Vaginal Microbiota in Short Cervix Pregnancy: Secondary Analysis of Pessary vs. Progesterone Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Collection, DNA Extraction, Library Preparation, and DNA Sequencing
2.3. Microbiome Profiling of the Vaginal Samples
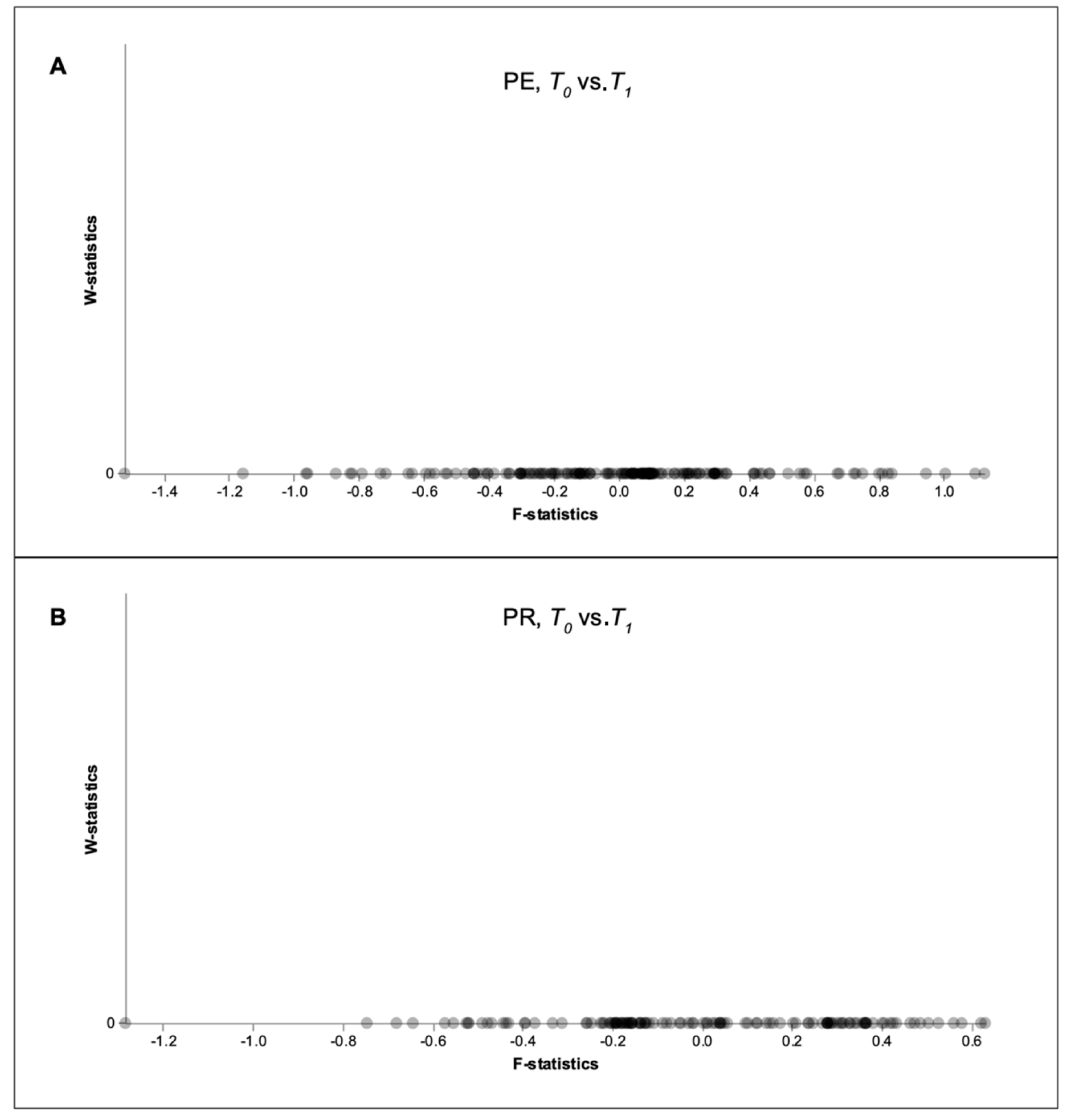
2.4. Statistical Analyses of Microbiomes
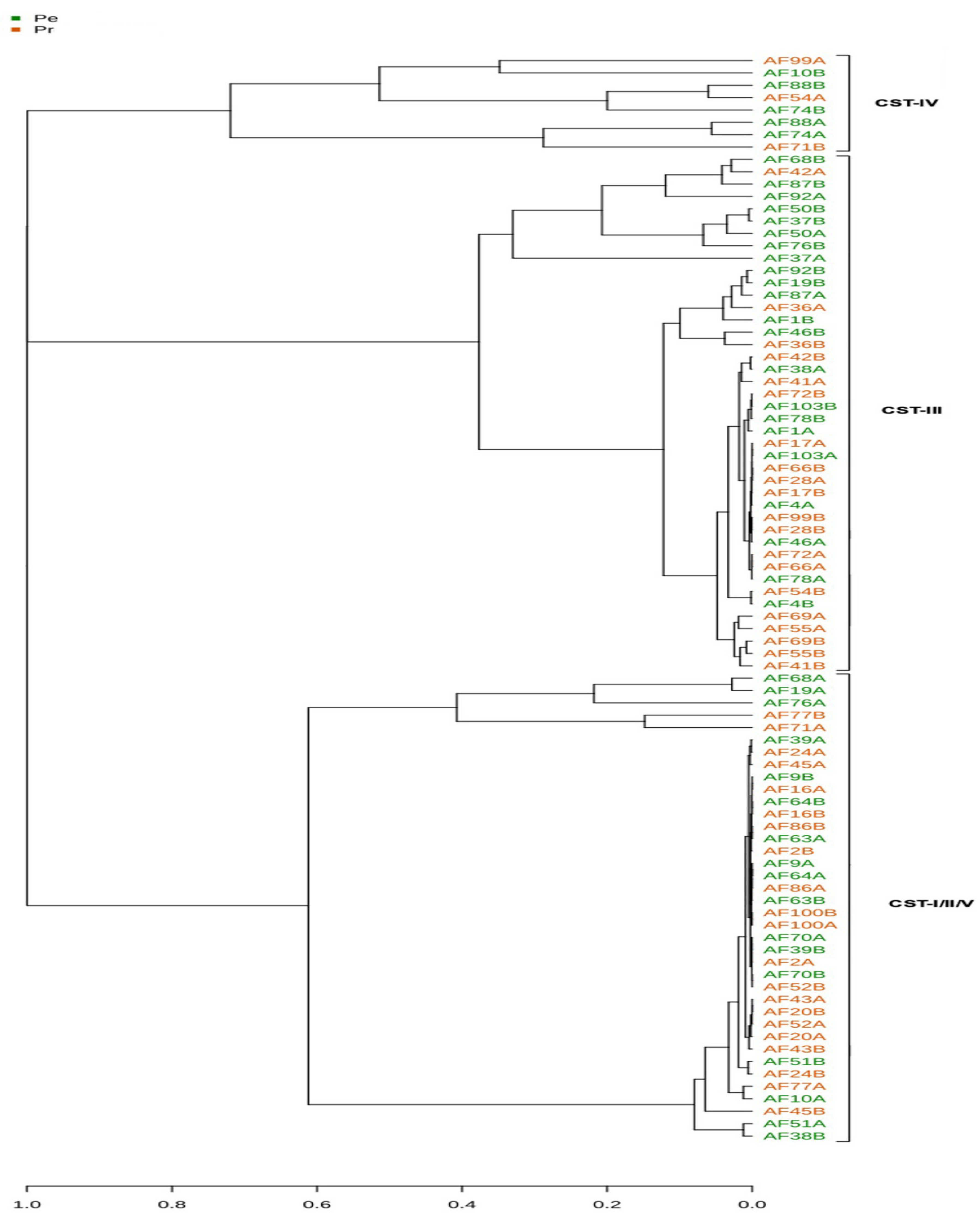
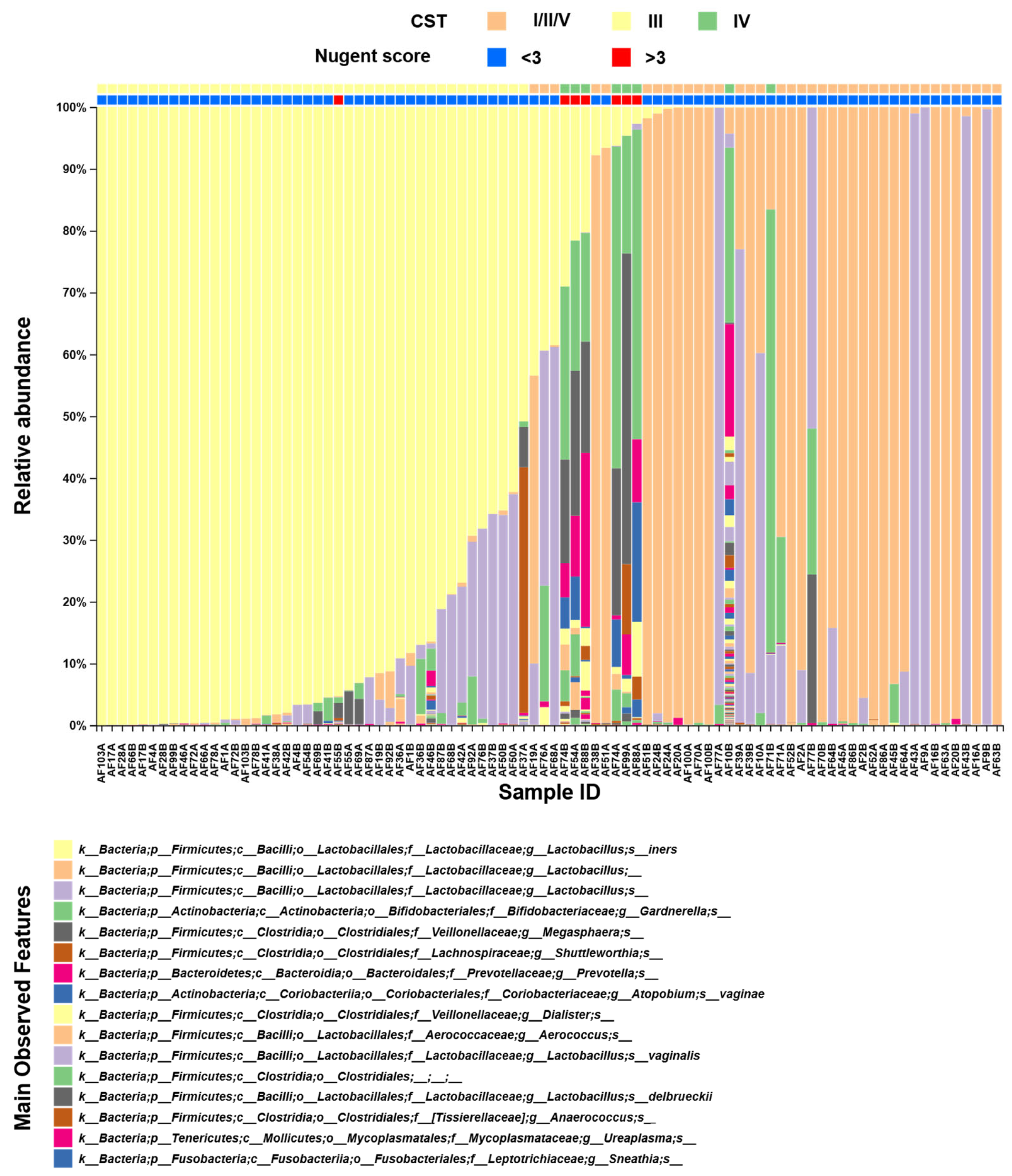
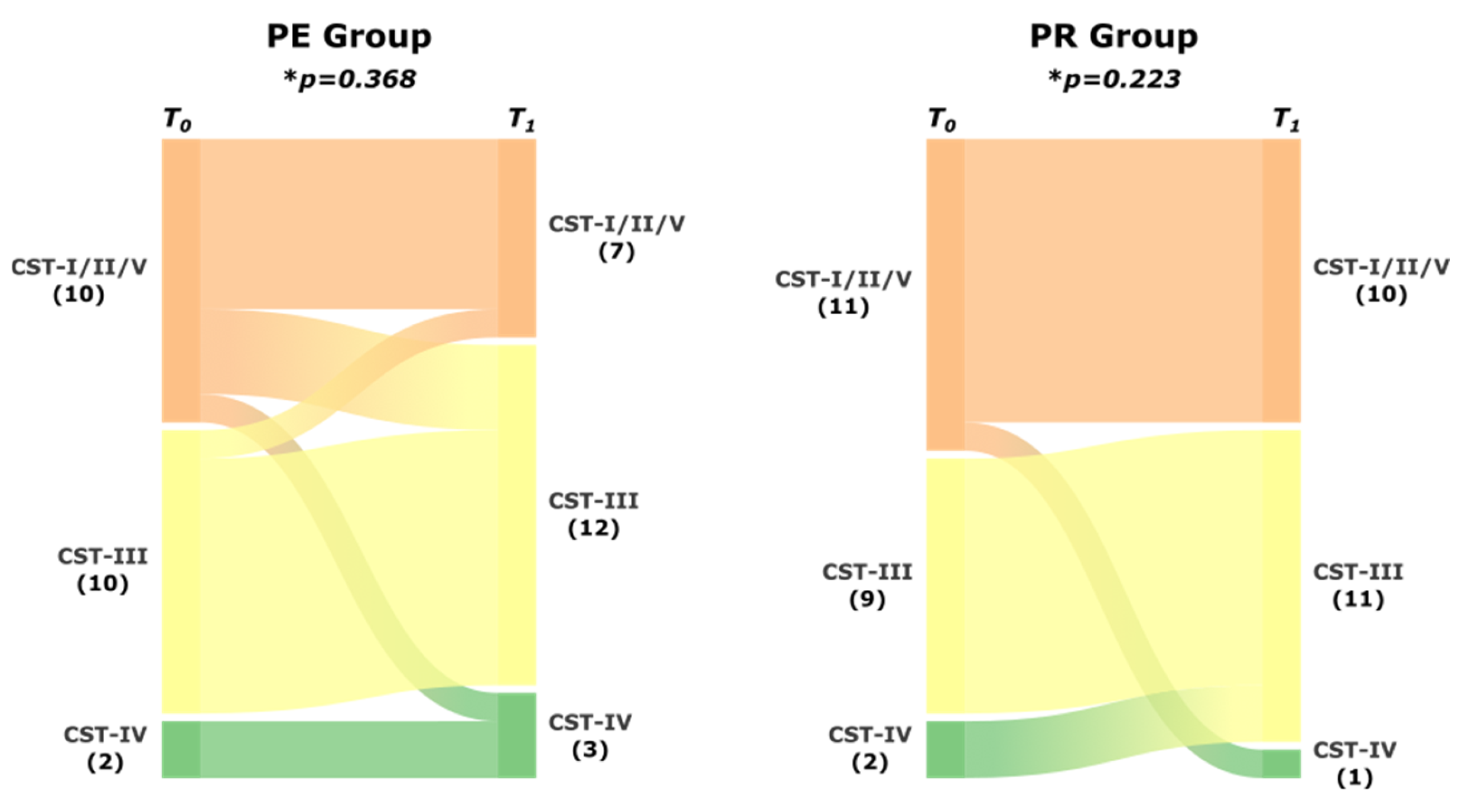
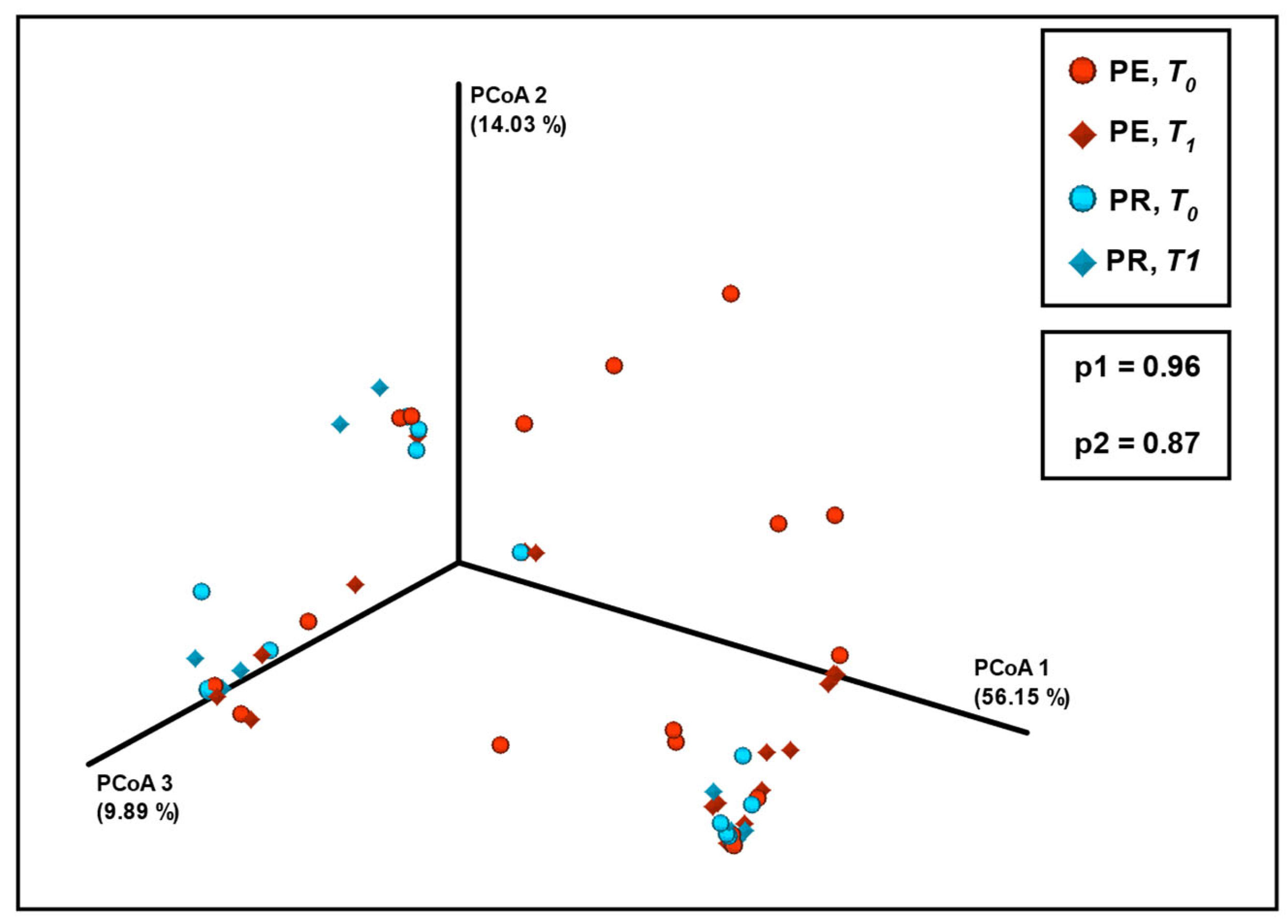
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASV | Amplicon sequence variant |
| BV | Bacterial vaginosis |
| CST | Community state type |
| D-La | D-lactic acid |
| L-La | L-lactic acid |
| OTU | Operational taxonomic unit |
| PCR | Polymerase chain reaction |
| PE | Pessary |
| PR | Progesterone |
| PTB | Preterm birth |
| sPTB | Spontaneous preterm birth |
| TVUS | Transvaginal ultrasound |
| QIIME | Quantitative Insights into Microbial Ecology |
Appendix A
Appendix A.1

Appendix A.2
| PRIMER | COMPLETE SEQUENCE | ADAPTER | BARCODE | 16S-V4 |
|---|---|---|---|---|
| R806_trP1_rev | CCTCTCTATGGGCAGTCGGTGATGGACTACHVGGGTWTCTAAT | |||
| F515_16S(for)-BC-01 | CCATCTCATCCCTGCGTGTCTCCGACTCAGCTAAGGTAACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCA | CTAAGGTAA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-02 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTAAGGAGAACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TAAGGAGAA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-03 | CCATCTCATCCCTGCGTGTCTCCGACTCAGAAGAGGATTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | AAGAGGATT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-04 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTACCAAGATCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TACCAAGAT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-05 | CCATCTCATCCCTGCGTGTCTCCGACTCAGCAGAAGGAACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | CAGAAGGAA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-06 | CCATCTCATCCCTGCGTGTCTCCGACTCAGCTGCAAGTTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | CTGCAAGTT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-07 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTTCGTGATTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TTCGTGATT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-08 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTTCCGATAACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TTCCGATAA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-09 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTGAGCGGAACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TGAGCGGAA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-10 | CCATCTCATCCCTGCGTGTCTCCGACTCAGCTGACCGAACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | CTGACCGAA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-11 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTCCTCGAATCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TCCTCGAAT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-12 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTAGGTGGTTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TAGGTGGTT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-13 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTCTAACGGACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TCTAACGGA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-14 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTTGGAGTGTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TTGGAGTGT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-15 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTCTAGAGGTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TCTAGAGGT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-16 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTCTGGATGACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TCTGGATGA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-17 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTCTATTCGTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TCTATTCGT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-18 | CCATCTCATCCCTGCGTGTCTCCGACTCAGAGGCAATTGCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | AGGCAATTG | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-19 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTTAGTCGGACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TTAGTCGGA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-20 | CCATCTCATCCCTGCGTGTCTCCGACTCAGCAGATCCATCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | CAGATCCAT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-21 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTCGCAATTACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TCGCAATTA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-22 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTTCGAGACGCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TTCGAGACG | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-23 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTGCCACGAACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TGCCACGAA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-24 | CCATCTCATCCCTGCGTGTCTCCGACTCAGAACCTCATTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | AACCTCATT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-25 | CCATCTCATCCCTGCGTGTCTCCGACTCAGCCTGAGATACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | CCTGAGATA | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-26 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTTACAACCTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TTACAACCT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-27 | CCATCTCATCCCTGCGTGTCTCCGACTCAGAACCATCCGCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | AACCATCCG | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-28 | CCATCTCATCCCTGCGTGTCTCCGACTCAGATCCGGAATCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | ATCCGGAAT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-29 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTCGACCACTCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TCGACCACT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-30 | CCATCTCATCCCTGCGTGTCTCCGACTCAGCGAGGTTATCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | CGAGGTTAT | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-31 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTCCAAGCTGCGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TCCAAGCTG | CGATGTGCCAGCMGCCGCGGTAA |
| F515_16S(for)-BC-32 | CCATCTCATCCCTGCGTGTCTCCGACTCAGTCTTACACACGATGTGCCAGCMGCCGCGGTAA | CCATCTCATCCCTGCGTGTCTCCGACTCAG | TCTTACACA | CGATGTGCCAGCMGCCGCGGTAA |
Appendix A.3
Appendix A.4
References
- Leal, M.D.; Esteves-Pereira, A.P.; Nakamura-Pereira, M.; Torres, J.A.; Theme-Filha, M.; Domingues, R.M.S.M.; Dias, M.A.B.; Moreira, M.E.; Gama, S.G. Prevalence and risk factors related to preterm birth in Brazil. Reprod. Health 2016, 13 (Suppl. S3), 127. [Google Scholar] [CrossRef]
- Romero, R.; Gomez-Lopez, N.; Winters, A.D.; Jung, E.; Shaman, M.; Bieda, J.; Panaitescu, B.; Pacora, P.; Erez, O.; Greenberg, J.M.; et al. Evidence that intra-amniotic infections are often the result of an ascending invasion—A molecular microbiological study. J. Perinat. Med. 2019, 47, 915–931. [Google Scholar] [CrossRef]
- Carvalho, M.H.B.D.; Bittar, R.E.; Maganha, P.P.D.A.; Pereira, S.V.; Zugaib, M. Associação da vaginose bacteriana com o parto prematuro espontâneo [The association between bacterial vaginosis and premature birth]. Rev. Bras. Gynecol. Obstet. 2001, 23, 529–533. [Google Scholar]
- de Carvalho, M.H.B.; Bittar, R.E.; Brizot, M.; Bicudo, C.; Zugaib, M. Prediction of preterm delivery in the second trimester. Obstet. Gynecol. 2005, 105, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Nicolaides, K.H.; Conde-Agudelo, A.; O’Brien, J.M.; Cetingoz, E.; Da Fonseca, E.; Creasy, G.W.; Hassan, S.S. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: An updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet. Gynecol. 2016, 48, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Saccone, G.; Ciardulli, A.; Xodo, S.; Dugoff, L.; Ludmir, J.; Pagani, G.; Visentin, S.; Gizzo, S.; Volpe, N.; Maruotti, G.M.; et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: A systematic review and meta-analysis. J. Ultrasound Med. 2017, 36, 1535–1543. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R.; Nicolaides, K.H. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 223, 42–65.e2. [Google Scholar] [CrossRef]
- Pacagnella, R.C.; Silva, T.V.; Cecatti, J.G.; Passini, R., Jr.; Fanton, T.F.; Borovac-Pinheiro, A.; Pereira, C.M.; Fernandes, K.G.; França, M.S.; Li, W.; et al. Pessary plus progesterone to prevent preterm birth in women with short cervixes: A randomized controlled trial. Obstet. Gynecol. 2022, 139, 41–51. [Google Scholar] [CrossRef]
- ACOG. Practice Bulletin No.142: Cerclage for the management of cervical insufficiency. Obstet. Gynecol. 2014, 123, 372–379. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, D.A.; Chandiramani, M.; Lee, Y.S.; Kindinger, L.; Smith, A.; Angelopoulos, N.; Lehne, B.; Arulkumaran, S.; Brown, R.; Teoh, T.G.; et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 2015, 5, 8988. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P.; et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef]
- Witkin, S.S.; Linhares, I.M. Why do lactobacilli dominate the human vaginal microbiota? BJOG 2017, 124, 606–611. [Google Scholar] [CrossRef]
- Callahan, B.J.; DiGiulio, D.B.; Goltsman, D.S.A.; Sun, C.L.; Costello, E.K.; Jeganathan, P.; Biggio, J.R.; Wong, R.J.; Druzin, M.L.; Shaw, G.M.; et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl. Acad. Sci. USA 2017, 114, 9966–9971. [Google Scholar] [CrossRef]
- Stout, M.J.; Zhou, Y.; Wylie, K.M.; Tarr, P.I.; Macones, G.A.; Tuuli, M.G. Early pregnancy vaginal microbiome trends and preterm birth. Am. J. Obstet. Gynecol. 2017, 217, 356.e1–356.e18. [Google Scholar] [CrossRef]
- Kindinger, L.M.; Bennett, P.R.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.G.; MacIntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef]
- Freitas, A.C.; Bocking, A.; Hill, J.E.; Money, D.M.; VOGUE Research Group. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018, 6, 117. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Feehily, C.; Crosby, D.; Walsh, C.J.; Lawton, E.M.; Higgins, S.; McAuliffe, F.M.; Cotter, P.D. Shotgun sequencing of the vaginal microbiome reveals both a species and functional potential signature of preterm birth. NPJ Biofilms Microbiomes 2020, 6, 50. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Baud, A.; Hillion, K.H.; Plainvert, C.; Tessier, V.; Tazi, A.; Mandelbrot, L.; Poyart, C.; Kennedy, S.P. Microbial diversity in the vaginal microbiota and its link to pregnancy outcomes. Sci. Rep. 2023, 13, 9061. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Yañez, F.; Elias, A.; Bernabeu, A.; Goya, M.; Xie, Z.; Farrás, A.; Sánchez, O.; Soler, Z.; Blasquez, C.; et al. Cervical pessary and cerclage placement for preterm birth prevention and cervicovaginal microbiome changes. Acta Obstet. Gynecol. Scand. 2022, 101, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Amorim Filho, A.G.; Martins, R.C.R.; Franco, L.A.M.; Marinelli, J.V.; Peres, S.V.; Francisco, R.P.; Carvalho, M.H. Effect of the Arabin Pessary and Natural Progesterone on the Vaginal Microbiome. Res. Sq. 2023; preprint (version 1). [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef]
- Ribeiro, R.M.; Souza-Basqueira, M.; Oliveira, L.C.; Salles, F.C.; Pereira, N.B.; Sabino, E.C. An alternative storage method for characterization of the intestinal microbiota through next generation sequencing. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e77. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Cole, M.; Morris, A.; Martinson, J.; Mckay, H.; Mimiaga, M.; Margolick, J.; Fitch, A.; Methe, B.; et al. Signature changes in gut microbiome are associated with increased susceptibility to HIV-1 infection in MSM. Microbiome 2021, 9, 237. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A tool for visualizing high-throughput microbial community data. GigaScience 2013, 2, 16. [Google Scholar] [CrossRef]
- Witkin, S.S.; Moron, A.F.; Ridenhour, B.J.; Minis, E.; Hatanaka, A.; Sarmento, S.G.P.; Franca, M.S.; Carvalho, F.H.C.; Hamamoto, T.K.; Mattar, R.; et al. Vaginal biomarkers that predict cervical length and dominant bacteria in the vaginal microbiomes of pregnant women. mBio 2019, 10, e02242-19. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G. Gestational shaping of the maternal vaginal microbiome. Nat. Med. 2019, 25, 882–883. [Google Scholar] [CrossRef]
- Golob, J.L.; Oskotsky, T.T.; Tang, A.S.; Roldan, A.; Chung, V.; Ha, C.W.Y.; Wong, R.J.; Flynn, K.J.; Parraga-Leo, A.; Wibrand, C.; et al. Microbiome preterm birth DREAM challenge: Crowdsourcing machine learning approaches to advance preterm birth research. Cell Rep. Med. 2024, 5, 101350. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | PE (n = 22) | PR (n = 22) | p |
|---|---|---|---|
| Demographics | |||
| Age, y, mean ± SD (range) | 30.1 ± 7.2 (15–42) | 28.1 ± 6.0 (17–37) | 0.315 1 |
| Ethnicity | |||
| White, n | 15 (68.2%) | 16 (72.7%) | 0.741 2 |
| Black/mixed, n | 7 (31.8%) | 6 (27.3%) | |
| BMI (kg/m2), mean ± SD (range) | 26.5 ± 4.3 (18.6–36.1) | 27.7 ± 5.1 (20.5–37.7) | 0.453 3 |
| Obstetric history | |||
| Nulliparous, n | 13 (59.1%) | 8 (36.4%) | 0.131 2 |
| Miscarriage, n | 7 (31.8%) | 8 (36.4%) | 0.750 2 |
| Preterm birth, n | 4 (18.2%) | 3 (13.6%) | 1.000 4 |
| Sample collection | |||
| Mean GA at T0 ± SD (range), wk | 22.3 ± 1.1 (20.6–23.9) | 22.9 ± 0.8 (21.6–24.9) | 0.107 3 |
| Mean cervical length at T0 ± SD (range), mm | 16.0 ± 6.0 (5.0–24.0) | 17.0 ± 5.0 (7.0–23.0) | 0.494 3 |
| Nugent score > 3, n | 4 (18.2%) | 3 (13.6%) | 1.000 4 |
| Pregnancy outcome | |||
| Mean GA at birth ± SD (range), wk | 37.4 ± 4.0 (25.4–40.3) | 37.4 ± 3.4 (25.9–40.7) | 0.677 3 |
| Spontaneous preterm birth, n | 2 (9.1%) | 6 (30.0%) | 0.123 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim Filho, A.G.; Martins, R.C.R.; Franco, L.A.M.; Marinelli, J.V.C.; Peres, S.V.; Francisco, R.P.V.; Carvalho, M.H.B. Vaginal Microbiota in Short Cervix Pregnancy: Secondary Analysis of Pessary vs. Progesterone Trial. Diseases 2025, 13, 338. https://doi.org/10.3390/diseases13100338
Amorim Filho AG, Martins RCR, Franco LAM, Marinelli JVC, Peres SV, Francisco RPV, Carvalho MHB. Vaginal Microbiota in Short Cervix Pregnancy: Secondary Analysis of Pessary vs. Progesterone Trial. Diseases. 2025; 13(10):338. https://doi.org/10.3390/diseases13100338
Chicago/Turabian StyleAmorim Filho, Antonio G., Roberta C. R. Martins, Lucas A. M. Franco, Juliana V. C. Marinelli, Stela V. Peres, Rossana P. V. Francisco, and Mário H. B. Carvalho. 2025. "Vaginal Microbiota in Short Cervix Pregnancy: Secondary Analysis of Pessary vs. Progesterone Trial" Diseases 13, no. 10: 338. https://doi.org/10.3390/diseases13100338
APA StyleAmorim Filho, A. G., Martins, R. C. R., Franco, L. A. M., Marinelli, J. V. C., Peres, S. V., Francisco, R. P. V., & Carvalho, M. H. B. (2025). Vaginal Microbiota in Short Cervix Pregnancy: Secondary Analysis of Pessary vs. Progesterone Trial. Diseases, 13(10), 338. https://doi.org/10.3390/diseases13100338








