The Role of Obesity in the Regulation of Immunosuppressive Cell Infiltration and Immunosurveillance in Cancers
Abstract
1. Introduction
2. Obesity-Associated Factors in Inflammation, Energy Metabolism, and Cancer
2.1. Adipokines
2.2. Cytokines
2.3. Chemokines
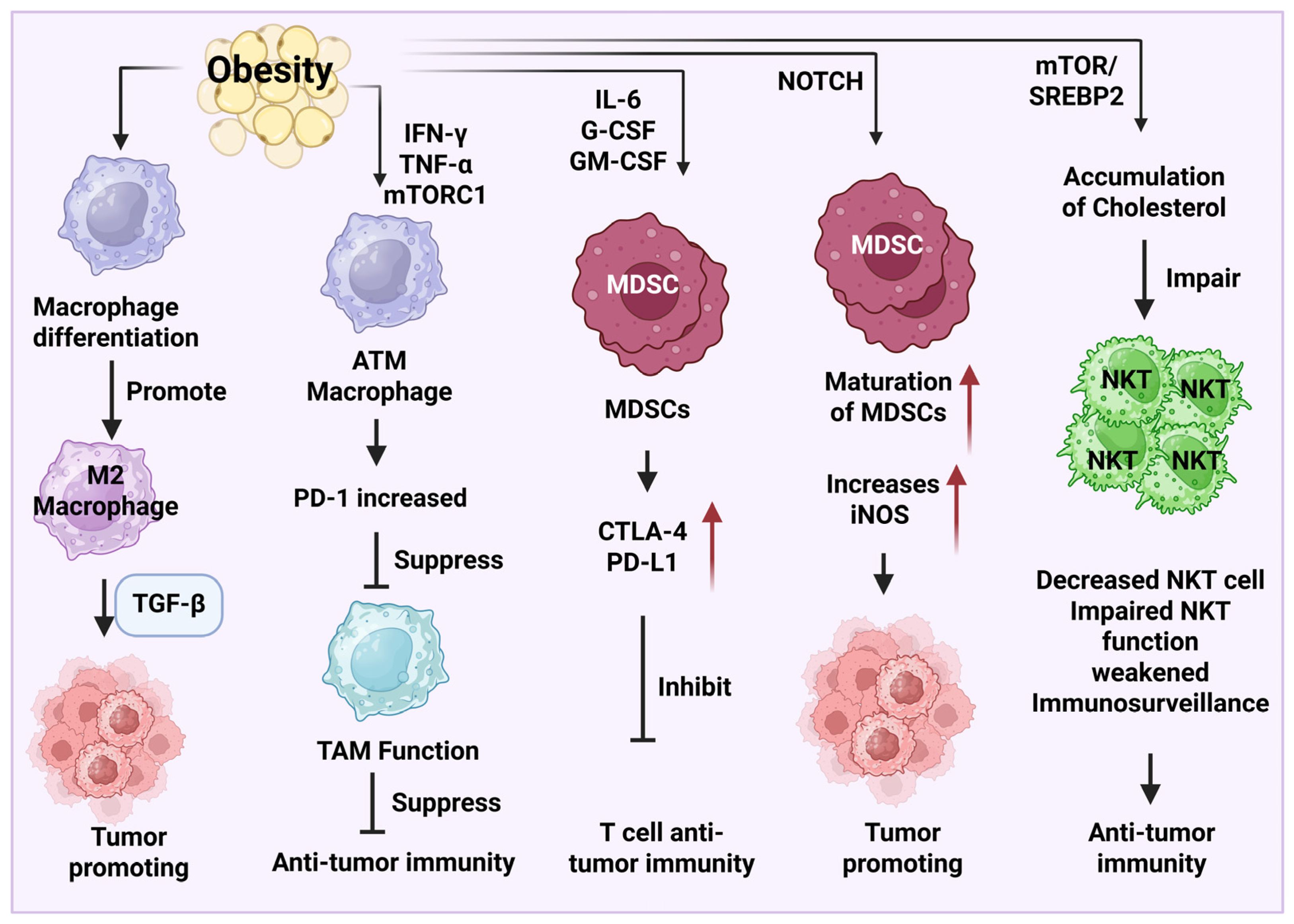
3. Obesity-Associated Immunosuppressive Microenvironment
3.1. CD8 T Cells
3.2. Macrophages, MDSCs, and NKT Cells
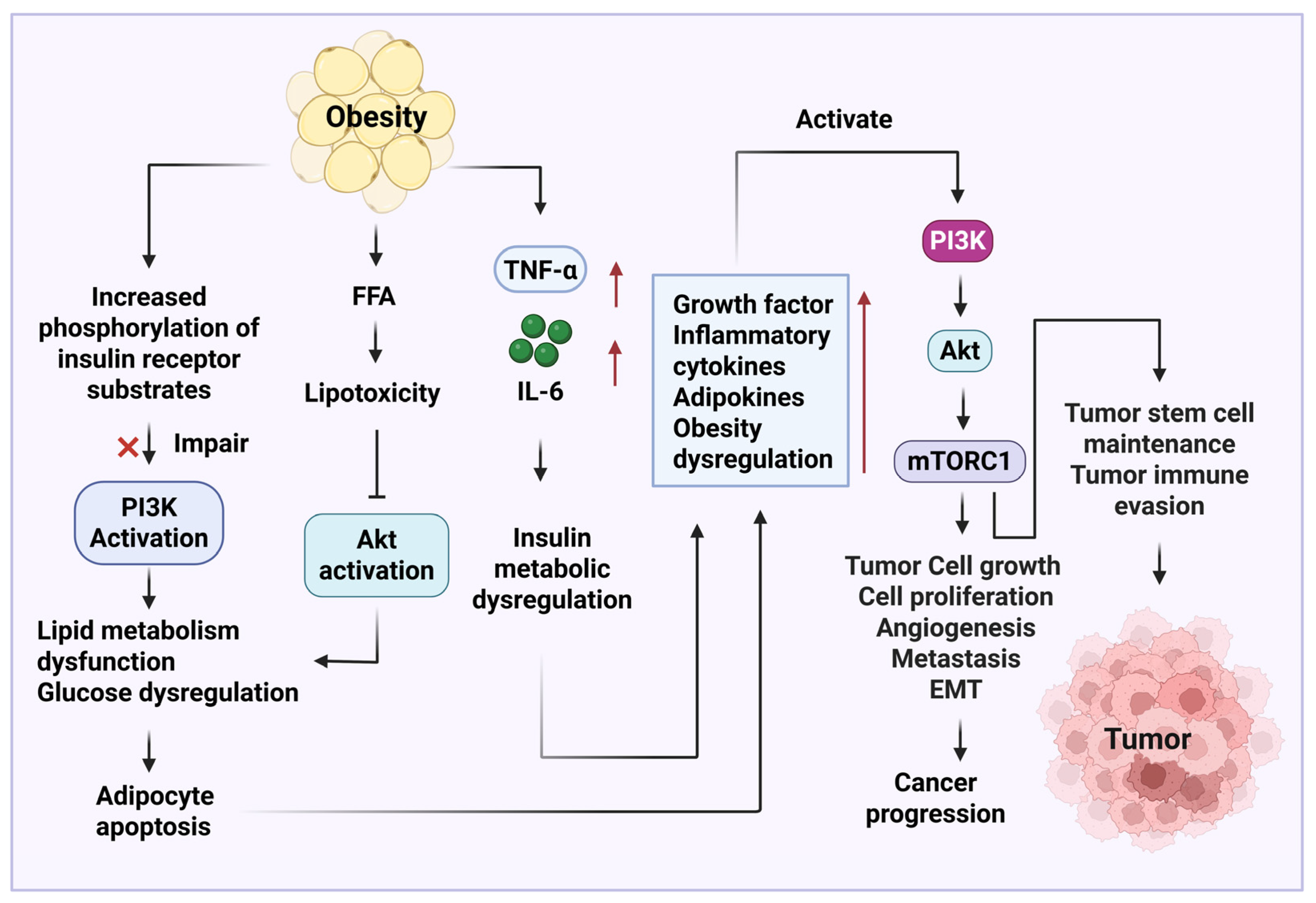
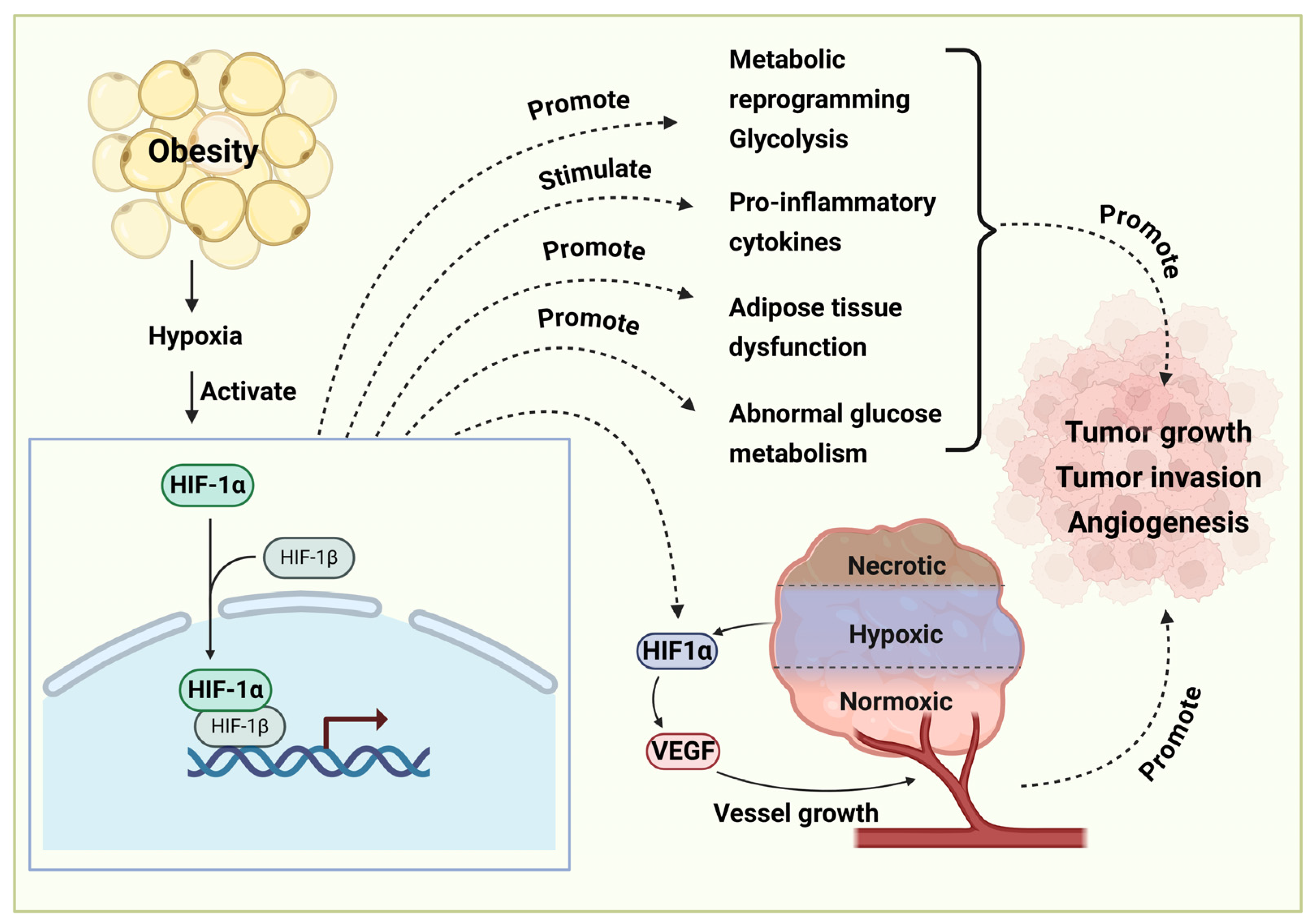
4. Important Signaling Pathways Involved in Obesity-Related Cancer Development and Therapy
4.1. JNK, IKK/NF-κB, and STAT3 Signaling Pathways
4.2. PI3K/AKT/mTOR Signaling Pathway
4.3. Wnt/β-Catenin Signaling Pathway
4.4. NOTCH Signaling Pathway
4.5. HIF-1α Signaling Pathway
5. Natural Products in Obesity-Associated Cancer Therapy
5.1. Ginsenosides
5.2. Artemisinin
5.3. Paclitaxel
5.4. Hesperidin
5.5. Quercetin
5.6. Celastrol
5.7. Curcumin
5.8. Ursolic Acid
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acs-2 | acyl-CoA synthetase |
| AGEs | Advanced glycation end products |
| AKT | Protein kinase B |
| AMPK | 5′-adenosine monophosphate (AMP)-activated protein kinase |
| ATMs | Adipose tissue macrophages |
| BAP1 | BRCA1-associated protein 1 |
| Bax | BCL2-associated X |
| Bcl-2 | B-cell lymphoma 2 |
| BMI | Body mass index |
| BRCA1 | Breast cancer susceptibility gene 1 |
| C/EBPβ | CCAAT enhancer-binding protein β |
| CCK | Cholecystokinin |
| CCL | Chemokine (C-C motif) ligand |
| CCR | C-C chemokine receptor |
| CD8 | Cluster of differentiation 8 |
| c-Jun | Jun proto-oncogene |
| CRP | C-reactive protein |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CVD | Cardiovascular diseases |
| CXCL | Chemokine (C-X-C motif) ligand |
| CXCR | Chemokine receptors |
| DTC | Differentiated thyroid cancer |
| EC | Endometrial cancer |
| EGFR | Epidermal growth factor receptor |
| ELAM-1 | Endothelial-leukocyte adhesion molecule 1 |
| EMT | Epithelial–mesenchymal transition |
| EOC | Epithelial ovarian cancer |
| ERK1/2 | Extracellular signal-related kinases 1 and 2 |
| ERS | Endoplasmic reticulum stress |
| FAT-6 | Stearoyl-CoA desaturase |
| FFA | Free fatty acid |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| G-MDSC | Granulocytic myeloid-derived suppressor cells |
| GzmB | Granzyme B |
| HCC | Hepatocellular carcinoma |
| HDL | High-density lipoprotein |
| HepG2 | Liver cancer cells |
| HIF1α | Hypoxia-inducible factor 1 alpha |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IFN-γ | Interferon gamma |
| IKK | Inhibitory-κB kinase |
| IL-6 | Interleukin 6 |
| iNOS | Inducible nitric oxide synthase |
| IR | Insulin resistance |
| IRS-1 | Insulin receptor substrate 1 |
| JAK2 | Janus kinase 2 |
| JNK | c-Jun N-terminal kinases |
| Kat-1 | 3-ketoacyl-CoA thiolase 1 |
| Ki-67 | Marker of proliferation Kiel 67 |
| KRAS | Kirsten rat sarcoma virus |
| Lag3 | Lymphocyte activation gene 3 protein |
| LAMs | Lipid-associated macrophages |
| LD | Linear dichroism |
| LDL | Low-density lipoprotein |
| MAPK | Mitogen-activated protein kinase |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MCF-7 | Michigan cancer foundation-7 cancer cell line |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDSC | Myeloid-derived suppressor cell |
| Mdt-15 | Mediator subunit MDT-15 |
| MHC-II | Major histocompatibility complex class II molecules |
| MIP-1α | Macrophage inflammatory protein (MIP)-1α |
| MIP-1β | Macrophage inflammatory protein (MIP)-1β |
| M-MDSCs | Monocytic myeloid-derived suppressor cells |
| mTOR | Mammalian target of rapamycin |
| mTORC | Mammalian target of rapamycin complex |
| MYC | Myelocytomatosis oncogene |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cell | Natural killer cell |
| NKT cell | Natural killer T cell |
| NLRP3 | NLR family pyrin domain containing 3 |
| NOTCH | Neurogenic locus notch homolog protein |
| NSCLC | Non-small cell lung cancer |
| p53 | Tumor protein p53 |
| PCNA | Proliferating cell nuclear antigen |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1-α |
| PI3K | Phosphoinositide 3-kinases |
| PKC | Protein kinase C |
| PLK-2 | Polo-like kinase 2 |
| pod-2 | Gene encoding acetyl-CoA carboxylase |
| PPARα | Peroxisome proliferator-activated receptor-alpha |
| PPAR-γ | Peroxisome proliferator-activated receptor γ |
| PRDM16 | PR domain containing 16 |
| RAB7 | Ras-related protein |
| ROS | Reactive oxygen species |
| S6 | Ribosomal protein S6 |
| SETD2 | SET domain-containing 2 protein |
| SHH | Sonic hedgehog protein |
| SLC7A5 | Solute carrier family 7 member 5 |
| SREBP1c | Sterol regulatory element binding protein 1c |
| SREBP2 | Sterol regulatory element-binding protein 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| STC-1 cells | Intestinal secretin tumor cell line |
| T2D | Type 2 diabetes |
| TGF-β | Transforming growth factor-β |
| TGR5 | Takeda G protein-coupled receptor 5 |
| TGs | Triglycerides |
| Th1 | Type 1 T helper cell |
| Th17 | T helper type 17 |
| TILs | Tumor-infiltrating lymphocytes |
| Tim3 | T-cell immunoglobulin and mucin domain 3 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| Treg | Regulatory T cell |
| Trem2 | Triggering receptor expressed on myeloid cells 2 |
| UCP1 | Uncoupling protein 1 |
| VAMP7 | Vesicle-associated membrane protein 7 |
| VAT | Visceral adipose tissue |
| VCAM-1 | Vascular-cell adhesion molecule-1 |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless-type MMTV integration site family |
| ZEB1 | Zinc finger E-box binding homeobox 1 |
References
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Ogden, C.L. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA 2018, 319, 1723–1725. [Google Scholar] [CrossRef]
- Tsigos, C.; Hainer, V.; Basdevant, A.; Finer, N.; Fried, M.; Mathus-Vliegen, E.; Micic, D.; Maislos, M.; Roman, G.; Schutz, Y.; et al. Management of obesity in adults: European clinical practice guidelines. Obes. Facts 2008, 1, 106–116. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. Economic Impact of Overweight and Obesity to Surpass $4 Trillion by 2035. Available online: https://www.worldobesity.org/ (accessed on 9 July 2025).
- Lopez-Jimenez, F.; Almahmeed, W.; Bays, H.; Cuevas, A.; Di Angelantonio, E.; le Roux, C.W.; Sattar, N.; Sun, M.C.; Wittert, G.; Pinto, F.J.; et al. Obesity and cardiovascular disease: Mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur. J. Prev. Cardiol. 2022, 29, 2218–2237. [Google Scholar] [CrossRef] [PubMed]
- Julián, M.T.; Arteaga, I.; Torán-Monserrat, P.; Pera, G.; Pérez-Montes de Oca, A.; Ruiz-Rojano, I.; Casademunt-Gras, E.; Chacón, C.; Alonso, N. The Link between Abdominal Obesity Indices and the Progression of Liver Fibrosis: Insights from a Population-Based Study. Nutrients 2024, 16, 1586. [Google Scholar] [CrossRef] [PubMed]
- Llévenes, P.; Chen, A.; Lawton, M.; Rondón-Ortiz, A.N.; Qiu, Y.; Seen, M.; Monti, S.; Denis, G.V. Plasma exosomes in insulin resistant obesity exacerbate progression of triple negative breast cancer. BMC Cancer 2025, 25, 1089. [Google Scholar] [CrossRef]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef]
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef]
- Singh, D.; Dhiman, V.K.; Pandey, M.; Dhiman, V.K.; Sharma, A.; Pandey, H.; Verma, S.K.; Pandey, R. Personalized medicine: An alternative for cancer treatment. Cancer Treat. Res. Commun. 2024, 42, 100860. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, T.; Xue, S.T.; Zhang, P.; Wang, F.; Li, Y.; Liu, Y.; Zhao, L.; Wu, J.; Yan, Y.; et al. Adipocyte-derived glutathione promotes obesity-related breast cancer by regulating the SCARB2-ARF1-mTORC1 complex. Cell Metab. 2025, 37, 692–707.e9. [Google Scholar] [CrossRef]
- Baek, A.E.; Yu, Y.A.; He, S.; Wardell, S.E.; Chang, C.Y.; Kwon, S.; Pillai, R.V.; McDowell, H.B.; Thompson, J.W.; Dubois, L.G.; et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 2017, 8, 864. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Yang, M. Hepatocellular Carcinoma and Obesity, Type 2 Diabetes Mellitus, Cardiovascular Disease: Causing Factors, Molecular Links, and Treatment Options. Front. Endocrinol. 2021, 12, 808526. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef]
- Oberman, B.; Khaku, A.; Camacho, F.; Goldenberg, D. Relationship between obesity, diabetes and the risk of thyroid cancer. Am. J. Otolaryngol. 2015, 36, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.D.; Miller, A.B.; Rohan, T.E. Obesity and colorectal cancer risk in women. Gut 2002, 51, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Stock, W.; Luger, S.M.; Advani, A.S.; Yin, J.; Harvey, R.C.; Mullighan, C.G.; Willman, C.L.; Fulton, N.; Laumann, K.M.; Malnassy, G.; et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: Results of CALGB 10403. Blood 2019, 133, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Dostal, A.; Menk, J.; Church, T.R. BMI Is a Risk Factor for Colorectal Cancer Mortality. Dig. Dis. Sci. 2017, 62, 2511–2517. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef]
- Bechtel, M.D.; Michel, C.; Srinivasan, P.; Chalise, P.; Parker, W.P.; Mirza, M.; Thrasher, B.; Gibbs, H.D.; DiGiovanni, J.; Hamilton-Reeves, J. Impact of Weight Management on Obesity-Driven Biomarkers of Prostate Cancer Progression. J. Urol. 2024, 211, 552–562. [Google Scholar] [CrossRef]
- Mita, M.M.; Mita, A.C.; Carver, B.J.; Shanahan, J.M.; Mayes, B.A.; Dufour, P.J.; Browning, D.; Anderson-Villaluz, A.; Petersen, J.S.; Turnquist, D.J.; et al. A Phase 1 Safety Study of Evexomostat (SDX-7320) in Patients with Late-Stage Cancer: An Antiangiogenic, Insulin-Sensitizing Drug Conjugate Targeting METAP2. Cancer Res. Commun. 2025, 5, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Wang, D.; Wu, Y.; Chen, X.; Shao, F.; Wei, Y.; Zhang, R.; Lange, T.; Ma, H.; Xu, H.; et al. Associations of genetic risk, BMI trajectories, and the risk of non-small cell lung cancer: A population-based cohort study. BMC Med. 2022, 20, 203. [Google Scholar] [CrossRef] [PubMed]
- Tangalakis, L.L.; Cortina, C.S.; Son, J.D.; Poirier, J.; Madrigrano, A. Obesity Does Not Influence Management of Advanced Breast Cancer in the Elderly. Clin. Breast Cancer 2019, 19, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Ianiro, G.; Gasbarrini, A.; Adolph, T.E. Adipokines: Masterminds of metabolic inflammation. Nat. Rev. Immunol. 2025, 25, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, Z.; Gomez-Salazar, M.; Alexaki, V.I. Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease. J. Innate Immun. 2022, 14, 4–30. [Google Scholar] [CrossRef]
- Beghini, M.; Metz, M.; Baumgartner, C.; Wolf, P.; Bastian, M.; Hackl, M.; Baumgartner-Parzer, S.; Marculescu, R.; Krebs, M.; Harreiter, J.; et al. Leptin acutely increases hepatic triglyceride secretion in patients with lipodystrophy. Metabolism 2025, 169, 156261. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Li, Z. Adipokines in glucose and lipid metabolism. Adipocyte 2023, 12, 2202976. [Google Scholar] [CrossRef]
- He, S.; Ryu, J.; Liu, J.; Luo, H.; Lv, Y.; Langlais, P.R.; Wen, J.; Dong, F.; Sun, Z.; Xia, W.; et al. LRG1 is an adipokine that mediates obesity-induced hepatosteatosis and insulin resistance. J. Clin. Investig. 2021, 131, e148545. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Yidilisi, A.; Xu, Y.; Dong, Q.; Jiang, J. Causal Associations Between Circulating Adipokines and Cardiovascular Disease: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e2572–e2580. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Li, Y.; Zhang, S.; Cai, X.; Zhang, R.; Gong, S.; Han, X.; Ji, L. Serum leptin, resistin, and adiponectin levels in obese and non-obese patients with newly diagnosed type 2 diabetes mellitus: A population-based study. Medicine 2020, 99, e19052. [Google Scholar] [CrossRef]
- Gelsomino, L.; Naimo, G.D.; Catalano, S.; Mauro, L.; Andò, S. The Emerging Role of Adiponectin in Female Malignancies. Int. J. Mol. Sci. 2019, 20, 2127. [Google Scholar] [CrossRef]
- Lohmann, A.E.; Soldera, S.V.; Pimentel, I.; Ribnikar, D.; Ennis, M.; Amir, E.; Goodwin, P.J. Association of Obesity With Breast Cancer Outcome in Relation to Cancer Subtypes: A Meta-Analysis. JNCI J. Natl. Cancer Inst. 2021, 113, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Zhang, Q.Y.; Yang, Z.C. The correlation between obesity and the occurrence and development of breast cancer. Eur. J. Med. Res. 2025, 30, 419. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, F.; Chen, Y.; Xing, Y.; Huang, J.; Cao, J.; Xiong, J.; Liu, Y.; Zhao, Q.; Luo, M.; et al. A positive feedback loop of OTUD1 and c-Jun driven by leptin expedites stemness maintenance in ovarian cancer. Oncogene 2025, 44, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Wei, X.H.; Li, S.J.; Liu, Y.; Hu, H.L.; Li, Z.Z.; Kuang, X.H.; Wang, L.; Shi, X.; Yuan, S.T.; et al. Adipocyte-derived IL-6 and leptin promote breast Cancer metastasis via upregulation of Lysyl Hydroxylase-2 expression. Cell Commun. Signal. 2018, 16, 100. [Google Scholar] [CrossRef]
- Kumar, R.; Mal, K.; Razaq, M.K.; Magsi, M.; Memon, M.K.; Memon, S.; Afroz, M.N.; Siddiqui, H.F.; Rizwan, A. Association of Leptin With Obesity and Insulin Resistance. Cureus 2020, 12, e12178. [Google Scholar] [CrossRef]
- Caruso, A.; Gelsomino, L.; Panza, S.; Accattatis, F.M.; Naimo, G.D.; Barone, I.; Giordano, C.; Catalano, S.; Andò, S. Leptin: A Heavyweight Player in Obesity-Related Cancers. Biomolecules 2023, 13, 1084. [Google Scholar] [CrossRef]
- Bocian-Jastrzębska, A.; Malczewska-Herman, A.; Kos-Kudła, B. Role of Leptin and Adiponectin in Carcinogenesis. Cancers 2023, 15, 4250. [Google Scholar] [CrossRef]
- Jiménez-Cortegana, C.; López-Saavedra, A.; Sánchez-Jiménez, F.; Pérez-Pérez, A.; Castiñeiras, J.; Virizuela-Echaburu, J.A.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Leptin, Both Bad and Good Actor in Cancer. Biomolecules 2021, 11, 913. [Google Scholar] [CrossRef]
- Murphy, W.J.; Longo, D.L. The Surprisingly Positive Association Between Obesity and Cancer Immunotherapy Efficacy. JAMA 2019, 321, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Frąk, M.; Grenda, A.; Krawczyk, P.; Kuźnar-Kamińska, B.; Pazdrowski, P.; Kędra, K.; Chmielewska, I.; Milanowski, J. The influence of nutritional status, lipid profile, leptin concentration and polymorphism of genes encoding leptin and neuropeptide Y on the effectiveness of immunotherapy in advanced NSCLC patients. BMC Cancer 2024, 24, 937. [Google Scholar] [CrossRef] [PubMed]
- Miethe, C.; Torres, L.; Zamora, M.; Price, R.S. Inhibition of PI3K/Akt and ERK signaling decreases visfatin-induced invasion in liver cancer cells. Horm. Mol. Biol. Clin. Investig. 2021, 42, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Srivastava, S.K.; Bhardwaj, A.; Singh, A.P.; Tyagi, N.; Marimuthu, S.; Dyess, D.L.; Dal Zotto, V.; Carter, J.E.; Singh, S. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget 2015, 6, 11231–11241. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, E.; Deng, L.; Zhu, Y.; Lu, X.; Li, X.; Li, F.; Yan, Y.; Han, J.Y.; Li, Y.; et al. Immunological roles for resistin and related adipokines in obesity-associated tumors. Int. Immunopharmacol. 2024, 142, 112911. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Srivastava, S.K.; Zubair, H.; Bhardwaj, A.; Tyagi, N.; Al-Ghadhban, A.; Singh, A.P.; Dyess, D.L.; Carter, J.E.; Singh, S. Resistin potentiates chemoresistance and stemness of breast cancer cells: Implications for racially disparate therapeutic outcomes. Cancer Lett. 2017, 396, 21–29. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, P.J.; Hsieh, Y.C.; Lo, S.; Lee, Y.C.; Chen, Y.C.; Tsai, C.H.; Chiu, W.C.; Chu-Sung Hu, S.; Lu, C.W.; et al. Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene 2018, 37, 589–600. [Google Scholar] [CrossRef]
- Cuachirria-Espinoza, R.L.; García-Miranda, A.; Hernández-Barragán, R.; Nava-Tapia, D.A.; Olea-Flores, M.; Navarro-Tito, N. Analysis of the relationship between resistin with prognosis, cell migration, and p38 and ERK1/2 activation in breast cancer. Biochimie 2025, 229, 19–29. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Li, M.Z.; Xu, H.; Wang, Q.; Song, J.; Lin, P.; Zhang, L.; Liu, Q.; Huang, Q.X.; et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin. Med. J. 2012, 125, 3440–3444. [Google Scholar]
- Sun, X.; Guo, Y. Chemerin Enhances Migration and Invasion of OC Cells via CMKLR1/RhoA/ROCK-Mediated EMT. Int. J. Endocrinol. 2024, 2024, 7957018. [Google Scholar] [CrossRef]
- Hu, X.; Jia, F.; Li, L.; Chen, W.; Zhang, L.; Pan, J.; Zhu, S.; Wang, Z.; Huang, J. Single-Cell and Single-Nuclei transcriptomics profiling reveals dynamic cellular features in tumor-related adipose microenvironment of breast cancer patients with high BMI. Transl. Oncol. 2025, 57, 102408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, L.; Meng, H.; Zhang, A.; Liang, Y.; Lu, J. Obesity alters immunopathology in cancers and inflammatory diseases. Obes. Rev. 2023, 24, e13638. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Bowers, L.W. The role of immune dysfunction in obesity-associated cancer risk, progression, and metastasis. Cell. Mol. Life Sci. 2021, 78, 3423–3442. [Google Scholar] [CrossRef]
- De Simone, V.; Franzè, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015, 34, 3493–3503. [Google Scholar] [CrossRef]
- Oh, K.; Lee, O.Y.; Park, Y.; Seo, M.W.; Lee, D.S. IL-1β induces IL-6 production and increases invasiveness and estrogen-independent growth in a TG2-dependent manner in human breast cancer cells. BMC Cancer 2016, 16, 724. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, B.; Kang, J.; Li, A.; Sun, J. Obesity Promotes Tumor Immune Evasion in Ovarian Cancer Through Increased Production of Myeloid-Derived Suppressor Cells via IL-6. Cancer Manag. Res. 2021, 13, 7355–7363. [Google Scholar] [CrossRef]
- Lacraz, G.; Rakotoarivelo, V.; Labbé, S.M.; Vernier, M.; Noll, C.; Mayhue, M.; Stankova, J.; Schwertani, A.; Grenier, G.; Carpentier, A.; et al. Deficiency of Interleukin-15 Confers Resistance to Obesity by Diminishing Inflammation and Enhancing the Thermogenic Function of Adipose Tissues. PLoS ONE 2016, 11, e0162995. [Google Scholar] [CrossRef]
- Barra, N.G.; Chew, M.V.; Reid, S.; Ashkar, A.A. Interleukin-15 treatment induces weight loss independent of lymphocytes. PLoS ONE 2012, 7, e39553. [Google Scholar] [CrossRef]
- Kochumon, S.; Al Madhoun, A.; Al-Rashed, F.; Thomas, R.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci. Rep. 2020, 10, 16364. [Google Scholar] [CrossRef]
- Ullah, A.; Ud Din, A.; Ding, W.; Shi, Z.; Pervaz, S.; Shen, B. A narrative review: CXC chemokines influence immune surveillance in obesity and obesity-related diseases: Type 2 diabetes and nonalcoholic fatty liver disease. Rev. Endocr. Metab. Disord. 2023, 24, 611–631. [Google Scholar] [CrossRef] [PubMed]
- Gallerand, A.; Stunault, M.I.; Merlin, J.; Luehmann, H.P.; Sultan, D.H.; Firulyova, M.M.; Magnone, V.; Khedher, N.; Jalil, A.; Dolfi, B.; et al. Brown adipose tissue monocytes support tissue expansion. Nat. Commun. 2021, 12, 5255. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Bilecz, A.J.; Lengyel, E. The adipocyte microenvironment and cancer. Cancer Metastasis Rev. 2022, 41, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.; Lu, C.H.; Chien, H.C.; Tian, Y.F.; Hsieh, P.S. Adipose Tissue-Derived CCL5 Enhances Local Pro-Inflammatory Monocytic MDSCs Accumulation and Inflammation via CCR5 Receptor in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2022, 23, 14226. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, J.; Li, J.; Zhu, Z.; Cui, Z.; Liu, R.; Lu, R.; Yao, Z.; Xu, Q. Cancer associated fibroblast-derived CCL5 promotes hepatocellular carcinoma metastasis through activating HIF1α/ZEB1 axis. Cell Death Dis. 2022, 13, 478. [Google Scholar] [CrossRef]
- Ban, Y.; Mai, J.; Li, X.; Mitchell-Flack, M.; Zhang, T.; Zhang, L.; Chouchane, L.; Ferrari, M.; Shen, H.; Ma, X. Targeting Autocrine CCL5-CCR5 Axis Reprograms Immunosuppressive Myeloid Cells and Reinvigorates Antitumor Immunity. Cancer Res. 2017, 77, 2857–2868. [Google Scholar] [CrossRef]
- Walsh, R.M.; Ambrose, J.; Jack, J.L.; Eades, A.E.; Bye, B.A.; Tannus Ruckert, M.; Messaggio, F.; Olou, A.A.; Chalise, P.; Pei, D.; et al. Depletion of tumor-derived CXCL5 improves T cell infiltration and anti-PD-1 therapy response in an obese model of pancreatic cancer. J. Immunother. Cancer 2025, 13, e010057. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Z.; Hong, J.; Wu, J.; Huang, O.; He, J.; Chen, W.; Li, Y.; Chen, X.; Shen, K. Targeting cancer-associated adipocyte-derived CXCL8 inhibits triple-negative breast cancer progression and enhances the efficacy of anti-PD-1 immunotherapy. Cell Death Dis. 2023, 14, 703. [Google Scholar] [CrossRef]
- He, W.; Wang, H.; Yang, G.; Zhu, L.; Liu, X. The Role of Chemokines in Obesity and Exercise-Induced Weight Loss. Biomolecules 2024, 14, 1121. [Google Scholar] [CrossRef]
- Li, H.; Wu, M.; Zhao, X. Role of chemokine systems in cancer and inflammatory diseases. MedComm 2022, 3, e147. [Google Scholar] [CrossRef]
- Bader, J.E.; Wolf, M.M.; Lupica-Tondo, G.L.; Madden, M.Z.; Reinfeld, B.I.; Arner, E.N.; Hathaway, E.S.; Steiner, K.K.; Needle, G.A.; Hatem, Z.; et al. Obesity induces PD-1 on macrophages to suppress anti-tumour immunity. Nature 2024, 630, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Ruiz, A.; Sánchez-Brena, F.; López-Pacheco, C.; Acevedo-Domínguez, N.A.; Soldevila, G. Obesity modulates the immune macroenvironment associated with breast cancer development. PLoS ONE 2022, 17, e0266827. [Google Scholar] [CrossRef] [PubMed]
- Dyck, L.; Prendeville, H.; Raverdeau, M.; Wilk, M.M.; Loftus, R.M.; Douglas, A.; McCormack, J.; Moran, B.; Wilkinson, M.; Mills, E.L.; et al. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J. Exp. Med. 2022, 219. [Google Scholar] [CrossRef] [PubMed]
- Piening, A.; Ebert, E.; Gottlieb, C.; Khojandi, N.; Kuehm, L.M.; Hoft, S.G.; Pyles, K.D.; McCommis, K.S.; DiPaolo, R.J.; Ferris, S.T.; et al. Obesity-related T cell dysfunction impairs immunosurveillance and increases cancer risk. Nat. Commun. 2024, 15, 2835. [Google Scholar] [CrossRef]
- Farag, K.I.; Makkouk, A.; Norian, L.A. Re-Evaluating the Effects of Obesity on Cancer Immunotherapy Outcomes in Renal Cancer: What Do We Really Know? Front. Immunol. 2021, 12, 668494. [Google Scholar] [CrossRef]
- Boi, S.K.; Orlandella, R.M.; Gibson, J.T.; Turbitt, W.J.; Wald, G.; Thomas, L.; Buchta Rosean, C.; Norris, K.E.; Bing, M.; Bertrand, L.; et al. Obesity diminishes response to PD-1-based immunotherapies in renal cancer. J. Immunother. Cancer 2020, 8, e000725. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- Donnelly, D.; Bajaj, S.; Yu, J.; Hsu, M.; Balar, A.; Pavlick, A.; Weber, J.; Osman, I.; Zhong, J. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J. Immunother. Cancer 2019, 7, 222. [Google Scholar] [CrossRef]
- Singh, A.; Mayengbam, S.S.; Yaduvanshi, H.; Wani, M.R.; Bhat, M.K. Obesity Programs Macrophages to Support Cancer Progression. Cancer Res. 2022, 82, 4303–4312. [Google Scholar] [CrossRef]
- Jaitin, D.A.; Adlung, L.; Thaiss, C.A.; Weiner, A.; Li, B.; Descamps, H.; Lundgren, P.; Bleriot, C.; Liu, Z.; Deczkowska, A.; et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178, 686–698.e14. [Google Scholar] [CrossRef]
- Sanchez-Pino, M.D.; Gilmore, L.A.; Ochoa, A.C.; Brown, J.C. Obesity-Associated Myeloid Immunosuppressive Cells, Key Players in Cancer Risk and Response to Immunotherapy. Obesity 2021, 29, 944–953. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Chen, X.; Wang, C.; Zhang, J.; Ren, X.; Wang, Y.; Tao, X.; Li, H.; Song, M.; et al. Diet-induced obesity accelerates oral carcinogenesis by recruitment and functional enhancement of myeloid-derived suppressor cells. Cell Death Dis. 2021, 12, 946. [Google Scholar] [CrossRef]
- Gibson, J.T.; Orlandella, R.M.; Turbitt, W.J.; Behring, M.; Manne, U.; Sorge, R.E.; Norian, L.A. Obesity-Associated Myeloid-Derived Suppressor Cells Promote Apoptosis of Tumor-Infiltrating CD8 T Cells and Immunotherapy Resistance in Breast Cancer. Front. Immunol. 2020, 11, 590794. [Google Scholar] [CrossRef]
- Bähr, I.; Goritz, V.; Doberstein, H.; Hiller, G.G.; Rosenstock, P.; Jahn, J.; Pörtner, O.; Berreis, T.; Mueller, T.; Spielmann, J.; et al. Diet-Induced Obesity Is Associated with an Impaired NK Cell Function and an Increased Colon Cancer Incidence. J. Nutr. Metab. 2017, 2017, 4297025. [Google Scholar] [CrossRef] [PubMed]
- Lynch, L.A.; O’Connell, J.M.; Kwasnik, A.K.; Cawood, T.J.; O’Farrelly, C.; O’Shea, D.B. Are natural killer cells protecting the metabolically healthy obese patient? Obesity 2009, 17, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Bähr, I.; Spielmann, J.; Quandt, D.; Kielstein, H. Obesity-Associated Alterations of Natural Killer Cells and Immunosurveillance of Cancer. Front. Immunol. 2020, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar] [CrossRef]
- Tang, W.; Zhou, J.; Yang, W.; Feng, Y.; Wu, H.; Mok, M.T.S.; Zhang, L.; Liang, Z.; Liu, X.; Xiong, Z.; et al. Aberrant cholesterol metabolic signaling impairs antitumor immunosurveillance through natural killer T cell dysfunction in obese liver. Cell. Mol. Immunol. 2022, 19, 834–847. [Google Scholar] [CrossRef]
- Tang, C.; Castillon, V.J.; Waters, M.; Fong, C.; Park, T.; Boscenco, S.; Kim, S.; Pekala, K.; Carrot-Zhang, J.; Hakimi, A.A.; et al. Obesity-dependent selection of driver mutations in cancer. Nat. Genet. 2024, 56, 2318–2321. [Google Scholar] [CrossRef]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Gatla, H.R.; Wu, S.; Wang, G.; Gabrielson, K.; Sears, C.L.; Ladle, B.H.; Sharma, D. Gut colonization with an obesity-associated enteropathogenic microbe modulates the premetastatic niches to promote breast cancer lung and liver metastasis. Front. Immunol. 2023, 14, 1194931. [Google Scholar] [CrossRef]
- Pingili, A.K.; Chaib, M.; Sipe, L.M.; Miller, E.J.; Teng, B.; Sharma, R.; Yarbro, J.R.; Asemota, S.; Al Abdallah, Q.; Mims, T.S.; et al. Immune checkpoint blockade reprograms systemic immune landscape and tumor microenvironment in obesity-associated breast cancer. Cell Rep. 2021, 35, 109285. [Google Scholar] [CrossRef]
- Zhang, T.; Li, S.; Chang, J.; Qin, Y.; Li, C. Impact of BMI on the survival outcomes of non-small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. BMC Cancer 2023, 23, 1023. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.K.; Veeraraghavan, V.P.; Palanisamy, C.P.; Cui, B.; Periyasamy, V.; Chandrasekar, K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules 2021, 26, 2101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Liu, S.; Yang, M. Antioxidant and anti-inflammatory agents in chronic liver diseases: Molecular mechanisms and therapy. World J. Hepatol. 2023, 15, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. Message Transmission Between Adipocyte and Macrophage in Obesity. Adv. Exp. Med. Biol. 2024, 1460, 273–295. [Google Scholar] [CrossRef]
- Pan, D.; Li, G.; Jiang, C.; Hu, J.; Hu, X. Regulatory mechanisms of macrophage polarization in adipose tissue. Front. Immunol. 2023, 14, 1149366. [Google Scholar] [CrossRef]
- Saraswat, N.; Wal, P.; Pal, R.S.; Wal, A.; Pal, Y.; Pharmacophore, D. Current review on IRS-1, JNK, NF-ΚB & m-TOR pathways in Insulin Resistance. Pharmacophores 2020, 11, 1–14. [Google Scholar]
- Zhao, M.; Cheng, Y.; Wang, X.; Cui, X.; Cheng, X.; Fu, Q.; Song, Y.; Yu, P.; Liu, Y.; Yu, Y. Hydrogen Sulfide Attenuates High-Fat Diet-Induced Obesity: Involvement of mTOR/IKK/NF-κB Signaling Pathway. Mol. Neurobiol. 2022, 59, 6903–6917. [Google Scholar] [CrossRef]
- Ahmad, A.; Biersack, B.; Li, Y.; Kong, D.; Bao, B.; Schobert, R.; Padhye, S.B.; Sarkar, F.H. Targeted regulation of PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their derivatives: Mechanistic details and biological implications for cancer therapy. Anticancer. Agents Med. Chem. 2013, 13, 1002–1013. [Google Scholar] [CrossRef]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef]
- Pande, M.; Bondy, M.L.; Do, K.A.; Sahin, A.A.; Ying, J.; Mills, G.B.; Thompson, P.A.; Brewster, A.M. Association between germline single nucleotide polymorphisms in the PI3K-AKT-mTOR pathway, obesity, and breast cancer disease-free survival. Breast Cancer Res. Treat. 2014, 147, 381–387. [Google Scholar] [CrossRef]
- Chen, J. Multiple signal pathways in obesity-associated cancer. Obes. Rev. 2011, 12, 1063–1070. [Google Scholar] [CrossRef]
- Ning, H.; Sun, Z.; Liu, Y.; Liu, L.; Hao, L.; Ye, Y.; Feng, R.; Li, J.; Li, Y.; Chu, X.; et al. Insulin Protects Hepatic Lipotoxicity by Regulating ER Stress through the PI3K/Akt/p53 Involved Pathway Independently of Autophagy Inhibition. Nutrients 2016, 8, 227. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Lin, S.; Chen, T.; Chang, D.; Sun, Y.; Wang, C.; Liu, Y.; Lu, Y.; Song, J.; et al. Advanced effect of curcumin and resveratrol on mitigating hepatic steatosis in metabolic associated fatty liver disease via the PI3K/AKT/mTOR and HIF-1/VEGF cascade. Biomed. Pharmacother. 2023, 165, 115279. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Si, Y.; Guo, T.; Zhao, W.; Zhang, L.; Wang, Y.; Wang, L.; Sun, K.; Feng, S. Ethoxychelerythrine as a potential therapeutic strategy targets PI3K/AKT/mTOR induced mitochondrial apoptosis in the treatment of colorectal cancer. Sci. Rep. 2025, 15, 6642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.M.Y.; Wellberg, E.A.; Kopp, J.L.; Johnson, J.D. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab. J. 2021, 45, 285–311. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, R.; Lehwald, N.; Tao, G.Z.; Liu, B.; Liu, B.; Koh, Y.; Sylvester, K.G. Wnt/β-catenin signaling activation promotes lipogenesis in the steatotic liver via physical mTOR interaction. Front. Endocrinol. 2023, 14, 1289004. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, H.; Zhuo, H.; Yu, C.; Liu, Y. High Fat Diet Induces Kidney Injury via Stimulating Wnt/β-Catenin Signaling. Front. Med. 2022, 9, 851618. [Google Scholar] [CrossRef]
- Das, B.; Das, M.; Kalita, A.; Baro, M.R. The role of Wnt pathway in obesity induced inflammation and diabetes: A review. J. Diabetes Metab. Disord. 2021, 20, 1871–1882. [Google Scholar] [CrossRef]
- Tsukanov, V.V.; Tonkikh, J.L.; Kasparov, E.V.; Vasyutin, A.V. Inhibition of M2 tumor-associated macrophages polarization by modulating the Wnt/β-catenin pathway as a possible liver cancer therapy method. World J. Gastroenterol. 2024, 30, 4399–4403. [Google Scholar] [CrossRef]
- Mortezaee, K. WNT/β-catenin regulatory roles on PD-(L)1 and immunotherapy responses. Clin. Exp. Med. 2024, 24, 15. [Google Scholar] [CrossRef]
- Muto, S.; Enta, A.; Maruya, Y.; Inomata, S.; Yamaguchi, H.; Mine, H.; Takagi, H.; Ozaki, Y.; Watanabe, M.; Inoue, T.; et al. Wnt/β-Catenin Signaling and Resistance to Immune Checkpoint Inhibitors: From Non-Small-Cell Lung Cancer to Other Cancers. Biomedicines 2023, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X.; Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch signaling pathway in cancer: From mechanistic insights to targeted therapies. Signal Transduct. Target. Ther. 2024, 9, 128. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Wang, Y.; Kaur, B.; Han, H.; Yu, J. The Notch signaling pathway: A potential target for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 45. [Google Scholar] [CrossRef]
- Pu, Q.; Yu, L.; Liu, X.; Yan, H.; Xie, Y.; Cai, X.; Wu, Y.; Du, J.; Yang, Z. Prognostic value of CD8(+)T cells related genes and exhaustion regulation of Notch signaling pathway in hepatocellular carcinoma. Front. Immunol. 2024, 15, 1375864. [Google Scholar] [CrossRef]
- Jiang, N.; Hu, Y.; Wang, M.; Zhao, Z.; Li, M. The Notch Signaling Pathway Contributes to Angiogenesis and Tumor Immunity in Breast Cancer. Breast Cancer Targets Ther. 2022, 14, 291–309. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Gao, Z.; Yin, J.; Zhang, J.; Yun, Z.; Ye, J. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: Adipogenesis, insulin, and hypoxia. Am. J. Physiol.-Endocrinol. Metab. 2011, 300, E877–E885. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Kang, S.C. Kaempferol promotes apoptosis and inhibits proliferation and migration by suppressing HIF-1α/VEGF and Wnt/β-catenin activation under hypoxic condition in colon cancer. Appl. Biol. Chem. 2025, 68, 19. [Google Scholar] [CrossRef]
- Teleb, E.K.; Mehanna, R.A.; Assem, N.M.; Houssen, M.E. Antitumor effects of dauricine on sorafenib-treated human lung cancer cell lines via modulation of HIF-1α signaling pathways. Med. Oncol. 2025, 42, 157. [Google Scholar] [CrossRef]
- Aird, R.; Wills, J.; Roby, K.F.; Bénézech, C.; Stimson, R.H.; Wabitsch, M.; Pollard, J.W.; Finch, A.; Michailidou, Z. Hypoxia-driven metabolic reprogramming of adipocytes fuels cancer cell proliferation. Front. Endocrinol. 2022, 13, 989523. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, R.; Ouyang, Y.; Shen, Y.; Hu, L.; Xu, C. Cancer stem cells: A target for overcoming therapeutic resistance and relapse. Cancer Biol. Med. 2023, 20, 985–1020. [Google Scholar] [CrossRef]
- Liu, X.; Peng, T.; Xu, M.; Lin, S.; Hu, B.; Chu, T.; Liu, B.; Xu, Y.; Ding, W.; Li, L.; et al. Spatial multi-omics: Deciphering technological landscape of integration of multi-omics and its applications. J. Hematol. Oncol. 2024, 17, 72. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Ginseng and obesity: Observations and understanding in cultured cells, animals and humans. J. Nutr. Biochem. 2017, 44, 1–10. [Google Scholar] [CrossRef]
- Dey, L.; Xie, J.T.; Wang, A.; Wu, J.; Maleckar, S.A.; Yuan, C.S. Anti-hyperglycemic effects of ginseng: Comparison between root and berry. Phytomedicine 2003, 10, 600–605. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Yamaguchi, K.; Choi, S.S.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Effects of Korean white ginseng extracts on obesity in high-fat diet-induced obese mice. Cytotechnology 2010, 62, 367–376. [Google Scholar] [CrossRef][Green Version]
- Song, M.Y.; Kim, B.S.; Kim, H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J. Ginseng Res. 2014, 38, 106–115. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y.; Szabo, A.; Han, M.; Huang, X.F. Central inflammation and leptin resistance are attenuated by ginsenoside Rb1 treatment in obese mice fed a high-fat diet. PLoS ONE 2014, 9, e92618. [Google Scholar] [CrossRef]
- Xiong, Y.; Shen, L.; Liu, K.J.; Tso, P.; Xiong, Y.; Wang, G.; Woods, S.C.; Liu, M. Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes 2010, 59, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Wang, C.Z.; Ni, M.; Wu, J.A.; Mehendale, S.R.; Aung, H.H.; Foo, A.; Yuan, C.S. American ginseng berry juice intake reduces blood glucose and body weight in ob/ob mice. J. Food Sci. 2007, 72, S590–S594. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Zhou, Y.P.; Dey, L.; Attele, A.S.; Wu, J.A.; Gu, M.; Polonsky, K.S.; Yuan, C.S. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine 2002, 9, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kong, G.; Tran, Q.; Kim, C.; Park, J.; Park, J. Relationship Between Ginsenoside Rg3 and Metabolic Syndrome. Front. Pharmacol. 2020, 11, 130. [Google Scholar] [CrossRef]
- Ha, K.S.; Jo, S.H.; Kang, B.H.; Apostolidis, E.; Lee, M.S.; Jang, H.D.; Kwon, Y.I. In vitro and in vivo antihyperglycemic effect of 2 amadori rearrangement compounds, arginyl-fructose and arginyl-fructosyl-glucose. J. Food Sci. 2011, 76, H188–H193. [Google Scholar] [CrossRef]
- Yang, C.; Qian, C.; Zheng, W.; Dong, G.; Zhang, S.; Wang, F.; Wei, Z.; Xu, Y.; Wang, A.; Zhao, Y.; et al. Ginsenoside Rh2 enhances immune surveillance of natural killer (NK) cells via inhibition of ERp5 in breast cancer. Phytomedicine 2024, 123, 155180. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Lyu, M.; Wong, Y.K.; Zhang, X.; Zhou, J.; Ma, N.; Wang, J. The potential of artemisinins as anti-obesity agents via modulating the immune system. Pharmacol. Ther. 2020, 216, 107696. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, F.C.; Qian, S.W.; Li, X.; Cui, Z.M.; Dang, Y.J.; Tang, Q.Q. Artemisinin derivatives prevent obesity by inducing browning of WAT and enhancing BAT function. Cell Res. 2016, 26, 1169–1172. [Google Scholar] [CrossRef]
- Sarder, A.; Pokharel, Y.R. Synthetic derivatives of artemisinin and cancer. Int. J. Med. Biomed. Sci. 2016, 1, 12–16. [Google Scholar] [CrossRef]
- Konstat-Korzenny, E.; Ascencio-Aragón, J.A.; Niezen-Lugo, S.; Vázquez-López, R. Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer. Med. Sci. 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kong, W.; Tucker, K.; Staley, A.; Fan, Y.; Sun, W.; Yin, Y.; Huang, Y.; Fang, Z.; Wang, J.; et al. SPR064, a pro-drug of paclitaxel, has anti-tumorigenic effects in endometrial cancer cell lines and mouse models. Am. J. Transl. Res. 2020, 12, 4264–4276. [Google Scholar]
- Zhao, Z.; Wang, J.; Kong, W.; Fang, Z.; Coleman, M.F.; Milne, G.L.; Burkett, W.C.; Newton, M.A.; Lara, O.; Lee, D.; et al. Intermittent energy restriction inhibits tumor growth and enhances paclitaxel response in a transgenic mouse model of endometrial cancer. Gynecol. Oncol. 2024, 186, 126–136. [Google Scholar] [CrossRef]
- Nagle, C.M.; Dixon, S.C.; Jensen, A.; Kjaer, S.K.; Modugno, F.; deFazio, A.; Fereday, S.; Hung, J.; Johnatty, S.E.; Fasching, P.A.; et al. Obesity and survival among women with ovarian cancer: Results from the Ovarian Cancer Association Consortium. Br. J. Cancer 2015, 113, 817–826. [Google Scholar] [CrossRef]
- Nowicka, A.; Marini, F.C.; Solley, T.N.; Elizondo, P.B.; Zhang, Y.; Sharp, H.J.; Broaddus, R.; Kolonin, M.; Mok, S.C.; Thompson, M.S.; et al. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS ONE 2013, 8, e81859. [Google Scholar] [CrossRef]
- Williams, M.E.; Howard, D.; Donnelly, C.; Izadi, F.; Parra, J.G.; Pugh, M.; Edwards, K.; Lutchman-Sigh, K.; Jones, S.; Margarit, L.; et al. Adipocyte derived exosomes promote cell invasion and challenge paclitaxel efficacy in ovarian cancer. Cell Commun. Signal. 2024, 22, 443. [Google Scholar] [CrossRef]
- Jeon, H.J.; Seo, M.J.; Choi, H.S.; Lee, O.H.; Lee, B.Y. Gelidium elegans, an edible red seaweed, and hesperidin inhibit lipid accumulation and production of reactive oxygen species and reactive nitrogen species in 3T3-L1 and RAW264.7 cells. Phytother. Res. 2014, 28, 1701–1709. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, M.; Kim, K.; Lee, Y.M.; Rhyu, M.R. Hesperetin Stimulates Cholecystokinin Secretion in Enteroendocrine STC-1 Cells. Biomol. Ther. 2013, 21, 121–125. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Lasa, A.; Abendaño, N.; Fernández-Quintela, A.; Mosqueda-Solís, A.; Garcia-Sobreviela, M.P.; Arbonés-Mainar, J.M.; Portillo, M.P. Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. J. Transl. Med. 2017, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent For Obesity. Drug Des. Devel Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.A.; Yang, C.; Song, J.; Song, G.; Jeong, W.; Lim, W. Hesperidin Suppresses the Proliferation of Prostate Cancer Cells by Inducing Oxidative Stress and Disrupting Ca(2+) Homeostasis. Antioxidants 2022, 11, 1633. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr. Res. 2021, 92, 21–31. [Google Scholar] [CrossRef]
- Wein, S.; Behm, N.; Petersen, R.K.; Kristiansen, K.; Wolffram, S. Quercetin enhances adiponectin secretion by a PPAR-gamma independent mechanism. Eur. J. Pharm. Sci. 2010, 41, 16–22. [Google Scholar] [CrossRef]
- Zhu, X.; Dai, X.; Zhao, L.; Li, J.; Zhu, Y.; He, W.; Guan, X.; Wu, T.; Liu, L.; Song, H.; et al. Quercetin activates energy expenditure to combat metabolic syndrome through modulating gut microbiota-bile acids crosstalk in mice. Gut Microbes 2024, 16, 2390136. [Google Scholar] [CrossRef]
- Sharma, A.; Kashyap, D.; Sak, K.; Tuli, H.S.; Sharma, A.K. Therapeutic charm of quercetin and its derivatives: A review of research and patents. Pharm. Pat. Anal. 2018, 7, 15–32. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Jabeen, S.; Attar, R.; Yaylim, I.; Xu, B. Quercetin-mediated regulation of signal transduction cascades and microRNAs: Natural weapon against cancer. J. Cell. Biochem. 2018, 119, 9664–9674. [Google Scholar] [CrossRef]
- Yang, X.; Wu, F.; Li, L.; Lynch, E.C.; Xie, L.; Zhao, Y.; Fang, K.; Li, J.; Luo, J.; Xu, L.; et al. Celastrol alleviates metabolic disturbance in high-fat diet-induced obese mice through increasing energy expenditure by ameliorating metabolic inflammation. Phytother. Res. 2021, 35, 297–310. [Google Scholar] [CrossRef]
- Liu, C.; Li, N.; Peng, M.; Huang, K.; Fan, D.; Zhao, Z.; Huang, X.; Liu, Y.; Chen, S.; Li, Z. Celastrol directly binds with VAMP7 and RAB7 to inhibit autophagy and induce apoptosis in preadipocytes. Front. Pharmacol. 2023, 14, 1094584. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Fan, N.; Zhang, X.; Ngo, F.Y.; Zhao, J.; Zhao, W.; Huang, M.; Li, D.; Wang, Y.; Rong, J. Covalent inhibition of endoplasmic reticulum chaperone GRP78 disconnects the transduction of ER stress signals to inflammation and lipid accumulation in diet-induced obese mice. eLife 2022, 11, e72182. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Kim, J.Y.; Lee, J.H.; Lee, B.W.; Park, K.H.; Shim, K.H.; Lee, M.K.; Seo, K.I. Celastrol isolated from Tripterygium regelii induces apoptosis through both caspase-dependent and -independent pathways in human breast cancer cells. Food Chem. Toxicol. 2011, 49, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.O.; Lee, S.K.; Kim, N.; You, G.Y.; Moon, J.W.; Sha, J.; Kim, S.J.; Park, S.H.; Kim, H.S. Celastrol suppresses breast cancer MCF-7 cell viability via the AMP-activated protein kinase (AMPK)-induced p53-polo like kinase 2 (PLK-2) pathway. Cell. Signal. 2013, 25, 805–813. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, W.; Yang, H.; Bi, J.; Xie, Y.; Cheng, B.; Wang, Y.; Chen, S. Celastrol Induces Necroptosis and Ameliorates Inflammation via Targeting Biglycan in Human Gastric Carcinoma. Int. J. Mol. Sci. 2019, 20, 5716. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Luo, W.; Chen, S.; Lin, F.; Zhang, X.; Fan, S.; Shen, X.; Wang, Y.; Liang, G. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics 2020, 10, 10290–10308. [Google Scholar] [CrossRef]
- Karthika, C.; Hari, B.; Mano, V.; Radhakrishnan, A.; Janani, S.K.; Akter, R.; Kaushik, D.; Rahman, M.H. Curcumin as a great contributor for the treatment and mitigation of colorectal cancer. Exp. Gerontol. 2021, 152, 111438. [Google Scholar] [CrossRef]
- Idoudi, S.; Bedhiafi, T.; Hijji, Y.M.; Billa, N. Curcumin and Derivatives in Nanoformulations with Therapeutic Potential on Colorectal Cancer. AAPS PharmSciTech 2022, 23, 115. [Google Scholar] [CrossRef]
- Kumar, A.; Singam, A.; Swaminathan, G.; Killi, N.; Tangudu, N.K.; Jose, J.; Gundloori Vn, R.; Dinesh Kumar, L. Combinatorial therapy using RNAi and curcumin nano-architectures regresses tumors in breast and colon cancer models. Nanoscale 2022, 14, 492–505. [Google Scholar] [CrossRef]
- Yan, S.-l.; Huang, C.-y.; Wu, S.-t.; Yin, M.-c. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. Vitr. 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Wei, L.; Shen, A.; Sferra, T.J.; Hong, Z.; Peng, J. Ursolic acid promotes colorectal cancer cell apoptosis and inhibits cell proliferation via modulation of multiple signaling pathways. Int. J. Oncol. 2013, 43, 1235–1243. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Ursolic acid-mediated changes in glycolytic pathway promote cytotoxic autophagy and apoptosis in phenotypically different breast cancer cells. Apoptosis 2017, 22, 800–815. [Google Scholar] [CrossRef]
- Li, X.; Asemi, A. A Novel AI-Driven Expert System for Obesity Diagnosis and Personalised Treatment. CAAI Trans. Intell. Technol. 2025. Early View. [Google Scholar]
- Liu, J.; Liu, Z.; Liu, C.; Sun, H.; Li, X.; Yang, Y. Integrating artificial intelligence in the diagnosis and management of metabolic syndrome: A comprehensive review. Diabetes/Metab. Res. Rev. 2025, 41, e70039. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Wang, H. Machine Learning and AI in Cancer Prognosis, Prediction, and Treatment Selection: A Critical Approach. J. Multidiscip. Heal. 2023, 16, 1779–1791. [Google Scholar] [CrossRef]
- Gangwal, A.; Lavecchia, A. Artificial intelligence in anti-obesity drug discovery: Unlocking next-generation therapeutics. Drug Discov. Today 2025, 30, 104333. [Google Scholar] [CrossRef]


| Cancers | Clinical Trials | Intervention | Functions | References |
|---|---|---|---|---|
| Differentiated thyroid cancer | Randomized controlled trial | Development | Patients with obesity have an increased risk of developing differentiated thyroid cancer (DTC). | [16] |
| Colorectal cancer | Canadian National Breast Screening Study | Development | Obesity (BMI ≥ 30 kg/m2) was associated with an approximately 2-fold increased risk of colorectal cancer among women who were premenopausal at baseline. | [17] |
| Acute lymphoblastic leukemia | NCT00558519 * | Prognosis | Pretreatment obesity (BMI ≥ 30 kg/m2) was significantly associated with worse overall survival rates of patients. | [18] |
| Colorectal cancer | Randomized controlled Trial | Prognosis | A higher BMI increased the risk of colorectal cancer mortality. | [19] |
| Breast cancer | NCT01140282 | Treatment | A 16-week aerobic and resistance exercise improved physical fitness and quality of life in ethnically diverse overweight or obese breast cancer survivors. | [20] |
| Prostate cancer | NCT03261271 | Treatment | A weight-loss intervention reduced obesity-related biomarkers of prostate cancer progression, such as insulin, cholesterol component, leptin/adiponectin ratio, visceral adipose tissue, C-reactive peptide, plasminogen-activator-inhibitor-1, and T cell count, as well as the quality of life of cancer patients. | [21] |
| Advanced refractory or late-stage solid tumors | NCT02743637 | Treatment | Treatment with evexomostat (SDX-7320), a novel antiangiogenic and antimetastatic drug with insulin-sensitizing and anti-obesity properties, decreased plasma levels of low-density lipoprotein (LDL) cholesterol and leptin and insulin resistance, but increased plasma levels of adiponectin and high-density lipoprotein (HDL) in patients. | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhu, K.; Liu, J.; Yang, M. The Role of Obesity in the Regulation of Immunosuppressive Cell Infiltration and Immunosurveillance in Cancers. Diseases 2025, 13, 271. https://doi.org/10.3390/diseases13080271
Zhang C, Zhu K, Liu J, Yang M. The Role of Obesity in the Regulation of Immunosuppressive Cell Infiltration and Immunosurveillance in Cancers. Diseases. 2025; 13(8):271. https://doi.org/10.3390/diseases13080271
Chicago/Turabian StyleZhang, Chunye, Keyao Zhu, Jiazheng Liu, and Ming Yang. 2025. "The Role of Obesity in the Regulation of Immunosuppressive Cell Infiltration and Immunosurveillance in Cancers" Diseases 13, no. 8: 271. https://doi.org/10.3390/diseases13080271
APA StyleZhang, C., Zhu, K., Liu, J., & Yang, M. (2025). The Role of Obesity in the Regulation of Immunosuppressive Cell Infiltration and Immunosurveillance in Cancers. Diseases, 13(8), 271. https://doi.org/10.3390/diseases13080271






