HBV Core Protein Enhances Cytokine Production
Abstract
:1. Introduction
2. HBV Core Protein and Cytokine Production
| Symbol | Name |
|---|---|
| CD180 | CD180 molecule |
| IL8 | Chemokine (C-X-C motif) ligand 8 |
| IL6 | Interleukin 6 |
| CLEC4E | C-type lectin domain family 4, member E |
| TLR10 | Toll-like receptor 10 |
| TLR8 | Toll-like receptor 8 |
| IFNG | Interferon, gamma |
| TLR2 | Toll-like receptor 2oll-like receptor 2 |
| IL12A | Interleukin 12A |
| MAPK8 | Mitogen-activated protein kinase 8 |
| LY96 | Lymphocyte antigen 96 |
| IFNB1 | Interferon, beta 1, fibroblast |
| TNF | Tumor necrosis factor |
| CHUK | Conserved helix-loop-helix ubiquitous kinase |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 |
| TLR3 | Toll-like receptor 3 |
| IFNA1 | Interferon, alpha 1 |
| PRKRA | Protein kinase, interferon-inducible double stranded RNA dependent activator |
| NFKBIA | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| NR2C2 | Nuclear receptor subfamily 2, group C, member 2 |
| IL2 | Interleukin 2 |
| ELK1 | ELK1, member of ETS oncogene family |
| TBK1 | TANK-binding kinase 1 |
| LY86 | Lymphocyte antigen 86 |
| HMGB1 | High mobility group box 1 |
| TLR7 | Toll-like receptor 7 |
| IRF3 | Interferon regulatory factor 3 |
| RIPK2 | Receptor-interacting serine-threonine kinase 2 |
| TOLLIP | Toll interacting protein |
| IKBKB | Inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta |
| IRAK2 | Interleukin-1 receptor-associated kinase 2 |
| PELI1 | Pellino E3 ubiquitin protein ligase 1 |
| MAP3K7 | Mitogen-activated protein kinase kinase kinase 7 |
| TRAF6 | TNF receptor-associated factor 6, E3 ubiquitin protein ligase |
| REL | v-rel avian reticuloendotheliosis viral oncogene homolog |
| CD86 | CD86 molecule |
| FOS | FBJ murine osteosarcoma viral oncogene homolog |
| MAP2K4 | Mitogen-activated protein kinase kinase 4 |
| CASP8 | Caspase 8, apoptosis-related cysteine peptidase |
| EIF2AK2 | Eukaryotic translation initiation factor 2-alpha kinase 2 |
| SARM1 | Sterile alpha and TIR motif containing 1 |
| CSF3 | Colony stimulating factor 3 (granulocyte) |
| MAP4K4 | Mitogen-activated protein kinase kinase kinase kinase 4 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 |
| UBE2N | Ubiquitin-conjugating enzyme |
| MAP3K1 | Mitogen-activated protein kinase kinase kinase 1, E3 ubiquitin protein ligase |
| NFRKB | Nuclear factor related to kappaB binding protein |
| HRAS | Harvey rat sarcoma viral oncogene homolog |
| IL1B | Interleukin 1, beta |
| JUN | Jun proto-oncogene |
| IL10 | Interleukin 10 |
| PPARA | Peroxisome proliferator-activated receptor alpha |
| CD80 | CD80 molecule |
| RELA | v-rel avian reticuloendotheliosis viral oncogene homolog A |
| MAPK8IP3 | Mitogen-activated protein kinase 8 interacting protein 3 |
| HSPD1 | Heat shock 60 kDa protein 1 (chaperonin) |
| TLR1 | Toll-like receptor 1 |
| TLR9 | Toll-like receptor 9 |
3. HBV Infection Induces Cytokine Production
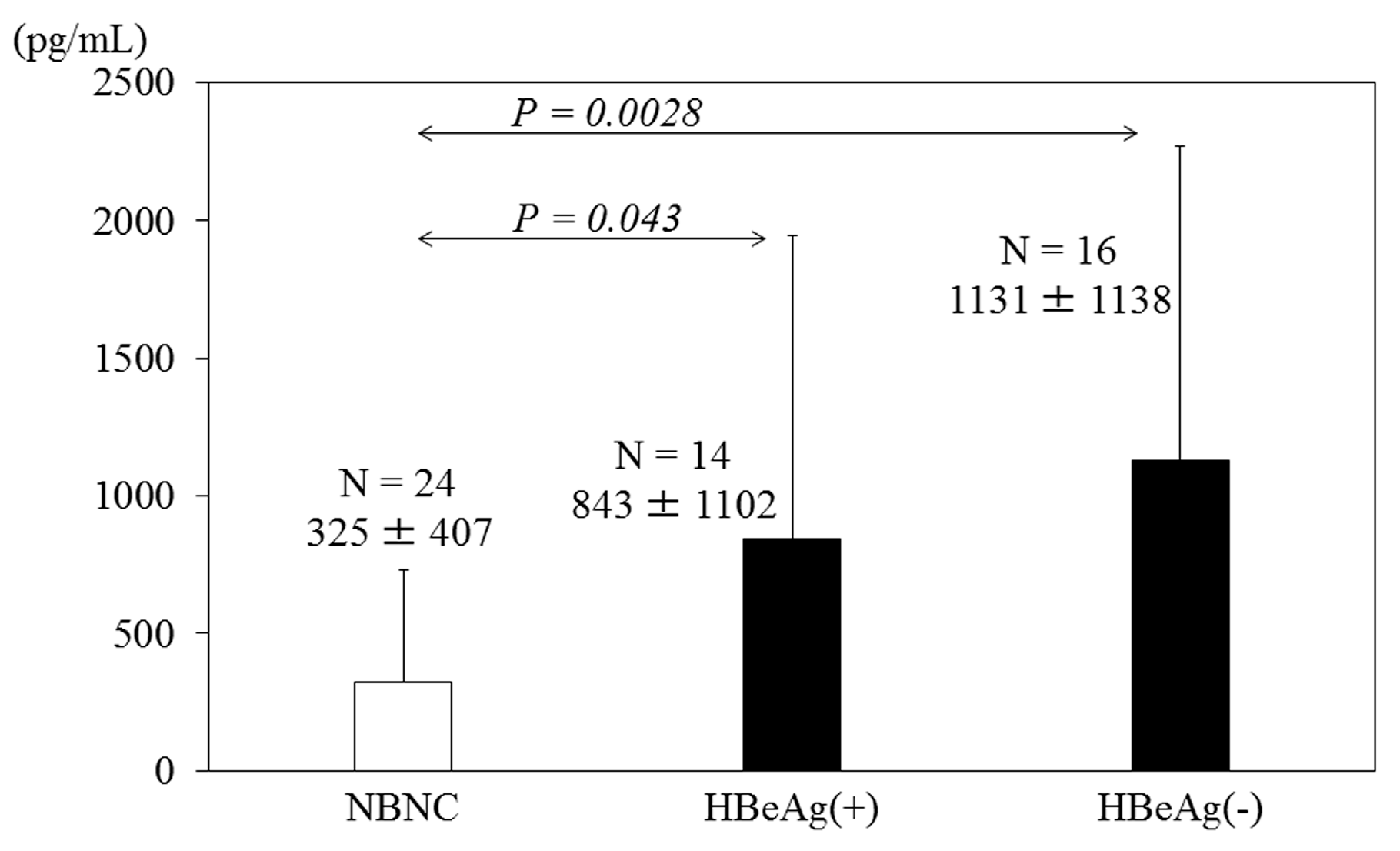
4. Targeting Therapies and Vaccines against HBV Core
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Locarnini, S.; Hatzakis, A.; Chen, D.S.; Lok, A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J. Hepatol. 2015, 62, S76–S86. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F. Anti-viral therapy in hepatitis B virus reactivation with acute-on-chronic liver failure. Hepatol. Int. 2014, 9, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lee, C.N.; Chang, C.H.; Ni, Y.H.; Shyu, M.K.; Chen, S.M.; Hu, J.J.; Lin, H.H.; Zhao, L.L.; Mu, S.C.; et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology 2015, 62, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, O.; Arai, M. Molecular biology of hepatitis B virus: Effect of nucleotide substitutions on the clinical features of chronic hepatitis B. Med. Mol. Morphol. 2006, 39, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Imazeki, F.; Yamada, Y.; Imamura, T.; Fukai, K.; Nagao, K.; Saisho, H. Hepatitis B virus X protein (HBx)-induced apoptosis in HuH-7 cells: Influence of HBV genotype and basal core promoter mutations. Scand. J. Gastroenterol. 2004, 39, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Koike, K.; Saito, I.; Miyamura, T.; Jay, G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature 1991, 351, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kanda, T.; Imazeki, F.; Arai, M.; Yonemitsu, Y.; Nakamoto, S.; Fujiwara, K.; Fukai, K.; Nomura, F.; Yokosuka, O. Hepatitis B virus e antigen downregulates cytokine production in human hepatoma cell lines. Viral Immunol. 2010, 23, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Pionek, K.; Unchwaniwala, N.; Maguire, M.L.; Loeb, D.D.; Zlotnick, A. The interface between hepatitis B virus capsid proteins affects self-assembly, pregenomic RNA packaging, and reverse transcription. J. Virol. 2015, 89, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kanda, T.; Imazeki, F.; Nakamoto, S.; Tanaka, T.; Arai, M.; Roger, T.; Shirasawa, H.; Nomura, F.; Yokosuka, O. Hepatitis B virus e antigen physically associates with receptor-interacting serine/threonine protein kinase 2 and regulates IL-6 gene expression. J. Infect. Dis. 2012, 206, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Gilles, P.N.; Fey, G.; Chisari, F.V. Tumor necrosis factor alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J. Virol. 1992, 66, 3955–3960. [Google Scholar] [PubMed]
- Guilhot, S.; Guidotti, L.G.; Chisari, F.V. Interleukin-2 downregulates hepatitis B virus gene expression in transgenic mice by a posttranscriptional mechanism. J. Virol. 1993, 67, 7444–7449. [Google Scholar] [PubMed]
- Guidotti, L.G.; Guilhot, S.; Chisari, F.V. Interleukin-2 and alpha/beta interferon down-regulate hepatitis B virus gene expression in vivo by tumor necrosis factor-dependent and -independent pathways. J. Virol. 1994, 68, 1265–1270. [Google Scholar] [PubMed]
- Cavanaugh, V.J.; Guidotti, L.G.; Chisari, F.V. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J. Virol. 1997, 71, 3236–3243. [Google Scholar] [PubMed]
- Pasquetto, V.; Guidotti, L.G.; Kakimi, K.; Tsuji, M.; Chisari, F.V. Host-virus interactions during malaria infection in hepatitis B virus transgenic mice. J. Exp. Med. 2000, 192, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.T.; Tsai, H.F.; Chyuan, I.T.; Liao, H.J.; Chen, C.J.; Chen, P.J.; Hsu, P.N. Tumor necrosis factor-alpha induced by hepatitis B virus core mediating the immune response for hepatitis B viral clearance in mice model. PLoS ONE 2014, 9, e103008. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Huang, L.R.; Yang, H.C.; Tzeng, H.T.; Hsu, P.N.; Wu, H.L.; Chen, P.J.; Chen, D.S. Hepatitis B virus core antigen determines viral persistence in a C57BL/6 mouse model. Proc. Natl. Acad. Sci. USA 2014, 107, 9340–9345. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.T.; Tsai, H.F.; Liao, H.J.; Lin, Y.J.; Chen, L.; Chen, P.J.; Hsu, P.N. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS ONE 2012, 7, e39179. [Google Scholar] [CrossRef] [PubMed]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [PubMed]
- Ye, B.; Liu, X.; Li, X.; Kong, H.; Tian, L.; Chen, Y. T-cell exhaustion in chronic hepatitis B infection: Current knowledge and clinical significance. Cell Death Dis. 2015, 6, e1694. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Takagi, T.; Nakamura, S.; Sawada, U.; Kura, Y.; Kodama, F.; Shimano, S.; Kudoh, I.; Nakamura, H.; Sawada, K.; et al. Hepatitis B virus carriers in the treatment of malignant lymphoma: An epidemiological study in Japan. Ann. Oncol. 1997, 8 (Suppl. 1), 107–910. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Imazeki, F.; Yoshida, S.; Suzuki, Y.; Nagao, K.; Saisho, H. Corticosteroids and lamivudine combined to treat acute severe flare-up in a chronic hepatitis B and C patient. J. Gastroenterol. Hepatol. 2004, 19, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, S.; Kanda, T.; Nakaseko, C.; Sakaida, E.; Ohwada, C.; Takeuchi, M.; Takeda, Y.; Mimura, N.; Iseki, T.; Wu, S.; et al. Reactivation of hepatitis B virus in hematopoietic stem cell transplant recipients in Japan: Efficacy of nucleos(t)ide analogues for prevention and treatment. Int. J. Mol. Sci. 2014, 15, 21455–21467. [Google Scholar] [CrossRef] [PubMed]
- Di Bisceglie, A.M.; Lok, A.S.; Martin, P.; Terrault, N.; Perrillo, R.P.; Hoofnagle, J.H. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: Just the tip of the iceberg? Hepatology 2015, 61, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kanda, T.; Nakamoto, S.; Haga, Y.; Sasaki, R.; Jiang, X.; Yasui, S.; Arai, M.; Yokosuka, O. Reappearance of serum HBV DNA in patients with hepatitis B surface antigen seroclearance. Hepatology 2015. [Google Scholar] [CrossRef]
- Prnewswire. Novira Therapeutics Announces Presentation of Preclinical Antiviral Data for NVR 3-778 at EASL. Available online: http://www.prnewswire.com/news-releases/novira-therapeutics-announces-presentation-of-preclinical-antiviral-data-for-nvr-3–778-at-easl-300072093.html (accessed on 9 May 2015).
- Klumpp, K.; Shimada, T.; Allweiss, L.; Volz, T.; Luetgehetman, M.; Flores, O.; Hartman, G.; Lam, A.; Dandri, M. High Antiviral Activity of the HBV Core Inhibitor NVR 3-778 in the Humanized uPA/SCID Mouse Model. J. Hepatol. 2015, 62, S252. [Google Scholar] [CrossRef]
- Heathcote, J.; McHutchison, J.; Lee, S.; Tong, M.; Benner, K.; Minuk, G.; Wright, T.; Fikes, J.; Livingston, B.; Sette, A.; et al. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatology 1999, 30, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.M.; Al-Mahtab, M.; Uddin, M.H.; Khan, M.S. HBsAg, HBcAg, and combined HBsAg/HBcAg-based therapeutic vaccines in treating chronic hepatitis B virus infection. Hepatobiliary Pancreat Dis. Int. 2013, 12, 363–369. [Google Scholar] [CrossRef]
- Saxena, R.; Kaur, J. Th1/Th2 cytokines and their genotypes as predictors of hepatitis B virus related hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.T.; Billaud, J.N.; Sällberg, M.; Guidotti, L.G.; Chisari, F.V.; Jones, J.; Hughes, J.; Milich, D.R. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc. Natl. Acad. Sci. USA 2004, 101, 14913–14918. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sällberg, M.; Hughes, J.; Jones, J.; Guidotti, L.G.; Chisari, F.V.; Billaud, J.N.; Milich, D.R. Immune tolerance split between hepatitis B virus precore and core proteins. J. Virol. 2005, 79, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Wu, S.; Sasaki, R.; Nakamura, M.; Haga, Y.; Jiang, X.; Nakamoto, S.; Yokosuka, O. HBV Core Protein Enhances Cytokine Production. Diseases 2015, 3, 213-220. https://doi.org/10.3390/diseases3030213
Kanda T, Wu S, Sasaki R, Nakamura M, Haga Y, Jiang X, Nakamoto S, Yokosuka O. HBV Core Protein Enhances Cytokine Production. Diseases. 2015; 3(3):213-220. https://doi.org/10.3390/diseases3030213
Chicago/Turabian StyleKanda, Tatsuo, Shuang Wu, Reina Sasaki, Masato Nakamura, Yuki Haga, Xia Jiang, Shingo Nakamoto, and Osamu Yokosuka. 2015. "HBV Core Protein Enhances Cytokine Production" Diseases 3, no. 3: 213-220. https://doi.org/10.3390/diseases3030213







