Modulated Electro-Hyperthermic (mEHT) Treatment in the Therapy of Inoperable Pancreatic Cancer Patients—A Single-Center Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Treatment Protocols and Matching of Cases and Controls
2.3. Hyperthermia Treatment
2.4. Statistical Analysis
3. Results
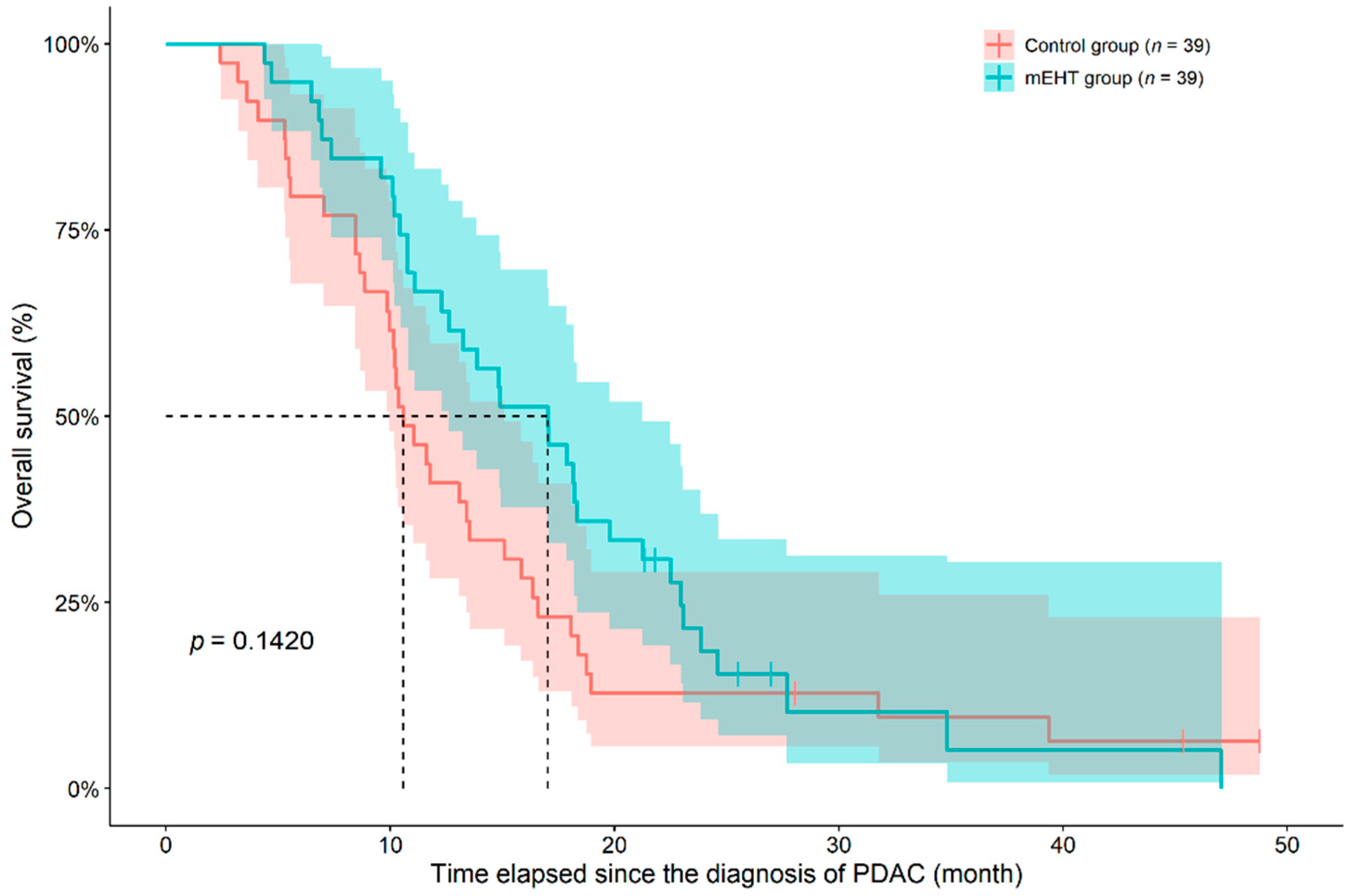
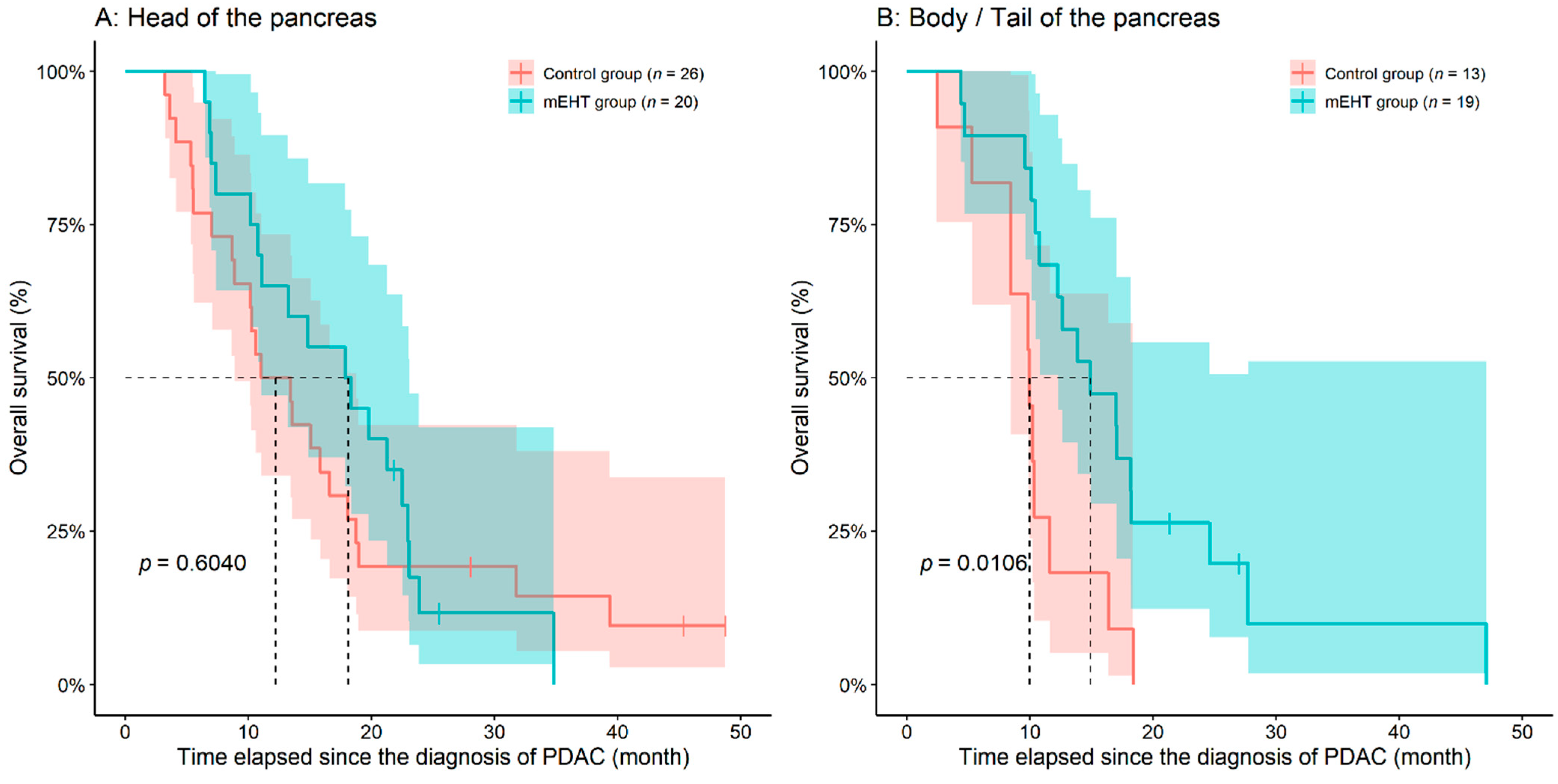
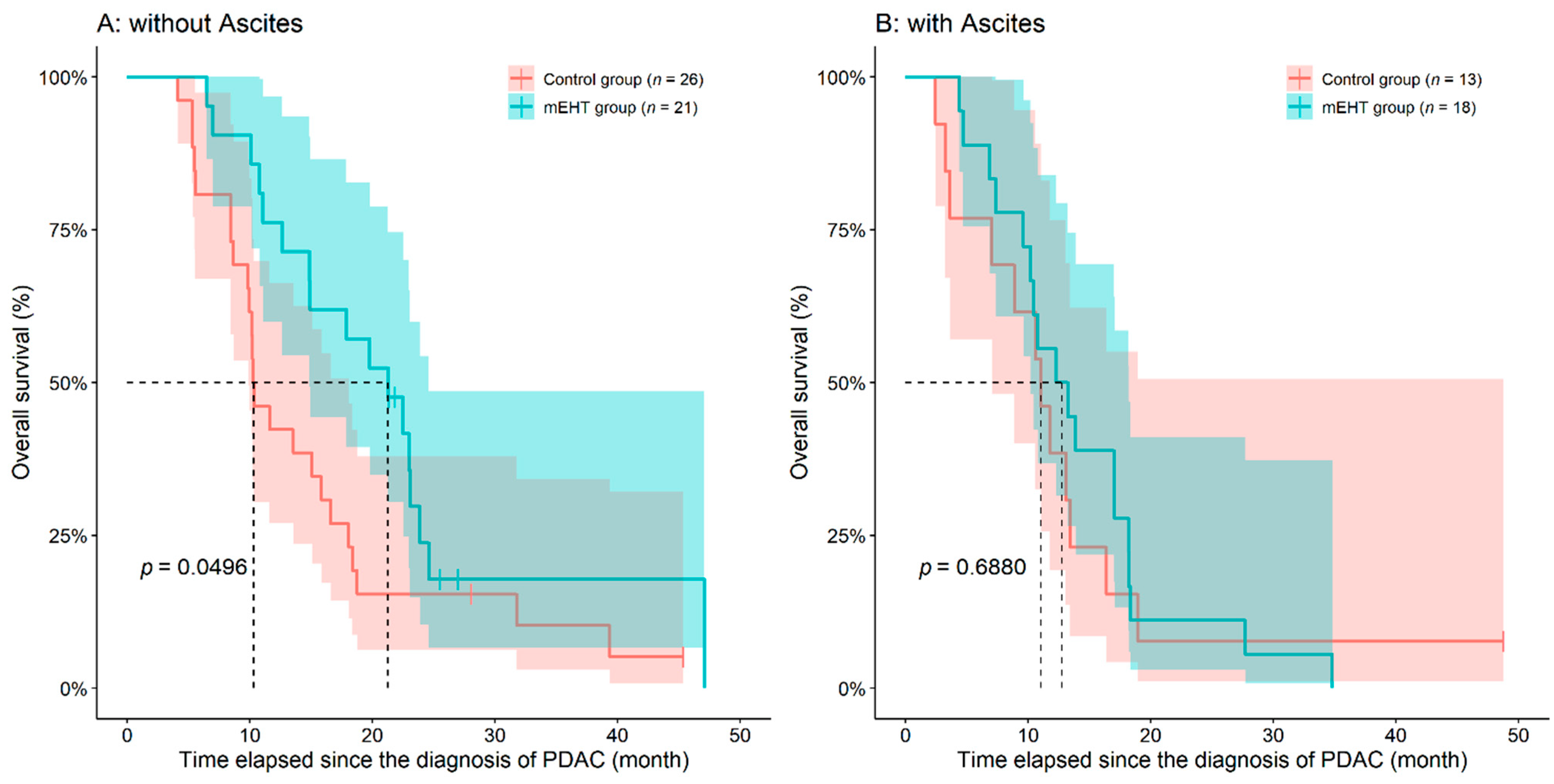
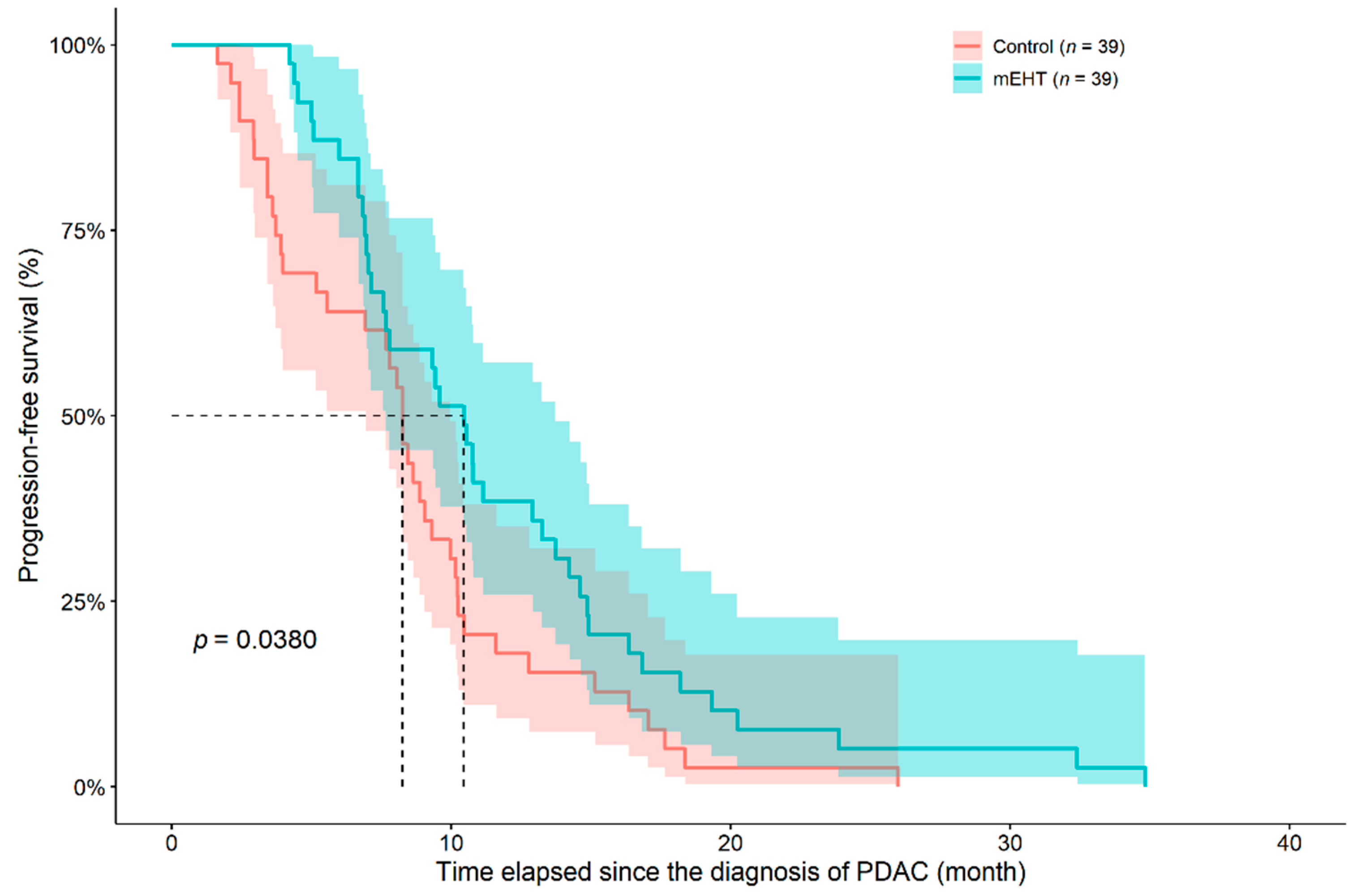
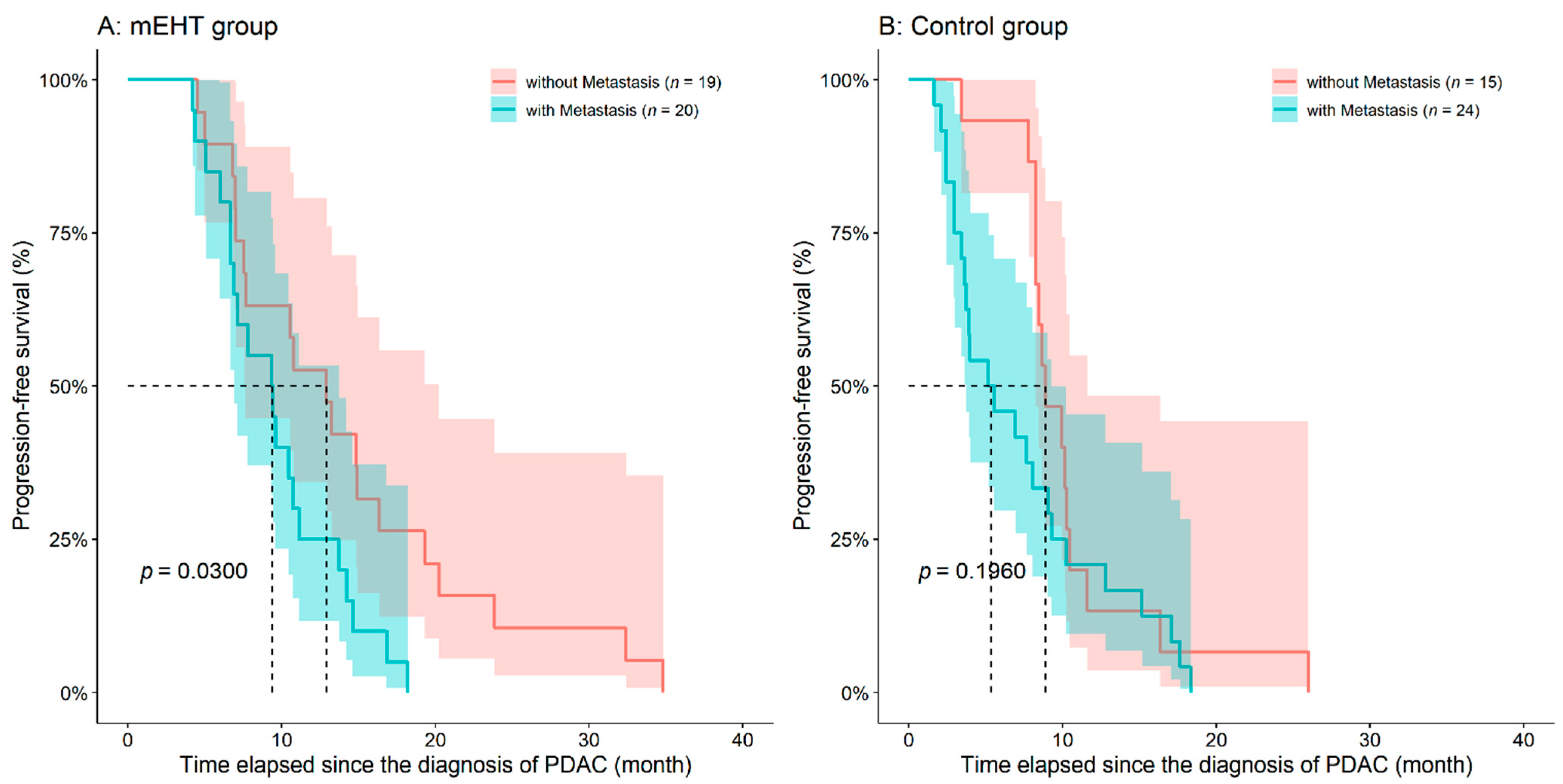
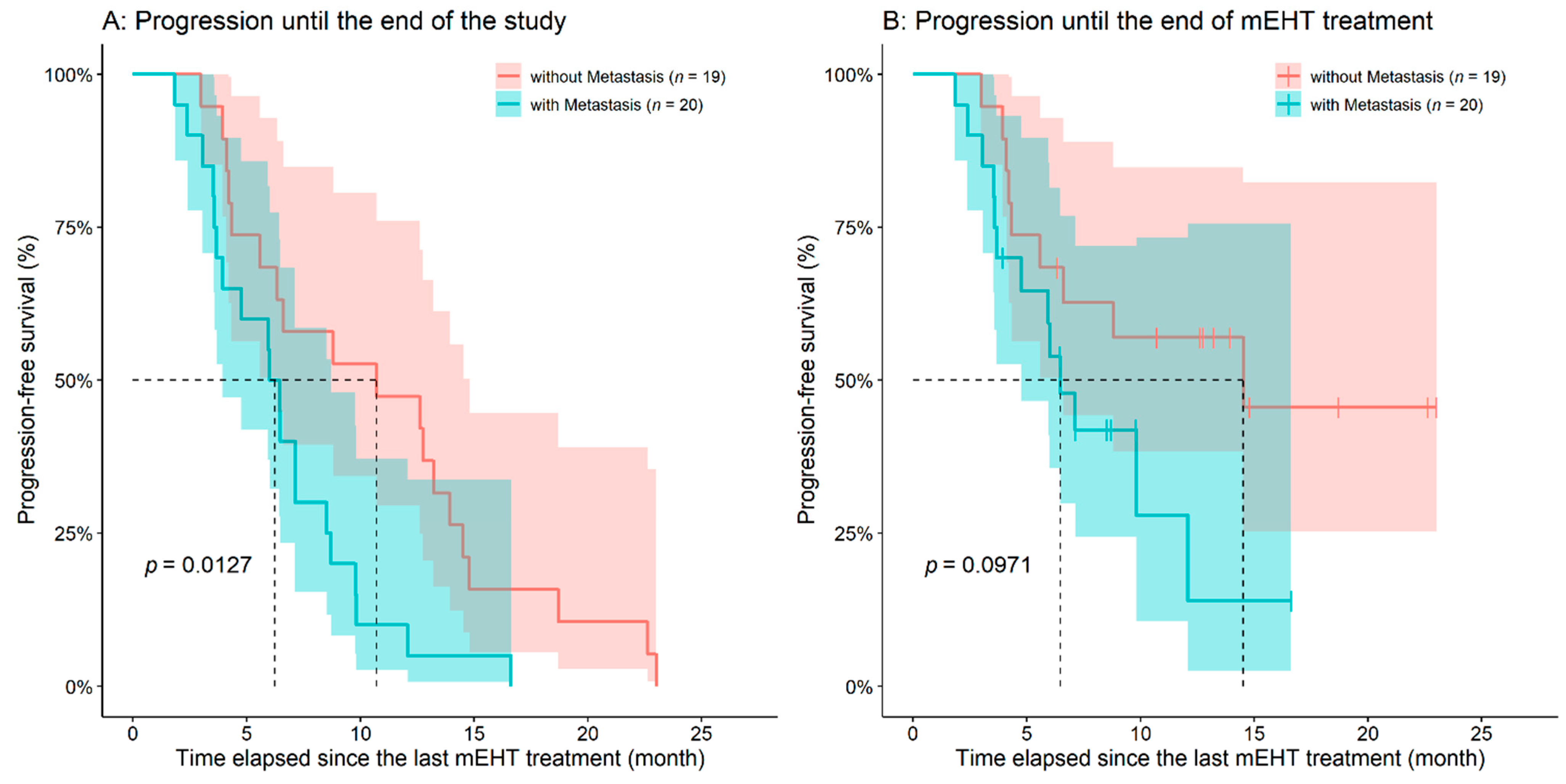
3.1. Analysis of Overall- and Progression-Free Survival
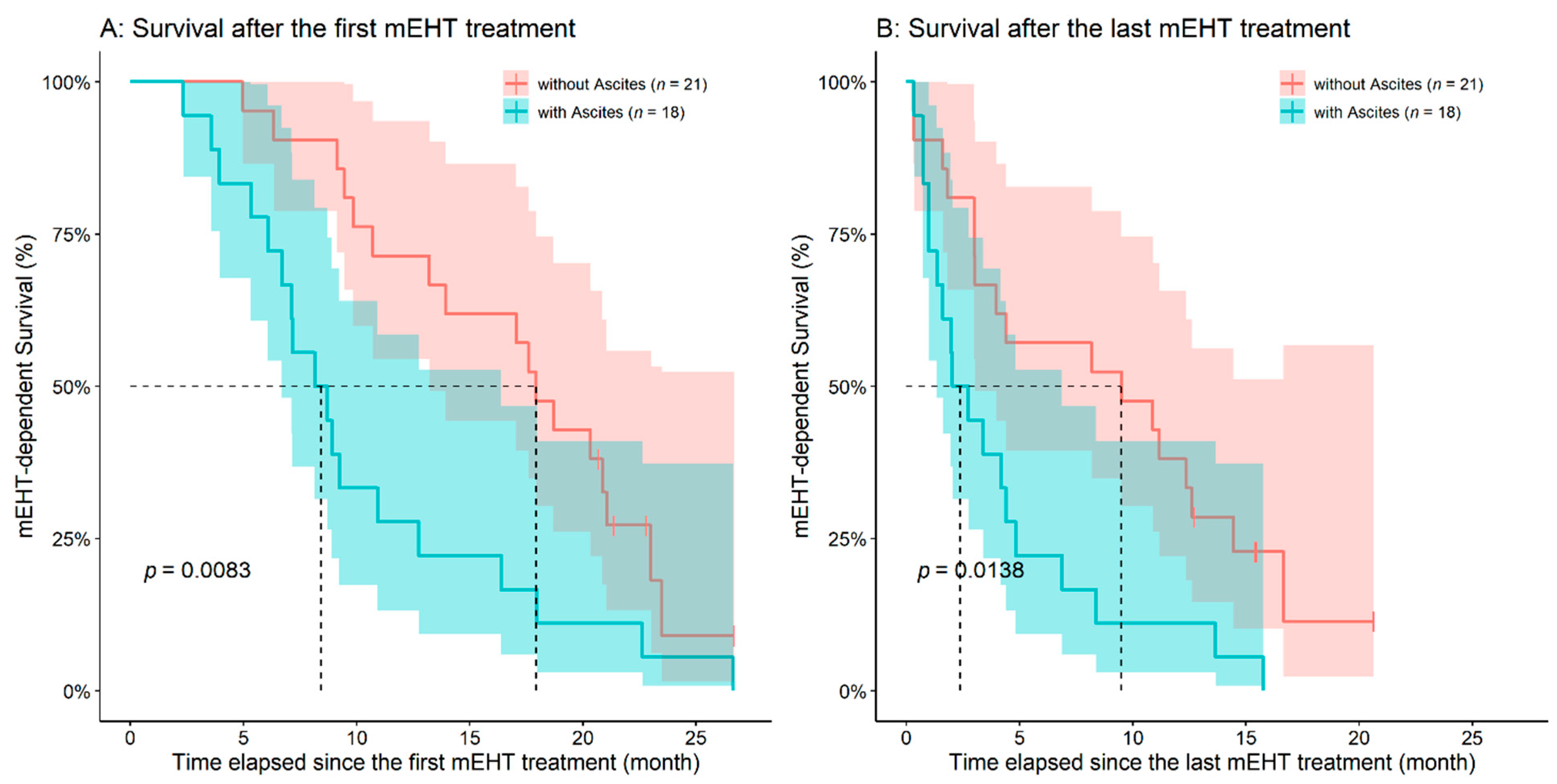
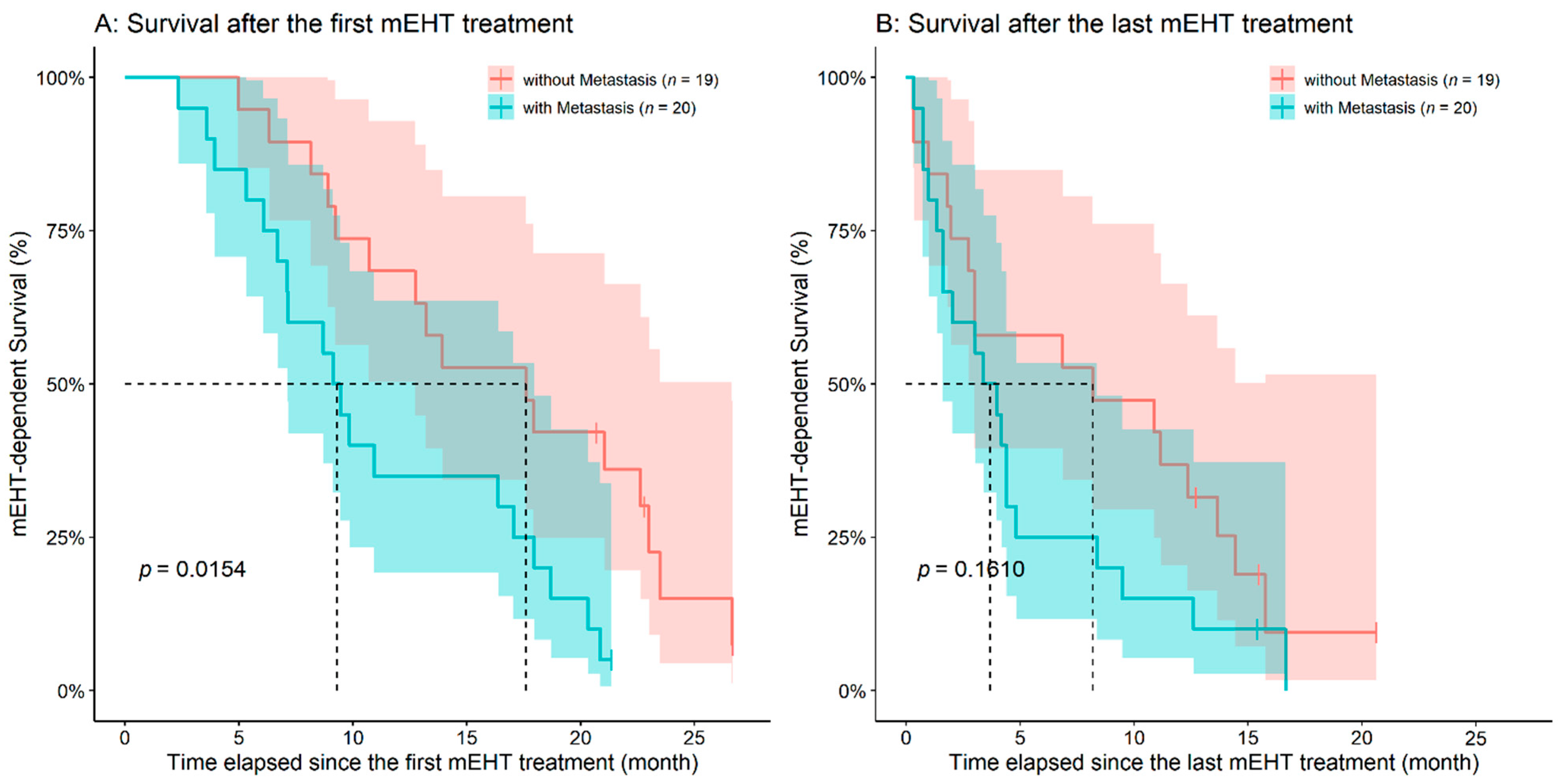
3.2. Analysis of Survival after the First and Last mEHT Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chin, V.; Nagrial, A.; Sjoquist, K.; O’Connor, C.A.; Chantrill, L.; Biankin, A.V.; Scholten, R.J.; Yip, D. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst. Rev. 2018, 3, CD011044. [Google Scholar] [CrossRef]
- Hruban, R.H.; Fukushima, N. Pancreatic adenocarcinoma: Update on the surgical pathology of carcinomas of ductal origin and PanINs. Mod. Pathol. 2007, 20 (Suppl. 1), S61–S70. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.; Honselmann, K.C.; Liss, A.S. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers 2018, 10, 316. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.T.; Cardin, D.B.; Berlin, J.D. Recent advances in the treatment of pancreatic cancer. F1000Res 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Dominguez, I.; Rodriguez, J.R.; Razo, O.; Thayer, S.P.; Ryan, D.P.; Warshaw, A.L.; Fernandez-del Castillo, C. Quality of life in pancreatic cancer: Analysis by stage and treatment. J. Gastrointest. Surg. 2008, 12, 783–793; discussion 784–793. [Google Scholar] [CrossRef]
- Gourgou-Bourgade, S.; Bascoul-Mollevi, C.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Boige, V.; et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial. J. Clin. Oncol. 2013, 31, 23–29. [Google Scholar] [CrossRef]
- Deng, Y.; Tu, H.; Pierzynski, J.A.; Miller, E.D.; Gu, X.; Huang, M.; Chang, D.W.; Ye, Y.; Hildebrandt, M.A.T.; Klein, A.P.; et al. Determinants and prognostic value of quality of life in patients with pancreatic ductal adenocarcinoma. Eur. J. Cancer 2018, 92, 20–32. [Google Scholar] [CrossRef]
- Saif, M.W. New Developments in the Treatment of Pancreatic Cancer: Highlights from the 44th ASCO Annual Virtual Meeting, May 29–31, 2020. JOP 2020, 21, 108–111. [Google Scholar]
- Fan, J.-q.; Wang, M.-F.; Chen, H.-L.; Shang, D.; Das, J.K.; Song, J. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol. Cancer 2020, 19, 32. [Google Scholar] [CrossRef]
- Oba, A.; Ho, F.; Bao, Q.R.; Al-Musawi, M.H.; Schulick, R.D.; Del Chiaro, M. Neoadjuvant Treatment in Pancreatic Cancer. Front. Oncol. 2020, 10, 245. [Google Scholar] [CrossRef]
- Yao, W.; Maitra, A.; Ying, H. Recent insights into the biology of pancreatic cancer. eBioMedicine 2020, 53, 102655. [Google Scholar] [CrossRef]
- Martin-Perez, E.; Dominguez-Munoz, J.E.; Botella-Romero, F.; Cerezo, L.; Matute Teresa, F.; Serrano, T.; Vera, R. Multidisciplinary consensus statement on the clinical management of patients with pancreatic cancer. Clin. Transl. Oncol. 2020, 22, 1963–1975. [Google Scholar] [CrossRef]
- Lidsky, M.E.; Sun, Z.; Nussbaum, D.P.; Adam, M.A.; Speicher, P.J.; Blazer, D.G.I. Going the Extra Mile: Improved Survival for Pancreatic Cancer Patients Traveling to High-volume Centers. Ann. Surg. 2017, 266, 333–338. [Google Scholar] [CrossRef]
- Datta, N.R.; Pestalozzi, B.; Clavien, P.A.; Siebenhuner, A.; Puric, E.; Khan, S.; Mamot, C.; Riesterer, O.; Knuchel, J.; Reiner, C.S.; et al. “HEATPAC”—A phase II randomized study of concurrent thermochemoradiotherapy versus chemoradiotherapy alone in locally advanced pancreatic cancer. Radiat. Oncol. 2017, 12, 183. [Google Scholar] [CrossRef]
- Rogers, S.J.; Datta, N.R.; Puric, E.; Timm, O.; Marder, D.; Khan, S.; Mamot, C.; Knuchel, J.; Siebenhuner, A.; Pestalozzi, B.; et al. The addition of deep hyperthermia to gemcitabine-based chemoradiation may achieve enhanced survival in unresectable locally advanced adenocarcinoma of the pancreas. Clin. Transl. Radiat. Oncol. 2021, 27, 109–113. [Google Scholar] [CrossRef]
- van der Horst, A.; Versteijne, E.; Besselink, M.G.H.; Daams, J.G.; Bulle, E.B.; Bijlsma, M.F.; Wilmink, J.W.; van Delden, O.M.; van Hooft, J.E.; Franken, N.A.P.; et al. The clinical benefit of hyperthermia in pancreatic cancer: A systematic review. Int. J. Hyperth. 2018, 34, 969–979. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Gadaleta, C.D.; Ballerini, M.; Fiorentini, C.; Garfagno, T.; Ranieri, G.; Guadagni, S. A Narrative Review of Regional Hyperthermia: Updates From 2010 to 2019. Integr. Cancer 2020, 19, 1534735420932648. [Google Scholar] [CrossRef]
- Chicheł, A.; Skowronek, J.; Kubaszewska, M.; Kanikowski, M. Hyperthermia—Description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007, 12, 267–275. [Google Scholar] [CrossRef]
- Szasz, A.; Szasz, N.; Szasz, O. Oncothermia: Principles and Practices; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Szasz, A. Thermal and nonthermal effects of radiofrequency on living state and applications as an adjuvant with radiation therapy. J. Radiat. Cancer Res. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Krenacs, T.; Meggyeshazi, N.; Forika, G.; Kiss, E.; Hamar, P.; Szekely, T.; Vancsik, T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int. J. Mol. Sci. 2020, 21, 6270. [Google Scholar] [CrossRef]
- Alshaibi, H.F.; Al-Shehri, B.; Hassan, B.; Al-Zahrani, R.; Assiss, T. Modulated Electrohyperthermia: A New Hope for Cancer Patients. Biomed. Res. Int. 2020, 2020, 8814878. [Google Scholar] [CrossRef] [PubMed]
- Jeung, T.S.; Ma, S.Y.; Yu, J.; Lim, S. Cases That Respond to Oncothermia Monotherapy. Conf. Pap. Med. 2013, 2013, 392480. [Google Scholar] [CrossRef]
- Minnaar, C.A.; Szigeti, G.P.; Kotzen, J.A. Modulated electro-hyperthermia as a monotherapy: A potential for further research? Oncothermia J. 2018, 24, 303–317. [Google Scholar]
- Pang, C.L.K.; Zhang, X.; Wang, Z.; Ou, J.; Lu, Y.; Chen, P.; Zhao, C.; Wang, X.; Zhang, H.; Roussakow, S.V. Local modulated electro-hyperthermia in combination with traditional Chinese medicine vs. intraperitoneal chemoinfusion for the treatment of peritoneal carcinomatosis with malignant ascites: A phase II randomized trial. Mol. Clin. Oncol. 2017, 6, 723–732. [Google Scholar] [CrossRef][Green Version]
- Falk, R.E.; Moffat, F.L.; Lawler, M.; Heine, J.; Makowka, L.; Falk, J.A. Combination therapy for resectable and unresectable adenocarcinoma of the pancreas. Cancer 1986, 57, 685–688. [Google Scholar] [CrossRef]
- Szasz, A. Towards the Immunogenic Hyperthermic Action: Modulated ElectroHyperthermia. Clin. Oncol. Res. 2020, 3, 2–6. [Google Scholar] [CrossRef]
- Chi, K.-H. Tumour-Directed Immunotherapy: Clinical Results of Radiotherapy with Modulated Electro-Hyperthermia. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 206–226. [Google Scholar]
- Fiorentini, G.; Sarti, D.; Casadei, V.; Milandri, C.; Dentico, P.; Mambrini, A.; Nani, R.; Fiorentini, C.; Guadagni, S. Modulated Electro-Hyperthermia as Palliative Treatment for Pancreatic Cancer: A Retrospective Observational Study on 106 Patients. Integr. Cancer 2019, 18, 1534735419878505. [Google Scholar] [CrossRef]
- Volovat, C.; Volovat, S.; Scripcaru, V.; Miron, L. Second-line chemotherapy with gemcitabine and oxaliplatin in combination with loco-regional hyperthermia (EHY-2000) in patients with refractory metastatic pancreatic cancer—Preliminary results of a prospective trial. Rom. Rep. Phys. 2014, 66, 166–174. [Google Scholar]
- Dani, A.; Varkonyi, A.; Magyar, T.; Kalden, M.; Szasz, A. Clinical study for advanced pancreas cancer treated by oncothermia. Oncothermia J. 2012, 6, 11–25. [Google Scholar]
- Douwes, F.R. Thermo-chemotherapy of the advanced pancreas carcinoma. Biol. Med. 2006, 35, 126–130. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Busing, F.M.; Weaver, B.; Dubois, S. 2 × 2 Tables: A note on Campbell’s recommendation. Stat. Med. 2016, 35, 1354–1358. [Google Scholar] [CrossRef]
- Kampinga, H.H. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int. J. Hyperth. 2006, 22, 191–196. [Google Scholar] [CrossRef]
- Rao, W.; Deng, Z.S.; Liu, J. A review of hyperthermia combined with radiotherapy/chemotherapy on malignant tumors. Crit Rev. Biomed. Eng. 2010, 38, 101–116. [Google Scholar] [CrossRef]
- Datta, N.R.; Ordonez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, G. Analysis of short and long term therapeutic effects of radiofrequency hyperthermia combined with conformal radiotherapy in hepatocellular carcinoma. J. BUON 2016, 21, 407–411. [Google Scholar]
- Kakehi, M.; Ueda, K.; Mukojima, T.; Hiraoka, M.; Seto, O.; Akanuma, A.; Nakatsugawa, S. Multi-institutional clinical studies on hyperthermia combined with radiotherapy or chemotherapy in advanced cancer of deep-seated organs. Int. J. Hyperth. 1990, 6, 719–740. [Google Scholar] [CrossRef] [PubMed]
- Marmor, J.B.; Hahn, G.M. Combined radiation and hyperthermia in superficial human tumors. Cancer 1980, 46, 1986–1991. [Google Scholar] [CrossRef]
- Szasz, A.M.; Minnaar, C.A.; Szentmartoni, G.; Szigeti, G.P.; Dank, M. Review of the Clinical Evidences of Modulated Electro-Hyperthermia (mEHT) Method: An Update for the Practicing Oncologist. Front. Oncol. 2019, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Bakshandeh-Bath, A.; Stoltz, A.S.; Homann, N.; Wagner, T.; Stolting, S.; Peters, S.O. Preclinical and clinical aspects of carboplatin and gemcitabine combined with whole-body hyperthermia for pancreatic adenocarcinoma. Anticancer Res. 2009, 29, 3069–3077. [Google Scholar]
- Bull, J.M.; Scott, G.L.; Strebel, F.R.; Nagle, V.L.; Oliver, D.; Redwine, M.; Rowe, R.W.; Ahn, C.W.; Koch, S.M. Fever-range whole-body thermal therapy combined with cisplatin, gemcitabine, and daily interferon-alpha: A description of a phase I-II protocol. Int. J. Hyperth. 2008, 24, 649–662. [Google Scholar] [CrossRef]
- Robins, H.I.; Cohen, J.D.; Schmitt, C.L.; Tutsch, K.D.; Feierabend, C.; Arzoomanian, R.Z.; Alberti, D.; d’Oleire, F.; Longo, W.; Heiss, C.; et al. Phase I clinical trial of carboplatin and 41.8 degrees C whole-body hyperthermia in cancer patients. J. Clin. Oncol. 1993, 11, 1787–1794. [Google Scholar] [CrossRef]
- Robins, H.I.; Rushing, D.; Kutz, M.; Tutsch, K.D.; Tiggelaar, C.L.; Paul, D.; Spriggs, D.; Kraemer, C.; Gillis, W.; Feierabend, C.; et al. Phase I clinical trial of melphalan and 41.8 degrees C whole-body hyperthermia in cancer patients. J. Clin. Oncol. 1997, 15, 158–164. [Google Scholar] [CrossRef]
- Cho, C.; Wust, P.; Hildebrandt, B.; Issels, R.D.; Sehouli, J.; Kerner, T.; Deja, M.; Budach, V.; Gellermann, J. Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: Evaluation of two annular-phased-array applicators. Int. J. Hyperth. 2008, 24, 399–408. [Google Scholar] [CrossRef]
- Ohguri, T.; Imada, H.; Yahara, K.; Narisada, H.; Morioka, T.; Nakano, K.; Korogi, Y. Concurrent chemoradiotherapy with gemcitabine plus regional hyperthermia for locally advanced pancreatic carcinoma: Initial experience. Radiat. Med. 2008, 26, 587–596. [Google Scholar] [CrossRef]
- Zhang, L.P.; Nie, Q.; Kang, J.B.; Wang, B.; Cai, C.L.; Li, J.G.; Qi, W.J. Efficacy of whole body gamma-knife radiotherapy combined with thermochemotherapy on locally advanced pancreatic cancer. Ai Zheng 2008, 27, 1204–1207. [Google Scholar]
- Ishikawa, T.; Kokura, S.; Sakamoto, N.; Ando, T.; Imamoto, E.; Hattori, T.; Oyamada, H.; Yoshinami, N.; Sakamoto, M.; Kitagawa, K.; et al. Phase II trial of combined regional hyperthermia and gemcitabine for locally advanced or metastatic pancreatic cancer. Int. J. Hyperth. 2012, 28, 597–604. [Google Scholar] [CrossRef]
- Hamazoe, R.; Maeta, M.; Murakami, A.; Yamashiro, H.; Kaibara, N. Heating efficiency of radiofrequency capacitive hyperthermia for treatment of deep-seated tumors in the peritoneal cavity. J. Surg. Oncol. 1991, 48, 176–179. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kokura, S.; Oyamada, H.; Inui, T.; Okita, M.; Isozaki, Y.; Nagao, Y.; Takagi, T.; Handa, O.; Ando, T.; et al. Effects of a Sequential Combination of Hyperthermia and Gemcitabine in the Treatment of Advanced Unresectable Pancreatic Cancer: A Retrospective Study. Therm. Med. 2008, 24, 131–139. [Google Scholar] [CrossRef][Green Version]
- Maebayashi, T.; Ishibashi, N.; Aizawa, T.; Sakaguchi, M.; Sato, T.; Kawamori, J.; Tanaka, Y. Treatment outcomes of concurrent hyperthermia and chemoradiotherapy for pancreatic cancer: Insights into the significance of hyperthermia treatment. Oncol. Lett. 2017, 13, 4959–4964. [Google Scholar] [CrossRef]
- Maluta, S.; Schaffer, M.; Pioli, F.; Dall’oglio, S.; Pasetto, S.; Schaffer, P.M.; Weber, B.; Giri, M.G. Regional hyperthermia combined with chemoradiotherapy in primary or recurrent locally advanced pancreatic cancer: An open-label comparative cohort trial. Strahlenther. Onkol. 2011, 187, 619–625. [Google Scholar] [CrossRef]
- Iyikesici, M.S. Long-Term Survival Outcomes of Metabolically Supported Chemotherapy with Gemcitabine-Based or FOLFIRINOX Regimen Combined with Ketogenic Diet, Hyperthermia, and Hyperbaric Oxygen Therapy in Metastatic Pancreatic Cancer. Complement. Med. Res. 2020, 27, 31–39. [Google Scholar] [CrossRef]
- Bonucci, M.; Pastore, C.; Ferrera, V.; Fiorentini, C.; Fabbri, A. Integrated Cancer Treatment in the Course of Metastatic Pancreatic Cancer: Complete Resolution in 2 Cases. Integr. Cancer 2018, 17, 994–999. [Google Scholar] [CrossRef]
- Werthmann, P.G.; Inter, P.; Welsch, T.; Sturm, A.K.; Grutzmann, R.; Debus, M.; Sterner, M.G.; Kienle, G.S. Long-term tumor-free survival in a metastatic pancreatic carcinoma patient with FOLFIRINOX/Mitomycin, high-dose, fever inducing Viscum album extracts and subsequent R0 resection: A case report. Medecine 2018, 97, e13243. [Google Scholar] [CrossRef]
- Seufferlein, T.; Bachet, J.B.; Van Cutsem, E.; Rougier, P.; Group, E.G.W. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. 7), vii33–vii40. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Barreto, S.G.; Shukla, P.J.; Shrikhande, S.V. Tumors of the Pancreatic Body and Tail. World J. Oncol. 2010, 1, 52–65. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Number of mEHT sessions | 59.18 ± 26.22 (64 (21–154)) |
| Elapsed time from diagnosis to mEHT treatment (days from pathological diagnosis) | 99.56 ± 147.88 (46 (2–718)) |
| Applied applicator (cm) | 30 |
| Average duration of sessions (min) | 59.5 |
| Frequency of treatments/patients (/week) | 2–3 |
| Protocol (fit to patient’s tolerability) | Step-up |
| Power (W) | From 60 to 150 |
| Parameter | mEHT Treated (n = 39) | Control (n = 39) | p-Value |
|---|---|---|---|
| Age (years) | 65.90 ± 9.90 (67 (45–84)) | 66.02 ± 8.73 (67 (45–78)) | 0.8927 1 |
| Male gender | 18 (46.2) | 18 (46.2) | matched |
Location of the tumor
| 20 (51.3) 14 (35.9) 5 (12.8) | 26 (66.7) 8 (20.5) 5 (12.8) | 0.3030 2 |
Chemotherapy protocol:
| 24 (61.5) 8 (20.5) 5 (12.8) 1 (2.6) 1 (2.6) | 24 (61.5) 8 (20.5) 5 (12.8) 1 (2.6) 1 (2.6) | matched matched matched matched matched |
| Without radiologically detected ascites | 21 (53.8) | 26 (66.7) | 0.2504 2 |
| Without distant metastasis | 19 (48.7) | 15 (38.4) | 0.3642 2 |
| Overall survival (month) | 16.96 ± 8.72 (17.02 (4.4–47.1)) | 14.19 ± 10.86 (10.58 (2.4–48.8)) | 0.0301 1 |
| Progression-free survival (month) | 11.87 ± 7.05 (10.45 (4.2–34.8)) | 8.53 ± 5.37 (8.25 (1.6–26.0)) | 0.0258 1 |
| One-year OS | 26 (66.7) | 16 (41.0) | 0.0240 2 |
| Two-year OS # | 6 (15.4) | 5 (12.8) | 0.6761 2 |
| Three-year OS # | 1 (2.6) | 3 (7.7) | 0.3481 2 |
| One-year PFS | 15 (38.5) | 7 (17.9) | 0.0455 2 |
| Two-year PFS | 1 (2.6) | 2 (5.1) | 0.5585 2 |
| OS after the first mEHT treatment (month) | 13.69 ± 7.11 (12.75 (2.3–26.7)) | – | – |
| OS after the last mEHT treatment (month) | 6.57 ± 5.83 (4.17 (0.3–20.6)) | – | – |
| PFS after the first mEHT treatment (month) | 8.60 ± 5.45 (6.60 (1.8–23.0)) | – | – |
| PFS after the last mEHT treatment (month) * | 1.48 ± 4.88 (0.72 (−9.0–15.8)) | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petenyi, F.G.; Garay, T.; Muhl, D.; Izso, B.; Karaszi, A.; Borbenyi, E.; Herold, M.; Herold, Z.; Szasz, A.M.; Dank, M. Modulated Electro-Hyperthermic (mEHT) Treatment in the Therapy of Inoperable Pancreatic Cancer Patients—A Single-Center Case-Control Study. Diseases 2021, 9, 81. https://doi.org/10.3390/diseases9040081
Petenyi FG, Garay T, Muhl D, Izso B, Karaszi A, Borbenyi E, Herold M, Herold Z, Szasz AM, Dank M. Modulated Electro-Hyperthermic (mEHT) Treatment in the Therapy of Inoperable Pancreatic Cancer Patients—A Single-Center Case-Control Study. Diseases. 2021; 9(4):81. https://doi.org/10.3390/diseases9040081
Chicago/Turabian StylePetenyi, Flora Greta, Tamas Garay, Dorottya Muhl, Blanka Izso, Adam Karaszi, Erika Borbenyi, Magdolna Herold, Zoltan Herold, Attila Marcell Szasz, and Magdolna Dank. 2021. "Modulated Electro-Hyperthermic (mEHT) Treatment in the Therapy of Inoperable Pancreatic Cancer Patients—A Single-Center Case-Control Study" Diseases 9, no. 4: 81. https://doi.org/10.3390/diseases9040081
APA StylePetenyi, F. G., Garay, T., Muhl, D., Izso, B., Karaszi, A., Borbenyi, E., Herold, M., Herold, Z., Szasz, A. M., & Dank, M. (2021). Modulated Electro-Hyperthermic (mEHT) Treatment in the Therapy of Inoperable Pancreatic Cancer Patients—A Single-Center Case-Control Study. Diseases, 9(4), 81. https://doi.org/10.3390/diseases9040081






