Abstract
Background/Objectives: Chronic widespread pain (CWP), a key feature of fibromyalgia (FM), has been increasingly associated with gut microbiota alterations, yet the specific changes in microbial composition and the therapeutic potential of probiotics or prebiotics in these patients remain unclear. This systematic review aimed to synthesize current evidence regarding gut microbiota alterations and the effects of microbiota-targeted interventions in individuals with CWP/FM. Methods: A comprehensive search across multiple databases, including PubMed, Web of Science, Cochrane Library, Ovid Embase, Medline, Ovid AMED, and Global Health. These studies were categorized into two primary themes: changes in gut microbiota composition at various taxonomic levels and the therapeutic impact of microbiota-involved treatments in patients with CWP/FM. Results: We finally identified 432 studies, of which 11 met the inclusion criteria. The findings indicate that while alterations in the gut microbiota have been observed in CWP patients, the evidence remains limited and heterogeneous. Conclusions: Preliminary indications suggest a potential role of dysbiosis in the pathophysiology of CWP, but further rigorously designed studies are needed to clarify the therapeutic efficacy of microbiota-based interventions in this patient population.
1. Introduction
Chronic widespread musculoskeletal pain (CWP) is a common disorder, affecting some 5–15% of the general population, and presents a sizeable economic burden in terms of disability, work absence, and healthcare costs [1], with a complex etiology. CWP is commonly associated with other physical symptoms such as tiredness, sleep disturbance, and concentration problems [2]. Characterized by CWP and tenderness, sleeping disorders, fatigue, and cognitive dysfunction, fibromyalgia (FM) is the most disabling type of CWP [3], involving a malfunction in pain signaling, resulting in heightened sensitivity (hyperalgesia), pain from normally innocuous stimuli (allodynia), and the spread of pain to larger areas [4], usually linked with other symptoms like stiffness in the muscles and joints, fatigue, poor sleep quality, cognitive problems, anxiety, depression, and irritable intestinal syndrome [5,6]. Growing evidence shows that altered diversity and abundance have been observed in patients with CWP [7,8,9]. Although the microbiota’s association with chronic pain has been fully confirmed [10,11], the exact molecular mechanism is unclear and requires further research.
The complex and multi-dimensional experience of chronic pain syndrome involves various mechanisms, including one of the most noteworthy mechanisms where the gut microbiota’s regulation of pain has been suggested based on the microbiota–gut–brain axis [12]. A diverse array of signaling molecules produced by the gut microbiota—including microbial metabolites, neurotransmitters, and neuromodulators—binds to specific receptors to significantly influence peripheral and central sensitization processes. These processes are key drivers of chronic pain [13]. In the periphery, these microbiota-derived compounds can directly or indirectly enhance the excitability of nociceptive neurons. Within the central nervous system, they can promote neuroinflammation by activating microglia, compromising the blood–brain barrier, and recruiting immune cells, thereby facilitating the establishment and persistence of central sensitization [13,14]. Animal studies have supported these molecular mechanisms in visceral pain [15,16,17,18], neuropathic pain [19,20], inflammatory pain [21,22], and opioid tolerance [23,24]. Human studies have largely focused on visceral pain and found consistent alterations of gut microbiota in individuals with irritable bowel syndrome and abdominal pain [25,26,27,28]. However, data on the possible role of the gut microbiota in the pathophysiology of extra-intestinal diseases like CWP remains scarce.
While the influence of the gut microbiome on the gut–brain axis and systemic health is widely recognized, its specific implications for CWP remain insufficiently synthesized. To our knowledge, a systematic appraisal linking gut microbial composition, derived metabolites, and microbiome-targeted therapeutic interventions specifically to CWP is lacking. This review aims to address this gap by critically evaluating current evidence on gut microbiome and metabolome profiles in CWP, alongside emerging interventional strategies targeting microbiota for pain management.
2. Methods
2.1. Data Source and Search Strategy
A systematic review was conducted that comprehensively describes the alterations of gut microbiota and microbiota treatments in adult patients with CWP/FM. The protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42023467275) [29]. The systematic review was conducted according to the latest Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [30]. A search of Medline/PubMed, Cochrane library, Amed, Global Health, Embase, and Web of Science databases were performed from database inception to 31 December 2024. We combined the keywords “chronic widespread pain”, “fibromyalgia”, and “microbiota” with the Boolean “AND” and “OR” in abovementioned selected databases (detailed in Appendix A).
2.2. Eligibility Criteria
Eligibility criteria of included articles were as follows: (1) clinical trial or pilot study or observational study; (2) alteration in the gut microbiota; (2) adult patients (≥18 years old) with CWP and/or FM; (3) and/or include microbiota treatment; and (4) until 31 December 2024.
2.3. Data Extraction
Records were managed in Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia; https://app.covidence.org/). Following the removal of duplicate entries, the records were screened to exclude conference abstracts, protocols, review articles, case reports, studies with participants under 18 years of age, and publications that were not pertinent to the review’s aims. The full text of the remaining articles was subsequently obtained and linked to its respective EndNote record. To ensure a comprehensive search, the reference lists of all included studies were also manually examined for any additional relevant publications.
2.4. Quality Assessment
The methodological quality of all included articles was independently appraised by two investigators (LHS and PYL). For this assessment, we employed two validated scales: the Physiotherapy Evidence Database (PEDro) Scale for controlled trials and the Methodological Index for Non-Randomized Studies (MINORS) for observational studies. Any discrepancies in assessment between the two reviewers were resolved through discussion to reach a consensus. This approach to quality evaluation was adapted from established methodological frameworks used in prior research [31,32,33].
3. Results
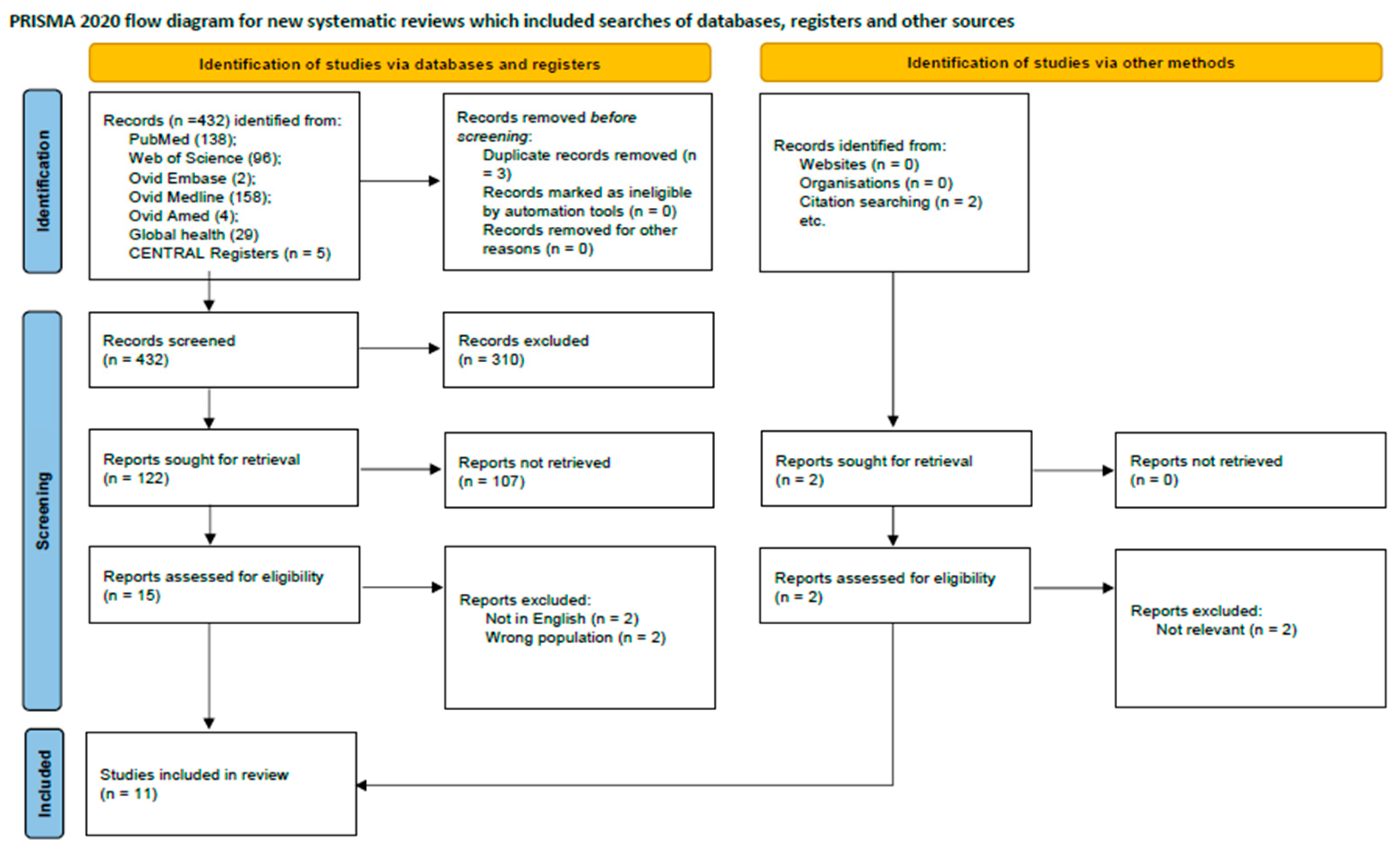
The initial literature search from PubMed, Web of Science, Cochrane library, Ovid Embase, Medline, Ovid Amed, and Global Health databases yielded 432 records, supplemented by two additional records identified from external sources. Following the removal of duplicates and a multi-stage screening process of titles, abstracts, and full-text articles, 11 studies met the eligibility criteria for inclusion. These studies collectively enrolled 20,807 participants. The research landscape was characterized by a high degree of methodological heterogeneity and variable quality among the included articles. The study selection process is detailed in the PRISMA flow diagram (Figure 1), and a summary of included studies is presented in Table 1. Geographically, eight studies were conducted in Europe (Spain, Canada, UK, Denmark) and three in Asia (China, Turkey). The methodological quality for clinical trials and observational studies were acceptable (Table 2 and Table 3).
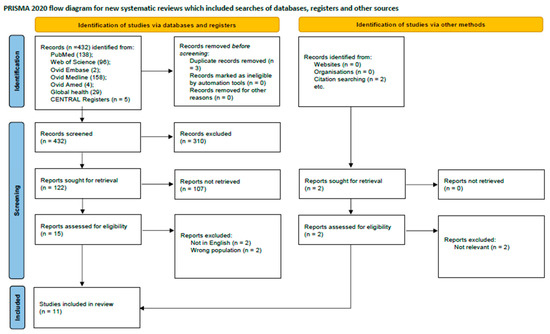
Figure 1.
PRISMA flow diagram showing study selection process.

Table 1.
Characteristics of the included studies investigating gut microbiota in subjects with CWP/FM.

Table 2.
Scale “Physiotherapy Evidence Database (PEDro)” to analyze the methodological quality of clinical control trial studies.

Table 3.
Scale “Methodological Index for Non-Randomized Studies (MINORS)” to analyze the methodological quality of non-randomized studies.
It would not be possible or appropriate to compare the difference in microbiota composition and measure the effect size of treatment with microbiota in these studies due to heterogeneous study designs (observational studies, case–control studies, and only five RCTs) and differences in patient populations and disease duration. Notwithstanding these limitations, this review summarizes and discusses evidence on the alterations of microbiota and treatment with microbiota in CWP/FM patients.
3.1. Alteration of Microbiota in CWP
Different Taxonomy Level Alteration
Alterations of microbiota taxonomy levels have been elaborated in Table 4. At diversity level, two studies found that the alpha diversity is reduced in FM patients [7,8].

Table 4.
Altered microbiota at different levels in CWP/FM patients.
At phylum level, only one study addressed the alteration of microbiota in FM [8], with increased Firmicutes and Bacteroidetes in these patients, but decreased microbiota at the same time (Firmicutes, Bacteroidetes, and Actinobacteria).
At genus level, four studies observed the alterations of microbiota in FM patients. [8,35,41,45] Bacteroides were the common microbiota altered in these studies, with other different genus observed. The abundance of Bacteroides was reduced in FM patients, as were Bifidobacterium, Eubacterium, and Clostridium. However, the abundances of the genera Dorea, Roseburia, Alistipes, Roseburia, Subdoligranulum, and Papillibacter were increased in this group. Of importance, one large observational study found the causal relationship between alteration of five genus in FM patients; they are Coprococcus2, Eggerthella, Lactobacillus, FamillyXIIIUCG001, and Olsenella [41].
At family level, two studies reported the relevant results. Only one bacterial family that was found to be diminished in CWP patients (decreased Ruminococcaceae) [7], with two families (Firmicutes and Lachnospiraceae) increased and decreased at the same time. Specifically for FM patients, two bacteria families were observed to be absent in the core microbiota of these patients, Bifidobacteriaceae and Bacteroidales [8], and other families were decreased. But the Rikenellaceae and Lachnospiraceae family showed an increased abundance in FM patients [8].
At class level, only one study addressed the alteration in which Actinobacteria was decreased in FM patients compared with controls [8].
At the species level, two studies elaborated that different microbiota were altered in FM [45] or CWP patients [7]. The increased butyriciproducens, F. plautii, B. desmolans, E. tayi, E. massiliensis, Parabacteroides merdae, Akkermansia muciniphila, and Clostridium scindens were observed in FM patients, while it was the reverse for F. prausnitzii, B. uniformis, Haemophilus, P. copri, and Blautia faecis. In CWP patients [7], massiliensis was shown to be the common one altered between CWP and FM. Increased odontolyticus and decreased excrementihominis, obeum, formicigenerans, splanchnicus, ureilytica, inulinivorans, and Coprococcus comes were reported.
3.2. Metabolic Function of Microbiota in CWP
For FM patients in the study by Minerbi et al. [45], F. prausnitzii decreased in FM patients as evident by the presence of circulating short-chain fatty acids (SCFA). Among the 19 species demonstrating significant differential abundance (DA) between FM patients and healthy controls, the level of scientific characterization varied widely. Species found to be depleted in FM patients, such as F. prausnitzii, B. uniformis, P. copri, and Blautia faecis, are generally well-documented in the literature [45]. In contrast, species exhibiting increased abundance in patients, including Intestinimonas butyriciproducens, Flavonifractor plautii, Butyricoccus desmolans, and members of the Eisenbergiella genus (E. tayi and E. massiliensis), are less extensively characterized [45]. Moreover, the abundance of the Bifidobacterium and Eubacterium genera (bacteria participating in the metabolism of neurotransmitters in the host) in FM patients was significantly reduced [8].
Of note, when looking at the association between altered microbiota and clinical functional performance in FM patients, Bacteroides spp. was positively correlated with total symptom score on the Fibromyalgia Impact Questionnaire (FIQ) [45].
3.3. Treatments with Microbiota in FM
There are only five studies investigating the effects of drugs with microbiota (probiotics or prebiotics) on FM patients (Table 5). A study by Aslan et al. [37] utilized a high-potency probiotic formulation (1 × 10^10 CFU) comprising a multi-strain consortium, including Lactobacillus acidophilus L1, Lactobacillus rhamnosus liobif, Bifidobacterium longum, and Saccharomyces boulardii. This intervention was associated with significant improvements in self-reported pain, sleep quality, and overall quality of life in patients with fibromyalgia. Furthermore, the administration of this probiotic blend was linked to a notable alleviation of depressive and anxiety symptoms, a finding consistent with earlier research in this population [46,47].

Table 5.
Treatment with microbiota in CWP/FM.
The efficacy of gut microbiota-based therapy was assessed using patient-reported outcomes. Two RCTs revealed no statistically significant differences between the intervention and control groups for scores on the visual analogue scale (VAS) or the Fibromyalgia Impact Questionnaire (FIQ) [39,40,43].
Of note, there is only one RCT investigating the potential efficiency of fecal microbiota transplantation (FMT) in FM patients; it seems FMT had improved the clinical symptoms of patients with FM after 6 and 12 months, including widespread pain intensity, anxiety, depression, and sleep. Moreover, certain altered neurotransmitters have been observed with FMT therapy in FM.
In addition, only one prospective cohort study lasting one month has shown the symbiotic intervention where probiotic and prebiotic strains decreased the levels of stress, anxiety as well as depression, and improved quality of life during patients’ daily activities. This intervention improvement was observed to dysregulate the inflammatory and stress responses, particularly in those without a previous chronic fatigue syndrome (CFS) diagnosis.
4. Discussion
We aim to systematically evaluate the literature reporting evidence associated with gut microbiota in people with CWP/FM. The review identified broad areas of research including alterations in microbiota composition and its relation to human functions as well as the effects of treatment with microbiota on CWP patients. Based on clinical trials and observational studies with acceptable methodological quality, we found significant alterations of microbiota composition in different taxonomy levels, but the effects of current probiotics or prebiotics are debatable in improving the symptoms of FM patients.
We found few common microbiota across different studies. The alterations of microbiota in different taxonomy levels are quite different across these included studies (Table 5). With rapid developments in microbiota technology over the last two decades, it is not surprising that a diverse set of methodological approaches were employed in the assessment of microbiota compositions, which may account for this observation in this review. We also noted that the diagnosis criteria of FM across included studies is different. For Clos-Garcia et al. [8], the different selections of FM patients may yield different compositions. For CWP patients, although only one study by Freidin et al. [7] reported the composition alterations, it is reasonable that different alterations of microbiota communities were observed in these milder patients, since FM patients who were deemed to be more severe with psychological involvement. However, the studies identified did not provide adequate substance for inclusion, and more future investigations are needed to address this field.
Gut microbiota has been closely linked to metabolic, immunological, and neurological functions in humans. Importantly, in FM patients, an abundance of butyrate producers (I. butyriciproducens, F. plautii, B. desmolans, E. tayi, Parabacteroides merdae, and E. massiliensis) has been detected [48,49,50,51,52,53,54]. While the relative abundance of certain butyrate-producing bacteria was reduced in FM patients, including F. prausnitzii and B. uniformis [45], an elevated abundance was observed for other microbial taxa with distinct metabolic functions. Notably, FM patients exhibited a higher abundance of Clostridium scindens and B. desmolans, two species implicated in steroid hormone metabolism through the activity of 20α-hydroxysteroid dehydrogenase, which facilitates the conversion of cortisol to androgens [55,56,57,58,59]. This analysis revealed a higher relative abundance of two specific species in FM patients: Parabacteroides merdae and Akkermansia muciniphila. This finding is intriguing given that these same species were previously identified by Olson et al. as critical mediators of the antiseizure effects of a ketogenic diet [60]. Species with a putative pro-inflammatory role such as P. copri, Bacteroides Uniformis, and Haemophilus parainfluenza were depleted in FM patients [34,61,62]. The alterations of composition observed in our review will be valuable to explore their link to different functions abovementioned.
Although some probiotic species (Lactobacillus rhamnosus GG, L. paracasei, L. acidophilus, and Bifidobacterium bifidus) have been used previously to improve functions related to the gut–brain axis [33,39,40], inclusive evidence observed in recent trials preclude the conclusion whether the probiotic or prebiotic treatment is effective in FM patients. However, Roman et al. reported that using probiotic group containing Lactobacillus acidophilus or Lactobacillus Rhamnosus GG ® did not significantly improve depressive or anxiety symptoms when compared to the placebo group [43]. Moreover, another preclinical study [36] reported that probiotics L. reuteri LR06 or Bifidobacterium BL5b had no significant antinociception effects in chronic pain rats.
Unfortunately, there is no study investigating the treatment effect with microbiota on CWP patients, which leaves a huge gap in this field. For CWP patients, an animal study has found that Lactobacillus rhamnosus GG (10 billion CFU/150 mL) can attenuate muscle mechanical hyperalgesia in early-life stress-induced widespread muscle pain in adult rats [38]. The different animal findings between FM and CWP may be ascribed to the different species used in animals, but it cannot be ruled out that their effects are different since FM is the severe type of CWP. In addition, whether the therapeutic effect of FMT on the clinical symptoms of FM patients could apply in CWP patients remains uncertain. Future larger cohorts and well-designed RCT are needed to confirm the effect of these therapies on CWP patients.
4.1. Interpretation of Findings and Causal Inference
This study identifies several significant alterations in the gut microbiota of individuals with FM/CMP compared to healthy controls, primarily observing a decrease in taxa such as Faecalibacterium prausnitzii, Ruminococcaceae, and Bifidobacteriaceae. The associations reported here do not establish causality; the observed dysbiosis could be a cause, a consequence, or a parallel phenomenon of the pathophysiology of FM/CWP. The functional implications of these taxonomic shifts are informed primarily by preclinical evidence. For instance, we observed a decreased abundance of Faecalibacterium prausnitzii, a species which in vitro and animal studies have shown to be a prominent producer of the anti-inflammatory short-chain fatty acid (SCFA) butyrate [45]. Mechanistic studies suggest that butyrate can modulate immune function by inhibiting histone deacetylase (HDAC) activity and promoting regulatory T-cell differentiation [63,64]. Therefore, it is plausible that the lower levels of F. prausnitzii we observed are associated with a reduced capacity for butyrate production, which may potentially contribute to a pro-inflammatory state that has been hypothesized in FM/CWP. Similarly, the literature from animal models indicates that certain Bifidobacterium species can influence host stress response and cortisol metabolism through the gut–brain axis [65,66]. While this provides a mechanistic rationale for a link between their absence and altered central pain processing, it remains a hypothetical link in humans with FM/CWP. Future intervention studies (e.g., probiotic supplementation) are required to test this causal relationship.
4.2. Strengths and Limitations
A significant strength of this review is that this is the first systematic review focusing on CWP patients regarding the alterations in microbiota composition and its relation to human functions as well as the effects of treatment with microbiota. However, methodical differences, heterogeneous study designs, and differences in criteria of patient populations pose limitations to our findings. Moreover, the small number of studies included in investigating microbiota and CWP precluded drawing adequate conclusions. In addition, limitations also included the risk of publication bias and selective reporting given the small number of RCTs and pilot designs. Lastly, although some positive findings have been reported for probiotics/synbiotics and results from one FMT trial and multiple RCTs were negative regarding VAS/FIQ, some limitations of the study designs cannot be neglected.
4.3. Future Direction
Future studies should prioritize investigating the functional implications of the sex-dependent microbial signatures identified here, particularly the elevated Ruminococcus and Pseudomonas in females [35]. While community structure (as reflected in calculated microbiota ratios Firmicutes/Bacteroidetes, Bacteroides/Prevotella, and Roseburia/Eubacterium) may not differ, these specific taxonomic shifts suggest a potential role in mediating the well-documented female predominance in FM prevalence and symptom severity. Targeted culturing and genomic analysis of these gender-specific taxa are needed to elucidate their mechanistic role in sex-biased pain pathways.
5. Conclusions
Despite these limitations and the heterogeneity of the studies included, this systematic review suggests that the gut microbiota is altered in different taxonomy levels in CWP patients, and the therapeutic potential of targeted interventions for alleviating fibromyalgia symptoms remains a subject of ongoing scientific discourse. To resolve this, rigorously controlled trials utilizing advanced methodologies, standardized diagnostic criteria, and robust statistical adjustment for confounding variables are critically needed.
Author Contributions
Conceptualization L.-H.S.; methodology L.-H.S. and P.-Y.L.; software L.-H.S. and P.-Y.L.; validation, L.-H.S.; formal analysis, L.-H.S. and P.-Y.L.; investigation, L.-H.S. and P.-Y.L.; resources, L.-H.S. and P.-Y.L.; data curation, L.-H.S.; writing—original draft preparation, L.-H.S. and P.-Y.L.; writing—review and editing, L.-H.S.; visualization, L.-H.S. and P.-Y.L.; supervision, L.-H.S.; project administration, L.-H.S.; funding acquisition, L.-H.S. and P.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available on request from authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Search Strategy Used for the Medline Database to Identify Literature Reporting on Terms Related to CWP/FM and Gut Microbiota
| Search term(s): |
| 1. (“fibromyalgia” [MeSH Terms] OR “fibromyositis” [All Fields] OR “fibrositis” [All Fields] OR “muscular rheumatism” [All Fields]) |
| 2. (“chronic pain, widespread” [All Fields] OR “chronic widespread pain” [All Fields]) |
| 3. (“microbiota” [MeSH Terms] OR “microbiota” [All Fields] OR “microbiota” [All Fields] OR “microbiota” [All Fields]) |
| 4. 1 or 2 |
| 5. 3 and 4 |
| 6. Limit 10 to English language, Adults |
| 7. Limit 11 to publication date: 1 January 1976 ^ to 31 December 2024 |
| ^ the year “fibromyalgia” became the official term for the condition [67]. |
References
- Mansfield, K.E.; Sim, J.; Jordan, J.L.; Jordan, K.P. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain 2016, 157, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Burri, A.; Ogata, S.; Lachance, G.; Williams, F. Chronic widespread pain: Clinical comorbidities and psychological correlates. Pain 2015, 156, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Di Paola, R.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef] [PubMed]
- Slim, M.; Calandre, E.P.; Rico-Villademoros, F. An insight into the gastrointestinal component of fibromyalgia: Clinical manifestations and potential underlying mechanisms. Rheumatol. Int. 2015, 35, 433–444. [Google Scholar] [CrossRef]
- Raffaeli, W.; Malafoglia, V.; Bonci, A.; Tenti, M.; Ilari, S.; Gremigni, P.; Iannuccelli, C.; Gioia, C.; Di Franco, M.; Mollace, V.; et al. Identification of MOR-Positive B Cell as Possible Innovative Biomarker (Mu Lympho-Marker) for Chronic Pain Diagnosis in Patients with Fibromyalgia and Osteoarthritis Diseases. Int. J. Mol. Sci. 2020, 21, 1499. [Google Scholar] [CrossRef]
- Ilari, S.; Passacatini, L.C.; Malafoglia, V.; Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Palma, E.; Tomino, C.; Fini, M.; Raffaeli, W.; et al. Tantali fibromyalgic supplicium: Is there any relief with the antidepressant employment? A systematic review. Pharmacol. Res. 2022, 186, 106547. [Google Scholar] [CrossRef]
- Freidin, M.B.; Stalteri, M.A.; Wells, P.M.; Lachance, G.; Baleanu, A.-F.; Bowyer, R.C.; Kurilshikov, A.; Zhernakova, A.; Steves, C.J.; Williams, F.M.K. An association between chronic widespread pain and the gut microbiome. Rheumatology 2021, 60, 3727–3737. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef]
- Marazzato, M.; Iannuccelli, C.; Guzzo, M.P.; Nencioni, L.; Lucchino, B.; Radocchia, G.; Gioia, C.; Bonfiglio, G.; Neroni, B.; Guerrieri, F.; et al. Gut Microbiota Structure and Metabolites, Before and After Treatment in Early Rheumatoid Arthritis Patients: A Pilot Study. Front. Med. 2022, 9, 921675. [Google Scholar] [CrossRef]
- Vendrik, K.E.W.; Ooijevaar, R.E.; De Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; Van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The Gut Microbiome in Adult and Pediatric Functional Gastrointestinal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef]
- Russo, R.; Cristiano, C.; Avagliano, C.; De Caro, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut-brain axis: Role of lipids in the regulation of inflammation, pain and CNS diseases. Curr. Med. Chem. 2018, 25, 3930–3952. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.-H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hua, D.; Wang, Q.; Yang, L.; Wang, X.; Luo, A.; Yang, C. The role of bacteria and its derived metabolites in chronic pain and depression: Recent findings and research progress. Int. J. Neuropsychopharmacol. 2020, 23, 26–41. [Google Scholar] [CrossRef] [PubMed]
- O’mAhony, S.; Felice, V.; Nally, K.; Savignac, H.; Claesson, M.; Scully, P.; Woznicki, J.; Hyland, N.; Shanahan, F.; Quigley, E.; et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014, 277, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Kannampalli, P.; Pochiraju, S.; Chichlowski, M.; Berg, B.M.; Rudolph, C.; Bruckert, M.; Miranda, A.; Sengupta, J.N. Probiotic L actobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol. Motil. 2014, 26, 1694–1704. [Google Scholar] [CrossRef]
- Miquel, S.; Martin, R.; Lashermes, A.; Gillet, M.; Meleine, M.; Gelot, A.; Eschalier, A.; Ardid, D.; Bermudez-Humaran, L.G.; Sokol, H.; et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci. Rep. 2016, 6, 19399. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef]
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017, 20, 1213–1216. [Google Scholar] [CrossRef]
- Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Li, S.; Bi, J.; Jiang, R.; Yang, L.; Miao, L.; Zhu, B.; et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry 2019, 9, 57. [Google Scholar] [CrossRef]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar]
- Vieira, A.T.; Macia, L.; Galvão, I.; Martins, F.S.; Canesso, M.C.C.; Amaral, F.A.; Garcia, C.C.; Maslowski, K.M.; De Leon, E.; Shim, D.; et al. A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol. 2015, 67, 1646–1656. [Google Scholar]
- Kang, M.; Mischel, R.A.; Bhave, S.; Komla, E.; Cho, A.; Huang, C.; Dewey, W.L.; Akbarali, H.I. The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci. Rep. 2017, 7, srep42658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, J.; Ban, Y.; Jalodia, R.; Chupikova, I.; Fernandez, I.; Brito, N.; Sharma, U.; Abreu, M.T.; Ramakrishnan, S.; et al. Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 13523–13532. [Google Scholar] [PubMed]
- Ringel-Kulka, T.; Goldsmith, J.R.; Carroll, I.M.; Barros, S.P.; Palsson, O.; Jobin, C.; Ringel, Y. Lactobacillus acidophilus NCFM affects colonic mucosal opioid receptor expression in patients with functional abdominal pain—A randomised clinical study. Aliment. Pharmacol. Ther. 2014, 40, 200–207. [Google Scholar] [PubMed]
- Saulnier, D.M.; Riehle, K.; Mistretta, T.A.; Diaz, M.A.; Mandal, D.; Raza, S.; Weidler, E.M.; Qin, X.; Coarfa, C.; Milosavljevic, A.; et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1782–1791. [Google Scholar] [CrossRef]
- Theodorou, V.; Ait-Belgnaoui, A.; Agostini, S.; Eutamene, H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes 2014, 5, 430–436. [Google Scholar] [CrossRef]
- Newlove-Delgado, T.; Abbott, R.A.; Martin, A.E. Probiotics for children with recurrent abdominal pain. JAMA Pediatr. 2019, 173, 183–184. [Google Scholar] [CrossRef]
- Centre for Reviews Dissemination University of York. PROSPERO: International Prospective Register of Systematic Reviews 2017. Available online: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 26 September 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar]
- Oliva, E.M.; Villafañe, J.H.; Pérez, J.L.A.; Sal, A.A.; Carlier, G.M.; García, A.Q.; Turroni, S.; Martínez-Pozas, O.; Izquierdo, N.V.; Romero, E.A.S. Effect of Exercise on Inflammation in Hemodialysis Patients: A Systematic Review. J. Pers. Med. 2022, 12, 1188. [Google Scholar] [CrossRef]
- Sanchez Romero, E.A.; Martínez-Pozas, O.; García-González, M.; De-Pedro, M.; González-Álvarez, M.E.; Esteban-González, P.; Cid-Verdejo, R.; Villafañe, J.H. Association between Sleep Disorders and Sleep Quality in Patients with Temporomandibular Joint Osteoarthritis: A Systematic Review. Biomedicines 2022, 10, 2143. [Google Scholar] [CrossRef]
- Schulté, B.; Nieborak, L.; Leclercq, F.; Villafañe, J.H.; Romero, E.A.S.; Corbellini, C. The Comparison of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training after Coronary Artery Bypass Graft: A Systematic Review of Recent Studies. J. Cardiovasc. Dev. Dis. 2022, 9, 328. [Google Scholar] [CrossRef]
- Hernandez, C.J. The Microbiome and Bone and Joint Disease. Curr. Rheumatol. Rep. 2017, 19, 77. [Google Scholar] [CrossRef]
- Ramirez-Tejero, J.A.; Durán-González, E.; Martínez-Lara, A.; del Amo, L.L.; Sepúlveda, I.; Huancas-Díaz, A.; Carvajal, M.; Cotán, D. Microbiota and Mitochondrial Sex-Dependent Imbalance in Fibromyalgia: A Pilot Descriptive Study. Neurol. Int. 2023, 15, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, C.; Wang, J.; Guo, Q.; Zou, W. Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats. Brain Behav. 2019, 9, e01260. [Google Scholar] [CrossRef] [PubMed]
- Aslan Çİn, N.N.; Açik, M.; Tertemİz, O.F.; Aktan, Ç.; Akçali, D.T.; Çakiroğlu, F.P.; Özçelİk, A.Ö. Effect of prebiotic and probiotic supplementation on reduced pain in patients with fibromyalgia syndrome: A double-blind, placebo-controlled randomized clinical trial. Psychol. Health Med. 2024, 29, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Green, P.G.; Alvarez, P.; Levine, J.D. A role for gut microbiota in early-life stress-induced widespread muscle pain in the adult rat. Mol. Pain 2021, 17, 17448069211022952. [Google Scholar] [CrossRef]
- Cardona, D.; Roman, P.; Cañadas, F.; Sánchez-Labraca, N. The Effect of Multiprobiotics on Memory and Attention in Fibromyalgia: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 3543. [Google Scholar] [CrossRef]
- Calandre, E.P.; Hidalgo-Tallon, J.; Molina-Barea, R.; Rico-Villademoros, F.; Molina-Hidalgo, C.; Garcia-Leiva, J.M.; Carrillo-Izquierdo, M.D.; Slim, M. The Probiotic VSL#3® Does Not Seem to Be Efficacious for the Treatment of Gastrointestinal Symptomatology of Patients with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals 2021, 14, 1063. [Google Scholar]
- Wang, Z.; Jiang, D.; Zhang, M.; Teng, Y.; Huang, Y. Causal association between gut microbiota and fibromyalgia: A Mendelian randomization study. Front. Microbiol. 2023, 14, 1305361. [Google Scholar] [CrossRef]
- Fang, H.; Hou, Q.; Zhang, W.; Su, Z.; Zhang, J.; Li, J.; Lin, J.; Wang, Z.; Yu, X.; Yang, Y.; et al. Fecal Microbiota Transplantation Improves Clinical Symptoms of Fibromyalgia: An Open-Label, Randomized, Nonplacebo-Controlled Study. J. Pain 2024, 25, 104535. [Google Scholar] [CrossRef]
- Roman, P.; Estévez, A.F.; Miras, A.; Sánchez-Labraca, N.; Cañadas, F.; Vivas, A.B.; Cardona, D. A Pilot Randomized Controlled Trial to Explore Cognitive and Emotional Effects of Probiotics in Fibromyalgia. Sci. Rep. 2018, 8, 10965. [Google Scholar] [CrossRef]
- Hinchado, M.D.; Quero-Calero, C.D.; Otero, E.; Gálvez, I.; Ortega, E. Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome. Nutrients 2023, 15, 1591. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Rashid, S. Functional and therapeutic potential of inulin: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–13. [Google Scholar] [PubMed]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Jin, T.-E.; Chang, D.-H.; Rhee, M.-S.; Kim, H.J.; Lee, S.J.; Park, D.-S.; Kim, B.-C. Agathobaculum butyriciproducens gen. nov. sp. nov., a strict anaerobic, butyrate-producing gut bacterium isolated from human faeces and reclassification of Eubacterium desmolans as Agathobaculum desmolans comb. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 3656–3661. [Google Scholar] [CrossRef]
- Amir, I.; Bouvet, P.; Legeay, C.; Gophna, U.; Weinberger, A. Eisenbergiella tayi gen. nov., sp. nov., isolated from human blood. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 3, 907–914. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Shetty, S.A.; Lagkouvardos, I.; Ritari, J.; Chamlagain, B.; Douillard, F.P.; de Vos, W.M. Comparative genomics and physiology of the butyrate-producing bacterium Intestinimonas butyriciproducens. Environ. Microbiol. Rep. 2016, 8, 1024–1037. [Google Scholar] [CrossRef]
- Carlier, J.-P.; Bedora-Faure, M.; K’OUas, G.; Alauzet, C.; Mory, F. Proposal to unify Clostridium orbiscindens Winter et al. 1991 and Eubacterium plautii (Seguin 1928) Hofstad and Aasjord 1982, with description of Flavonifractor plautii gen. nov., comb. nov., and reassignment of Bacteroides capillosus to Pseudoflavonifractor capillosus gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 585–590. [Google Scholar] [CrossRef]
- Kläring, K.; Hanske, L.; Bui, N.; Charrier, C.; Blaut, M.; Haller, D.; Plugge, C.M.; Clavel, T. Intestinimonas butyriciproducens gen. nov., sp. nov., a butyrate-producing bacterium from the mouse intestine. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 12, 4606–4612. [Google Scholar] [CrossRef]
- Takada, T.; Watanabe, K.; Makino, H.; Kushiro, A. Reclassification of Eubacterium desmolans as Butyricicoccus desmolans comb. nov., and description of Butyricicoccus faecihominis sp. nov., a butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 2016, 66, 4125–4131. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.H.; Khelaifia, S.; Bittar, F.; Maraninchi, M.; Raoult, D.; Million, M. ‘Eisenbergiella massiliensis’, a new species isolated from human stool collected after bariatric surgery. New Microbes New Infect. 2016, 13, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Bloem, L.M.; Storbeck, K.-H.; Schloms, L.; Swart, A.C. 11β-hydroxyandrostenedione returns to the steroid arena: Biosynthesis, metabolism and function. Molecules 2013, 18, 13228–13244. [Google Scholar] [CrossRef] [PubMed]
- Devendran, S.; Mendez-Garcia, C.; Ridlon, J.M. Identification and characterization of a 20β-HSDH from the anaerobic gut bacterium Butyricicoccus desmolans ATCC 43058. J. Lipid Res. 2017, 58, 916–925. [Google Scholar] [CrossRef]
- Morris, D.J.; Ridlon, J.M. Glucocorticoids and gut bacteria: “The GALF Hypothesis” in the metagenomic era. Steroids 2017, 125, 1–13. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Ikegawa, S.; Alves, J.M.P.; Zhou, B.; Kobayashi, A.; Iida, T.; Mitamura, K.; Tanabe, G.; Serrano, M.; De Guzman, A.; et al. Clostridium scindens: A human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 2013, 54, 2437–2449. [Google Scholar] [CrossRef]
- Swart, A.C.; Storbeck, K.H. 11beta-Hydroxyandrostenedione: Downstream metabolism by 11betaHSD, 17betaHSD and SRD5A produces novel substrates in familiar pathways. Mol. Cell Endocrinol. 2015, 408, 114–123. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Li, S.; Yang, L.; Zhu, D.; Wang, Y.; Wang, H.; Wang, T.; Shi, B.; Gai, Z.; et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci. Rep. 2018, 8, 14305. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133 (Suppl. S7), 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Inanici, F.; Yunus, M.B. History of fibromyalgia: Past to present. Curr. Pain Headache Rep. 2004, 8, 369–378. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).