Prognostic Factors, Survival Analyses and the Risk of Second Primary Cancer: A Population-Based Study on Burkitt Lymphoma/Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Data Source and Cohort Selection
2.2. Variables
2.3. Statistical Analyses
2.3.1. Descriptive Data
2.3.2. Prognostic Factors and Survival Analyses
2.3.3. Risk Factor Analyses for the Development of Second Primary Cancers (SPCs)
3. Results
3.1. Population Characteristics
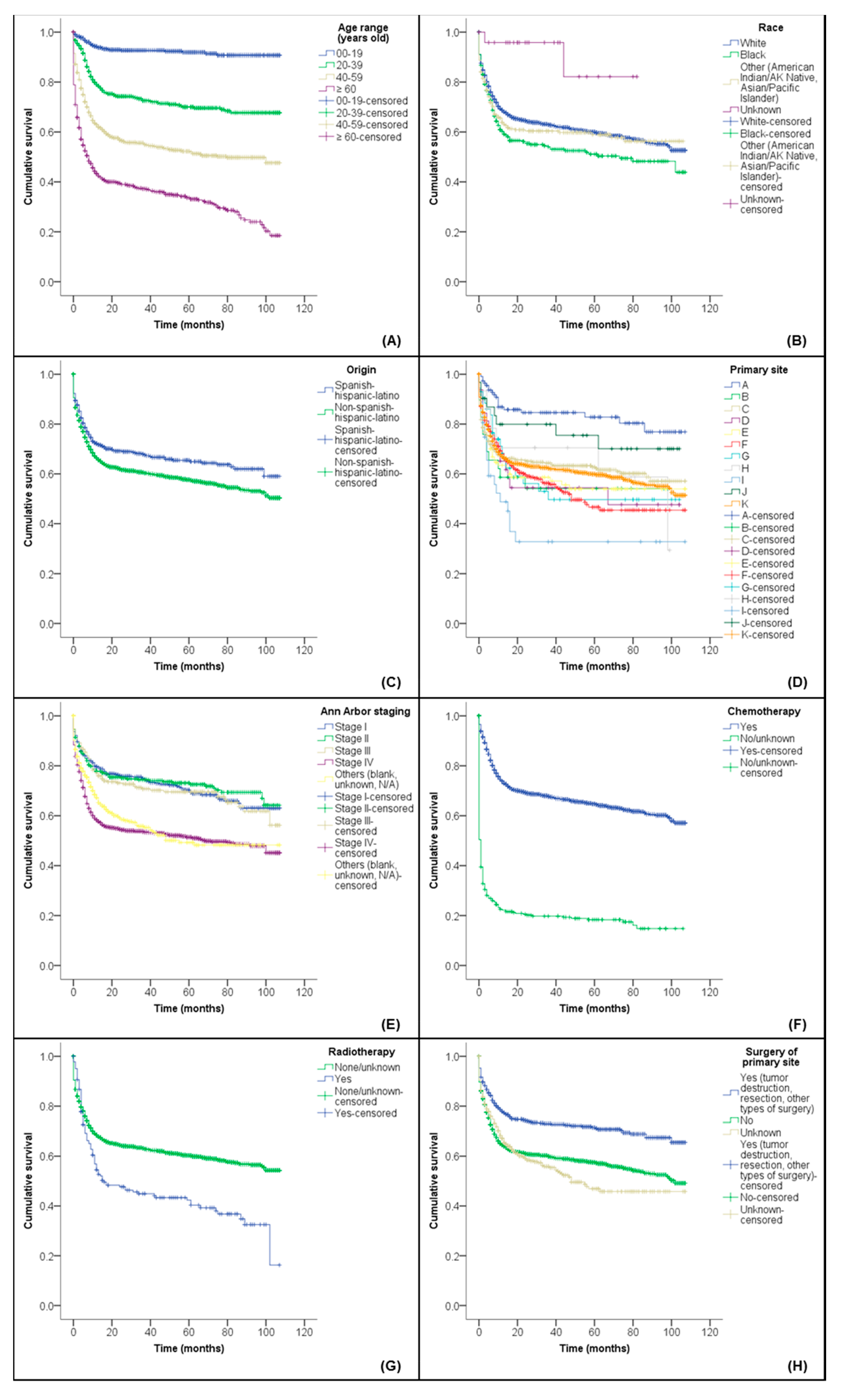
3.2. Influence of Prognostic Factors on the Overall Survival (OS)
3.3. Assessment of the Development Risk of Second Primary Cancers (SPCs)
4. Discussion
4.1. Prognostic Factors and Overall Survival (OS)
4.2. Risk of Second Primary Cancers (SPCs)
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2017; pp. 330–341. [Google Scholar]
- PathologyOutlines.com. Lymphoma & Related Disorders-General-WHO Classification-B Cell. Available online: https://www.pathologyoutlines.com/topic/lymphomabcellwho.html (accessed on 5 March 2021).
- Jaffe, E.S.; Harris, N.L.; Stein, H.; Vardiman, J.W. WHO Classification of Tumours-Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues, 3rd ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2001; pp. 181–184. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2008; pp. 262–266. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Dunleavy, K.; Little, R.F.; Wilson, W.H. Update on Burkitt Lymphoma. Hematol. Oncol. Clin. N. Am. 2016, 30, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, E.M.; Rochford, R.; Griffin, B.; Newton, R.; Jackson, G.; Menon, G.; Harrison, C.J.; Israels, T.; Bailey, S. Burkitt’s lymphoma. Lancet 2012, 379, 1234–1244. [Google Scholar] [CrossRef]
- Patte, C.; Auperin, A.; Gerrard, M.; Michon, J.; Pinkerton, R.; Sposto, R.; Weston, C.; Raphael, M.; Perkins, S.L.; McCarthy, K.; et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: It is possible to reduce treatment for the early responding patients. Blood 2007, 109, 2773–2780. [Google Scholar] [CrossRef]
- Gerrard, M.; Cairo, M.S.; Weston, C.; Auperin, A.; Pinkerton, R.; Lambilliote, A.; Sposto, R.; McCarthy, K.; Lacombe, M.J.; Perkins, S.L.; et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: Results of the FAB/LMB 96 international study. Br. J. Haematol. 2008, 141, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.; LaCasce, A. How I treat Burkitt lymphoma in adults. Blood 2014, 124, 2913–2920. [Google Scholar] [CrossRef]
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef]
- Hesseling, P.B.; Molyneux, E.; Tchintseme, F.; Welbeck, J.; McCormick, P.; Pritchard-Jones, K.; Wagner, H.P. Treating Burkitt’s lymphoma in Malawi, Cameroon, and Ghana. Lancet Oncol. 2008, 9, 512–513. [Google Scholar] [CrossRef]
- Depani, S.; Banda, K.; Bailey, S.; Israels, T.; Chagaluka, G.; Molyneux, E. Outcome is unchanged by adding vincristine upfront to the Malawi 28-day protocol for endemic Burkitt lymphoma. Pediatr. Blood Cancer 2015, 62, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Nature Communications Editors. Epidemiology is a science of high importance. Nat. Commun. 2018, 9, 1703. [Google Scholar] [CrossRef]
- Castillo, J.J.; Winer, E.S.; Olszewski, A.J. Population-based prognostic factors for survival in patients with Burkitt lymphoma: An analysis from the Surveillance, Epidemiology, and End Results database. Cancer 2013, 119, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Xavier, A.C.; Wahlquist, A.E.; Hill, E.G. Trends in survival of patients with Burkitt lymphoma/leukemia in the USA: An analysis of 3691 cases. Blood 2013, 121, 4861–4866. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Jegu, J.; Mounier, M.; Dandoit, M.; Colonna, M.; Daubisse-Marliac, L.; Tretarre, B.; Ganry, O.; Guizard, A.V.; Bara, S.; et al. Risk assessment of second primary cancer according to histological subtype of non-Hodgkin lymphoma. Leuk. Lymphoma 2015, 56, 2876–2882. [Google Scholar] [CrossRef] [PubMed]
- Pirani, M.; Marcheselli, R.; Marcheselli, L.; Bari, A.; Federico, M.; Sacchi, S. Risk for second malignancies in non-Hodgkin’s lymphoma survivors: A meta-analysis. Ann. Oncol. 2011, 22, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.M. Therapy-related myeloid neoplasms: When genetics and environment collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute. Overview of the SEER Program. Available online: https://seer.cancer.gov/about/overview.html (accessed on 4 August 2019).
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence-SEER 18 Regs Custom Data (with Additional Treatment Fields), Nov 2018 Sub (1975–2016 Varying)-Linked To County Attributes-Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Released April 2019, Based on the November 2018 Submission. Available online: https://seer.cancer.gov/seerstat/ (accessed on 29 April 2019).
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence-SEER 18 Regs Custom Data (with Additional Treatment Fields), Nov 2018 Sub (2000–2016) <Katrina/Rita Population Adjustment>-Linked to County Attributes-Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Released April 2019, Based on the November 2018 Submission. Available online: https://seer.cancer.gov/seerstat/ (accessed on 15 November 2019).
- Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute. SEER*Stat Software-Latest Release: Version 8.3.5-6 March 2018. Available online: https://seer.cancer.gov/seerstat/ (accessed on 19 February 2019).
- Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute. Documentation for the ASCII Text Data Files. 2019. Available online: https://seer.cancer.gov/data-software/documentation/seerstat/nov2018/TextData.FileDescription.pdf (accessed on 22 May 2019).
- Schemper, M.; Wakounig, S.; Heinze, G. The estimation of average hazard ratios by weighted Cox regression. Stat. Med. 2009, 28, 2473–2489. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC), World Health Organization (WHO), International Association of Cancer Registries (IACR), European Network of Cancer Registrier (ENCR). International Rules for Multiple Primary Cancers (ICD-O Third Edition). Internal Report No. 2004/02. Available online: http://www.iacr.com.fr/images/doc/MPrules_july2004.pdf (accessed on 5 March 2021).
- National Cancer Institute (NCI). NIH. NCI Dictionary of Cancer Terms. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms (accessed on 29 October 2019).
- Mazzone, E.; Mistretta, F.A.; Knipper, S.; Palumbo, C.; Tian, Z.; Pecoraro, A.; Preisser, F.; Gallina, A.; Shariat, S.F.; Saad, F.; et al. Long-term incidence of secondary bladder and rectal cancer in patients treated with brachytherapy for localized prostate cancer: A large-scale population-based analysis. BJU Int. 2019, 124, 1006–1013. [Google Scholar] [CrossRef]
- Malmgren, J.A.; Calip, G.S.; Pyott, S.M.; Atwood, M.K.; Kaplan, H.G. Therapy-related myelodysplastic syndrome following primary breast cancer. Leuk. Res. 2016, 47, 178–184. [Google Scholar] [CrossRef]
- Jamy, O.; Sarmad, R.; Costa, L. Risk and outcomes of second malignant neoplasms in chronic myeloid leukemia survivors. Leuk. Res. 2019, 82, 1–6. [Google Scholar] [CrossRef]
- DePinho, R.A. The age of cancer. Nature 2000, 408, 248–254. [Google Scholar] [CrossRef]
- Kalisz, K.; Alessandrino, F.; Beck, R.; Smith, D.; Kikano, E.; Ramaiya, N.H.; Tirumani, S.H. An update on Burkitt lymphoma: A review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Burau, K.D.; Fang, S.; Wang, H.; Du, X.L. Ethnic variations in diagnosis, treatment, socioeconomic status, and survival in a large population-based cohort of elderly patients with non-Hodgkin lymphoma. Cancer 2008, 113, 3231–3241. [Google Scholar] [CrossRef]
- Liu, Z.L.; Liu, P.P.; Bi, X.W.; Lei, D.X.; Wang, Y.; Li, Z.M.; Jiang, W.Q.; Xia, Y. Trends in survival of patients with stage I/II Burkitt lymphoma in the United States: A SEER database analysis. Cancer Med. 2019, 8, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xiao, Y.; Shi, H.; Ke, Z.; Liu, Y.; Liang, Y.; Han, A. Sporadic Burkitt lymphoma in southern China: 12 years’ experience in a single institution in Guangzhou. J. Clin. Pathol. 2011, 64, 1132–1135. [Google Scholar] [CrossRef]
- Carrillo-Cruz, E.; Marin-Oyaga, V.A.; Sole Rodriguez, M.; Borrego-Dorado, I.; de la Cruz Vicente, F.; Quiroga Cantero, E.; Manzanares Perez, M.; Capote, F.J.; Ramirez Sanchez, M.J.; Espigado Tocino, I.; et al. Role of 18F-FDG-PET/CT in the management of Burkitt lymphoma. Eur. J. Haematol. 2015, 94, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Crossley-May, H.; Vigneau, F.D.; Brown, K.; Banerjee, M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control 2003, 14, 761–766. [Google Scholar] [CrossRef]
- Howell, J.M.; Auer-Grzesiak, I.; Zhang, J.; Andrews, C.N.; Stewart, D.; Urbanski, S.J. Increasing incidence rates, distribution and histological characteristics of primary gastrointestinal non-Hodgkin lymphoma in a North American population. Can. J. Gastroenterol. 2012, 26, 452–456. [Google Scholar] [CrossRef]
- Hill, Q.A.; Owen, R.G. CNS prophylaxis in lymphoma: Who to target and what therapy to use. Blood Rev. 2006, 20, 319–332. [Google Scholar] [CrossRef]
- Han, X.; Kilfoy, B.; Zheng, T.; Holford, T.R.; Zhu, C.; Zhu, Y.; Zhang, Y. Lymphoma survival patterns by WHO subtype in the United States, 1973-2003. Cancer Causes Control 2008, 19, 841–858. [Google Scholar] [CrossRef]
- Allan, J.M.; Travis, L.B. Mechanisms of therapy-related carcinogenesis. Nat. Rev. Cancer 2005, 5, 943–955. [Google Scholar] [CrossRef]
- Mukhtar, F.; Ilozumba, M.; Utuama, O.; Cimenler, O. Change in Pattern of Secondary Cancers After Kaposi Sarcoma in the Era of Antiretroviral Therapy. JAMA Oncol. 2018, 4, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Jiamsakul, A.; Polizzotto, M.; Wen-Wei Ku, S.; Tanuma, J.; Hui, E.; Chaiwarith, R.; Kiertiburanakul, S.; Avihingasanon, A.; Yunihastuti, E.; Kumarasamy, N.; et al. Brief Report: Malignancies in Adults Living With HIV in Asia. J. Acquir. Immune Defic. Syndr. 2019, 80, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.L.; Jiang, Y.Z.; Shao, Z.M. Survival and chemotherapy-related risk of second primary malignancy in breast cancer patients: A SEER-based study. Int. J. Clin. Oncol. 2019, 24, 934–940. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n (%) (Total: n = 3094) |
|---|---|
| Sociodemographic characteristics | |
| Age range (years old) | |
| 00–19 | 702 (22.7) |
| 20–39 | 633 (20.5) |
| 40–59 | 887 (28.7) |
| ≥60 | 872 (28.2) |
| Sex | |
| Male | 2255 (72.9) |
| Female | 839 (27.1) |
| Race | |
| White | 2458 (79.4) |
| Black | 326 (10.5) |
| Other (American Indian/Alaska Native, Asian/Pacific Islander) | 284 (9.2) |
| Unknown | 26 (0.8) |
| Origin | |
| Non-Spanish-Hispanic-Latino | 2451 (79.2) |
| Spanish-Hispanic-Latino | 643 (20.8) |
| Clinical characteristics | |
| Primary site 1 | |
| Lymph nodes | 1798 (58.1) |
| Hematopoietic and reticuloendothelial systems | 467 (15.1) |
| GIT and attachments | 433 (14.0) |
| Lip, oral cavity and pharynx | 112 (3.6) |
| Skin, bones, joints, articular cartilage and other tissues/sites | 98 (3.2) |
| Breast and female/male genital organs | 43 (1.4) |
| Eye, adnexa, brain and other parts of the CNS | 36 (1.2) |
| Respiratory system and cardiac system and attachments | 32 (1.0) |
| Glands (thyroid and adrenal) | 31 (1.0) |
| Urinary system | 15 (0.5) |
| Unknown primary site and other and ill-defined sites | 29 (0.9) |
| Ann Arbor staging 2 | |
| Stage I | 448 (14.5) |
| Stage II | 373 (12.1) |
| Stage III | 270 (8.7) |
| Stage IV | 1248 (40.3) |
| Others (Blank, Unknown, N/A) | 755 (24.4) |
| Treatment characteristics | |
| Chemotherapy | |
| No/Unknown | 389 (12.6) |
| Yes | 2705 (87.4) |
| Radiotherapy 1 | |
| No/Unknown | 2912 (94.1) |
| Yes | 182 (5.9) |
| Surgery of primary site 1 | |
| No | 1973 (63.8) |
| Yes (tumor destruction, resection, other types of surgery) | 614 (19.8) |
| Unknown | 507 (16.4) |
| Characteristics | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|
| Bivariate Analysis | Multivariate Analysis | Deaths (%) | |||||
| HR | 95% CI | p-Value * | HR | 95% CI | p-Value * | ||
| Sociodemographic characteristics | |||||||
| Age range (years old) | |||||||
| 00–19 | 1.0 | - | - | 1.0 | - | - | 7.0 |
| 20–39 | 3.9 | 2.9–5.4 | <0.001 | 3.5 | 2.5–4.8 | <0.001 | 26.2 |
| 40–59 | 7.7 | 5.6–10.4 | <0.001 | 6.1 | 4.5–8.4 | <0.001 | 45.2 |
| ≥60 | 14.0 | 10.2–19.3 | <0.001 | 10.5 | 7.5–14.5 | <0.001 | 64.9 |
| Sex | |||||||
| Male | 1.0 | - | - | 1.0 | - | - | 37.1 |
| Female | 1.1 | 0.9–1.2 | 0.390 | 0.9 | 0.8–1.0 | 0.028 | 40.9 |
| Race | |||||||
| White | 1.0 | - | - | 1.0 | - | - | 37.4 |
| Black | 1.4 | 1.1–1.6 | 0.001 | 1.6 | 1.3–1.9 | <0.001 | 45.8 |
| Other 1 | 1.1 | 0.9–1.3 | 0.463 | 1.1 | 0.9–1.3 | 0.508 | 38.9 |
| Unknown | 0.2 | 0.1–0.8 | 0.025 | 0.2 | 0.1–0.8 | 0.024 | 7.7 |
| Origin | |||||||
| Spanish-Hispanic-Latino | 1.0 | - | - | 1.0 | - | - | 31.1 |
| Non-Spanish-Hispanic-Latino | 1.2 | 1.0–1.4 | 0.014 | 1.0 | 0.8–1.1 | 0.592 | 40.0 |
| Clinical characteristics | |||||||
| Histology/Behavior (ICD-O-3) | |||||||
| 9687/3: Burkitt lymphoma | 1.0 | - | - | 1.0 | - | - | 38.0 |
| 9826/3: Burkitt cell leukemia | 1.1 | 1.0–1.4 | 0.110 | 0.8 | 0.5–1.3 | 0.346 | 39.1 |
| Primary site 2 | |||||||
| Sites in lip, oral cavity and pharynx | 1.0 | - | - | 1.0 | - | - | 17.0 |
| GIT and attachments | 2.4 | 1.5–3.8 | <0.001 | 1.5 | 0.9–2.4 | 0.102 | 35.6 |
| Respiratory system and cardiac system (with attachments) | 3.1 | 1.6–6.2 | 0.001 | 2.1 | 1.1–4.2 | 0.028 | 46.9 |
| Skin, bones, joints, articular cartilage and other tissues/sites | 2.7 | 1.6–4.7 | <0.001 | 1.6 | 0.9–2.7 | 0.096 | 42.9 |
| Hematopoietic and reticuloendothelial systems | 3.0 | 1.9–4.8 | <0.001 | 0.8 | 0.2–3.1 | 0.699 | 41.3 |
| Breast and female/male genital organs | 2.5 | 1.3–4.7 | 0.005 | 1.5 | 0.8–2.8 | 0.224 | 44.2 |
| Urinary system | 2.1 | 0.8–5.2 | 0.123 | 1.1 | 0.4–2.7 | 0.899 | 40.0 |
| Eye, adnexa, brain and other parts of the CNS | 4.7 | 2.5–8.7 | <0.001 | 2.2 | 1.2–4.2 | 0.015 | 58.3 |
| Glands (thyroid and adrenal) | 1.1 | 0.5–2.6 | 0.789 | 0.8 | 0.4–1.9 | 0.642 | 25.8 |
| Lymph nodes | 2.4 | 1.6–3.9 | <0.001 | 1.4 | 0.9–2.3 | 0.127 | 38.4 |
| Unknown primary site and other and ill-defined sites | 3.2 | 1.6–6.5 | 0.001 | 0.5 | 0.1–2.3 | 0.382 | 44.8 |
| Ann Arbor staging 3 | |||||||
| Stage I | 1.0 | - | - | 1.0 | - | - | 29.8 |
| Stage II | 0.9 | 0.7–1.2 | 0.664 | 1.1 | 0.8–1.4 | 0.564 | 27.3 |
| Stage III | 1.1 | 0.8–1.4 | 0.592 | 1.2 | 0.9–1.6 | 0.140 | 31.5 |
| Stage IV | 1.9 | 1.5–2.3 | <0.001 | 1.8 | 1.5–2.2 | <0.001 | 48.0 |
| Others (Blank, Unknown, N/A) | 1.7 | 1.4–2.1 | <0.001 | 1.5 | 1.1–2.0 | 0.004 | 34.6 |
| Treatment characteristics | |||||||
| Chemotherapy | |||||||
| Yes | 1.0 | - | - | 1.0 | - | - | 32.5 |
| No/Unknown | 4.9 | 4.3–5.7 | <0.001 | 4.0 | 3.5–4.6 | <0.001 | 78.4 |
| Radiotherapy 2 | |||||||
| Yes | 1.0 | - | - | 1.0 | - | - | 58.2 |
| No/Unknown | 0.7 | 0.6–0.8 | <0.001 | 0.7 | 0.6–0.9 | 0.001 | 36.9 |
| Surgery of primary site 2 | |||||||
| Yes (tumor destruction, resection, other types of surgery) | 1.0 | - | - | 1.0 | - | - | 27.2 |
| No | 1.6 | 1.3–1.9 | <0.001 | 1.4 | 1.1–1.6 | 0.001 | 40.7 |
| Unknown | 1.8 | 1.5–2.2 | <0.001 | 3.5 | 1.0–12.8 | 0.058 | 41.8 |
| Cancers | First Primary Cancer | Second Primary Cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| BL/L-2nd (%) | XCA-Non-BL/L-2nd (%) | RR (95% CI) | p-Value * | BL/L-1st (%) | XCA-Non-BL/L-1st (%) | RR (95% CI) | p-Value * | |
| Aleukemic, subleukemic and NOS | 1 (0.7) | 184 (0.1) | 9.5 (1.3–68.3) | 0.026 | - | - | - | - |
| AML | - | - | - | - | 8 (12.1) | 1909 (1.2) | 4.6 (2.1–10.4) | <0.001 |
| Anus, anal canal and anorectum | 3 (2.0) | 1615 (0.5) | 3.2 (1.0–10.4) | 0.048 | 2 (3.0) | 736 (0.5) | 3.0 (0.7–12.8) | 0.134 |
| Breast | 14 (9.5) | 43,177 (12.9) | 0.6 (0.3–1.0) | 0.059 | 1 (1.5) | 9899 (6.4) | 0.1 (0.0–0.8) | 0.032 |
| HL—extranodal | - | - | - | - | 1 (1.5) | 14 (0.01) | 74.3 (10.0–549.8) | <0.001 |
| HL—nodal | 10 (6.8) | 2288 (0.7) | 7.6 (3.9–15.0) | <0.001 | - | - | - | - |
| Kaposi sarcoma | 9 (6.1) | 454 (0.1) | 34.0 (16.8–68.9) | <0.001 | 4 (6.1) | 123 (0.1) | 35.1 (12.1–101.4) | <0.001 |
| Liver | 4 (2.7) | 2079 (0.6) | 3.4 (1.2–9.3) | 0.020 | - | - | - | - |
| Lung and bronchus | 4 (2.7) | 20,642 (6.2) | 0.3 (0.1–0.9) | 0.037 | 3 (4.5) | 26,604 (17.1) | 0.1 (0.0–0.4) | 0.001 |
| Melanoma of the skin | 4 (2.7) | 15,905 (4.8) | 0.4 (0.2–1.2) | 0.113 | - | - | - | - |
| Trachea, mediastinum and other respiratory organs | 1 (0.7) | 110 (0.03) | 15.8 (2.2–113.9) | 0.006 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Rocca, A.M.; Tonin, F.S.; Fachi, M.M.; Cobre, A.F.; Ferreira, V.L.; Leonart, L.P.; Steffenello-Durigon, G.; Del Moral, J.A.G.; Lenzi, L.; Pontarolo, R. Prognostic Factors, Survival Analyses and the Risk of Second Primary Cancer: A Population-Based Study on Burkitt Lymphoma/Leukemia. Diseases 2021, 9, 43. https://doi.org/10.3390/diseases9020043
Della Rocca AM, Tonin FS, Fachi MM, Cobre AF, Ferreira VL, Leonart LP, Steffenello-Durigon G, Del Moral JAG, Lenzi L, Pontarolo R. Prognostic Factors, Survival Analyses and the Risk of Second Primary Cancer: A Population-Based Study on Burkitt Lymphoma/Leukemia. Diseases. 2021; 9(2):43. https://doi.org/10.3390/diseases9020043
Chicago/Turabian StyleDella Rocca, Ana M., Fernanda S. Tonin, Mariana M. Fachi, Alexandre F. Cobre, Vinicius L. Ferreira, Letícia P. Leonart, Giovanna Steffenello-Durigon, Joanita A. G. Del Moral, Luana Lenzi, and Roberto Pontarolo. 2021. "Prognostic Factors, Survival Analyses and the Risk of Second Primary Cancer: A Population-Based Study on Burkitt Lymphoma/Leukemia" Diseases 9, no. 2: 43. https://doi.org/10.3390/diseases9020043
APA StyleDella Rocca, A. M., Tonin, F. S., Fachi, M. M., Cobre, A. F., Ferreira, V. L., Leonart, L. P., Steffenello-Durigon, G., Del Moral, J. A. G., Lenzi, L., & Pontarolo, R. (2021). Prognostic Factors, Survival Analyses and the Risk of Second Primary Cancer: A Population-Based Study on Burkitt Lymphoma/Leukemia. Diseases, 9(2), 43. https://doi.org/10.3390/diseases9020043






