Current Neuroethical Perspectives on Deep Brain Stimulation and Neuromodulation for Neuropsychiatric Disorders: A Scoping Review of the Past 10 Years
Abstract
1. Introduction
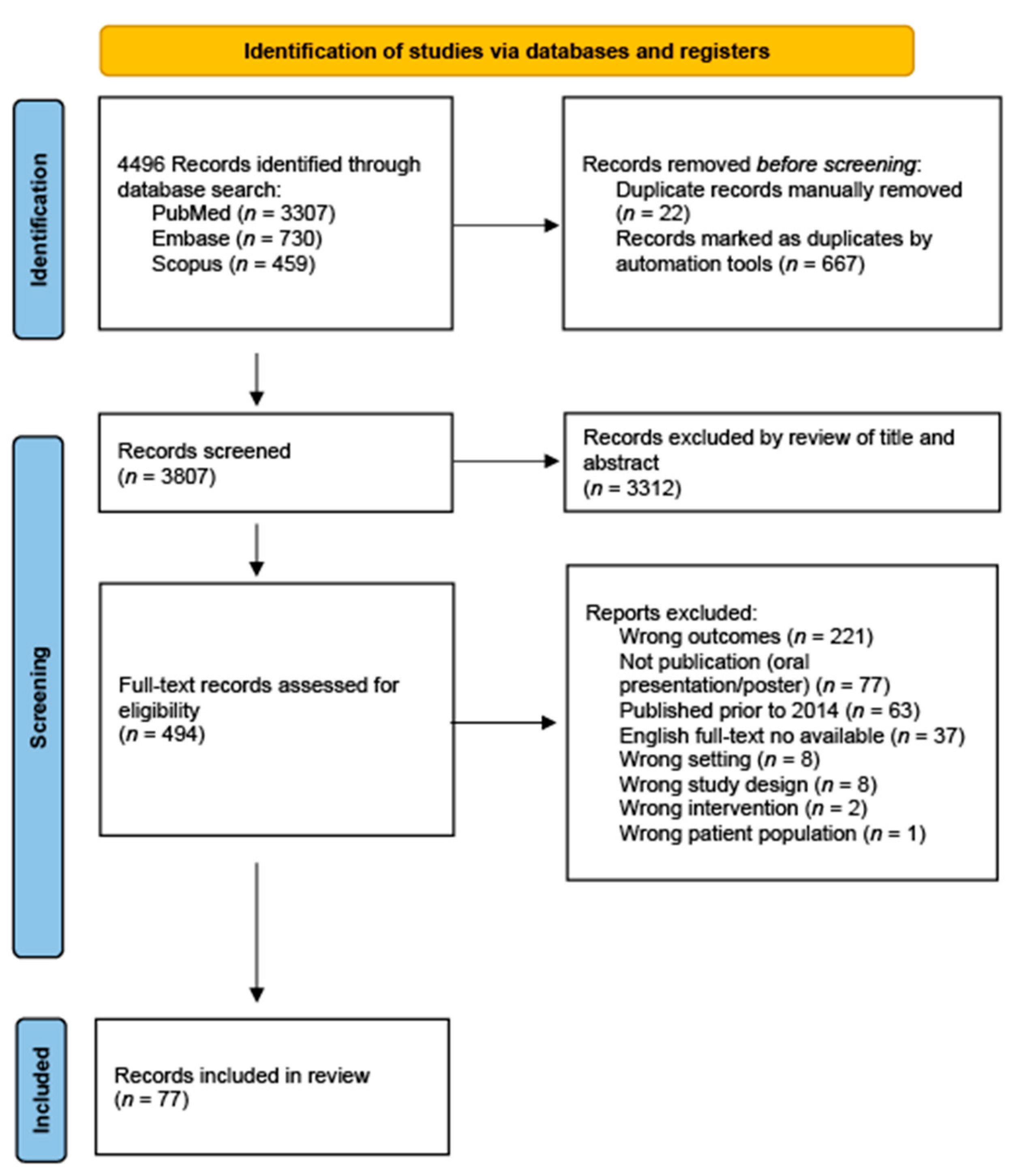
2. Methods
2.1. Search Strategy and Study Eligibility
2.2. Data Extraction
3. Results
3.1. Personal Identity and Authenticity
3.2. Autonomy and Informed Consent
3.3. Beneficence and Non-Maleficence
3.4. Justice
3.5. Privacy and Responsibility
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Data Accessibility Statement
Conflicts of Interest
Abbreviations
| DBS | Deep Brain Stimulation |
| TMS | Transcranial Magnetic Stimulation |
| tDCS | Transcranial Direct Current Stimulation |
| ECT | Electroconvulsive Therapy |
| BCI | Brain-Computer Interface |
| FDA | Food and Drug Administration |
| OCD | Obsessive Compulsive Disorder |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PIAAS | Personality, Agency, Authenticity, Autonomy, and Self |
| ADHD | Attention Deficit Hyperactivity Disorder |
| TS | Tourette’s Syndrome |
| TRD | Treatment-Resistant Depression |
| PTSD | Post Traumatic Stress Disorder |
References
- Thomson, S. History of Neuromodulation. Neuromodulation.com. Available online: https://www.neuromodulation.com/brief-history-neuromodulation (accessed on 5 July 2025).
- Zhao, H.; Qiao, L.; Fan, D.; Zhang, S.; Turel, O.; Li, Y.; Li, J.; Xue, G.; Chen, A.; He, Q. Modulation of Brain Activity with Noninvasive Transcranial Direct Current Stimulation (tDCS): Clinical Applications and Safety Concerns. Front. Psychol. 2017, 8, 685. [Google Scholar] [CrossRef]
- Delaloye, S.; Holtzheimer, P. Deep Brain Stimulation in the Treatment of Depression. Dialogues Clin. Neurosci. 2014, 16, 83–91. [Google Scholar] [CrossRef]
- Deli, A.; Green, A.L. Deep Brain Stimulation for Consciousness Disorders; Technical and Ethical Considerations. Neuroethics 2024, 17, 1–9. [Google Scholar] [CrossRef]
- Scelzo, E.; Beghi, E.; Rosa, M.; Angrisano, S.; Antonini, A.; Bagella, C.; Bianchi, E.; Caputo, E.; Lena, F.; Lopiano, L.; et al. Deep brain stimulation in Parkinson's disease: A multicentric, long-term, observational pilot study. J. Neurol. Sci. 2019, 405, 116411. [Google Scholar] [CrossRef]
- McDonald, W.M.; Weiner, R.D.; Fochtmann, L.J.M.; McCall, W.V. The FDA and ECT. J. ECT 2016, 32, 75–77. [Google Scholar] [CrossRef]
- Walsh, S. FDA in Brief: FDA Takes Action to Ensure Regulation of Electroconvulsive Therapy Devices Better Protects Patients, Reflects Current Understanding of Safety and Effectiveness; Food & Drug Administration. 2019. Available online: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-takes-action-ensure-regulation-electroconvulsive-therapy-devices-better-protects (accessed on 5 July 2025).
- Food & Drug Administration. FDA Permits Marketing of Transcranial Magnetic Stimulation for Treatment of Obsessive Compulsive Disorder; Food & Drug Administration. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-transcranial-magnetic-stimulation-treatment-obsessive-compulsive-disorder (accessed on 5 July 2025).
- Mahoney, J.J.; Koch-Gallup, N.; Scarisbrick, D.M.; Berry, J.H.; Rezai, A.R. Deep brain stimulation for psychiatric disorders and behavioral/cognitive-related indications: Review of the literature and implications for treatment. J. Neurol. Sci. 2022, 437, 120253. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S.; Gillett, G.; Manning, P.; Glue, P.; Langguth, B. Psychosurgery Reduces Uncertainty and Increases Free Will? A Review. Neuromodulation Technol. Neural Interface 2016, 19, 239–248. [Google Scholar] [CrossRef]
- Bergeron, D.; Iorio-Morin, C.; Bonizzato, M.; Lajoie, G.; Gaucher, N.O.; Racine, É.; Weil, A.G. Use of Invasive Brain-Computer Interfaces in Pediatric Neurosurgery: Technical and Ethical Considerations. J. Child Neurol. 2023, 38, 223–238. [Google Scholar] [CrossRef]
- Desmoulin-Canselier, S. DBS: A compelling example for ethical and legal reflection-a French perspective on ethical and legal concerns about DBS. Monash Bioeth. Rev. 2020, 38, 15–34. [Google Scholar] [CrossRef]
- Eijkholt, M.; Cabrera, L.Y.; Ramirez-Zamora, A.; Pilitsis, J.G. Shaking Up the Debate: Ensuring the Ethical Use of DBS Intervention Criteria for Mid-Stage Parkinson’s Patients. Neuromodul. Technol. Neural Interface 2017, 20, 411–416. [Google Scholar] [CrossRef]
- Lo, C.; Mane, M.; Kim, J.H.; Berk, M.; Sharp, R.R.; Lee, K.H.; Yuen, J. Treating addiction with deep brain stimulation: Ethical and legal considerations. Int. J. Drug Policy 2023, 113, 103964. [Google Scholar] [CrossRef]
- Beasleypat, N.; Auvichayapat, P. Transcranial Direct Current Stimulation in Treatment of Child Neuropsychiatric Disorders: Ethical Considerations. Front. Hum. Neurosci. 2022, 16, 842013. [Google Scholar] [CrossRef]
- Beasley, K.D. Commentary: Old Self vs. New Self. Camb. Q. Heal. Ethic. 2016, 25, 751–753. [Google Scholar] [CrossRef]
- Beeker, T.; Schlaepfer, T.E.; Coenen, V.A. Autonomy in Depressive Patients Undergoing DBS-Treatment: Informed Consent, Freedom of Will and DBS’ Potential to Restore It. Front. Integr. Neurosci. 2017, 11, 11. [Google Scholar] [CrossRef]
- Bewernick, B.; Schlaepfer, E.T. Update on Neuromodulation for Treatment-Resistant Depression. F1000Research 2015, 4, 1389. [Google Scholar] [CrossRef]
- Bittlinger, M.; Müller, S. Opening the debate on deep brain stimulation for Alzheimer disease—A critical evaluation of rationale, shortcomings, and ethical justification. BMC Med. Ethic. 2018, 19, 41. [Google Scholar] [CrossRef]
- Bluhm, R.; Cabrera, L.; McKenzie, R. What we (Should) Talk about when we Talk about Deep Brain Stimulation and Personal Identity. Neuroethics 2019, 13, 289–301. [Google Scholar] [CrossRef]
- Bluhm, R.; Cabrera, L.Y. Self-implant ambiguity? Understanding self-related changes in deep brain stimulation. Philos. Explor. 2022, 25, 367–385. [Google Scholar] [CrossRef]
- Cabrera, L.Y.; Miller, M.M.; Achtyes, E.D.; McCright, A.M.; Bluhm, R. Jumping through the hoops: Barriers and other ethical concerns regarding the use of psychiatric electroceutical interventions. Psychiatry Res. 2022, 313, 114612. [Google Scholar] [CrossRef]
- Cabrera, L.Y.; Heuvel, O.A.v.D. Ethical Considerations Regarding the Use of Transcranial Magnetic Stimulation in Mental Health Practice. Biol. Psychiatry 2023, 95, 491–493. [Google Scholar] [CrossRef]
- Coman, A. Emerging Technologies in the Treatment of Anorexia Nervosa and Ethics: Sufferers’ Accounts of Treatment Strategies and Authenticity. Heal. Care Anal. 2014, 25, 212–224. [Google Scholar] [CrossRef]
- Dalton, B.; Dornik, J.; McClelland, J.; Bartholdy, S.; Kekic, M.; Campbell, I.C.; Schmidt, U. Clinicians’ views on neuromodulation as a treatment for eating disorders: A qualitative study. Neuropsychiatrie 2020, 35, 84–91. [Google Scholar] [CrossRef]
- Davidson, B.; Elkaim, L.M.; Lipsman, N.; Ibrahim, G.M. Editorial. An ethical framework for deep brain stimulation in children. Neurosurg. Focus 2018, 45, E11. [Google Scholar] [CrossRef] [PubMed]
- Erler, A. Discussions of DBS in Neuroethics: Can We Deflate the Bubble Without Deflating Ethics? Neuroethics 2019, 14, 75–81. [Google Scholar] [CrossRef]
- Gallagher, S. Deep Brain Stimulation, Self and Relational Autonomy. Neuroethics 2018, 14, 31–43. [Google Scholar] [CrossRef]
- Gault, J.M.; Hosokawa, P.; Kramer, D.; Saks, E.R.; Appelbaum, P.S.; Thompson, J.A.; Olincy, A.; Cascella, N.; Sawa, A.; Goodman, W.; et al. Postsurgical morbidity and mortality favorably informs deep brain stimulation for new indications including schizophrenia and schizoaffective disorder. Front. Surg. 2023, 10, 958452. [Google Scholar] [CrossRef]
- Gilbert, F. Self-Estrangement & Deep Brain Stimulation: Ethical Issues Related to Forced Explantation. Neuroethics 2014, 8, 107–114. [Google Scholar] [CrossRef]
- Glannon, W. Neuromodulation, Agency and Autonomy. Brain Topogr. 2013, 27, 46–54. [Google Scholar] [CrossRef]
- Goddard, E. Deep Brain Stimulation Through the “Lens of Agency”: Clarifying Threats to Personal Identity from Neurological Intervention. Neuroethics 2017, 10, 325–335. [Google Scholar] [CrossRef]
- González-Márquez, C. Neuromodulation and memory: Exploring ethical ramifications in memory modification treatment via implantable neurotechnologies. Front. Psychol. 2023, 14, 1282634. [Google Scholar] [CrossRef]
- Grant, R.A.; Halpern, C.H.; Baltuch, G.H.; O’Reardon, J.P.; Caplan, A. Ethical considerations in deep brain stimulation for psychiatric illness. J. Clin. Neurosci. 2014, 21, 1–5. [Google Scholar] [CrossRef]
- de Haan, S.; Rietveld, E.; Stokhof, M.; Denys, D.; Schmahl, C. Becoming more oneself? Changes in personality following DBS treatment for psychiatric disorders: Experiences of OCD patients and general considerations. PLoS ONE 2017, 12, e0175748. [Google Scholar] [CrossRef]
- Hansen, R.T.B.; Dubey, A.; Smith, C.; Henry, P.J.; Mammis, A. Paediatric deep brain stimulation: Ethical considerations in malignant Tourette syndrome. J. Med. Ethic. 2020, 46, 668–673. [Google Scholar] [CrossRef]
- Hübner, D.; White, L. Neurosurgery for Psychopaths? An Ethical Analysis. AJOB Neurosci. 2016, 7, 140–149. [Google Scholar] [CrossRef]
- Klein, E.; Brown, T.; Sample, M.; Truitt, A.R.; Goering, S. Engineering the Brain: Ethical Issues and the Introduction of Neural Devices. Hast. Cent. Rep. 2015, 45, 26–35. [Google Scholar] [CrossRef]
- Klein, E.; Goering, S.; Gagne, J.; Shea, C.V.; Franklin, R.; Zorowitz, S.; Dougherty, D.D.; Widge, A.S. Brain-computer interface-based control of closed-loop brain stimulation: Attitudes and ethical considerations. Brain-Comput. Interfaces 2016, 3, 140–148. [Google Scholar] [CrossRef]
- Kostick-Quenet, K.M.; Kalwani, L.; Torgerson, L.N.; Munoz, K.; Sanchez, C.; Storch, A.E.; Blumenthal-Barby, J.S.; Lazáro-Muñoz, G. Deep Brain Stimulation for Pediatric Dystonia: Clinicians’ Perspectives on the Most Pressing Ethical Challenges. Ster. Funct. Neurosurg. 2023, 101, 301–313. [Google Scholar] [CrossRef]
- Kubu, C.S.; Ford, P.J. Clinical Ethics in the Context of Deep Brain Stimulation for Movement Disorders. Arch. Clin. Neuropsychol. 2017, 32, 829–839. [Google Scholar] [CrossRef]
- Lázaro-Muñoz, G.; Yoshor, D.; Beauchamp, M.S.; Goodman, W.K.; McGuire, A.L. Continued access to investigational brain implants. Nat. Rev. Neurosci. 2018, 19, 317–318. [Google Scholar] [CrossRef]
- Lewis, C.J.; Maier, F.; Horstkötter, N.; Zywczok, A.; Witt, K.; Eggers, C.; Meyer, T.D.; Dembek, T.A.; Maarouf, M.; Moro, E.; et al. Subjectively perceived personality and mood changes associated with subthalamic stimulation in patients with Parkinson's disease. Psychol. Med. 2014, 45, 73–85. [Google Scholar] [CrossRef]
- Mandarelli, G.; Moretti, G.; Pasquini, M.; Nicolò, G.; Ferracuti, S. Informed Consent Decision-Making in Deep Brain Stimulation. Brain Sci. 2018, 8, 84. [Google Scholar] [CrossRef]
- Maslen, H.; Pugh, J.; Savulescu, J. The Ethics of Deep Brain Stimulation for the Treatment of Anorexia Nervosa. Neuroethics 2015, 8, 215–230. [Google Scholar] [CrossRef]
- Müller, S.; Walter, H.; Christen, M. When benefitting a patient increases the risk for harm for third persons—The case of treating pedophilic Parkinsonian patients with deep brain stimulation. Int. J. Law Psychiatry 2014, 37, 295–303. [Google Scholar] [CrossRef]
- Müller, S.; Bittlinger, M.; Walter, H. Threats to Neurosurgical Patients Posed by the Personal Identity Debate. Neuroethics 2017, 10, 299–310. [Google Scholar] [CrossRef]
- Müller, S.; van Oosterhout, A.; Bervoets, C.; Christen, M.; Martínez-Álvarez, R.; Bittlinger, M. Concerns About Psychiatric Neurosurgery and How They Can Be Overcome: Recommendations for Responsible Research. Neuroethics 2022, 15, 1–26. [Google Scholar] [CrossRef]
- Muñoz, K.A.; Kostick, K.; Torgerson, L.; Zuk, P.; Kalwani, L.; Sanchez, C.; Blumenthal-Barby, J.; Storch, E.A.; Lázaro-Muñoz, G. Pressing ethical issues in considering pediatric deep brain stimulation for obsessive-compulsive disorder. Brain Stimul. 2021, 14, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, K.A.; Blumenthal-Barby, J.; Storch, E.A.; Torgerson, L.; Lázaro-Muñoz, G. Pediatric Deep Brain Stimulation for Dystonia: Current State and Ethical Considerations. Camb. Q. Heal. Ethic. 2020, 29, 557–573. [Google Scholar] [CrossRef]
- Muñoz, K.A.; Kostick, K.; Sanchez, C.; Kalwani, L.; Torgerson, L.; Hsu, R.; Sierra-Mercado, D.; Robinson, J.O.; Outram, S.; Koenig, B.A.; et al. Researcher Perspectives on Ethical Considerations in Adaptive Deep Brain Stimulation Trials. Front. Hum. Neurosci. 2020, 14, 578695. [Google Scholar] [CrossRef]
- Noda, Y. The New Ethics of Neuromodulation with Transcranial Magnetic Stimulation: A Critical Appraisal. J. Integr. Neurosci. 2024, 23, 112. [Google Scholar] [CrossRef]
- Nyholm, S.; O’Neill, E. Deep Brain Stimulation, Continuity over Time, and the True Self. Camb. Q. Heal. Ethic. 2016, 25, 647–658. [Google Scholar] [CrossRef]
- Nyholm, S.; O’Neill, E. Deep Brain Stimulation, Authenticity and Value. Camb. Q. Heal. Ethic. 2017, 26, 658–670. [Google Scholar] [CrossRef][Green Version]
- Park, R.J.; Singh, I.; Pike, A.C.; Tan, J.O.A. Deep Brain Stimulation in Anorexia Nervosa: Hope for the Hopeless or Exploitation of the Vulnerable? The Oxford Neuroethics Gold Standard Framework. Front. Psychiatry 2017, 8, 44. [Google Scholar] [CrossRef]
- Pugh, J. Clarifying the Normative Significance of ‘Personality Changes’ Following Deep Brain Stimulation. Sci. Eng. Ethic. 2020, 26, 1655–1680. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.; Maslen, H.; Savulescu, J. Deep Brain Stimulation, Authenticity and Value. Camb. Q. Heal. Ethic. 2017, 26, 640–657. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.; Pycroft, L.; Maslen, H.; Aziz, T.; Savulescu, J. Evidence-Based Neuroethics, Deep Brain Stimulation and Personality—Deflating, but not Bursting, the Bubble. Neuroethics 2018, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.; Aziz, T.; Herring, J.; Savulescu, J. Deep brain stimulation and revising the Mental Health Act: The case for intervention-specific safeguards. Br. J. Psychiatry 2018, 214, 133–136. [Google Scholar] [CrossRef]
- Rainey, S. Datafied Brains and Digital Twins: Lessons from Industry, Caution for Psychiatry. Philos. Psychiatry Psychol. 2022, 29, 29–42. [Google Scholar] [CrossRef]
- Roskies, A.L. Agency and intervention. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140215. [Google Scholar] [CrossRef]
- Schönau, A.; Dasgupta, I.; Brown, T.; Versalovic, E.; Klein, E.; Goering, S. Mapping the Dimensions of Agency. AJOB Neurosci. 2021, 12, 172–186. [Google Scholar] [CrossRef]
- Shlobin, N.A.; Rosenow, J.M. Ethical Considerations in the Implantation of Neuromodulatory Devices. Neuromodulation Technol. Neural Interface 2021, 25, 222–231. [Google Scholar] [CrossRef]
- Shlobin, N.A.; Campbell, J.M.; Rosenow, J.M.; Rolston, J.D. Ethical considerations in the surgical and neuromodulatory treatment of epilepsy. Epilepsy Behav. 2022, 127, 108524. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.M.; Barrett, M.S.; Bhati, M.T.; Rosen, A. Deep Brain Stimulation for Alzheimer’s Disease: Ethical Challenges for Clinical Research. J. Alzheimer's Dis. 2016, 56, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Skorburg, J.; Sinnott-Armstrong, W. Some Ethics of Deep Brain Stimulation. In Global Mental Health and Neuroethics; Stein, D., Singh, I., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 117–132. [Google Scholar] [CrossRef]
- Smeets, A.Y.J.M.; Duits, A.A.; Horstkötter, D.; Verdellen, C.; de Wert, G.; Temel, Y.; Ackermans, L.; Leentjens, A.F.G. Ethics of Deep Brain Stimulation in Adolescent Patients with Refractory Tourette Syndrome: A Systematic Review and Two Case Discussions. Neuroethics 2018, 11, 143–155. [Google Scholar] [CrossRef]
- Smith, J.N.; Dorfman, N.; Hurley, M.; Cenolli, I.; Kostick-Quenet, K.; Storch, E.A.; Lázaro-Muñoz, G.; Blumenthal-Barby, J. Adolescent OCD Patient and Caregiver Perspectives on Identity, Authenticity, and Normalcy in Potential Deep Brain Stimulation Treatment. Camb. Q. Heal. Ethic. 2024, 33, 507–520. [Google Scholar] [CrossRef]
- Sullivan, L.S. Insight and the no-self in deep brain stimulation. Bioethics 2018, 33, 487–494. [Google Scholar] [CrossRef]
- Stevens, I.; Gilbert, F. Ethical examination of deep brain stimulation’s ‘last resort’ status. J. Med. Ethic. 2021, 47, e68. [Google Scholar] [CrossRef]
- Takamiya, A.; Bouckaert, F.; Sienaert, P.; Uchida, T.; Kudo, S.; Yamagata, B.; Kishimoto, T.; Mimura, M.; Hirano, J. Electroconvulsive Therapy for Patients with Depression Who Lack Capacity for Consent. J. ECT 2021, 37, 171–175. [Google Scholar] [CrossRef]
- Thomson, C.; Carter, A. Ethical issues in experimental treatments for psychiatric disorders: Lessons from deep brain stimulation. Transl. Issues Psychol. Sci. 2020, 6, 240–246. [Google Scholar] [CrossRef]
- Thomson, C.J.; Segrave, R.A.; Fitzgerald, P.B.; Richardson, K.E.; Racine, E.; Carter, A. “Nothing to Lose, Absolutely Everything to Gain”: Patient and Caregiver Expectations and Subjective Outcomes of Deep Brain Stimulation for Treatment-Resistant Depression. Front. Hum. Neurosci. 2021, 15, 755276. [Google Scholar] [CrossRef]
- Thomson, C.J.; Segrave, R.A.; Fitzgerald, P.B.; Richardson, K.E.; Racine, E.; Carter, A.; Ahmed, F.R. Personal and relational changes following deep brain stimulation for treatment-resistant depression: A prospective qualitative study with patients and caregivers. PLoS ONE 2023, 18, e0284160. [Google Scholar] [CrossRef]
- Unterrainer, M.; Oduncu, F.S. The ethics of deep brain stimulation (DBS). Med. Heal. Care Philos. 2015, 18, 475–485. [Google Scholar] [CrossRef]
- Versalovic, E.; Klein, E.; Goering, S.; Ngo, Q.; Gliske, K.; Boulicault, M.; Sullivan, L.S.; Thomas, M.J.; Widge, A.S. Deep Brain Stimulation for Substance Use Disorders? An Exploratory Qualitative Study of Perspectives of People Currently in Treatment. J. Addict. Med. 2023, 17, e246–e254. [Google Scholar] [CrossRef]
- Viaña, J.N.M.; Gilbert, F. Deep brain stimulation for people with Alzheimer’s disease: Anticipating potential effects on the tripartite self. Dementia 2018, 18, 2836–2855. [Google Scholar] [CrossRef]
- Voigt, J.S. Bodily Felt Freedom: An Ethical Perspective on Positive Aspects of Deep Brain Stimulation. Neuroethics 2018, 14, 45–57. [Google Scholar] [CrossRef]
- Warner, N.S.; Tung, E.E.; DeMartino, E.S.; Kissoon, N.R. Ethics of neuromodulation in adults with cognitive impairment and chronic pain. Pain Med. 2023, 24, S3–S5. [Google Scholar] [CrossRef]
- Wexler, A.; Choi, R.J.; Ramayya, A.G.; Sharma, N.; McShane, B.J.; Buch, L.Y.; Donley-Fletcher, M.P.; Gold, J.I.; Baltuch, G.H.; Goering, S.; et al. Ethical Issues in Intraoperative Neuroscience Research: Assessing Subjects’ Recall of Informed Consent and Motivations for Participation. AJOB Empir. Bioeth. 2021, 13, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.; Stümpel, J.; Woopen, C. Caregiver burden and the medical ethos. Med. Heal. Care Philos. 2017, 20, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zohny, H.; Lyreskog, D.M.; Singh, I.; Savulescu, J. The Mystery of Mental Integrity: Clarifying Its Relevance to Neurotechnologies. Neuroethics 2023, 16, 20. [Google Scholar] [CrossRef]
- Zuk, P.; Lázaro-Muñoz, G. DBS and Autonomy: Clarifying the Role of Theoretical Neuroethics. Neuroethics 2019, 14, 83–93. [Google Scholar] [CrossRef]
- Gilbert, F.; Viaña, J.N.M.; Ineichen, C. Deflating the “DBS causes personality changes” bubble. Neuroethics 2018, 14, 1–17. [Google Scholar] [CrossRef]
| Author | Year | Country | Neuroethic Topics |
|---|---|---|---|
| Auvichayapat [15] | 2022 | Thailand | Non-Maleficence |
| Beasley [16] | 2016 | United States | Personal Identity, Autonomy |
| Beeker et al. [17] | 2017 | Germany | Autonomy |
| Bergeron et al. [11] | 2023 | Canada | Autonomy, Beneficence |
| Bewernick et al. [18] | 2022 | Germany, United States | Autonomy, Justice, Beneficence |
| Bittlinger et al. [19] | 2018 | Germany | Autonomy, Maleficence |
| Bluhm et al. [20] | 2020 | United States | Personal Identity |
| Bluhm et al. [21] | 2022 | United States | Personal Identity |
| Cabrera et al. [22] | 2022 | United States | Justice, Autonomy |
| Cabrera et al. [23] | 2024 | United States | Non-Maleficence, Beneficence, Justice, Autonomy |
| Coman [24] | 2017 | Norway | Personal Identity, Authenticity |
| Dalton [25] | 2021 | United Kingdom | Non-Maleficence, Beneficence, Autonomy, Personal Identity |
| Davidson [26] | 2018 | Canada | Autonomy, Non-Maleficence, Beneficence |
| Delaloye [3] | 2014 | United States | Autonomy, Non-Maleficence, Beneficence |
| Deli [4] | 2024 | United Kingdom, United States | Non-Maleficence, Beneficence, Autonomy, Personal Identity, Privacy |
| Desmoulin-Canselier [12] | 2020 | France | Autonomy, Personal Identity, Privacy, Justice |
| Eijkholt et al. [13] | 2017 | United States | Non-Maleficence, Beneficence, Autonomy, Justice |
| Erler [27] | 2021 | Hong Kong | Autonomy, Personal Identity |
| Gallagher [28] | 2021 | United States | Autonomy, Personal Identity |
| Gault et al. [29] | 2023 | United States | Non-Maleficence, Beneficence |
| Gilbert [30] | 2015 | Australia | Personal Identity, Autonomy |
| Gilbert [30] | 2021 | United States, Australia, Switzerland | Personal Identity, Autonomy |
| Glannon [31] | 2014 | Canada | Autonomy |
| Goddard [32] | 2017 | Australia | Personal Identity, Autonomy |
| Gonzalez-Marquez [33] | 2023 | United Kingdom | Personal Identity, Justice |
| Grant [34] | 2014 | United States | Personal Identity, Justice, Autonomy |
| de Haan [35] | 2017 | Germany, Netherlands | Personal Identity |
| Hansen et al. [36] | 2020 | United States, Australia | Non-Maleficence, Beneficence, autonomy |
| Hubner et al. [37] | 2016 | Germany | Non-Maleficence, Beneficence, autonomy |
| Klein et al. [38] | 2015 | United States | Autonomy, Privacy, Personal Identity |
| Klein et al. [39] | 2016 | United States | Autonomy, Informed Consent |
| Kostick-Quenet et al. [40] | 2023 | United States | Beneficence, Non-Maleficence |
| Kubu [41] | 2017 | United States | Autonomy |
| Lazaro-Munoz et al. [42] | 2018 | United States | Justice |
| Lewis [43] | 2014 | Germany, United Kingdom, France, Canada | Personal Identity |
| Lo et al. [14] | 2023 | United States, Australia | Autonomy, Beneficence, Non-Maleficence, Justice, DBS |
| Mandarelli et al. [44] | 2018 | Italy | Informed Consent |
| Maslen et al. [45] | 2015 | United Kingdom | Autonomy, Personal Identity, DBS |
| Muller et al. [46] | 2014 | Germany, Switzerland | Beneficence, Non-Maleficence, Autonomy, DBS |
| Muller et al. [47] | 2017 | Germany | Personal Identity |
| Muller et al. [48] | 2022 | Germany, Belgium, Switzerland, Spain | Beneficence, Non-Maleficence, Personal Identity, DBS |
| Munoz et al. [49] | 2021 | United States | Autonomy, Informed Consent, Non-Maleficence, Beneficence, DBS |
| Munoz et al. [50] | 2020a | United States | Pediatric DBS, Beneficence, Non-Maleficence, Justice, Personal Identity |
| Munoz et al. [51] | 2020b | United States | Non-Maleficence, Beneficence, Autonomy, Justice, Personal Identity, DBS |
| Noda [52] | 2024 | Japan | Autonomy, Informed Consent, Beneficence, Non-Maleficence, Justice, Neuromodulation |
| Nyholm et al. [53] | 2016 | Netherlands | Personal Identity |
| Nyholm et al. [54] | 2017 | Netherlands | Personal Identity |
| Park et al. [55] | 2017 | United Kingdom | Informed Consent, Autonomy, Beneficence, Non-Maleficence, DBS |
| Pugh [56] | 2020 | United Kingdom | Personal Identity, Autonomy, DBS |
| Pugh et al. [57] | 2017 | United Kingdom | Personal Identity, Autonomy, Beneficence, DBS |
| Pugh et al. [58] | 2018a | United Kingdom | Personal Identity, Autonomy, DBS |
| Pugh et al. [59] | 2019 | United Kingdom | Informed Consent |
| Rainey [60] | 2022 | United States | Privacy |
| Ridder et al. [10] | 2016 | New Zealand, United States, Germany | Autonomy |
| Roskies [61] | 2015 | United States | Autonomy |
| Schonau et al. [62] | 2021 | United States | Autonomy, Privacy, Responsibility |
| Shlobin et al. [63] | 2020 | United States | Beneficence, Non-Maleficence, Justice, Autonomy, Informed Consent |
| Shlobin et al. [64] | 2022 | United States | Beneficence, Non-Maleficence, Justice, Autonomy, Informed Consent |
| Siegel et al. [65] | 2017 | United States | Autonomy |
| Skorburg et al. [66] | 2020 | United States | Identity |
| Smeets et al. [67] | 2018 | Netherlands | Beneficence, Autonomy, Identity |
| Smith et al. [68] | 2024 | United States | Identity |
| Specker [69] | 2019 | United States | Identity |
| Stevens et al. [70] | 2021 | Australia | Beneficence, Autonomy |
| Takamiya et al. [71] | 2021 | Belgium, Japan | Beneficence |
| Thomson et al. [72] | 2020 | Australia | Autonomy, Non-Maleficence, Beneficence, Justice |
| Thomson et al. [73] | 2021 | Australia, Canada | Personal Identity, Non-Maleficence, Beneficence, Autonomy |
| Thomson et al. [74] | 2023 | Australia, Canada | Personal Identity, Autonomy |
| Unterrainer et al. [75] | 2015 | Germany | Autonomy, Informed Consent, Personal Identity, Authenticity |
| Versalovic et al. [76] | 2023 | United States | Autonomy, Informed Consent, Beneficence, Non-Maleficence |
| Viana et al. [77] | 2019 | Australia | Beneficence, Non-Maleficence, Personal Identity, Authenticity |
| Voigt [78] | 2021 | Germany | Beneficence, Non-Maleficence, Personal Identity, Authenticity |
| Warner et al. [79] | 2023 | United States | Autonomy, Informed Consent, Privacy, Responsibility, Personal Identity, Authenticity |
| Wexler et al. [80] | 2021 | United States | Autonomy, Informed Consent, Beneficence, Non-Maleficence |
| Witt et al. [81] | 2017 | Germany | Autonomy, Informed Consent, Privacy, Responsibility, Personal Identity, Authenticity |
| Zohny et al. [82] | 2023 | United Kingdom | Autonomy, Informed Consent, Privacy, Responsibility, Personal Identity, Authenticity |
| Zuk et al. [83] | 2019 | United States | Autonomy, Informed Consent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, J.; Pyreddy, S.; Rosendahl, C.; Lai, C.; Ton, E.; Carter, R. Current Neuroethical Perspectives on Deep Brain Stimulation and Neuromodulation for Neuropsychiatric Disorders: A Scoping Review of the Past 10 Years. Diseases 2025, 13, 262. https://doi.org/10.3390/diseases13080262
Shaw J, Pyreddy S, Rosendahl C, Lai C, Ton E, Carter R. Current Neuroethical Perspectives on Deep Brain Stimulation and Neuromodulation for Neuropsychiatric Disorders: A Scoping Review of the Past 10 Years. Diseases. 2025; 13(8):262. https://doi.org/10.3390/diseases13080262
Chicago/Turabian StyleShaw, Jonathan, Sagar Pyreddy, Colton Rosendahl, Charles Lai, Emily Ton, and Rustin Carter. 2025. "Current Neuroethical Perspectives on Deep Brain Stimulation and Neuromodulation for Neuropsychiatric Disorders: A Scoping Review of the Past 10 Years" Diseases 13, no. 8: 262. https://doi.org/10.3390/diseases13080262
APA StyleShaw, J., Pyreddy, S., Rosendahl, C., Lai, C., Ton, E., & Carter, R. (2025). Current Neuroethical Perspectives on Deep Brain Stimulation and Neuromodulation for Neuropsychiatric Disorders: A Scoping Review of the Past 10 Years. Diseases, 13(8), 262. https://doi.org/10.3390/diseases13080262






