Gut Microbiota in Adults with Chronic Widespread Pain: A Systematic Review
Abstract
1. Introduction
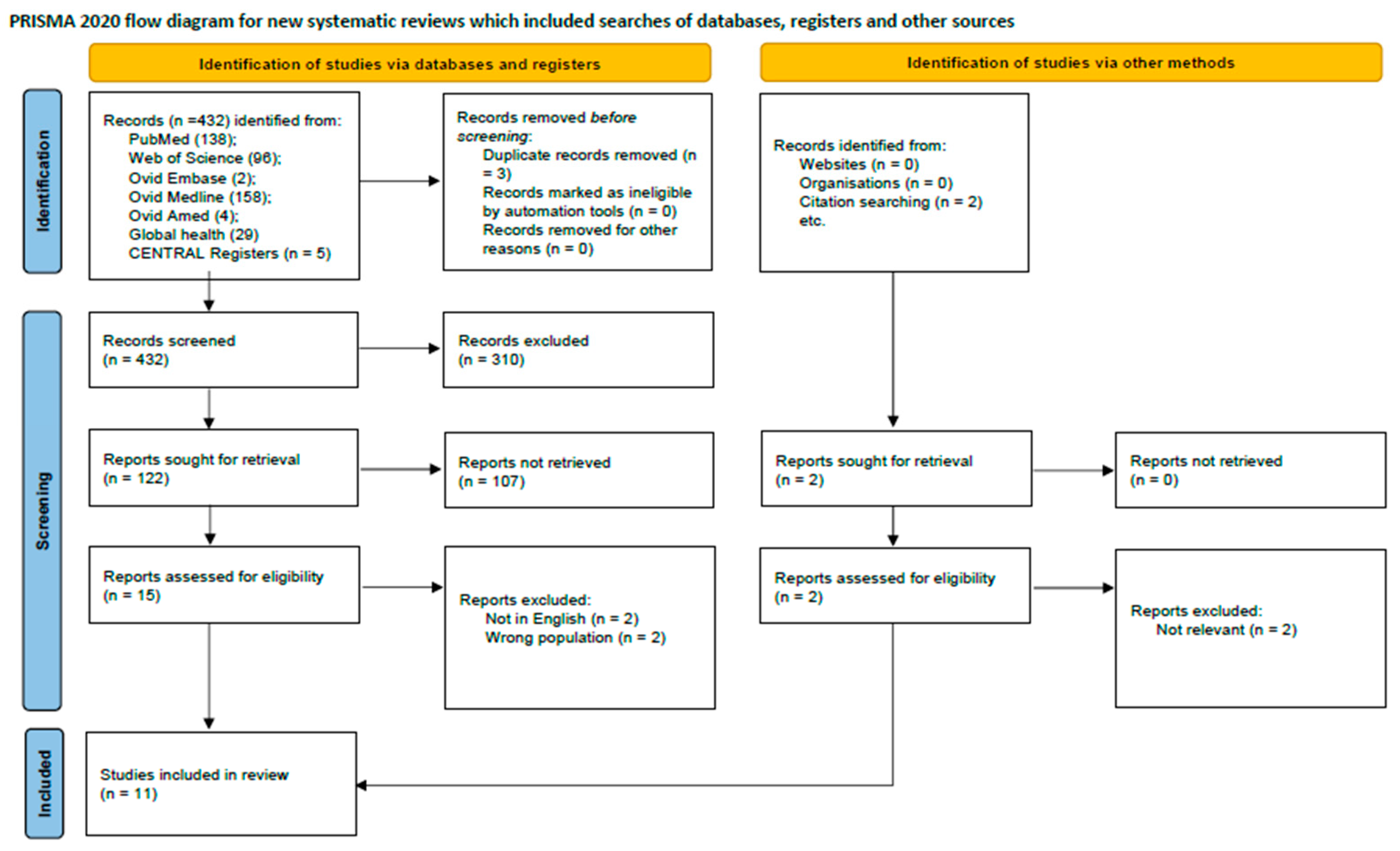
2. Methods
2.1. Data Source and Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Alteration of Microbiota in CWP
Different Taxonomy Level Alteration
3.2. Metabolic Function of Microbiota in CWP
3.3. Treatments with Microbiota in FM
4. Discussion
4.1. Interpretation of Findings and Causal Inference
4.2. Strengths and Limitations
4.3. Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy Used for the Medline Database to Identify Literature Reporting on Terms Related to CWP/FM and Gut Microbiota
| Search term(s): |
| 1. (“fibromyalgia” [MeSH Terms] OR “fibromyositis” [All Fields] OR “fibrositis” [All Fields] OR “muscular rheumatism” [All Fields]) |
| 2. (“chronic pain, widespread” [All Fields] OR “chronic widespread pain” [All Fields]) |
| 3. (“microbiota” [MeSH Terms] OR “microbiota” [All Fields] OR “microbiota” [All Fields] OR “microbiota” [All Fields]) |
| 4. 1 or 2 |
| 5. 3 and 4 |
| 6. Limit 10 to English language, Adults |
| 7. Limit 11 to publication date: 1 January 1976 ^ to 31 December 2024 |
| ^ the year “fibromyalgia” became the official term for the condition [67]. |
References
- Mansfield, K.E.; Sim, J.; Jordan, J.L.; Jordan, K.P. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain 2016, 157, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Burri, A.; Ogata, S.; Lachance, G.; Williams, F. Chronic widespread pain: Clinical comorbidities and psychological correlates. Pain 2015, 156, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Di Paola, R.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef] [PubMed]
- Slim, M.; Calandre, E.P.; Rico-Villademoros, F. An insight into the gastrointestinal component of fibromyalgia: Clinical manifestations and potential underlying mechanisms. Rheumatol. Int. 2015, 35, 433–444. [Google Scholar] [CrossRef]
- Raffaeli, W.; Malafoglia, V.; Bonci, A.; Tenti, M.; Ilari, S.; Gremigni, P.; Iannuccelli, C.; Gioia, C.; Di Franco, M.; Mollace, V.; et al. Identification of MOR-Positive B Cell as Possible Innovative Biomarker (Mu Lympho-Marker) for Chronic Pain Diagnosis in Patients with Fibromyalgia and Osteoarthritis Diseases. Int. J. Mol. Sci. 2020, 21, 1499. [Google Scholar] [CrossRef]
- Ilari, S.; Passacatini, L.C.; Malafoglia, V.; Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Palma, E.; Tomino, C.; Fini, M.; Raffaeli, W.; et al. Tantali fibromyalgic supplicium: Is there any relief with the antidepressant employment? A systematic review. Pharmacol. Res. 2022, 186, 106547. [Google Scholar] [CrossRef]
- Freidin, M.B.; Stalteri, M.A.; Wells, P.M.; Lachance, G.; Baleanu, A.-F.; Bowyer, R.C.; Kurilshikov, A.; Zhernakova, A.; Steves, C.J.; Williams, F.M.K. An association between chronic widespread pain and the gut microbiome. Rheumatology 2021, 60, 3727–3737. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef]
- Marazzato, M.; Iannuccelli, C.; Guzzo, M.P.; Nencioni, L.; Lucchino, B.; Radocchia, G.; Gioia, C.; Bonfiglio, G.; Neroni, B.; Guerrieri, F.; et al. Gut Microbiota Structure and Metabolites, Before and After Treatment in Early Rheumatoid Arthritis Patients: A Pilot Study. Front. Med. 2022, 9, 921675. [Google Scholar] [CrossRef]
- Vendrik, K.E.W.; Ooijevaar, R.E.; De Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; Van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The Gut Microbiome in Adult and Pediatric Functional Gastrointestinal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef]
- Russo, R.; Cristiano, C.; Avagliano, C.; De Caro, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut-brain axis: Role of lipids in the regulation of inflammation, pain and CNS diseases. Curr. Med. Chem. 2018, 25, 3930–3952. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.-H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hua, D.; Wang, Q.; Yang, L.; Wang, X.; Luo, A.; Yang, C. The role of bacteria and its derived metabolites in chronic pain and depression: Recent findings and research progress. Int. J. Neuropsychopharmacol. 2020, 23, 26–41. [Google Scholar] [CrossRef] [PubMed]
- O’mAhony, S.; Felice, V.; Nally, K.; Savignac, H.; Claesson, M.; Scully, P.; Woznicki, J.; Hyland, N.; Shanahan, F.; Quigley, E.; et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014, 277, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Kannampalli, P.; Pochiraju, S.; Chichlowski, M.; Berg, B.M.; Rudolph, C.; Bruckert, M.; Miranda, A.; Sengupta, J.N. Probiotic L actobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol. Motil. 2014, 26, 1694–1704. [Google Scholar] [CrossRef]
- Miquel, S.; Martin, R.; Lashermes, A.; Gillet, M.; Meleine, M.; Gelot, A.; Eschalier, A.; Ardid, D.; Bermudez-Humaran, L.G.; Sokol, H.; et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci. Rep. 2016, 6, 19399. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef]
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017, 20, 1213–1216. [Google Scholar] [CrossRef]
- Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Li, S.; Bi, J.; Jiang, R.; Yang, L.; Miao, L.; Zhu, B.; et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry 2019, 9, 57. [Google Scholar] [CrossRef]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar]
- Vieira, A.T.; Macia, L.; Galvão, I.; Martins, F.S.; Canesso, M.C.C.; Amaral, F.A.; Garcia, C.C.; Maslowski, K.M.; De Leon, E.; Shim, D.; et al. A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol. 2015, 67, 1646–1656. [Google Scholar]
- Kang, M.; Mischel, R.A.; Bhave, S.; Komla, E.; Cho, A.; Huang, C.; Dewey, W.L.; Akbarali, H.I. The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci. Rep. 2017, 7, srep42658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, J.; Ban, Y.; Jalodia, R.; Chupikova, I.; Fernandez, I.; Brito, N.; Sharma, U.; Abreu, M.T.; Ramakrishnan, S.; et al. Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 13523–13532. [Google Scholar] [PubMed]
- Ringel-Kulka, T.; Goldsmith, J.R.; Carroll, I.M.; Barros, S.P.; Palsson, O.; Jobin, C.; Ringel, Y. Lactobacillus acidophilus NCFM affects colonic mucosal opioid receptor expression in patients with functional abdominal pain—A randomised clinical study. Aliment. Pharmacol. Ther. 2014, 40, 200–207. [Google Scholar] [PubMed]
- Saulnier, D.M.; Riehle, K.; Mistretta, T.A.; Diaz, M.A.; Mandal, D.; Raza, S.; Weidler, E.M.; Qin, X.; Coarfa, C.; Milosavljevic, A.; et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1782–1791. [Google Scholar] [CrossRef]
- Theodorou, V.; Ait-Belgnaoui, A.; Agostini, S.; Eutamene, H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes 2014, 5, 430–436. [Google Scholar] [CrossRef]
- Newlove-Delgado, T.; Abbott, R.A.; Martin, A.E. Probiotics for children with recurrent abdominal pain. JAMA Pediatr. 2019, 173, 183–184. [Google Scholar] [CrossRef]
- Centre for Reviews Dissemination University of York. PROSPERO: International Prospective Register of Systematic Reviews 2017. Available online: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 26 September 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar]
- Oliva, E.M.; Villafañe, J.H.; Pérez, J.L.A.; Sal, A.A.; Carlier, G.M.; García, A.Q.; Turroni, S.; Martínez-Pozas, O.; Izquierdo, N.V.; Romero, E.A.S. Effect of Exercise on Inflammation in Hemodialysis Patients: A Systematic Review. J. Pers. Med. 2022, 12, 1188. [Google Scholar] [CrossRef]
- Sanchez Romero, E.A.; Martínez-Pozas, O.; García-González, M.; De-Pedro, M.; González-Álvarez, M.E.; Esteban-González, P.; Cid-Verdejo, R.; Villafañe, J.H. Association between Sleep Disorders and Sleep Quality in Patients with Temporomandibular Joint Osteoarthritis: A Systematic Review. Biomedicines 2022, 10, 2143. [Google Scholar] [CrossRef]
- Schulté, B.; Nieborak, L.; Leclercq, F.; Villafañe, J.H.; Romero, E.A.S.; Corbellini, C. The Comparison of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training after Coronary Artery Bypass Graft: A Systematic Review of Recent Studies. J. Cardiovasc. Dev. Dis. 2022, 9, 328. [Google Scholar] [CrossRef]
- Hernandez, C.J. The Microbiome and Bone and Joint Disease. Curr. Rheumatol. Rep. 2017, 19, 77. [Google Scholar] [CrossRef]
- Ramirez-Tejero, J.A.; Durán-González, E.; Martínez-Lara, A.; del Amo, L.L.; Sepúlveda, I.; Huancas-Díaz, A.; Carvajal, M.; Cotán, D. Microbiota and Mitochondrial Sex-Dependent Imbalance in Fibromyalgia: A Pilot Descriptive Study. Neurol. Int. 2023, 15, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, C.; Wang, J.; Guo, Q.; Zou, W. Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats. Brain Behav. 2019, 9, e01260. [Google Scholar] [CrossRef] [PubMed]
- Aslan Çİn, N.N.; Açik, M.; Tertemİz, O.F.; Aktan, Ç.; Akçali, D.T.; Çakiroğlu, F.P.; Özçelİk, A.Ö. Effect of prebiotic and probiotic supplementation on reduced pain in patients with fibromyalgia syndrome: A double-blind, placebo-controlled randomized clinical trial. Psychol. Health Med. 2024, 29, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Green, P.G.; Alvarez, P.; Levine, J.D. A role for gut microbiota in early-life stress-induced widespread muscle pain in the adult rat. Mol. Pain 2021, 17, 17448069211022952. [Google Scholar] [CrossRef]
- Cardona, D.; Roman, P.; Cañadas, F.; Sánchez-Labraca, N. The Effect of Multiprobiotics on Memory and Attention in Fibromyalgia: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 3543. [Google Scholar] [CrossRef]
- Calandre, E.P.; Hidalgo-Tallon, J.; Molina-Barea, R.; Rico-Villademoros, F.; Molina-Hidalgo, C.; Garcia-Leiva, J.M.; Carrillo-Izquierdo, M.D.; Slim, M. The Probiotic VSL#3® Does Not Seem to Be Efficacious for the Treatment of Gastrointestinal Symptomatology of Patients with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals 2021, 14, 1063. [Google Scholar]
- Wang, Z.; Jiang, D.; Zhang, M.; Teng, Y.; Huang, Y. Causal association between gut microbiota and fibromyalgia: A Mendelian randomization study. Front. Microbiol. 2023, 14, 1305361. [Google Scholar] [CrossRef]
- Fang, H.; Hou, Q.; Zhang, W.; Su, Z.; Zhang, J.; Li, J.; Lin, J.; Wang, Z.; Yu, X.; Yang, Y.; et al. Fecal Microbiota Transplantation Improves Clinical Symptoms of Fibromyalgia: An Open-Label, Randomized, Nonplacebo-Controlled Study. J. Pain 2024, 25, 104535. [Google Scholar] [CrossRef]
- Roman, P.; Estévez, A.F.; Miras, A.; Sánchez-Labraca, N.; Cañadas, F.; Vivas, A.B.; Cardona, D. A Pilot Randomized Controlled Trial to Explore Cognitive and Emotional Effects of Probiotics in Fibromyalgia. Sci. Rep. 2018, 8, 10965. [Google Scholar] [CrossRef]
- Hinchado, M.D.; Quero-Calero, C.D.; Otero, E.; Gálvez, I.; Ortega, E. Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome. Nutrients 2023, 15, 1591. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Rashid, S. Functional and therapeutic potential of inulin: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–13. [Google Scholar] [PubMed]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Jin, T.-E.; Chang, D.-H.; Rhee, M.-S.; Kim, H.J.; Lee, S.J.; Park, D.-S.; Kim, B.-C. Agathobaculum butyriciproducens gen. nov. sp. nov., a strict anaerobic, butyrate-producing gut bacterium isolated from human faeces and reclassification of Eubacterium desmolans as Agathobaculum desmolans comb. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 3656–3661. [Google Scholar] [CrossRef]
- Amir, I.; Bouvet, P.; Legeay, C.; Gophna, U.; Weinberger, A. Eisenbergiella tayi gen. nov., sp. nov., isolated from human blood. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 3, 907–914. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Shetty, S.A.; Lagkouvardos, I.; Ritari, J.; Chamlagain, B.; Douillard, F.P.; de Vos, W.M. Comparative genomics and physiology of the butyrate-producing bacterium Intestinimonas butyriciproducens. Environ. Microbiol. Rep. 2016, 8, 1024–1037. [Google Scholar] [CrossRef]
- Carlier, J.-P.; Bedora-Faure, M.; K’OUas, G.; Alauzet, C.; Mory, F. Proposal to unify Clostridium orbiscindens Winter et al. 1991 and Eubacterium plautii (Seguin 1928) Hofstad and Aasjord 1982, with description of Flavonifractor plautii gen. nov., comb. nov., and reassignment of Bacteroides capillosus to Pseudoflavonifractor capillosus gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 585–590. [Google Scholar] [CrossRef]
- Kläring, K.; Hanske, L.; Bui, N.; Charrier, C.; Blaut, M.; Haller, D.; Plugge, C.M.; Clavel, T. Intestinimonas butyriciproducens gen. nov., sp. nov., a butyrate-producing bacterium from the mouse intestine. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 12, 4606–4612. [Google Scholar] [CrossRef]
- Takada, T.; Watanabe, K.; Makino, H.; Kushiro, A. Reclassification of Eubacterium desmolans as Butyricicoccus desmolans comb. nov., and description of Butyricicoccus faecihominis sp. nov., a butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 2016, 66, 4125–4131. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.H.; Khelaifia, S.; Bittar, F.; Maraninchi, M.; Raoult, D.; Million, M. ‘Eisenbergiella massiliensis’, a new species isolated from human stool collected after bariatric surgery. New Microbes New Infect. 2016, 13, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Bloem, L.M.; Storbeck, K.-H.; Schloms, L.; Swart, A.C. 11β-hydroxyandrostenedione returns to the steroid arena: Biosynthesis, metabolism and function. Molecules 2013, 18, 13228–13244. [Google Scholar] [CrossRef] [PubMed]
- Devendran, S.; Mendez-Garcia, C.; Ridlon, J.M. Identification and characterization of a 20β-HSDH from the anaerobic gut bacterium Butyricicoccus desmolans ATCC 43058. J. Lipid Res. 2017, 58, 916–925. [Google Scholar] [CrossRef]
- Morris, D.J.; Ridlon, J.M. Glucocorticoids and gut bacteria: “The GALF Hypothesis” in the metagenomic era. Steroids 2017, 125, 1–13. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Ikegawa, S.; Alves, J.M.P.; Zhou, B.; Kobayashi, A.; Iida, T.; Mitamura, K.; Tanabe, G.; Serrano, M.; De Guzman, A.; et al. Clostridium scindens: A human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 2013, 54, 2437–2449. [Google Scholar] [CrossRef]
- Swart, A.C.; Storbeck, K.H. 11beta-Hydroxyandrostenedione: Downstream metabolism by 11betaHSD, 17betaHSD and SRD5A produces novel substrates in familiar pathways. Mol. Cell Endocrinol. 2015, 408, 114–123. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Li, S.; Yang, L.; Zhu, D.; Wang, Y.; Wang, H.; Wang, T.; Shi, B.; Gai, Z.; et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci. Rep. 2018, 8, 14305. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133 (Suppl. S7), 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Inanici, F.; Yunus, M.B. History of fibromyalgia: Past to present. Curr. Pain Headache Rep. 2004, 8, 369–378. [Google Scholar] [CrossRef]
| Author (Year) | Country | Sample Size | Participants (CWP/FM), n (%) | Study Design | Diagnosis Criteria | Intervention | Controls, n | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Çin (2024) [34] | Turkey | 53 | 53 (100) | RCT | NA | Probiotics and prebiotic | 18 Probiotics 17 Prebiotic 18 Placebo | Clinical measurements |
| Calandre 2021 [7] | Canada | 110 | 54 (49.1) | RCT | NA | Probiotic | 56 HC | Clinical measurements |
| Cardona (2021) [35] | Spain | 31 | 16 (51.6) | Pilot RCT | ACR 1990 ACR 2016 | Probiotics | 15 Placebo | Cognitive and memory measurements |
| Clos-Garcia (2019) [8] | Spain | 159 | 105 (66) | Case–control | ACR 1990 | NA | 54 HC | Microbiota Metabolomics |
| Fang H (2024) [36] | China | 45 | 45 (100) | RCT | ACR 2016 | FMT | 23 FM | Clinical measurements, Metabolomics |
| Freidin (2020) [37] | UK | 1736 | 113 (6.5) | Case–control | Modified version of the London Fibromyalgia Epidemiology Study Screening Questionnaire (LFESSQ) in UK | NA | 1623 HC | Microbiota Metabolomics |
| Hinchado (2023) [38] | Spain | 15 | 15 (100) | Cohort | ACR 2016 | Synbiotic | 7 FM with CFS 8 FM without CFS | Clinical measurements, Inflammatory and stress biomarkers |
| Minerbi (2019) [39] | Canada | 156 | 77 (49.4) | Case–control | ACObR 2016 | NA | 11 FC 20 HM 48 UC | Microbiota Metabolites |
| Ramírez-Tejero (2023) [40] | Spain | 41 | 26 (100) | Pilot descriptive | ACR 2016 | NA | 15 Male | Microbiota Metabolites |
| Roman (2018) [41] | Spain | 31 | 16 (51.6) | Pilot RCT | NA | Probiotics | 15 Placebo | Cognitive and clinical measurements |
| Wang (2024) [42] | China | 18,430 | 18,430 (100) | Observational genetic study | NA | NA | NA | Microbiota |
| Author (year) | Specified Selection Criteria | Randomization of Subjects | Allocation Was Concealed | Similar Groups at Baseline | Blinded Subjects | Blinded Therapists | Blinded Assessors | Outcomes Obtained 85% | Treatment or Intervention to Treat | Comparison Between Groups | Points Measure Variability | * Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aslan et al. (2023) [37] | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | 10 |
| Calandre 2021 [40] | yes | yes | yes | no | yes | yes | yes | yes | yes | yes | yes | 9 |
| Cardona et al. (2021) [39] | yes | yes | yes | yes | yes | yes | yes | no | yes | yes | yes | 9 |
| Fang H 2024 [42] | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | 10 |
| Roman et al. (2018) [43] | yes | yes | yes | no | yes | yes | yes | yes | yes | yes | yes | 9 |
| Items and Author (Year) | Hinchado (2023) [44] | Minerbi et al. (2019) [45] | Clos-Garcia et al. (2019) [8] | Wang (2024) [41] | Ramírez-Tejero et al. (2023) [35] | Freidin et al. (2020) [7] |
|---|---|---|---|---|---|---|
| A clearly stated aim | 2 | 2 | 2 | 2 | 2 | 2 |
| Inclusion of consecutive patients | 2 | 2 | 2 | 2 | 2 | 2 |
| Prospective collection of data | 2 | 0 | 0 | 0 | 0 | 0 |
| Endpoint appropriate to the study aim | 2 | 2 | 2 | 2 | 2 | 2 |
| Unbiased evaluation of endpoints | 2 | 2 | 2 | 2 | 2 | 2 |
| Follow-up period appropriate to the major endpoint | 2 | 0 | 0 | 0 | 0 | 0 |
| Loss to follow-up not exceeding 5% | 2 | 2 | 2 | 2 | 2 | 2 |
| ^ A control group having the gold standard intervention | 2 | 2 | 2 | 0 | 2 | 2 |
| ^ Contemporary groups | 2 | 2 | 2 | 2 | 2 | 2 |
| ^ Baseline equivalence of groups | 2 | 2 | 2 | 2 | 2 | 2 |
| ^ Prospective calculation of the sample size | 0 | 0 | 0 | 0 | 0 | 0 |
| ^ Statistical analyses adapted to the study design | 2 | 2 | 2 | 2 | 2 | 2 |
| Outcome | 22 | 18 | 18 | 16 | 18 | 18 |
| Author | Study Design | Disease | Level | Increased Microbiota | Decreased Microbiota | Function or Correlation to Human |
|---|---|---|---|---|---|---|
| Clos-Garcia 2019 [8] | Cross-sectional study | FM | diversity | alpha diversity | ||
| phylum | Firmicutes | Firmicutes | ||||
| Bacteroidetes | Bacteroidetes | |||||
| Actinobacteria | ||||||
| genus | Dorea | Bifidobacterium | ||||
| Roseburia | Eubacterium | |||||
| Alistipes | Bacteroides | |||||
| Roseburia | Clostridium | |||||
| Subdoligranulum | ||||||
| Papillibacter | ||||||
| family | Rikenellaceae | Bifidobacteriaceae (absent) | ||||
| Lachnospiraceae | Bacteroidales (absent) | |||||
| Erysipelotichaceae | ||||||
| Bacteroidales Prevotella | ||||||
| Eubacterium | ||||||
| Ruminococcaceae | ||||||
| class | Actinobacteria | |||||
| Minerbi 2019 [45] | Cross-sectional study | FM | genus | Intestinimonas | Bacteroides | Bacteroides positively correlated with total symptom score on the Fibromyalgia Impact Questionnaire (FIQ) |
| Flavonifractor | Faecalibacterium | |||||
| Butyricoccus | ||||||
| Eisenbergiella | ||||||
| Enterobacter | ||||||
| species | F. prausnitzii (Faecalibacterium prausnitzii) | Butyrate producers, antinociceptive as well as anti-inflammatory, enhance the intestinal barrier function, certain short-chain fatty acids (SCFA) producing bacteria | ||||
| B. uniformis (Bacteroides uniformis) | Butyrate producers, certain short-chain fatty acids (SCFA) producing bacteria | |||||
| Haemophilus parainfluenzae | Butyrate producers, a putative pro-inflammatory role | |||||
| P. copri (Prevotella copri) | Butyrate producers, a putative pro-inflammatory role | |||||
| Blautia faecis | Butyrate producers | |||||
| Butyriciproducens (Intestinimonas butyriciproducens) | Butyrate producers | |||||
| F. plautii (Flavonifractor plautii) | Butyrate producers | |||||
| B. desmolans (Butyricicoccus desmolans) | Butyrate producers | |||||
| E. tayi (Eisenbergiella tayi) | Butyrate producers | |||||
| E. massiliensis (Eisenbergiella massiliensis) | Butyrate producers | |||||
| Parabacteroides merdae | Antiepileptic effect | |||||
| Akkermansia muciniphila | Ketogenic diet effect on seizures | |||||
| Clostridium scindens | Converting cortisol to androgens by 20α-hydroxysteroid dehydrogenase activity | |||||
| Freidin 2023 [7] | Cross-sectional study | CWP | diversity | alpha diversity | ||
| family | Firmicutes | Firmicutes | ||||
| Lachnospiraceae | Lachnospiraceae | |||||
| Ruminococcaceae | ||||||
| species | IOdontolyticus | Excrementihominis | ||||
| Massiliensis | Obeum | |||||
| Formicigenerans | ||||||
| Splanchnicus | ||||||
| Ureilytica | ||||||
| Inulinivorans | ||||||
| Coprococcus comes | butyrate-producing and anti-inflammatory bacteria | |||||
| Ramírez-Tejero 2023 [35] | Pilot descriptive study | FM | genus | Ruminococcus | ||
| Pseudomonas | ||||||
| Wang 2023 [41] | Observational genetic study | FM | genus | Coprococcus2 | FamilyXIIIUCG001 | |
| Eggerthella | Olsenella | |||||
| Lactobacillus |
| Author | Study Type | Study Design | Patients | Intervention | Contents | Outcome | Summary of Results |
|---|---|---|---|---|---|---|---|
| Çin 2024 [37] | Human | RCT | FM | Probiotics | 4 × 10^10 CFUs per day (Lactobacillus acidophilus L1 (2.9 × 109) and Lactobacillus rhamnosus liobif (2.9 × 109), Bifidobacterium longum (2.9 × 109), and Saccharomyces boulardii (1.3 × 109) | Pain, quality of sleep, quality of life, anxiety, and depressive symptoms | The probiotic group significantly decreased self-reported pain and increased both quality of life and sleep quality, and depressive symptoms and anxiety levels in FM |
| Prebiotics | 10 g dose inulin per day | ||||||
| Calandre 2021 [40] | Human | RCT | FM | Probiotic | Multi-strain probiotic, VSL#3® | Mean change from the baseline to the endpoint in the composite severity score of the three main gastrointestinal symptoms reported by patients with fibromyalgia (abdominal pain, abdominal bloating, and meteorism) | This study could not demonstrate any beneficial effects of VSL#3® either on the composite score of severity of abdominal pain, bloating, and meteorism or in any of the secondary outcome variables |
| Cardona 2021 [39] | Human | Pilot RCT | FM | Probiotics | Selected probiotic species (Lactobacillus rhamnosus GG, L. paracasei, L. acidophilus, and Bifidobacterium bifidus), revivification of 6 million germs per capsule, 4 capsules per day | Cognitive functions (memory and attention), a tendency to reduce errors of omission (Go trials) during the Go/No-Go Task | Treatment with a multispecies probiotic produced an improvement in attention by reducing errors on an attention task, but it had no effect on memory. More specifically, a tendency to reduce errors of omission (Go trials) during the Go/No-Go Task was observed after treatment |
| Fang H 2024 [42] | Human | RCT | FM | FMT | NA | Pain, sleep quality, quality of life, anxiety, depressive symptoms and metabolites | FMT can effectively improve the clinical symptoms of FM. The close relations between the changes in neurotransmitters and FM, certain neurotransmitters may serve as a diagnostic marker or potential target for FM patients |
| Roman 2018 [43] | Human | Pilot RCT | FM | Probiotics | Lactobacillus acidophilus or Lactobacillus Rhamnosus GG ® | Pain, impact of FMS, quality of life, anxiety and depressive symptoms, computerized cognitive tasks, urinary cortisol | The present results indicate that probiotic treatment did not significantly improve depressive or anxiety symptoms when compared to the placebo group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leong, P.-Y.; Shi, L.-H. Gut Microbiota in Adults with Chronic Widespread Pain: A Systematic Review. Diseases 2025, 13, 299. https://doi.org/10.3390/diseases13090299
Leong P-Y, Shi L-H. Gut Microbiota in Adults with Chronic Widespread Pain: A Systematic Review. Diseases. 2025; 13(9):299. https://doi.org/10.3390/diseases13090299
Chicago/Turabian StyleLeong, Pui-Ying, and Lin-Hong Shi. 2025. "Gut Microbiota in Adults with Chronic Widespread Pain: A Systematic Review" Diseases 13, no. 9: 299. https://doi.org/10.3390/diseases13090299
APA StyleLeong, P.-Y., & Shi, L.-H. (2025). Gut Microbiota in Adults with Chronic Widespread Pain: A Systematic Review. Diseases, 13(9), 299. https://doi.org/10.3390/diseases13090299








