Cotton-Based Rotation, Intercropping, and Alternate Intercropping Increase Yields by Improving Root–Shoot Relations
Abstract
:1. Introduction
2. How Cotton-Based Rotation Improves Productivity
2.1. Productivity and Economic Benefits
2.2. Soil Improvement and Root Growth
2.3. Resource Utilization
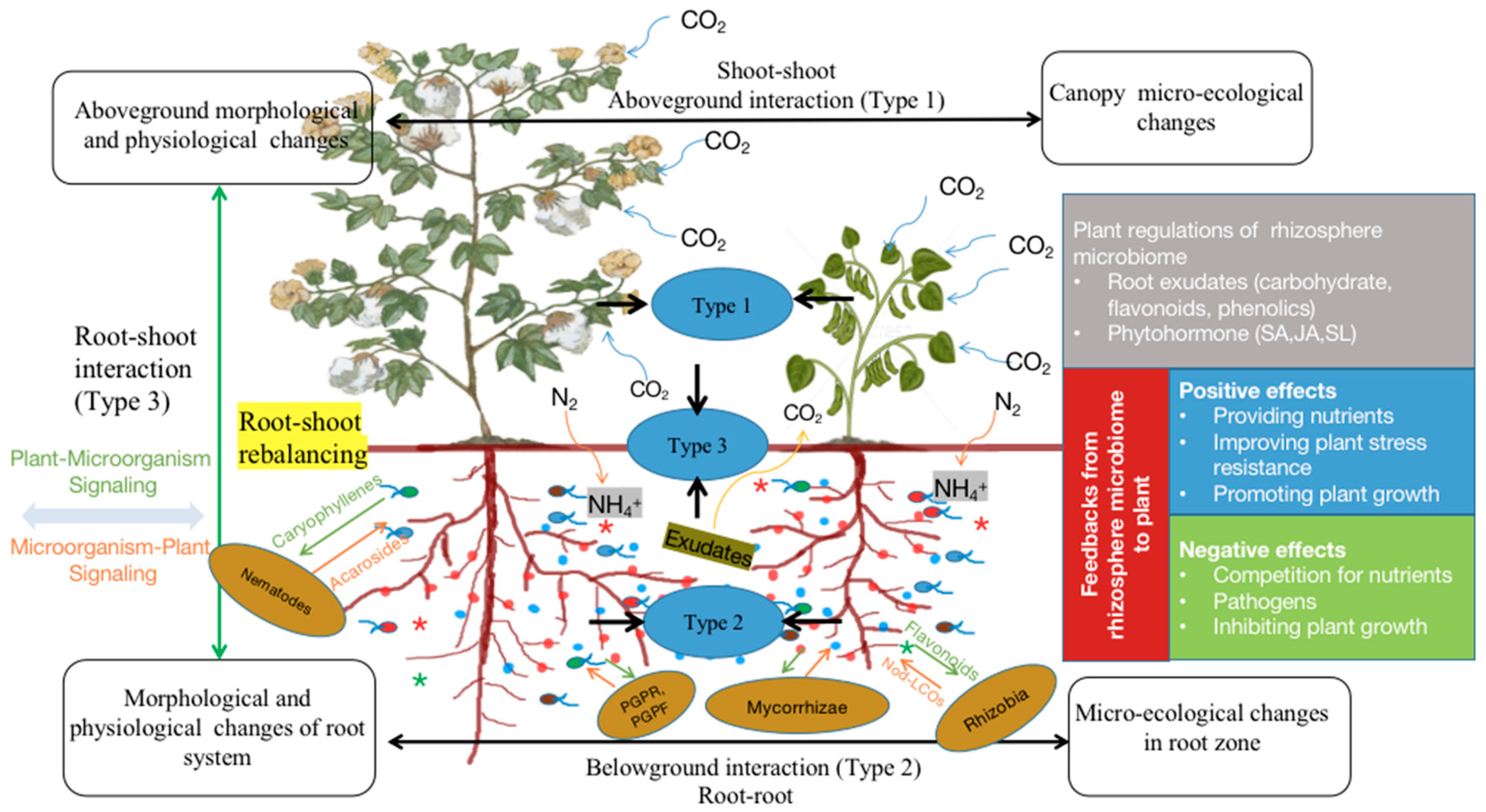
2.4. Root–Shoot Interaction
2.5. Pest and Disease Control
3. Cotton-based Intercropping
3.1. Productivity and Economic Benefits
3.2. Rhizosphere Microbial Community
3.3. Resource Utilization
3.4. Pest Control
4. How Alternate Intercropping Improves Crop Productivity
4.1. Productivity and Economic Benefits
4.2. Soil and Rhizosphere Microbial Community
4.3. Resource Utilization
4.4. Interactions under Alternate Intercropping
4.4.1. Three Types of Interactions
4.4.2. Root–Shoot Interaction
4.4.3. Signaling in Interactions
5. Discussion and Conclusions
5.1. Modeling of Intercropping and Rotation
5.2. Root–Shoot Signal Transmission
5.3. Integration of Cultivar, Agronomy, and Machinery
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chi, B.J.; Zhang, Y.J.; Zhang, D.M.; Zhang, X.J.; Dong, H.Z. Wide-strip intercropping of cotton and peanut combined with strip rotation increases crop productivity and economic returns. Field Crop. Res. 2019, 243, 107617. [Google Scholar] [CrossRef]
- Gao, C.; Wei, C.; Zhang, L.; Han, D.X.; Liu, H.X.; Yu, X.F.; Wang, G.P. Historical (1880s–2000s) impact of wind erosion on wetland patches in semi-arid regions: A case study in the western Songnen Plain (China). Aeolian Res. 2019, 38, 13–23. [Google Scholar] [CrossRef]
- Huang, D.; Wang, K.; Wu, W. Problems and strategies for sustainable development of farming and animal husbandry in the Agro-Pastoral Transition Zone in Northern China (APTZNC). Int. J. Sustain. Dev. World Ecolog. 2007, 14, 391–399. [Google Scholar] [CrossRef]
- Zhu, S.W.; Gao, T.P.; Liu, Z.; Ning, T.Y. Rotary and subsoiling tillage rotations influence soil carbon and nitrogen sequestration and crop yield. Plant Soil Environ. 2022, 68, 89–97. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, X.K.; Zhang, J.L.; Gao, F.; Zhang, F.; Yang, C.T.; Wang, Y.Y.; Li, X.D. Effects of continuous cropping on agronomic traits and physiological characteristics of peanut and its regulation under plastic mulching. J. Peanut Sci. 2012, 41, 16–20. (In Chinese) [Google Scholar]
- Wang, C.B.; Wu, Z.F.; Cheng, B.; Zheng, Y.P.; Wan, S.B.; Guo, F.; Chen, D.X. Effect of continuous cropping on photosynthesis and metabolism of reactive oxygen in peanut. Acta Agron. Sin. 2007, 33, 1304–1309. [Google Scholar]
- Liu, W.; Wang, Q.; Wang, B.; Wang, X.; Franks, A.E.; Teng, Y.; Li, Z.; Luo, Y. Changes in the abundance and structure of bacterial communities under long-term fertilization treatments in a peanut monocropping system. Plant Soil. 2015, 395, 415–427. [Google Scholar] [CrossRef]
- Afrin, S.; Latif, A.; Banu, N.M.A.; Kabir, M.M.M.; Haque, S.S.; Ahmed, M.E.; Tonu, N.N.; Ali, M.P. Intercropping empower reduces insect pests and increases biodiversity in agro-ecosystem. Agric. Sci. 2017, 8, 1120–1134. [Google Scholar] [CrossRef] [Green Version]
- David, T.; Christian, B.; Jason, H.; Belinda, L.B. Global food demand and the sustainable intensifcation of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar]
- Johnson, A.W.; Dowler, C.C.; Handoo, Z.A. Population dynamics of Meloidogyne incognita, M. arenaria, and other nematodes and crop yields in rotations of cotton, peanut, and wheat under minimum tillage. J. Nematol. 2000, 32, 52–61. [Google Scholar] [PubMed]
- Ci, D.W.; Yang, J.S.; Ding, H.; Qin, F.F.; Shi, W.W.; Dai, L.X.; Zhang, Z.M. Effects of plants allocation on yield and economic benefit under peanut-cotton intercropping system. J. Peanut Sci. 2017, 46, 22–25. (In Chinese) [Google Scholar]
- Feike, T.; Doluschitz, R.; Chen, Q.; Graeff, S.; Claupein, W. How to overcome the slow death of intercropping in the North China Plain. Sustainability 2012, 4, 2550–2565. [Google Scholar] [CrossRef] [Green Version]
- Cheriere, T.; Lorin, M.; Corre-Hellou, G. Species choice and spatial arrangement in soybean-based intercropping: Levers that drive yield and weed control. Field Crop. Res. 2020, 256, 107963. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Li, X. Intercropping to maximize root–root interactions in agricultural plants: Agronomic aspects. In The Root Systems in Sustainable Agricultural Intensification; Rengel, Z., Djalovic, I., Eds.; Wiley: New York, NY, USA, 2021; pp. 309–328. [Google Scholar]
- Qi, H.; Wang, S.L.; Wang, Y.; Zhang, Q.; Feng, G.Y.; Lin, Y.Z.; Liang, Q.L. Effects of rotation and soil deep ploughing on cotton development traits and yield. Tianjin Agric. Sci. 2016, 22, 113–116. (In Chinese) [Google Scholar]
- Li, C.J.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Zhang, C.C.; Li, H.G.; Zhang, F.S.; van der Werf, W. Syndromes of production in intercropping impact yield gains. Nature Plants 2020, 6, 653–660. [Google Scholar] [CrossRef]
- Liu, C.; Daniel, P.B.; Jeffrey, A.C.H.; Kutcher, R.; Beckie, H.J.; Wang, L.; Floc’h, J.B.; Hamel, C.; Siddique, K.H.M.; Li, L.L.; et al. Diversifying crop rotations enhances agroecosystem services and resilience. Adv Agron. 2022, 173, 299–335. [Google Scholar]
- Mao, L.L.; Zhang, L.Z.; Zhang, S.P.; Jochem, B.E.; van der Wopke, W.; Wang, J.J.; Sun, H.Q.; Su, Z.C.; Huub, S. Resource use efficiency, ecological intensification and sustainability of intercropping systems. J. Integr. Agric. 2015, 14, 1542–1550. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, Z.S. Assessing effect of cotton and rice rotation on rotated cotton yield using observational data from remote sensing satellite. Earth Environ. Sci. 2020, 470, 012005. [Google Scholar] [CrossRef]
- Dhaliwal, N.S.; Sandhu, B.S. Yield production and economics of different cropping system in south-western part of Punjab. Int. Res. J. Econ. Stat. 2015, 6, 414–418. [Google Scholar] [CrossRef]
- Feng, L.; Wang, G.P.; Han, Y.C.; Li, Y.B.; Zhu, Y.; Zhou, Z.G.; Cao, W.X. Effects of planting pattern on growth and yield and economic benefifits of cotton in a wheat-cotton double cropping system versus monoculture cotton. Field Crop. Res. 2017, 213, 100–108. [Google Scholar] [CrossRef]
- Forster, D.; Andres, C.; Verma, R.; Zundel, C.; Messmer, M.; Mäder, P. Productivity and profitability of cotton-based production systems under organic and conventional management in India. In Proceedings of the 4th ISOFAR Scientifific Conference. ‘Building Organic Bridges’, at the Organic World Congress, Istanbul, Turkey, 13–15 October 2014; Rahmann, G., Aksoy, U., Eds.; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2014; pp. 647–650. [Google Scholar]
- Turkhede, A.B.; Nagdeve, M.B.; Karunakar, A.P.; Gabhane, V.V.; Mali, R.S. Diversification in cotton-based cropping system under mechanization in rainfed condition of vidarbha of Maharashtra, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2189–2206. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.J.; Rochester, I.; Constable, G. Maximizing the Profitability of Cotton Cropping Systems with Legumes; CRDC: Tianjin, China, 2011. [Google Scholar]
- Rochester, I.J.; Peoples, M.B.; Hullugalle, N.R.; Gault, R.R.; Constable, G.A. Using legumes to enhance nitrogen fertility and improve soil condition in cotton cropping systems. Field Crop. Res. 2001, 70, 27–41. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; Mcdaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Eeusha, N.; Heidi, W.; Isaac, D.; Nafi, E.; Webber, H.; Danso, I.; Naab, J.B.; Frei, M.; Gaiser, T. Interactive effects of conservation tillage, residue management, and nitrogen fertilizer application on soil properties under maize-cotton rotation system on highly weathered soils of West Africa. Soil Till. Res. 2020, 196, 104473. [Google Scholar]
- Martínez, V.A.; Upchurch, D.R.; Schubert, A.M.; Porter, D.; Wheeler, T. Early impacts of cotton and peanut cropping systems on selected soil chemical, physical, microbiological and biochemical properties. Biol. Fertil. Soils 2004, 40, 44–54. [Google Scholar] [CrossRef]
- Feng, G.; Haile, T.; Zhang, B.B.; Buehring, N.W.; Adeli, A. Soil physical and hydrological properties as affected by a five-year history of poultry litter applied to a cotton–corn–soybean rotation system. Soil Sci. Soc. Am. J. 2021, 85, 800–813. [Google Scholar] [CrossRef]
- Kumar, N.; Nath, C.P.; Hazra, K.K.; Das, K.; Venkatesh, M.S.; Singh, M.K.; Singh, S.S.; Praharaj, C.S.; Singh, N.P. Impact of zero-till residue management and crop diversification with legumes on soil aggregation and carbon sequestration. Soil Tillage Res. 2019, 189, 158–167. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Luo, Y.; Zhan, Y.N.; Zhou, Z.G. Biochar increases 15N fertilizer retention and indigenous soil N uptake in a cotton-barley rotation system. Geoderma 2020, 357, 113944. [Google Scholar] [CrossRef]
- Katsvairo, T.W.; Wright, D.L.; Marois, J.J.; Hartzog, D.L.; Balkcom, K.B.; Wiatrak, P.P.; Rich, J.R. Cotton roots, earthworms, and infiltration characteristics in sod-peanut-cotton cropping systems. Agron. J. 2007, 99, 390–398. [Google Scholar] [CrossRef]
- Katsvairo, T.W.; Wright, D.L.; Marois, J.J.; Rich, J.R.; Wiatrak, P.P. Comparative plant growth and development in two cotton rotations under irrigated and non-irrigated conditions. Crop Sci. 2009, 49, 2233–2245. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.J.; Liu, S.D.; Liu, R.H.; Zhang, S.P.; Yang, J.; Pang, C.Y. Effects of crop rotation on bacterial communities in cotton rhizosphere soil. Biotechnol. Bull. 2020, 36, 117–124. (In Chinese) [Google Scholar]
- Xi, H.; Zhang, X.K.; Qu, Z.; Yang, D.; Zhu, L. Effects of cotton–maize rotation on soil microbiome structure. Mol. Plant Pathol. 2020, 22, 673–682. [Google Scholar] [CrossRef]
- Amsili, J.P.; Kaye, J.P. Root traits of cover crops and carbon inputs in an organic grain rotation. Renew. Agric. Food Syst. 2020, 36, 182–191. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Tiemann, L.K.; Grandy, A.S. Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis. Ecol. Appl. 2014, 24, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Alvey, S.; Yang, C.H.; Buerkert, A.; Crowley, D.E. Cereal/legume rotation effects on rhizosphere bacterial community structure in west African soils. Biol. Fertil. Soils 2003, 37, 73–82. [Google Scholar] [CrossRef]
- Johnson, M.J.; Lee, K.Y.; Scow, K.M. DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities. Geoderma 2003, 114, 279–303. [Google Scholar] [CrossRef]
- Yin, C.; Jones, K.; Peterson, D.E.; Garrett, K.A.; Hulbert, S.H.; Paulitz, T.C. Members of soil bacterial communities sensitive to tillage and crop rotation. Soil Biol. Biochem. 2010, 42, 2111–2118. [Google Scholar] [CrossRef]
- González-Chávez, M.D.C.A.; Aitkenhead-Peterson, J.A.; Gentry, T.J.; Zuberer, D.; Hons, F.; Loeppert, R. Soil microbial community, C, N, and P responses to long-term tillage and crop rotation. Soil Till. Res. 2010, 106, 285–293. [Google Scholar] [CrossRef]
- Suzuki, C.; Takenaka, M.; Oka, N.; Nagaoka, K.; Karasawa, T. A DGGE analysis shows that crop rotation systems influence the bacterial and fungal communities in soils. Soil Sci. Plant Nutr. 2012, 58, 288–296. [Google Scholar] [CrossRef]
- Tang, C.C.; Yu, Q.; Weng, J.; Chen, G.; Zhu, J. Effects of different fertilization modes on crop yields and soil nutrients under rapeseed-cotton rotation. Hum. Agric. Sci. 2020, 7, 36–40. (In Chinese) [Google Scholar]
- Lv, X.B.; Wang, Z.; Ma, L.J.; Cao, N.; Zhou, Z.G. Crop residue incorporation combined with potassium fertilizer increased cotton canopy apparent photosynthesis and seed cotton yield in barley-cotton rotation system. Arch. Agron. Soil Sci. 2021, 67, 300–312, (Online). [Google Scholar] [CrossRef]
- Sorensen, R.B.; Lamb, M.C.; Butts, C.L. Crop rotation, irrigation system, and irrigation rate on cotton yield in southwestern Georgia. Crop Forage Turfgrass Manag. 2020, 6, e20053. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Wang, J.J.; Xie, J.H.; Tian, H.Y.; Niu, Y.; Yang, X.K. Photosynthetic characteristics of cotton under crop rotation conditions. Agric. Res. Arid. Areas 2022, 40, 2. (In Chinese) [Google Scholar]
- Marimuthua, S.; Surendran, U.; Subbian, P. Productivity, nutrient uptake and post-harvest soil fertility as inflfluenced by cotton-based cropping system with integrated nutrient management practices in semi-arid tropics. Arch. Agron. Soil Sci. 2014, 60, 87–101. [Google Scholar] [CrossRef]
- Wang, L.; Cutforth, H.; Lal, R.; Chai, Q.; Zhao, C.; Gan, Y.; Siddique, K.H.M. ‘Decoupling’ land productivity and greenhouse gas footprints: A review. Land Degr. Dev. 2018, 29, 4348–4361. [Google Scholar] [CrossRef]
- Singh, R.J.; Ahlawat, I.P.S.; Sharma, N.K. Effects of peanut-cotton intercropping ratio on soil nutrien, crop yield and income. Shandong Agric. Sci. 2021, 53, 33–36. (In Chinese) [Google Scholar]
- Tewolde, H.; Buehring, N.; Way, T.R.; Feng, G.; He, Z.Q.; Sistani, K.; Jenkins, J.N. Yield and nutrient removal of cotton-corn-soybean rotation systems fertilized with poultry litter. Agron. J. 2022, 113, 5483–5498. [Google Scholar] [CrossRef]
- Yakudu, H.; Kwari, J.D.; Sandade, M.K. Effect of phosphorus on nitrogen fixation by some grain legume varieties in Sudan Asahelian zone of north. Niger. J. Basic Appl. Sci. 2010, 189, 19–26. [Google Scholar]
- Hulugalle, N.R.; Nachimuthu, G.; Kirkby, K.; Lonergan, P.; Heimoana, V.; Watkins, M.D.; Finlay, L.A. Sowing maize as a rotation crop in irrigated cotton cropping systems in a Vertosol: Effects on soil properties, greenhouse gas emissions, black root rot incidence, cotton lint yield and fibre quality. Soil Res. 2020, 58, 137–150. [Google Scholar] [CrossRef]
- Chi, B.J.; Zhang, D.M.; Dong, H.Z. Control of cotton pests and diseases by intercropping: A review. J. Integr. Agric. 2021, 20, 2–13. [Google Scholar] [CrossRef]
- Balloux, F.; van Dorp, L. Q&a: What are pathogens, and what have they done to and for us? BMC Biol. 2017, 15, 91. [Google Scholar]
- Smith, E.G.; Kutcher, H.R.; Brandt, S.A.; Ulrich, D.; Malhi, S.S.; Johnston, A.M. The profitability of short-duration canola and pea rotations in western Canada. Can. J. Plant Sci. 2013, 93, 933–940. [Google Scholar] [CrossRef]
- Ouyang, F.; Su, W.W.; Zhang, Y.S.; Liu, X.H.; Su, J.W.; Zhang, Q.Q.; Men, X.Y.; Ju, Q.; Ge, F. Ecological control service of the predatory natural enemy and its maintaining mechanism in rotation-intercropping ecosystem via wheat-maize-cotton. Agric. Ecosyst. Environ. 2020, 301, 107024. [Google Scholar] [CrossRef]
- Botir, B.; Sanjar, B. Management methods of harmful pests in the cotton-wheat crop rotation system. E3S Web Conf. 2021, 244, 02049. [Google Scholar]
- Wang, T.L.; Wang, X.J.; Liu, E.K. Advantages and disadvantages of crop rotation and research prospects. Anhui Agric. Sci. Bull. 2021, 27, 24. (In Chinese) [Google Scholar]
- Li, L.; Tilman, D.; Lambers, H.; Zhang, F.S. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Afzal, M.N.; Muhammad, D.; Ahmad, S.; Shahzad, A.N.; Kiran, A.; Wakeel, A. Relationship of tissue potassium content with yield and fiber quality components of Bt cotton as influenced by potassium application methods. Field Crop. Res. 2018, 229, 37–43. [Google Scholar] [CrossRef]
- Surendran, U.; Subramoniam, S.R.; Raja, P.; Kumar, V.; Murugappan, V. Budgeting of major nutrients and the mitigation options for nutrient mining in semi-arid tropical agro-ecosystem of Tamil Nadu, India using NUT-MON model. Environ. Monit. Assess. 2016, 188, 250. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Khan, M.; Hussain, M.; Farooq, M.; Jabran, K.; Lee, D.-J. Bio-economic assessment of different wheat-canola intercropping systems. Int. J. Agric. Biol. 2012, 14, 769–774. [Google Scholar]
- Saeed, M.; Shahid, M.R.M.; Jabbar, A. Agro-economic assessment of different cotton-based inter/relay cropping systems in two geometrical patterns. Int. J. Agric. Biol. 1999, 4, 234–237. [Google Scholar]
- Ali, H.; Afzal, M.N.; Ahmad, F.S.; Atif, R. Effect of sowing dates, plant spacing and nitrogen application on growth and productivity on cotton crop. Int. J. Sci. Eng. Res. 2011, 2, 1–6. [Google Scholar]
- Wu, L.K.; Lin, X.M.; Lin, W.X. Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chin. J. Plant Ecol. 2014, 38, 298–310. (In Chinese) [Google Scholar]
- Lin, W.W.; Li, N.; Chen, L.S.; Wu, Z.Y.; Lin, W.X.; Shen, L.H. Effects of maize and soybean interspecific interactions on rhizospheric bacteria community structure and diversity. Chin. J. Eco Agric. 2022, 30, 26–37. (In Chinese) [Google Scholar]
- Ulbrich, T.C.; Rivas-Ubach, A.; Tiemann, L.K.; Friesen, M.L.; Evans, S.E. Plant root exudates and rhizosphere bacterial communities shift with neighbor context. Soil Biol. Biochem. 2022, 172, 108753. [Google Scholar] [CrossRef]
- Vora, S.M.; Joshi, P.; Belwalkar, M.G.A. Root exudates influence chemotaxis and colonization of diverse plant growth promoting rhizobacteria in the pigeon pea-maize intercropping system. Rhizosphere 2021, 18, 100331. [Google Scholar] [CrossRef]
- Wang, R.N.; Sun, Z.X.; Bai, W.; Wang, E.L.; Wang, Q.; Zhang, D.S.; Zhang, Y.; Yang, N.; Liu, Y.; Nie, J.Y.; et al. Canopy heterogeneity with border-row proportion affects light interception and use efficiency in maize/peanut strip intercropping. Field Crop. Res. 2021, 271, 108239. [Google Scholar] [CrossRef]
- Jo, S.G.; Kang, Y.I.; Om, K.S.; Cha, Y.H.; Ri, S.Y. Growth, photosynthesis and yield of soybean in ridge furrow intercropping system of soybean and flax. Field Crop. Res. 2022, 275, 108329. [Google Scholar] [CrossRef]
- Yao, X.; Lan, Y.; Liao, L.; Huang, Y.; Yu, S.; Ye, S.; Yang, M. Effects of nitrogen supply rate on photosynthesis, nitrogen uptake and growth of seedlings in a Eucalyptus/Dalbergia odorifera intercropping system. Plant Biol. 2022, 24, 192–204. [Google Scholar] [CrossRef]
- Carandang, D.A.; Council, A.P. Resource Utilization in Integrated Farming System with Crops as the Major Enterprise. Extension Bulletin Food & Fertilizer Technology Center. 1980. Available online: https://www.cabdirect.org/cabdirect/abstract/19826740211 (accessed on 1 January 2023).
- Guo, Z.P.; Luo, C.S.; Dong, Y.; Dong, K.; Zhu, J.H.; Ma, L.K. Effect of nitrogen regulation on the epidemic characteristics of intercropping faba bean rust disease primarily depends on the canopy microclimate and nitrogen nutrition. Field Crop. Res. 2021, 274, 108339. [Google Scholar] [CrossRef]
- Ai, P.R.; Ma, Y.J.; Hai, Y. Influence of jujube/cotton intercropping on soil temperature and crop evapotranspiration in an arid area. Agric. Water Manag. 2021, 256, 107118. [Google Scholar] [CrossRef]
- Yu, R.P.; Yang, H.; Xing, Y.; Zhang, W.P.; Lambers, H.; Li, L. Belowground processes and sustainability in agroecosystems with intercropping. Plant Soil 2022, 476, 263–288. [Google Scholar] [CrossRef]
- Homulle, Z.; George, T.S.; Karley, A.J. Root traits with team benefits: Understanding belowground interactions in intercropping systems. Plant Soil 2021, 471, 1–26. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Z.G.; Bao, X.G. Long-term increased grain yield and soil fertility from intercropping. Nat. Sustain. 2021, 4, 943–950. [Google Scholar] [CrossRef]
- Liang, J.P.; Shi, W.J. Cotton/halophytes intercropping decreases salt accumulation and improves soil physicochemical properties and crop productivity in saline-alkali soils under mulched drip irrigation: A three-year field experiment. Field Crop. Res. 2021, 262, 108027. [Google Scholar] [CrossRef]
- Liang, J.P.; He, Z.J.; Shi, W.J. Cotton/mung bean intercropping improves crop productivity, water use efficiency, nitrogen uptake, and economic benefits in the arid area of Northwest China. Agric. Water Manag. 2020, 240, 106277. [Google Scholar] [CrossRef]
- Suman, D.; Pala, R.; Krishna, R. Effect of intercropping on the parasitoids, Encarsia spp. and Trichogramma spp. in cotton fields, India. Egypt. J. Biol. Pest Control 2020, 30, 71. [Google Scholar]
- Guo, J.Y.; Wan, F.H.; Hu, Y.H.; Yan, Y. Effects of crop arrangement patterns on arthropod community structure in transgenic boll-worm-resistant cotton fields. Chin. J. Appl. Ecol. 2007, 18, 2061–2068. (In Chinese) [Google Scholar]
- Wang, C.; Xia, J.; Cui, J.; Luo, J. Community structure and diversity of arthropod in different cotton fields in north Xinjiang. Cotton Sci. 2004, 16, 112–116. (In Chinese) [Google Scholar]
- Chen, M.; Luo, J.C.; Li, G.Q. Evaluating alfalfa cutting as a potential measure to enhance predator abundance of Aphis gossypii (Homoptera: Aphididae) in cotton-alfalfa intercropping system. Acta Agrestia Sin. 2011, 19, 922–926. (In Chinese) [Google Scholar]
- Fernandes, F.S.; Godoy, W.A.C.; Ramalho, F.S.; Malaquias, J.B.; Santos, B.D.B. The behavior of Aphis gossypii and Aphis craccivora (Hemiptera: Aphididae) and of their predator Cycloneda sanguinea (Coleoptera: Coccinellidae) in cotton-cowpea intercropping systems. An. Acad. Bras. Cienc. 2018, 90, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Yao, J.; Li, H.B.; Zhang, Y.; Wang, D.; Ma, G.L. Comparative study on conservation of natural enemy in cotton field by different rapes in Xinjiang. Plant Prot. Sci. 2011, 37, 142–145. (In Chinese) [Google Scholar]
- Luo, J.; Zhang, S.; Wang, C.; Limin, L.; Li, C.; Cui, J. Ecological effects of different trap crop to sucking pests and natural enemies in cotton fields. China Cotton 2014, 41, 14–16. (In Chinese) [Google Scholar]
- Wang, Y. Scientific intercropping can reduce pests and diseases. Pestic. Mark. News 2015, 10, 47. (In Chinese) [Google Scholar]
- Boudreaua, M.A.; Shewb, B.B.; Duffifie Andrakoand, L.E. Impact of intercropping on epidemics of groundnut leaf spots: Defifining constraints and opportunities through a 7-year fifield study. Plant Pathol. 2016, 65, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yao, J.; Zhang, Y.; Liu, H. Effect of apricot trees on insect pests and their natural enemies in nearby cotton fields in southern Xinjiang. Chin. J. Appl. Entomol. 2012, 49, 951–956. (In Chinese) [Google Scholar]
- Guo, R.S.; Tian, L.W.; Lin, T.; Cui, J.P.; Xu, H.J.; Tang, Q.X. Effect of jujube–cotton intercropping on characteristics of microclimate during cotton flowering and boll-setting period and cotton yield. Acta Agric. Boreali Occident. Sin. 2014, 23, 92–98. (In Chinese) [Google Scholar]
- Xu, J.H. Reasons for the serious damage of cotton spider mite in jujube–cotton intercropping and comprehensive control measures. Xinjiang Agric. Sci. Tech. 2011, 5, 33. (In Chinese) [Google Scholar]
- Cui, A.H.; Du, C.L.; Huang, G.Q. Preliminary study on microclimate effects of cotton field intercropping in upland red soil. Meteorol. Disaster Reduct. Res. 2016, 39, 290–294. (In Chinese) [Google Scholar]
- Zhang, R.; Wang, W.; Liu, H.; Yao, J. Effect of the occurrence and damage of Lygus pratensis (Linnaeus) on cotton under almond–cotton interplanting. Plant Prot. Sci. 2018, 44, 172–176. (In Chinese) [Google Scholar]
- Zou, X.X.; Shi, P.X.; Zhang, C.J.; Tong, S.; Wang, Y.F.; Zhang, X.J.; Yu, X.N.; Wang, H.X.; Wang, M.L. Rotational strip intercropping of maize and peanuts has multiple benefits for agricultural production in the northern agropastoral ecotone region of China. Eur. J. Agron. 2021, 129, 126304. [Google Scholar] [CrossRef]
- Shi, A.D.; Li, J.W.; Yuan, L. Effects of rotation and intercropping systems on yield-quality of flue-cured tobacco and soil nutrients. Plant Nutr. Fertil. Sci. 2011, 17, 411–418. (In Chinese) [Google Scholar]
- Yang, L.; Guo, R.; Liu, Y.; Qian, R.; Liang, X.; Zhang, P.; Cai, T.; Ren, X.L.; Jia, Z.K. Effects of maize-soybean intercropping and strip rotation on water, nitrogen utilization and yield of maize in Yellow River irrigated area of Ningxia. Acta Agric. Boreali-Occident. Sin. 2022, 31, 755–765. (In Chinese) [Google Scholar]
- Daryanto, S.; Fu, B.; Zhao, W.; Wang, S.; Jacinthe, P.A.; Wang, L. Ecosystem service provision of grain legume and cereal intercropping in Africa. Agric. Syst. 2020, 178, 102761. [Google Scholar] [CrossRef]
- Kayikcioglu, H.H.; Duman, I.; Asciogul, T.K.; Bozokalfa, M.K.; Elmaci, O.L. Effects of tomato-based rotations with diversified pre-planting on soil health in the Mediterranean soils of Western Turkey. Agric. Ecosyst. Environ. 2020, 299, 106986. [Google Scholar] [CrossRef]
- Cappelli, S.L.; Domeignoz-Horta, L.A.; Loaiza, V.; Laine, A.L. Plant biodiversity promotes sustainable agriculture directly and via belowground effects. Trends Plant Sci. 2022, 27, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.X.; Liu, Y.; Huang, M.M.; Li, F.; Si, T.; Wang, Y.F.; Yu, X.N.; Zhang, X.J.; Wang, H.X.; Shi, P.X. Rotational strip intercropping of maize and peanut enhances productivity by improving crop photosynthetic production and optimizing soil nutrients and bacterial communities. Field Crop. Res. 2023, 291, 108770. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, K.; Wang, X.Y.; Zou, X.X.; Zhang, X.J.; Yu, X.N.; Wang, Y.F.; Si, T. Peanut/Cotton intercropping increases productivity and economic returns by regulating nutrient accumulation and soil microbial communities under both normal and saline soil conditions. BMC Plant Biol. 2022, 22, 121. [Google Scholar] [CrossRef]
- Jiao, N.Y.; Wang, J.T.; Ma, C.; Zhang, C.C.; Guo, D.Y.; Zhang, F.S.; Jensen, E.S. The importance of aboveground and belowground interspecific interactions in determining crop growth and advantages of peanut/maize intercropping. Crop J. 2021, 9, 1460–1469. [Google Scholar] [CrossRef]
- Wang, R.N.; Sun, Z.X.; Zhang, L.Z.; Yang, N.; Werf, W.V.D. Border-row proportion determines strength of interspecific interactions and crop yields in maize/peanut strip intercropping. Field Crop. Res. 2020, 253, 107819. [Google Scholar] [CrossRef]
- Kiar, L.P.; Weisbach, A.N.; Weiner, J. Root and shoot competition: A meta-analysis. J. Ecol. 2013, 101, 1298–1312. [Google Scholar] [CrossRef]
- Yang, F.; Liao, D.; Wu, X.; Gao, R.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; Liu, J. Effect of aboveground and belowground interactions on the intercrop yields in maize-soybean relay intercropping systems. Field Crop. Res. 2017, 203, 16–23. [Google Scholar] [CrossRef]
- Nadarajah, K.; Abdul, R.N.S.N. Plant–microbe interaction: Aboveground to belowground, from the good to the bad. Int. J. Mol. Sci. 2021, 22, 10388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.S.; Li, L.; Sun, J.H. Contribution of above- and below-Ground Interactions to Intercropping; Springer: Amsterdam, The Netherlands, 2001; pp. 73–92. [Google Scholar]
- Chen, G.D.; Chai, Q.; Huang, G.B.; Yu, A.; Feng, F.; Mu, Y.; Kong, X.; Huang, P. Belowground interspecies interaction enhances productivity and water use efficiency in maize-pea intercropping systems. Crop Sci. 2015, 55, 420–428. [Google Scholar] [CrossRef]
- Spiertz, J.H.J.; Li, B.; Zhang, L.; Zhang, S.; van der Werf, W.; Bastiaans, L. Light interception and utilization in relay intercrops of wheat and cotton. Field Crop. Res. 2008, 107, 29–42. [Google Scholar]
- Zhu, J.Q.; van der Werf, W.; Anten, N.P.R.; Vos, J.; Evers, J.B. The contribution of phenotypic plasticity to complementary light capture in plant mixtures. New Phytol. 2015, 207, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Li, X.Z.; Yao, M.J. Research progress on assembly of plant rhizosphere microbial community. Acta Microbiologica Sinica. 2021, 61, 231–246. [Google Scholar]
- Huang, Y.; Wu, Q.; Deng, S.Y.; He, X.Q.; Jin, Y.; Wang, J.; Luo, K.; Xie, C.; Wang, T.; Yong, T.W. Effects of root interaction on root growth, leaf photosynthetic characteristics and biomass of maize under two intercropping systems of maize and legumes. J. Sichuan Agric. Univ. 2020, 38, 514–527. (In Chinese) [Google Scholar]
- Mariotti, M.; Masoni, A.; Ercoli, L.; Arduini, I. Above- and below-ground competition between barley, wheat, lupin and vetch in a cereal and legume intercropping system. Grass Forage Sci. 2009, 64, 401–412. [Google Scholar] [CrossRef]
- Inal, A.; Gunes, A.; Zhang, F.; Cakmak, I. Peanut/maize intercropping induced changes in rhizosphere and nutrient concentrations in shoots. Plant Physiol. Bioch. 2007, 45, 350–356. [Google Scholar] [CrossRef]
- Chen, X.B.; Yao, Q.F.; Gao, X.H.; Jiang, C.F.; Harberd, N.P.; Fu, X.D. Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 2016, 26, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Raven, J.A. Interactions between above and below ground plant structures:mechanisms and ecosystem services. Front. Agric. Sci. Eng. 2022, 9, 197–213. [Google Scholar]
- Venturi, V.; Keel, C. Signaling in the Rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef]
- Albacete, A.; Martínez-Andújar, C.; Ghanem, M.E.; Acosta, M.; Sánchez-Bravo, J.; Asins, M.J.; Cuartero, J.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Root-stock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato. Plant Cell Environ. 2009, 32, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.L.; Dong, H.Z. Stem girdling influences concentrations of endogenous cytokinins and abscisic acid in relation to leaf senescence in cotton. Acta Physiol. Plant. 2011, 33, 1697–1705. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Wu, K.; Fu, X.D. Nitrogen signaling and use efficiency in plants: What’s new? Curr. Opin. Plant Biol. 2015, 27, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y. The Research of Root-Shoot Interaction Mechanism on Cotton Leaf Senescence; China Agricultural University: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Dong, H.Z.; Niu, Y.H.; Li, W.J.; Zhang, D.M. Effects of cotton rootstock on endogenous cytokinins and abscisic acid in xylem sap and leaves in relation to leaf senescence. J. Exp. Bot. 2008, 59, 1295–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Z.; Dong, H.Z. Mechanisms and regulation of senescence and maturity performance in cotton. Field Crop. Res. 2016, 189, 1–9. [Google Scholar] [CrossRef]
- Faiss, M.; Zalubìlová, J.; Strnad, M.; Schmülling, T. Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant J. 1997, 12, 401–415. [Google Scholar] [CrossRef] [Green Version]
- Osterlund, M.T.; Hardtke, C.S.; Wei, N.; Deng, X.W. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 2000, 405, 462–466. [Google Scholar] [CrossRef]
- Zafar, M.M.; Zhang, Y.; Farooq, M.A.; Ali, A.; Firdous, H.; Haseeb, M.; Fiaz, S.; Shakeel, A.; Razzaq, A.; Ren, M. Biochemical and associated agronomic traits in Gossypium hirsutum L. under high temperature stress. Agronomy 2022, 12, 1310. [Google Scholar] [CrossRef]
- Zafar, M.M.; Manan, A.; Razzaq, A.; Zulfqar, M.; Saeed, A.; Kashif, M.; Khan, A.I.; Sarfraz, Z.; Mo, H.; Iqbal, M.S.; et al. Exploiting agronomic and biochemical traits to develop heat resilient cotton cultivars under climate change scenarios. Agronomy 2021, 11, 1885. [Google Scholar] [CrossRef]
- Zafar, M.M.; Jia, X.; Shakeel, A.; Sarfraz, Z.; Manan, A.; Imran, A.; Mo, H.; Ali, A.; Youlu, Y.; Razzaq, A.; et al. Unraveling heat tolerance in upland cotton (Gossypium hirsutum L.) using univariate and multivariate analysis. Front. Plant Sci. 2022, 12, 727835. [Google Scholar] [CrossRef]
- Zhang, L.; Spiertz, J.H.J.; Zhang, S.P. Nitrogen economy in relay intercropping system of wheat and cotton. Plant Soil. 2008, 303, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Sievänen, R.; Mäkelä, A.; Nikinmaa, E. Special issue on functional-structural tree models: Preface. Silva Fenn. 1997, 31, 237–238. [Google Scholar]
- Sievänen, R.; Nikinmaa, E.; Nygren, P. Components of functional-structural tree models. Ann. For. Sci. 2000, 57, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Guo, R.; Hussain, S.; Guo, S.Q.; Cai, T.; Zhang, P.; Jia, Z.K.; Naseer, M.A.; Saqib, M.; Chen, X.L.; et al. Rotation of planting strips and reduction in nitrogen fertilizer application can reduce nitrogen loss and optimize its balance in maize–peanut intercropping. Eur. J. Agron. 2023, 143, 126707. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Q.; Chi, B.; He, N.; Zhang, D.; Dai, J.; Zhang, Y.; Dong, H. Cotton-Based Rotation, Intercropping, and Alternate Intercropping Increase Yields by Improving Root–Shoot Relations. Agronomy 2023, 13, 413. https://doi.org/10.3390/agronomy13020413
Lv Q, Chi B, He N, Zhang D, Dai J, Zhang Y, Dong H. Cotton-Based Rotation, Intercropping, and Alternate Intercropping Increase Yields by Improving Root–Shoot Relations. Agronomy. 2023; 13(2):413. https://doi.org/10.3390/agronomy13020413
Chicago/Turabian StyleLv, Qingqing, Baojie Chi, Ning He, Dongmei Zhang, Jianlong Dai, Yongjiang Zhang, and Hezhong Dong. 2023. "Cotton-Based Rotation, Intercropping, and Alternate Intercropping Increase Yields by Improving Root–Shoot Relations" Agronomy 13, no. 2: 413. https://doi.org/10.3390/agronomy13020413




