar] [CrossRef]
- Malek, A.; De la Hoz, A.; Gomez-Villegas, S.I.; Nowbakht, C.; Arias, C.A. Lactococcus garvieae, an unusual pathogen in infective endocarditis: Case report and review of the literature. BMC Infect. Dis. 2019, 19, 301. [Google Scholar] [CrossRef]
- Wang, C.Y.C.; Shie, H.S.; Chen, S.C.; Huang, J.P.; Hsieh, I.C.; Wen, M.S.; Lin, F.C.; Wu, D. Lactococcus garvieae infections in humans: Possible association with aquaculture outbreaks. Int. J. Clin. Pract. 2007, 61, 68–73. [Google Scholar] [CrossRef]
- Chan, J.F.W.; Woo, P.C.Y.; Teng, J.L.L.; Lau, S.K.P.; Leung, S.S.M.; Tam, F.C.C.; Yuen, K.Y. Primary infective spondylodiscitis caused by Lactococcus garvieae and a review of human L. garvieae infections. Infection 2011, 39, 259–264. [Google Scholar] [CrossRef]
- Mofredj, A.; Baraka, D.; Kloeti, G.; Dumont, J.L. Lactococcus garvieae septicemia with liver abscess in an immunosuppressed patient. Am. J. Med. 2000, 109, 513–514. [Google Scholar] [CrossRef]
- Gibello, A.; Galán-Sánchez, F.; Blanco, M.M.; Rodríguez-Iglesias, M.; Domínguez, L.; Fernández-Garayzábal, J.F. The zoonotic potential of Lactococcus garvieae: An overview on microbiology, epidemiology, virulence factors and relationship with its presence in foods. Res. Vet. Sci. 2016, 109, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Björk Werner, J.; Dolk, M.; Christensson, B. Lactococcus garvieae endocarditis presenting with subdural haematoma. BMC Cardiovasc. Disord. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nakai, K.; Sakata, M.; Nagaoka, Y.; Yoshida, K.; Katsumata, U.; Chiba, T.; Matsubayashi, K. Recipient sepsis caused by Lactococcus garvieae contamination of platelets from a donor with colon cancer. Vox Sang. 2019, 114, 182–184. [Google Scholar] [CrossRef]
- Sahu, K.K.; Sherif, A.A.; Syed, M.P.; Rajendran, A.; Mishra, A.K.; Davaro, R. A rare cause of sepsis: Lactococcus garvieae. QJM Int. J. Med. 2019, 112, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Colagrossi, L.; Costabile, V.; Scutari, R.; Agosta, M.; Onori, M.; Mancinelli, L.; Lucignano, B.; Muda, A.O.; Baldo, G.D.; Mastronuzzi, A.; et al. Evidence of pediatric sepsis caused by a drug resistant contaminated platelet concentrate. Emerg. Microbes Infect. 2022, 11, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Westberg, M.; Brekke, H.; Hermansen, N.O.; Flatøy, B. Late onset periprosthetic infection of the hip caused by the fish pathogen in a patient not associated with fish exposure. J. Bone Jt. Infect. 2020, 5, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, J.L.; Vela, A.I.; Gibello, A.; Casamayor, A.; Domínguez, L.; Fernández-Garayzábal, J.F. A genetic comparison of pig, cow and trout isolates of Lactococcus garvieae by PFGE analysis. Lett. Appl. Microbiol. 2011, 53, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Billen, F.; Boyen, F.; Duprez, J.-N.; Quenault, H.; Touzain, F.; Blanchard, Y.; Clercx, C.; Mainil, J.G. Genomic relatedness of a canine Lactococcus garvieae to human, animal and environmental isolates. Res. Vet. Sci. 2021, 137, 170–173. [Google Scholar] [CrossRef]
- Vela, A.I.; Vázquez, J.; Gibello, A.; Blanco, M.M.; Moreno, M.A.; Liébana, P.; Albendea, C.; Alcalá, B.; Mendez, A.; Domínguez, L.; et al. Phenotypic and genetic characterization of Lactococcus garvieae isolated in Spain from lactococcosis outbreaks and comparison with isolates of other countries and sources. J. Clin. Microbiol. 2000, 38, 3791–3795. [Google Scholar] [CrossRef]
- Eraclio, G.; Ricci, G.; Moroni, P.; Santisteban, C.; Plumed-Ferrer, C.; Bennett, J.; Fortina, M.G. Sand bedding as a reservoir for Lactococcus garvieae dissemination in dairy farms. Can. J. Microbiol. 2019, 65, 84–89. [Google Scholar] [CrossRef]
- Altın, G.; Nikerel, E.; Şahin, F. Draft Genome Sequence of Magnesium-Dissolving A1, Isolated from Soil. Genome Announc. 2017, 5, e00386-17. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, M.; Yoshida, T.; Kijima, M.; Yagyu, K.; Nakai, T.; Okada, S.; Endo, A.; Murakami, M.; Suzuki, S.; Morita, H. Characterization of Lactococcus garvieae isolated from radish and broccoli sprouts that exhibited a KG+ phenotype, lack of virulence and absence of a capsule. Lett. Appl. Microbiol. 2007, 44, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Ricci, G.; Borgo, F.; Rollando, A.; Fortina, M.G. Genetic investigation within Lactococcus garvieae revealed two genomic lineages. FEMS Microbiol. Lett. 2012, 332, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Farrow, J.A.; Phillips, B.A.; Kandler, O. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. Microbiology 1983, 129, 3427–3431. [Google Scholar] [CrossRef]
- Teixeira, L.M.; Merquior, V.L.; Vianni, M.C.; Carvalho, M.G.; Fracalanzza, S.E.; Steigerwalt, A.G.; Brenner, D.J.; Facklam, R.R. Phenotypic and genotypic characterization of atypical Lactococcus garvieae strains isolated from water buffalos with subclinical mastitis and confirmation of L. garvieae as a senior subjective synonym of Enterococcus seriolicida. Int. J. Syst. Evol. Microbiol. 1996, 46, 664–668. [Google Scholar] [CrossRef]
- Klijn, N.; Weerkamp, A.H.; de Vos, W.M. Detection and characterization of lactose-utilizing Lactococcus spp. in natural ecosystems. Appl. Environ. Microbiol. 1995, 61, 788–792. [Google Scholar] [CrossRef]
- Lafarge, V.; Ogier, J.-C.; Girard, V.; Maladen, V.; Leveau, J.-Y.; Gruss, A.; Delacroix-Buchet, A. Raw cow milk bacterial population shifts attributable to refrigeration. Appl. Environ. Microbiol. 2004, 70, 5644–5650. [Google Scholar] [CrossRef]
- Abdelfatah, E.N.; Mahboub, H.H.H. Studies on the effect of Lactococcus garvieae of dairy origin on both cheese and Nile tilapia (O. niloticus). Int. J. Vet. Sci. Med. 2018, 6, 201–207. [Google Scholar] [CrossRef]
- Alegría, A.; Alvarez-Martín, P.; Sacristán, N.; Fernández, E.; Delgado, S.; Mayo, B. Diversity and evolution of the microbial populations during manufacture and ripening of Casín, a traditional Spanish, starter-free cheese made from cow’s milk. Int. J. Food Microbiol. 2009, 136, 44–51. [Google Scholar] [CrossRef]
- Akabanda, F.; Owusu-Kwarteng, J.; Tano-Debrah, K.; Parkouda, C.; Jespersen, L. The Use of Lactic Acid Bacteria Starter Culture in the Production of Nunu, a Spontaneously Fermented Milk Product in Ghana. Int. J. Food Sci. 2014, 2014, 721067. [Google Scholar] [CrossRef]
- Ricci, G.; Ferrario, C.; Borgo, F.; Eraclio, G.; Fortina, M.G. Genome Sequences of Two Lactococcus garvieae Strains Isolated from Meat. Genome Announc. 2013, 1, e00018-12. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.X.; Lima, S.F.; Higgins, C.H.; Canniatti-Brazaca, S.G.; Bicalho, R.C. The Lactococcus genus as a potential emerging mastitis pathogen group: A report on an outbreak investigation. J. Dairy Sci. 2016, 99, 9864–9874. [Google Scholar] [CrossRef] [PubMed]
- Ture, M.; Altinok, I. Detection of putative virulence genes of Lactococcus garvieae. Dis. Aquat. Org. 2016, 119, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Klemm, P.; Schembri, M.A. Bacterial adhesins: Function and structure. Int. J. Med Microbiol. 2000, 290, 27–35. [Google Scholar] [CrossRef]
- Miyauchi, E.; Toh, H.; Nakano, A.; Tanabe, S.; Morita, H. Comparative Genomic Analysis of Lactococcus garvieae Strains Isolated from Different Sources Reveals Candidate Virulence Genes. Int. J. Microbiol. 2012, 2012, 728276. [Google Scholar] [CrossRef]
- Morita, H.; Toh, H.; Oshima, K.; Yoshizaki, M.; Kawanishi, M.; Nakaya, K.; Suzuki, T.; Miyauchi, E.; Ishii, Y.; Tanabe, S.; et al. Complete genome sequence and comparative analysis of the fish pathogen Lactococcus garvieae. PLoS ONE 2011, 6, e23184. [Google Scholar] [CrossRef]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Assessing the antimicrobial susceptibility of bacteria obtained from animals. Vet. Microbiol. 2010, 141, 601–604. [Google Scholar] [CrossRef]
- Burgos, J.M.; Ellington, B.A.; Varela, M.F. Presence of multidrug-resistant enteric bacteria in dairy farm topsoil. J. Dairy Sci. 2005, 88, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Langoni, H.; Hulland, C.; Ruegg, P.L. Minimum inhibitory concentrations of Staphylococcus aureus recovered from clinical and subclinical cases of bovine mastitis. J. Dairy Sci. 2012, 95, 1913–1920. [Google Scholar] [CrossRef]
- Su, F.-J.; Chen, M.-M. Protective Efficacy of Novel Oral Biofilm Vaccines against Infection in Mullet. Vaccines 2021, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 5th ed.; CLSI document; VET01SEd05E; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Liu, G.; Yin, J.; Han, B.; Barkema, H.W.; Shahid, M.; De Buck, J.; Cobo, E.R.; Kastelic, J.P.; Gao, J. Adherent/invasive capacities of bovine-associated Aerococcus viridans contribute to pathogenesis of acute mastitis in a murine model. Vet. Microbiol. 2019, 230, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A.; Facklam, R.R. Antimicrobial susceptibilities of Lactococcus lactis and Lactococcus garvieae and a proposed method to discriminate between them. J. Clin. Microbiol. 1996, 34, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Mi, K.; Li, M.; Sun, L.; Hou, Y.; Zhou, K.; Hao, H.; Pan, Y.; Liu, Z.; Xie, C.; Huang, L. Determination of Susceptibility Breakpoint for Cefquinome against in Pigs. Antibiotics 2021, 10, 958. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, R.; Jedrey, H.; Kim, S.; Connell, R.; Ma, S.; Chippada Venkata, U.D.; Chakravorty, S.; Gupta, A.; Sizemore, E.E.; Diem, L.; et al. Bacterial Factors That Predict Relapse after Tuberculosis Therapy. N. Engl. J. Med. 2018, 379, 823–833. [Google Scholar] [CrossRef]
- Villani, F.; Aponte, M.; Blaiotta, G.; Mauriello, G.; Pepe, O.; Moschetti, G. Detection and characterization of a bacteriocin, garviecin L1-5, produced by Lactococcus garvieae isolated from raw cow’s milk. J. Appl. Microbiol. 2001, 90, 430–439. [Google Scholar] [CrossRef]
- Sorge, U.S.; Huber-Schlenstedt, R.; Schierling, K. In vitro antimicrobial resistance profiles of Streptococcus uberis, Lactococcus spp., and Enterococcus spp. from quarter milk samples of cows between 2015 and 2019 in Southern Germany. J. Dairy Sci. 2021, 104, 5998–6012. [Google Scholar] [CrossRef]
- Kawanishi, M.; Kojima, A.; Ishihara, K.; Esaki, H.; Kijima, M.; Takahashi, T.; Suzuki, S.; Tamura, Y. Drug resistance and pulsed-field gel electrophoresis patterns of Lactococcus garvieae isolates from cultured Seriola (yellowtail, amberjack and kingfish) in Japan. Lett. Appl. Microbiol. 2005, 40, 322–328. [Google Scholar] [CrossRef]
- Walther, C.; Rossano, A.; Thomann, A.; Perreten, V. Antibiotic resistance in Lactococcus species from bovine milk: Presence of a mutated multidrug transporter mdt(A) gene in susceptible Lactococcus garvieae strains. Vet. Microbiol. 2008, 131, 348–357. [Google Scholar] [CrossRef]
- Cheng, J.; Qu, W.; Barkema, H.W.; Nobrega, D.B.; Gao, J.; Liu, G.; De Buck, J.; Kastelic, J.P.; Sun, H.; Han, B. Antimicrobial resistance profiles of 5 common bovine mastitis pathogens in large Chinese dairy herds. J. Dairy Sci. 2019, 102, 2416–2426. [Google Scholar] [CrossRef]
- Scillieri Smith, J.C.; Moroni, P.; Santisteban, C.G.; Rauch, B.J.; Ospina, P.A.; Nydam, D.V. Distribution of Lactococcus spp. in New York State dairy farms and the association of somatic cell count resolution and bacteriological cure in clinical mastitis samples. J. Dairy Sci. 2020, 103, 1785–1794. [Google Scholar] [CrossRef]
- Sol, J.; Sampimon, O.C.; Barkema, H.W.; Schukken, Y.H. Factors associated with cure after therapy of clinical mastitis caused by Staphylococcus aureus. J. Dairy Sci. 2000, 83, 278–284. [Google Scholar] [CrossRef]
- Oliver, S.P.; Gillespie, B.E.; Headrick, S.J.; Moorehead, H.; Lunn, P.; Dowlen, H.H.; Johnson, D.L.; Lamar, K.C.; Chester, S.T.; Moseley, W.M. Efficacy of extended ceftiofur intramammary therapy for treatment of subclinical mastitis in lactating dairy cows. J. Dairy Sci. 2004, 87, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Truchetti, G.; Bouchard, E.; Descôteaux, L.; Scholl, D.; Roy, J.-P. Efficacy of extended intramammary ceftiofur therapy against mild to moderate clinical mastitis in Holstein dairy cows: A randomized clinical trial. Can. J. Vet. Res. 2014, 78, 31–37. [Google Scholar] [PubMed]
- McMullen, C.K.; Sargeant, J.M.; Kelton, D.F.; Churchill, K.J.; Cousins, K.S.; Winder, C.B. Modifiable management practices to improve udder health in dairy cattle during the dry period and early lactation: A scoping review. J. Dairy Sci. 2021, 104, 10143–10157. [Google Scholar] [CrossRef] [PubMed]



| Target Gene | Abbreviation of Target Gene | Primer Name | Primer Sequence (5′ to 3′) | Product Size |
|---|---|---|---|---|
| Hemolysin 1 | hly1 | H1 F | CCTCCTCCGACTAGGAACCA | 521 |
| H1 R | GAAAAGCCAGCTTCTCGTGC | |||
| Hemolysin 2 | hly2 | H2 F | TCTCGTGCACACCGATGAAA | 492 |
| H2 R | TGAACTTCGGCTTCTGCGAT | |||
| Hemolysin 3 | hly3 | H3 F | AACGCGAGAACAGGCAAAAC | 291 |
| H3 R | CCCACGTCGAGAGCATAGAC | |||
| NADH oxidase | NADHO | NADHO F | TGCGATGGGTTCAAGACCAA | 331 |
| NADHO R | GCCTTTAAAAGCCTCGGCAG | |||
| Superoxide dismutase | SOD | SOD F | GCAGCGATTGAAAAACACCCA | 80 |
| SOD R | TCTTCTGGCAAACGGTCCAA | |||
| Phosphoglucomutase | pgm | PG F | AAGTTTACGGCGAAGACGGT | 997 |
| PG R | TTTTCTGGTGCATTGGCACG | |||
| Adhesin Pav | pav | AP F | CCTGTCGGGCGCTTTTATTG | 232 |
| AP R | TCCCGGAAGAAGAGTACGGT | |||
| Adhesin PsaA | PsaA | APSA F | GTTGCAACAGCTGGACACAG | 180 |
| APSA R | ATACGGTTGAGTTGGGCTGG | |||
| Enolase | eno | E F | CAAGAGCGATCATTGCACGG | 201 |
| E R | CATTCGGACGCGGTATGGTA | |||
| LPxTG-1 | LP1 | LP1-F | GTGAACGTGGAGCTTCCAGA | 878 |
| LP1-R | CCACTCACATGGGGGAGTTC | |||
| LPxTG-2 | LP2 | LP2 F | GCCAGTGAGAGAACCGTTGA | 767 |
| LP2 R | CAGGTTCAAGTGCAACTGCC | |||
| LPxTG-3 | LP3 | LP3 F | TTAAGCACAACGGCAACAGC | 231 |
| LP3 R | CACGCGAAATGATGGTGCAT | |||
| LPxTG-4 | LP4 | LP4-F | GGGAGCACCGGATTCACTTT | 928 |
| LP4-R | ACAAAGCCGCAGACCTTACA | |||
| Adhesin cluster 1 | AC1 | AC1 F | TTGGGCACATCAGACTGGAC | 264 |
| AC1 R | AGCATCATCAGCTGCCAAGT | |||
| Adhesin cluster 2 | AC2 | AC2 F | CTGCGAGTGGCATCTCCATT | 160 |
| AC2 R | TCAACACTGCGACCTTCTGT | |||
| Adhesin | AF | AF F | CAGCCAGCACCAGGTTATGA | 358 |
| AF R | CTCCTGCGTTGACATGGACT | |||
| capsule gene cluster A | CGC A | 1020-F | ACCTTCACTTGCATTCATAGGGT | 304 |
| 1323-R | TTGTCCCAGAGGGTTCTCCT | |||
| capsule gene cluster B | CGC B | 851-F | TAGGAGGTGTTCCTGGGAGG | 549 |
| 1399-R | TGTCCCACTCCTACTGTCGT | |||
| capsule gene cluster C | CGC C | 6329-F | AAAAACGGAGGGCAACAAGC | 785 |
| 7175-R | CACTTGTACAGGCCACTGGT | |||
| capsule gene cluster D | CGC D | 5358-F | TGGAGGGTATTGCCTACCGA | 650 |
| 6007-R | CCACAGCAGCTTCTTCACCT | |||
| conserved hypotherical protein | CHP | CHP F | CTGCTGATCAAGTCCAAGC | 303 |
| CHP R | GAGAAACGACCTTAGCTCCA | |||
| exopolysaccharide A | EpsA | EpsA F | TTATAGCCTCCCCAGTTTACAC | 299 |
| EpsA R | TTTAGCAGTCTCGTCTGCAATC | |||
| exopolysaccharide B | EpsB | EpsB F | CGCAAGTGCTAATCTAGCTG | 317 |
| EpsB R | AGAGAGGCGGAGTATCAATC | |||
| exopolysaccharide C | EpsC | EpsC F | TAACAACTATCACTGCGACTCC | 343 |
| EpsC R | TCAGGGTTCTCAATGATTCCAC | |||
| exopolysaccharide D | EpsD | EpsD F | TTTCTTATTGCGGCTGCATTGC | 270 |
| EpsD R | CTCATCAATTGAGTGTCGTCTG | |||
| exopolysaccharide L | EpsL | EpsL F | ACCAATCGTACAGATCAACG | 473 |
| EpsL R | CTTGAGCCACCACTATCAAG | |||
| exopolysaccharide R | EpsR | EpsR F | TTTTACCACCGGCTAAAGGAAC | 211 |
| EpsR R | TTGCAGAACTGTCATTAGGCTC | |||
| exopolysaccharide X | EpsX | EpsX F | TATTGAAGCAACAGCCTCACTG | 198 |
| EpsX R | TTTTTGTCTGGGTAACTAGCCC | |||
| rhamnosyltransferase | RIT | RIT F | TTGATGGTAAATCCTGATGG | 307 |
| RIT R | GAACAAACCGACCTACAACA | |||
| 30S rRNA gene | 30s rRNA gene | 30s F | TACGAACACCGTATCCTTGAC | 207 |
| 30s R | TTGTGTTGGTTCGATGATGTCG |
| GPCN Cocci | Number of Isolates | Percent of Isolates a (%) |
|---|---|---|
| Lactococcus garvieae | 49 | 19.76% |
| Lactococcus lactis | 41 | 16.53% |
| Streptococcus agalactiae | 34 | 13.71% |
| Streptococcus dysgalactiae | 26 | 10.48% |
| Streptococcus uberis | 25 | 10.08% |
| Streptococcus lutetiensis | 20 | 8.06% |
| Aerococcus viridans | 19 | 7.66% |
| Enterococcus faecium | 15 | 6.05% |
| Enterococcus faecalis | 13 | 5.24% |
| Trueperella pyogenes | 6 | 2.42% |
| total | 248 | 100.00% |
| Ribose | Sucrose | Lactose | Liquid Gelatin | Sorbitol | Maltose | Esculin | VP | Galactose | Trehalose | Glucose | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of positives | 0 | 0 | 47 | 0 | 0 | 47 | 39 | 38 | 40 | 47 | 47 |
| Percentage positive | 0.00% | 0.00% | 95.92% | 0.00% | 0.00% | 95.92% | 79.59% | 77.55% | 81.63% | 95.92% | 95.92% |
| MIC (μg/mL) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | Breakpoint | >16 | 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.12 | 0.06 | 0.03 | Resistance Rate | MIC50 (μg/mL) | MIC90 (μg/mL) |
| Penicillin | 16 a | 8 | 35 | 5 | 1 | 0.00% | 0.25 | 0.5 | |||||||
| Cephalexin | 16 b | 6 | 28 | 13 | 1 | 1 | 12.24% | 8 | 16 | ||||||
| Ampicillin | 8 a | 5 | 17 | 19 | 7 | 0.00% | 0.12 | 0.5 | |||||||
| Ceftiofur | 2 c | 1 | 21 | 21 | 3 | 1 | 0.00% | 0.5 | 1 | ||||||
| Cefquinome | 1 d | 1 | 3 | 15 | 29 | 1 | 0.00% | 0.12 | 0.25 | ||||||
| Lincomycin | 4 e | 40 | 9 | 100.00% | 16 | 16 | |||||||||
| Oxytetracycline | 8 f | 12 | 4 | 20 | 8 | 4 | 1 | 73.47% | 4 | 16 | |||||
| Marbofloxacin | 8 g | 4 | 31 | 14 | 0.00% | 0.5 | 0.5 | ||||||||
| Rifaximin | 1 h | 49 | 100.00% | ≥16 | ≥16 | ||||||||||
| Vancomycin | 32 i | 2 | 32 | 14 | 1 | 0.00% | 0.25 | 0.25 | |||||||
| Genes | Number of Positives | Percentage Positive |
|---|---|---|
| hly1 | 49 | 100.00% |
| hly2 | 48 | 97.96% |
| hly3 | 0 | 0.00% |
| NADHO | 49 | 100.00% |
| SOD | 49 | 100.00% |
| pgm | 15 | 30.61% |
| Pav | 49 | 100.00% |
| PsaA | 49 | 100.00% |
| eno | 49 | 100.00% |
| LP1 | 0 | 0.00% |
| LP2 | 0 | 0.00% |
| LP3 | 11 | 22.45% |
| LP4 | 0 | 0.00% |
| AC1 | 49 | 100.00% |
| AC2 | 49 | 100.00% |
| Adh | 0 | 0.00% |
| 1020-F, 1323-R | 2 | 4.08% |
| 851-F, 1399-R | 0 | 0.00% |
| 6329-F, 7175-R | 24 | 48.98% |
| 5358-F, 6007-R | 0 | 0.00% |
| CHP | 44 | 89.80% |
| EpsA | 49 | 100.00% |
| EpsB | 46 | 93.88% |
| EpsC | 49 | 100.00% |
| EpsD | 35 | 71.43% |
| EpsL | 42 | 85.71% |
| EspR | 49 | 100.00% |
| EspX | 49 | 100.00% |
| ORUP | 0 | 0.00% |
| RIF | 29 | 59.18% |
| 30S gene | 49 | 100.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Pan, Z.; Yu, Y.; Yu, L.; Wu, F.; Dong, J.; Wang, T.; Li, L. Prevalence, Virulence, and Antibiotics Gene Profiles in Lactococcus garvieae Isolated from Cows with Clinical Mastitis in China. Microorganisms 2023, 11, 379. https://doi.org/10.3390/microorganisms11020379
Xie X, Pan Z, Yu Y, Yu L, Wu F, Dong J, Wang T, Li L. Prevalence, Virulence, and Antibiotics Gene Profiles in Lactococcus garvieae Isolated from Cows with Clinical Mastitis in China. Microorganisms. 2023; 11(2):379. https://doi.org/10.3390/microorganisms11020379
Chicago/Turabian StyleXie, Xinmei, Zihao Pan, Yong Yu, Lirong Yu, Fan Wu, Jing Dong, Tiancheng Wang, and Lin Li. 2023. "Prevalence, Virulence, and Antibiotics Gene Profiles in Lactococcus garvieae Isolated from Cows with Clinical Mastitis in China" Microorganisms 11, no. 2: 379. https://doi.org/10.3390/microorganisms11020379
APA StyleXie, X., Pan, Z., Yu, Y., Yu, L., Wu, F., Dong, J., Wang, T., & Li, L. (2023). Prevalence, Virulence, and Antibiotics Gene Profiles in Lactococcus garvieae Isolated from Cows with Clinical Mastitis in China. Microorganisms, 11(2), 379. https://doi.org/10.3390/microorganisms11020379







