DOPS Adjuvant Confers Enhanced Protection against Malaria for VLP-TRAP Based Vaccines
Abstract
1. Introduction
2. Material and Methods
2.1. PfTRAP Protein Production
2.2. Vaccines Preparation
2.3. Immunization Protocol
2.4. Kinetics of Antibody Production
2.5. Assessment of TCD4 Cells Polarization
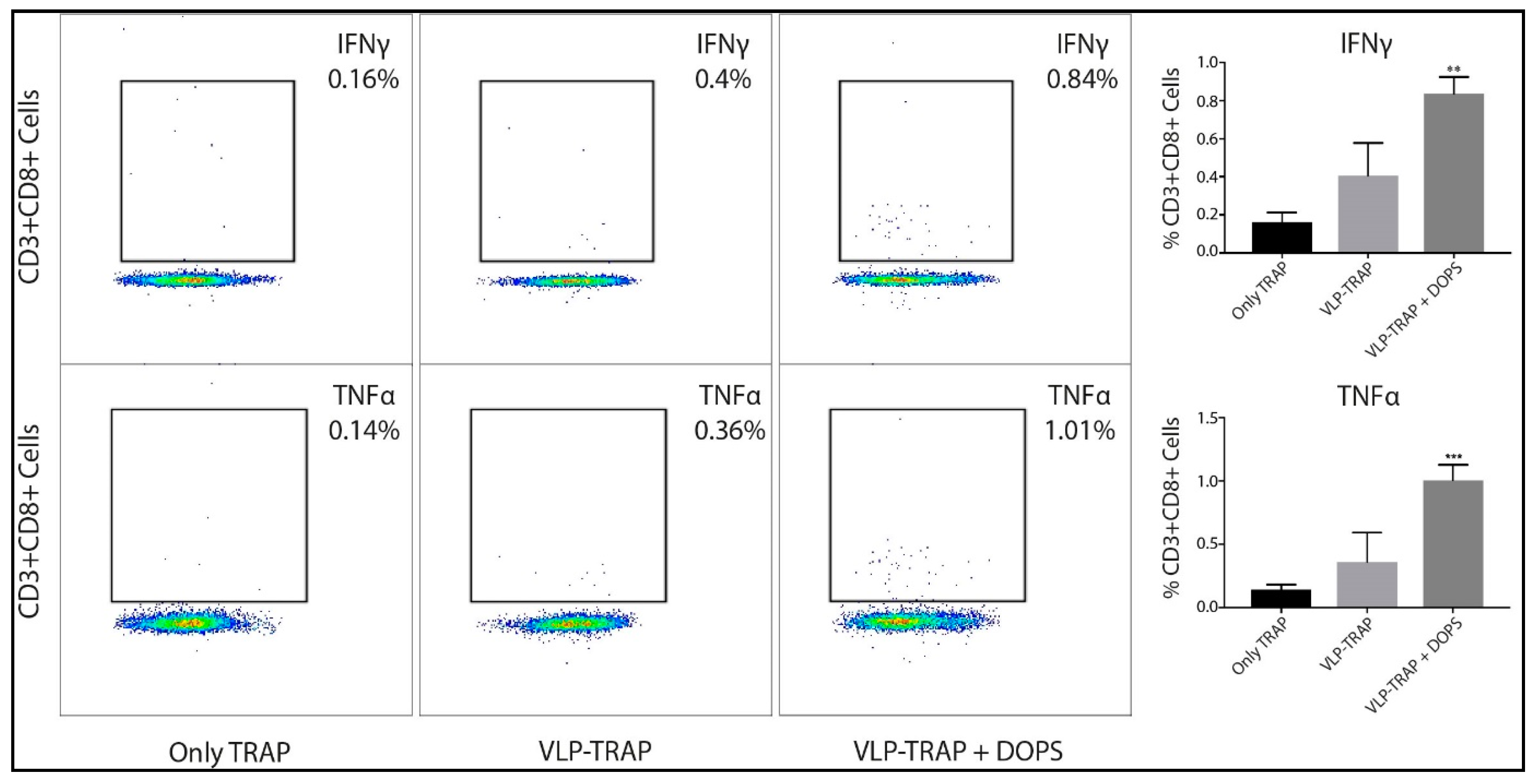
2.6. Evaluation of CD8+ T Cells by Intracellular Cytokine Staining (ICS)
2.7. Animals
2.8. Ethics Statement
2.9. Parasite Production
2.10. Parasites
2.11. Generation of DNA Constructs and Genotyping of the Transgenic Parasites
2.12. Phenotyping of Reporter and Transgenic Parasites.
2.13. Efficacy Studies: Determination of Liver Parasite Liver Load by Real Time Imaging and Determination Prepatent Period (after Challenge of Immunized Mice with Transgenic Sporozoites)
2.14. Statistical Analysis and Parasitemia Prediction
3. Results
3.1. DOPS Promotes a Th1 Response in TRAP-Vaccinated BALB/c Mice
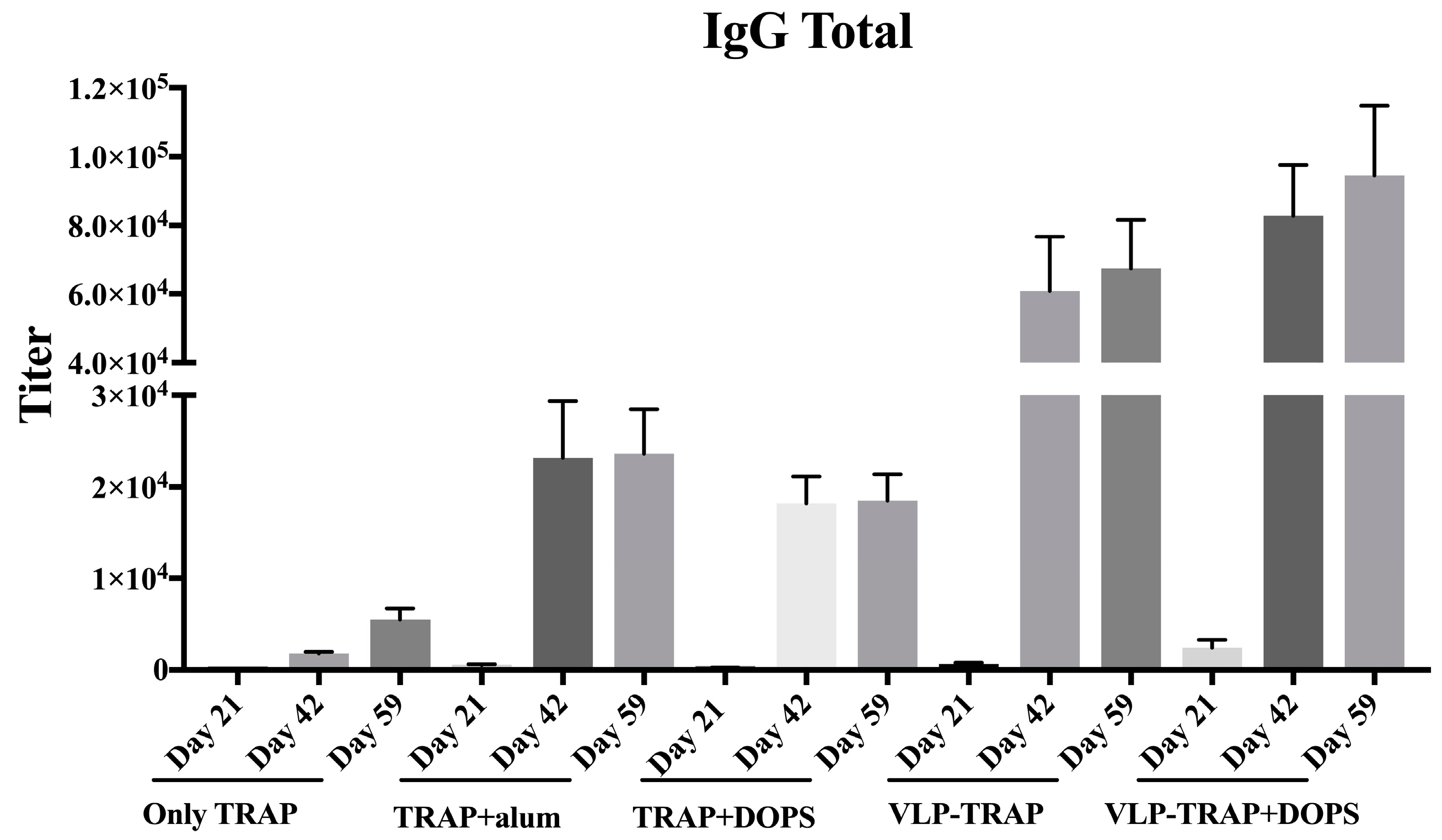
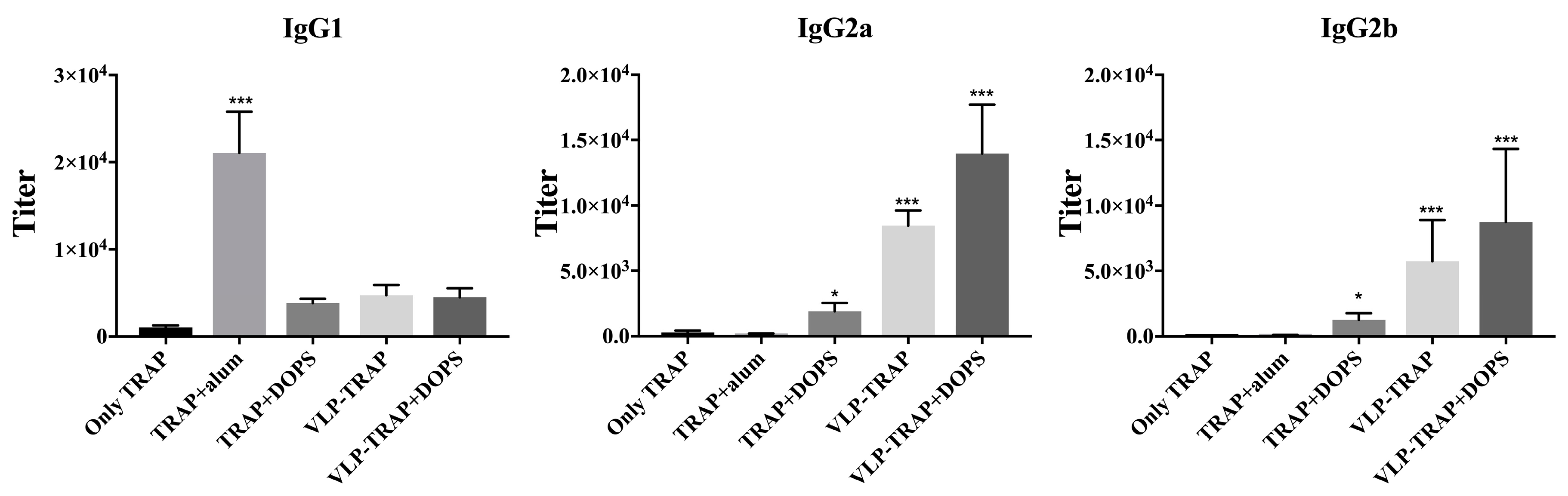
3.2. Antibody Production by Vaccinated BALB/c Mice
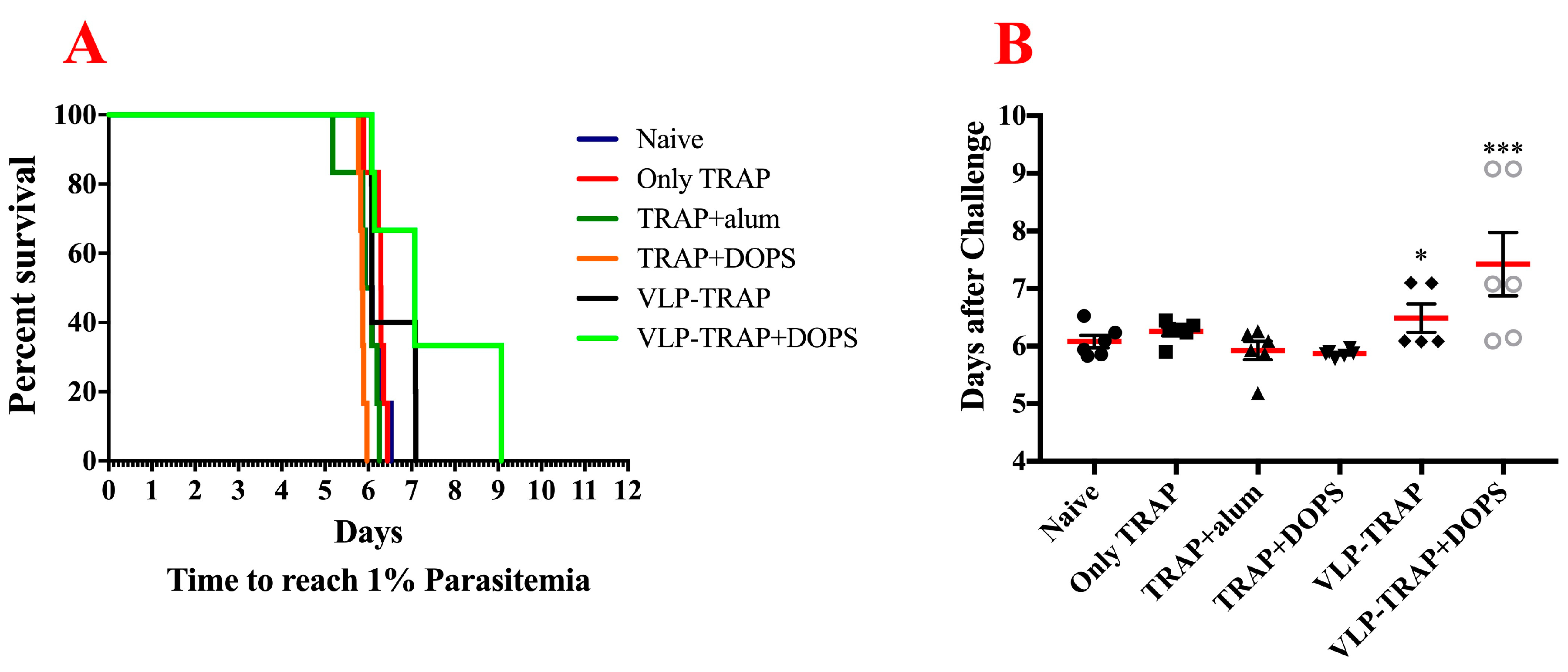
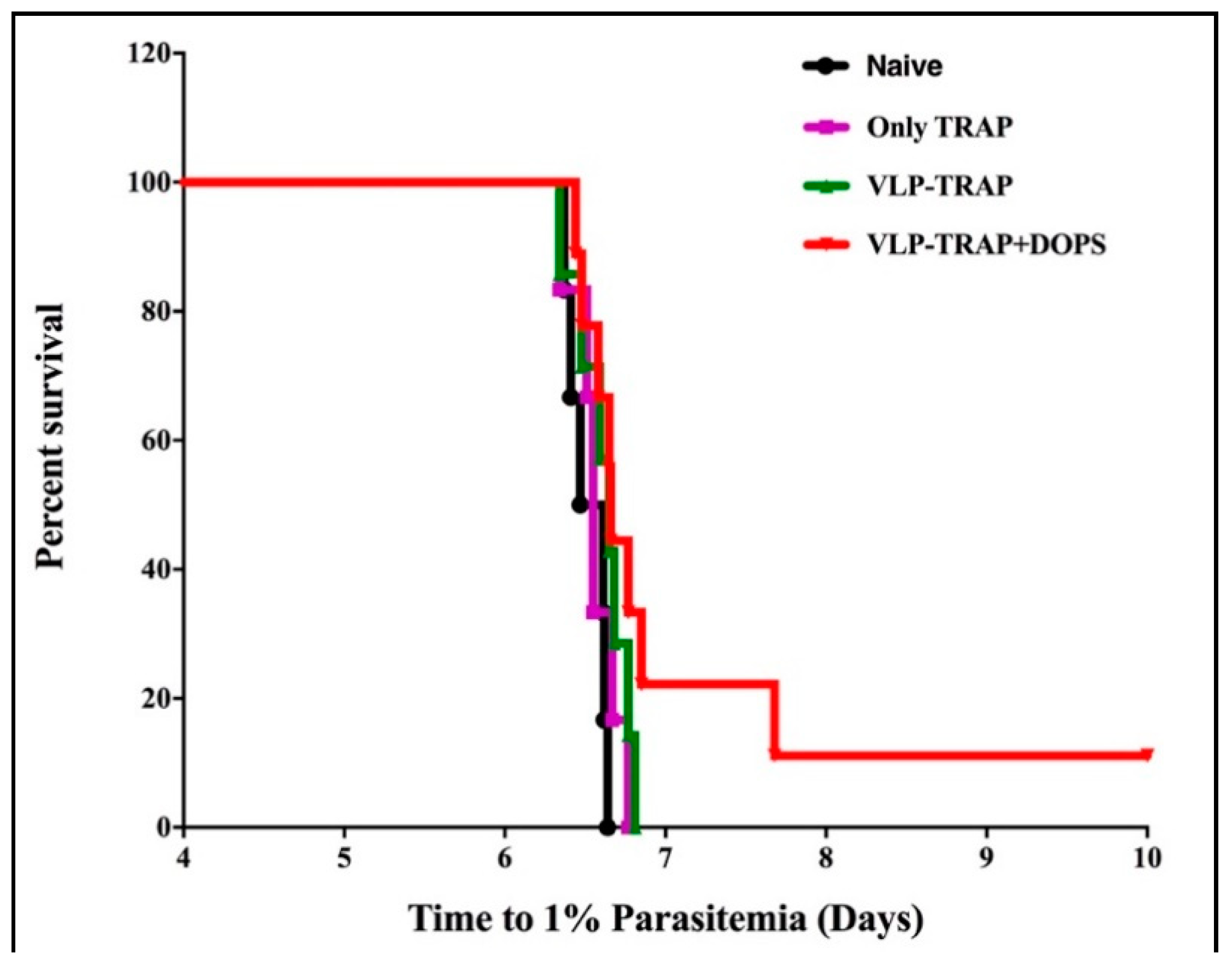
3.3. TRAP-CuMVtt+DOPS Induces Best Protection Against Infection with P. Bergei Expressing PfTRAP
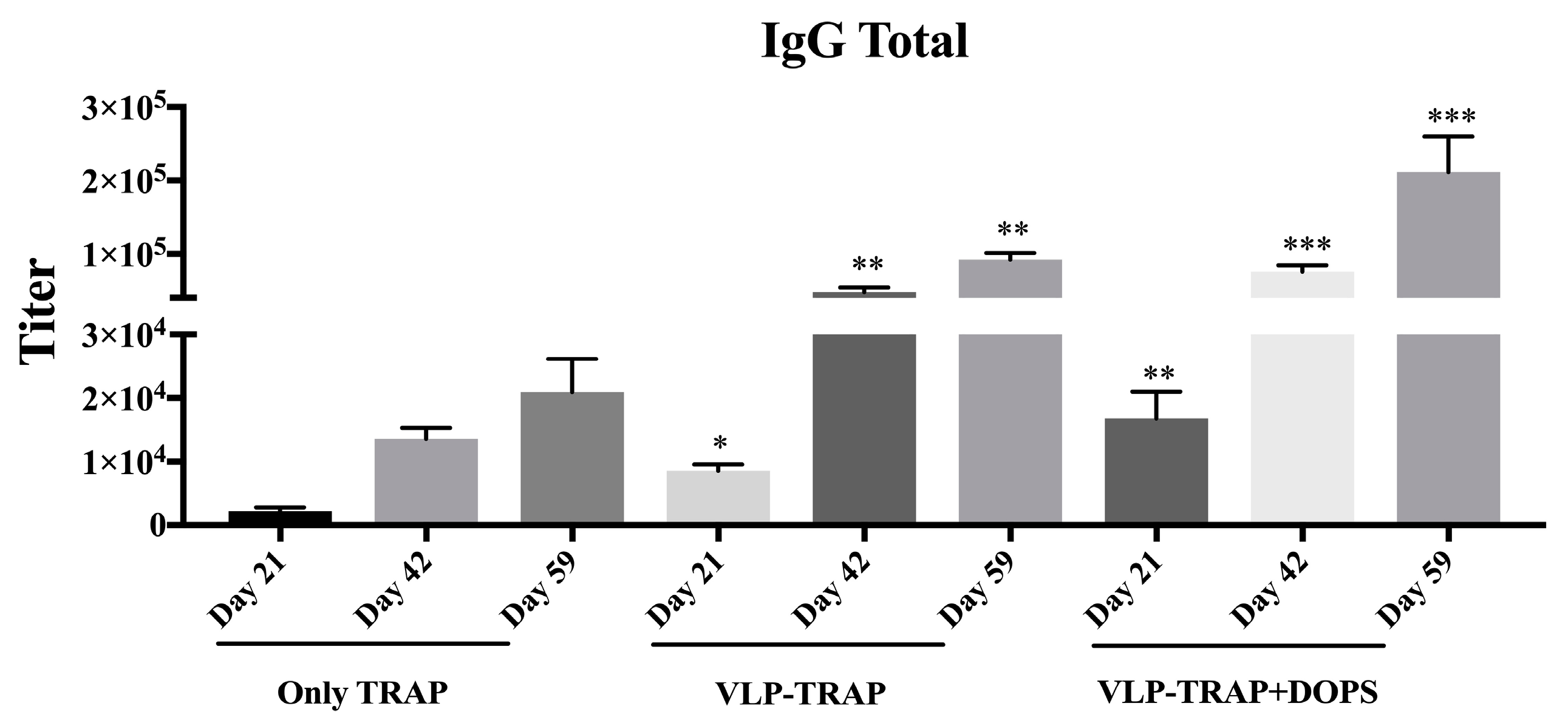
3.4. Assessing the Efficacy of Vaccine Formulated with TRAP-VLP + DOPS in CD1 Mice
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Garner, P.; Gelband, H.; Graves, P.; Jones, K.; Maclehose, H.; Olliaro, P. Systematic Reviews in Malaria: Global Policies Need Global Reviews. Infect. Dis. Clin. North Am. 2009, 23, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Gething, P.W.; Elyazar, I.R.; Moyes, C.L.; Smith, D.L.; Battle, K.E.; Guerra, C.A.; Patil, A.P.; Tatem, A.J.; Howes, R.E.; Myers, M.F.; et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl. Trop Dis. 2012, 6, e1814. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S. Epidemiological aspects of vivax and falciparum malaria: Global spectrum. Asian Pac. J. Trop Dis. 2014, 4, S13–S26. [Google Scholar] [CrossRef]
- WHO. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed on 15 December 2015).
- Bousema, T.; Drakeley, C. Epidemiology and Infectivity of Plasmodium falciparum and Plasmodium vivax Gametocytes in Relation to Malaria Control and Elimination. Clin. Microbiol. Rev. 2011, 24, 377–410. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: http://www.who.int/ith/diseases/malaria/en/ (accessed on 10 January 2018).
- RTS,S Clinical Trials Partnership. Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A. Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites. PLoS Med. 2014, 11, E1001685. [Google Scholar] [CrossRef]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Ejigiri, I.; Ragheb, D.R.T.; Pino, P.; Coppi, A.; Bennett, B.L.; Soldati-Favre, D.; Sinnis, P. Shedding of TRAP by a Rhomboid Protease from the Malaria Sporozoite Surface Is Essential for Gliding Motility and Sporozoite Infectivity. PLoS Pathog. 2012, 8, E1002725. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Miranda, G.; Heath, M.D.; Mohsen, M.O.; Gomes, A.C.; Engeroff, P.; Flaxman, A.; Leoratti, F.M.S.; El-Turabi, A.; Reyes-Sandoval, A.; Skinner, M.A.; et al. Virus-Like Particle (VLP) Plus Microcrystalline Tyrosine (MCT) Adjuvants Enhance Vaccine Efficacy Improving T and B Cell Immunogenicity and Protection against Plasmodium berghei/vivax. Vaccines 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.J.; Bauza, K.; Ewer, K.J.; Hill, A.V.S.; Spencer, A.J. Development of an In Vitro Assay and Demonstration of Plasmodium berghei Liver-Stage Inhibition by TRAP-Specific CD8+ T Cells. PLoS ONE 2015, 10, E0119880. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.J.; Cottingham, M.G.; Jenks, J.A.; Longley, R.J.; Capone, S.; Colloca, S.; Folgori, A.; Cortese, R.; Nicosia, A.; Bregu, M.; et al. Enhanced Vaccine-Induced CD8+ T Cell Responses to Malaria Antigen METRAP by Fusion to MHC Class II Invariant Chain. PLoS ONE 2014, 9, E100538. [Google Scholar] [CrossRef] [PubMed]
- White, M.T.; Griffin, J.T.; Riley, E.M.; Drakeley, C.J.; Moorman, A.M.; Sumba, P.O.; Kazura, J.W.; Ghani, A.C.; John, C.C. Efficacy model for antibody-mediated pre-erythrocytic malaria vaccines. Proc. R. Soc. B 2011, 278, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G. Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine 2010, 28, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, T.R. The mechanisms of action of vaccines containing aluminum adjuvants: An in vitro vs in vivo paradigm. Springer Plus 2015, 4, 181. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.M.; Shi, Y. Alum: An old dog with new tricks. Emerg. Microbes Infect. 2016, 5, e25. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Leroux-Roels, G.; Wen-Fang, C. Vaccine adjuvants. In Understanding Modern Vaccines: Perspectives in Vaccinology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, pp. 89–113. [Google Scholar]
- Garçon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Miranda, G.; Heath, M.D.; Gomes, A.C.; Mohsen, M.O.; Montoya-Diaz, E.; Salman, A.M.; Atcheson, E.; Skinner, M.A.; Kramer, M.F.; Reyes-Sandoval, A.; et al. Microcrystalline Tyrosine (MCT®): A Depot Adjuvant in Licensed Allergy Immunotherapy Offers New Opportunities in Malaria. Vaccines 2017, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.F.; Heath, M.D. Aluminum in allergen-specific subcutaneous immunotherapy—A German perspective. Vaccine 2014, 32, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- Bergfors, E.; Trollfors, B.; Inerot, A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminum in children after the use of adsorbed vaccines from a single manufacturer. Vaccine 2003, 22, 64–69. [Google Scholar] [CrossRef]
- Exley, C. Aluminum adjuvants and adverse events in sub-cutaneous allergy immunotherapy. Allergy Asthma Clin. Immunol. 2014, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, R.K.; Eidi, H.; Crépeaux, G.; Authier, F.J.; Cadusseau, J. Biopersistence and brain translocation of aluminum adjuvants of vaccines. Front. Neurol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.; Skinner, M.; Philips, G.; Berry, T.; Wheeler, A. Phosphatidyl serine (PS) derivatives alter immune responses in a brown Norway rat model of allergic sensitisation. Allergy 2007, 62, 456. [Google Scholar]
- Berry, A.R.; Wheeler, A.W. Composition comprising phosphatidyl serine and an antigen or allergen and the use thereof. U.S. patent US 8,765,721 B2, 2 December 2004. [Google Scholar]
- Zabel, F.; Mohanan, D.; Bessa, J.; Link, A.; Fettelschoss, A.; Saudan, P.; Kündig, T.M.; Bachmann, M.F. Viral Particles Drive Rapid Differentiation of Memory B Cells into Secondary Plasma Cells Producing Increased Levels of Antibodies. J. Immunol. 2014, 192, 5499–5508. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Rollier, C.S.; Hill, A.V.S.; Reyes-Sandoval, A. Influence of adenovirus and MVA vaccines on the breadth and hierarchy of T cell responses. Vaccine 2016, 34, 4470–4474. [Google Scholar] [CrossRef] [PubMed]
- Janse, C.J.; Franke-Fayard, B.; Waters, A.P. Selection by flow-sorting of genetically transformed, GFP-expressing blood stages of the rodent malaria parasite, Plasmodium berghei. Nat. Protoc. 2006, 1, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J.; Pedersen, G.; Madhun, A.S.; Svindland, S.; Sævik, M.; Breakwell, L.; Hoschler, K.; Willemsen, M.; Campitelli, L.; Nøstbakken, J.K.; et al. Evaluation of a virosomal H5N1 vaccine formulated with Matrix M adjuvant in a phase I clinical trial. Vaccine 2011, 29, 8049–8059. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.j.; Salman, A.M.; Cottingham, M.G.; Ewer, K.; Janse, C.J.; Khan, S.M.; Spencer, A.J.; Hill, A.V. Comparative assessment of vaccine vectors encoding ten malaria antigens identifies two protective liver-stage candidates. Sci. Rep. 2015, 5, 11820. [Google Scholar] [CrossRef] [PubMed]
- Menard, R.; Janse, C. Gene targeting in malaria parasites. Methods 1997, 13, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.W.; Annoura, T.; Sajid, M.; Chevalley-Maurel, S.; Ramesar, J.; Klop, O.; Franke-Fayard, B.M.; Janse, C.J.; Khan, S.M. A novel ‘gene insertion/marker out’ (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PLoS ONE 2011, 6, e29289. [Google Scholar] [CrossRef] [PubMed]
- Orr, R.Y.; Philip, N.; Waters, A.P. Improved negative selection protocol for Plasmodium berghei in the rodent malarial model. Malar. J. 2012, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.M.; Montoya-Díaz, E.; West, H.; Lall, A.; Atcheson, E.; Lopez-Camacho, C.; Ramesar, J.; Bauza, K.; Collins, K.A.; Brod, F.; et al. Rational development of a highly protective P. vivax vaccine evaluated using transgenic rodent parasite challenge models. Sci. Rep. 2017, 7, 46482. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.J.; Reyes-Sandoval, A.; Montoya-Díaz, E.; Dunachie, S.; Kumpitak, C.; Nguitragool, W.; Mueller, I.; Sattabongkot, J. Acquisition and Longevity of Antibodies to Plasmodium vivax Preerythrocytic Antigens in Western Thailand. Clin. Vaccine Immunol. 2015, 23, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sandoval, A.; Wyllie, D.H.; Bauza, K.; Milicic, A.; Forbes, E.K.; Rollier, C.S.; Hill, A.V. CD8+ T effector memory cells protect against liver-stage malaria. J. Immunol. 2011, 187, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Ndungu, F.M.; Bull, P.C.; Ross, A.; Lowe, B.S.; Kabiru, E.; Marsh, K. Naturally acquired immunoglobulin (Ig) G subclass antibodies to crude asexual Plasmodium falciparum lysates: Evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol. 2002, 24, 77–82. [Google Scholar] [CrossRef] [PubMed]
- John, C.C.; Tande, A.J.; Moormann, A.M.; Sumba, P.O.; Lanar, D.E.; Min, X.M.; Kazura, J.W. Antibodies to Pre- erythrocytic Plasmodium falciparum Antigens and Risk of Clinical Malaria in Kenyan Children. J. Infect. Dis. 2008, 197, 519–526. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B. Protective immunity against malaria after vaccination. Parasite Immunol. 2014, 36, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Favila-Castillo, L.; Monroy Ostria, A.; Hirunpetcharat, C.; Good, M.F. The spleen, IgG antibody subsets and immunity to Plasmodium berghei in rats. Immunol. Cell Biol. 1997, 75, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wipasa, J.; Elliott, S.; Xu, H.; Good, M.F. Immunity to asexual blood stage malaria and vaccine approaches. Immunol. Cell Biol. 2002, 80, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Soe, S.; Theisen, M.; Roussilhon, C.; Aye, K.S.; Druilhe, P. Association between Protection against Clinical Malaria and Antibodies to Merozoite Surface Antigens in an Area of Hyperendemicity in Myanmar: Complementarity between Responses to Merozoite Surface Protein 3 and the 220-Kilodalton Glutamate-Rich Protein. Infect. Immun. 2004, 72, 247–252. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B.; Ling, I.T.; Ogun, S.A.; Holder, A.A.; Playfair, J.H.L. Cytokines and Antibody Subclass Associated with Protective Immunity against Blood-Stage Malaria in Mice Vaccinated with the C Terminus of Merozoite Surface Protein 1 plus a Novel Adjuvant. Infect. Immun. 1996, 64, 3532–3536. [Google Scholar] [PubMed]
- Svensson, A.; Sandberg, T.; Siesjö, P.; Eriksson, H. Sequestering of damage-associated molecular patterns (DAMPs): A possible mechanism affecting the immune-stimulating properties of aluminum adjuvants. Immunol. Res. 2017, 65, 1164–1175. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral-Miranda, G.; M. Salman, A.; O. Mohsen, M.; L. Storni, F.; S. Roesti, E.; A. Skinner, M.; D. Heath, M.; F. Kramer, M.; M. Khan, S.; J. Janse, C.; et al. DOPS Adjuvant Confers Enhanced Protection against Malaria for VLP-TRAP Based Vaccines. Diseases 2018, 6, 107. https://doi.org/10.3390/diseases6040107
Cabral-Miranda G, M. Salman A, O. Mohsen M, L. Storni F, S. Roesti E, A. Skinner M, D. Heath M, F. Kramer M, M. Khan S, J. Janse C, et al. DOPS Adjuvant Confers Enhanced Protection against Malaria for VLP-TRAP Based Vaccines. Diseases. 2018; 6(4):107. https://doi.org/10.3390/diseases6040107
Chicago/Turabian StyleCabral-Miranda, Gustavo, Ahmed M. Salman, Mona O. Mohsen, Federico L. Storni, Elisa S. Roesti, Murray A. Skinner, Matthew D. Heath, Matthias F. Kramer, Shahid M. Khan, Chris J. Janse, and et al. 2018. "DOPS Adjuvant Confers Enhanced Protection against Malaria for VLP-TRAP Based Vaccines" Diseases 6, no. 4: 107. https://doi.org/10.3390/diseases6040107
APA StyleCabral-Miranda, G., M. Salman, A., O. Mohsen, M., L. Storni, F., S. Roesti, E., A. Skinner, M., D. Heath, M., F. Kramer, M., M. Khan, S., J. Janse, C., V. S. Hill, A., & F. Bachmann, M. (2018). DOPS Adjuvant Confers Enhanced Protection against Malaria for VLP-TRAP Based Vaccines. Diseases, 6(4), 107. https://doi.org/10.3390/diseases6040107





