Association Between Dietary Patterns and Lifestyle Habits with Vascular Inflammatory Responses in Individuals with Hypertension Living in PM2.5-Polluted Areas: A Cross-Sectional Pilot Study in Chiang Mai Province, Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Ethics Approval
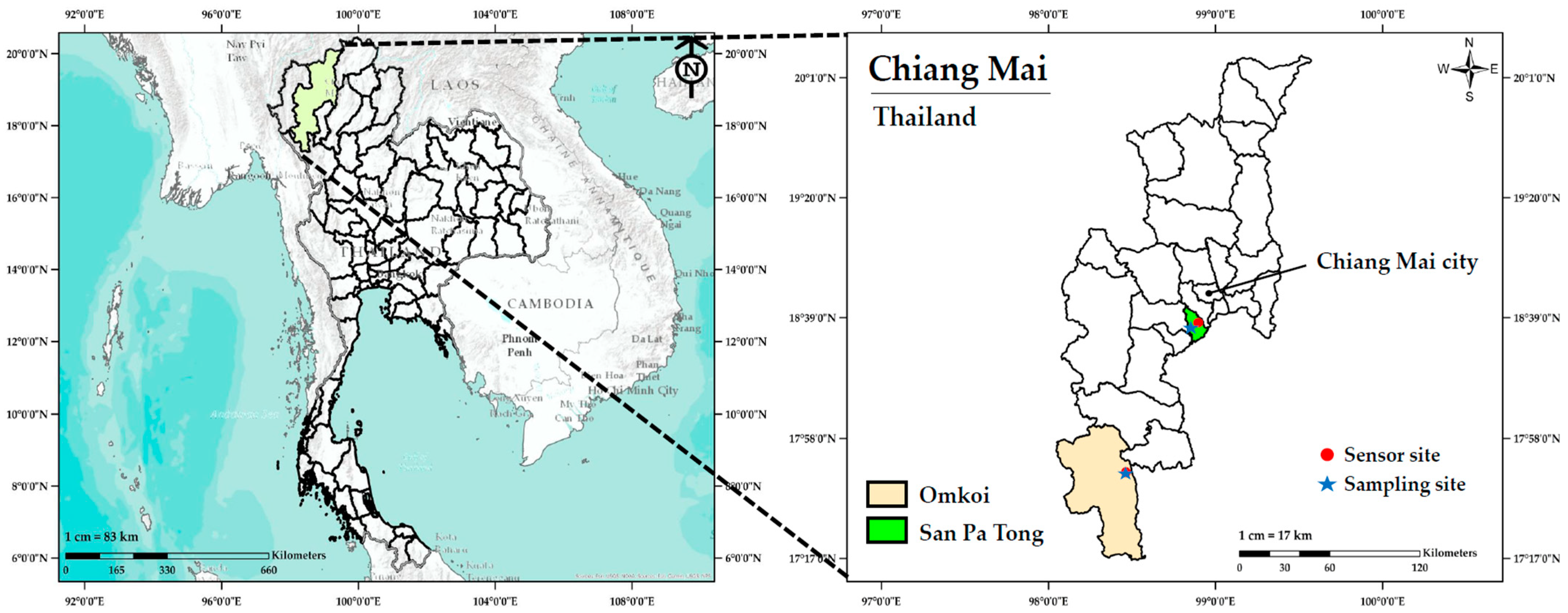
2.2. Study Locations and Ambient PM2.5 Monitoring Data
2.3. Study Subjects
2.4. Study Design
2.5. Assessment of Vascular Inflammation Markers
2.6. Assessment of Nutrient Intake
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants in Rural and Peri-Urban Areas
3.2. Physical Examination Parameters of Participants in Rural and Peri-Urban Areas
3.3. Blood Chemistry Parameters of Participants in Rural and Peri-Urban Areas
3.4. Vascular Inflammatory Biomarkers of Participants in Rural and Peri-Urban Areas
3.5. Nutrient Intake Among Participants in Rural and Peri-Urban Areas
3.6. Associations Between Lifestyle Behaviors and Vascular Inflammatory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| H3K9me3 | Trimethylation of lysine 9 on histone H3 |
| H3K27me3 | Trimethylation of lysine 27 on histone H3 |
| CD40LG | CD40 ligand gene |
| lncRNA | Long non-coding RNA |
| NTAQHI | Northern Thailand Air Quality and Health Index |
| PEAMIR | PEA15-interacting microRNA regulatory lncRNA |
| ELISA | Enzyme-linked immunosorbent assay |
| RIHES | Research Institute for Health Sciences |
| 8-OHdG | 8-Hydroxydeoxyguanosine |
| ICAM-1 | Intercellular adhesion molecule-1 |
| LINE-1 | Long interspersed nuclear element-1 |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| DASH | Dietary Approaches to Stop Hypertension |
| TNF-α | Tumor necrosis factor-alpha |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| ACE | Angiotensin-converting enzyme |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| BUN | Blood urea nitrogen |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| DBP | Diastolic blood pressure |
| FBG | Fasting blood glucose |
| HEC | Human Experimentation Committee |
| miR | MicroRNA |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| WHO | World Health Organization |
| WHR | Waist-to-hip ratio |
| NF-κB | Nuclear factor kappa-B |
| IL-6 | Interleukin-6 |
| PM2.5 | Fine particulate matter |
| TG | triglyceride |
| TC | Total cholesterol |
| WC | Waist circumference |
References
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef]
- Prado, A.F.; Batista, R.I.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix Metalloproteinases and Arterial Hypertension: Role of Oxidative Stress and Nitric Oxide in Vascular Functional and Structural Alterations. Biomolecules 2021, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, S.M.; da Silva, J.F.; Priviero, F.; Webb, R.C.; Eguchi, S.; Tostes, R.C. Vascular Stress Signaling in Hypertension. Circ. Res. 2021, 128, 969–992. [Google Scholar] [CrossRef] [PubMed]
- Wu, O.; Yuan, C.; Leng, J.; Zhang, X.; Liu, W.; Yang, F.; Zhang, H.; Li, J.; Khederzadeh, S.; Jiang, Z.; et al. Colorable role of interleukin (IL)-6 in obesity hypertension: A hint from a Chinese adult case-control study. Cytokine 2023, 168, 156226. [Google Scholar] [CrossRef]
- Fu, M.; Lv, M.; Guo, J.; Mei, A.; Qian, H.; Yang, H.; Wu, W.; Liu, Z.; Zhong, J.; Wei, Y.; et al. The clinical significance of T-cell regulation in hypertension treatment. Front. Immunol. 2025, 16, 1550206. [Google Scholar] [CrossRef] [PubMed]
- Smolgovsky, S.; Ibeh, U.; Tamayo, T.P.; Alcaide, P. Adding insult to injury—Inflammation at the heart of cardiac fibrosis. Cell Signal. 2021, 77, 109828. [Google Scholar] [CrossRef]
- Bei, Y.R.; Zhang, S.C.; Song, Y.; Tang, M.L.; Zhang, K.L.; Jiang, M.; He, R.C.; Wu, S.G.; Liu, X.H.; Wu, L.M.; et al. EPSTI1 promotes monocyte adhesion to endothelial cells in vitro via upregulating VCAM-1 and ICAM-1 expression. Acta Pharmacol. Sin. 2023, 44, 71–80. [Google Scholar] [CrossRef]
- Taurone, S.; Santarelli, M.T.; De Santis, E.; Di Gioia, C.; Pompili, E.; Pellegrino, F.; Familiari, P.; Papa, V.; Zanza, C.; Coppola, L.; et al. Porcine coronary arteries: Immunohistochemical profile of TNF-alpha, IL-1beta, TGF-beta1 and ICAM-1. Folia Morphol. 2023, 82, 119–126. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef]
- Bialecka, M.; Rac, M.; Dziedziejko, V.; Safranow, K.; Chlubek, D.; Rać, M.E. An Evaluation of Plasma TNF, VEGF-A, and IL-6 Determination as a Risk Marker of Atherosclerotic Vascular Damage in Early-Onset CAD Patients. J. Clin. Med. 2024, 13, 1742. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, Z.M.; Chen, L.; Zhao, X.; Cai, M.; Wang, C.; Zou, H.; Wu, Y.; Zhang, Z.; Li, H.; et al. Exposure to Air Pollution during Pre-Hypertension and Subsequent Hypertension, Cardiovascular Disease, and Death: A Trajectory Analysis of the UK Biobank Cohort. Environ. Health Perspect. 2023, 131, 17008. [Google Scholar] [CrossRef]
- Qin, P.; Luo, X.; Zeng, Y.; Zhang, Y.; Li, Y.; Wu, Y.; Han, M.; Qie, R.; Wu, X.; Liu, D.; et al. Long-term association of ambient air pollution and hypertension in adults and in children: A systematic review and meta-analysis. Sci. Total Environ. 2021, 796, 148620. [Google Scholar] [CrossRef] [PubMed]
- de Bont, J.; Jaganathan, S.; Dahlquist, M.; Persson, Å.; Stafoggia, M.; Ljungman, P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J. Intern. Med. 2022, 291, 779–800. [Google Scholar] [CrossRef] [PubMed]
- Chanda, F.; Lin, K.X.; Chaurembo, A.I.; Huang, J.Y.; Zhang, H.J.; Deng, W.H.; Xu, Y.J.; Li, Y.; Fu, L.D.; Cui, H.D.; et al. PM2.5-mediated cardiovascular disease in aging: Cardiometabolic risks, molecular mechanisms and potential interventions. Sci. Total Environ. 2024, 954, 176255. [Google Scholar] [CrossRef]
- Ding, R.; Huang, L.; Yan, K.; Sun, Z.; Duan, J. New insight into air pollution-related cardiovascular disease: An adverse outcome pathway framework of PM2.5-associated vascular calcification. Cardiovasc. Res. 2024, 120, 699–707. [Google Scholar] [CrossRef]
- Parasin, N.; Amnuaylojaroen, T. Effect of PM2.5 on burden of mortality from non-communicable diseases in northern Thailand. PeerJ 2024, 12, e18055. [Google Scholar] [CrossRef]
- Supasri, T.; Gheewala, S.H.; Macatangay, R.; Chakpor, A.; Sedpho, S. Association between ambient air particulate matter and human health impacts in northern Thailand. Sci. Rep. 2023, 13, 12753. [Google Scholar] [CrossRef]
- Zhao, T.; Qi, W.; Yang, P.; Yang, L.; Shi, Y.; Zhou, L.; Ye, L. Mechanisms of cardiovascular toxicity induced by PM2.5: A review. Environ. Sci. Pollut. Res. Int. 2021, 28, 65033–65051. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Q.; Sun, J.; Liang, Y.; Zhang, M.; Zhao, M.; Zhang, K.; Dong, C.; Ma, Q.; Liu, W.; et al. Extracellular vesicles derived from PM2.5-exposed alveolar epithelial cells mediate endothelial adhesion and atherosclerosis in ApoE-/- mice. FASEB J. 2022, 36, e22161. [Google Scholar] [CrossRef]
- Simo, L. The effects of PM2.5 air pollution on human health: A narrative review with a focus on cerebrovascular diseases. Environ. Dis. 2024, 9, 75–78. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, F.; Yin, H.; Shi, X.; Chen, Y.; Wang, H.; Wang, Y.; Bai, B.; Liu, Y.; Liu, Q.; et al. Trends in unhealthy lifestyle factors in US NHANES respondents with cardiovascular disease for the period between 1999 and 2018. Front. Cardiovasc. Med. 2023, 10, 1169036. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Dastmalchi, L.N.; Gulati, M.; Michos, E.D. A Heart-Healthy Diet for Cardiovascular Disease Prevention: Where Are We Now? Vasc. Health Risk Manag. 2023, 19, 237–253. [Google Scholar] [CrossRef]
- Pant, N.; Aasuri, N.; Shaikh, M.A. Impact of Modern Food Style on Cardiovascular Health in Young Adults. Arch. Med. Rep. 2024, 1, 14–20. [Google Scholar]
- Angelico, F.; Baratta, F.; Coronati, M.; Ferro, D.; Del Ben, M. Diet and metabolic syndrome: A narrative review. Intern. Emerg. Med. 2023, 18, 1007–1017. [Google Scholar] [CrossRef]
- Jayasinghe, S.; Byrne, N.M.; Hills, A.P. Cultural influences on dietary choices. Prog. Cardiovasc. Dis. 2025, 90, 22–26. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Kaur, P.; Dahiya, R.; Buttar, H.S.; Wilson, D.W.; De Meester, F.; Telessy, I.G. Antiatherogenic Effects of Vitamins, Mediterranean Diet and DASH Diet: An Overview for the Prevention of Cardiovascular Diseases. In Hydrophilic Vitamins in Health and Disease. Advances in Biochemistry in Health and Disease; Shah, A.K., Tappia, P.S., Dhalla, N.S., Eds.; Springer: Cham, Switzerland, 2024; Volume 29, pp. 45–66. [Google Scholar] [CrossRef]
- Siviroj, P.; Wungrath, J.; Ongprasert, K. Associated Factors of Dietary Patterns among Adolescents in the Rural Northern Region of Thailand: A Community-Based Cross-Sectional Study. Healthcare 2024, 12, 1215. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, M.; Misra, A. Heterogeneity of Dietary practices in India: Current status and implications for the prevention and control of type 2 diabetes. Eur. J. Clin. Nutr. 2023, 77, 145–155. [Google Scholar] [CrossRef]
- Pirard, C.; Charoenpanwutikul, A. Comprehensive review of the annual haze episode in Northern Thailand. arXiv 2023. [Google Scholar] [CrossRef]
- Dhingra, R.; Vasan, R.S. Age as a risk factor. Med. Clin. N. Am. 2012, 96, 87–91. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Clayton, Z.S.; Hutton, D.A.; Brunt, V.E.; VanDongen, N.S.; Ziemba, B.P.; Casso, A.G.; Greenberg, N.T.; Mercer, A.N.; Rossman, M.J.; Campisi, J.; et al. Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H185–H196. [Google Scholar] [CrossRef] [PubMed]
- Tylutka, A.; Walas, Ł.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and age-related diseases: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Kang, P.M. A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases. Antioxidants 2024, 13, 923. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, S.; Lichtenstein, A.H.; Chen, S.; Na, M.; Veldheer, S.; Xing, A.; Wang, Y.; Wu, S.; et al. Alcohol consumption and risk of cardiovascular disease, cancer and mortality: A prospective cohort study. Nutr. J. 2021, 20, 13. [Google Scholar] [CrossRef]
- Lopez-Jaramillo, P.; Joseph, P.; Lopez-Lopez, J.P.; Lanas, F.; Avezum, A.; Diaz, R.; Camacho, P.A.; Seron, P.; Oliveira, G.; Orlandini, A.; et al. Risk factors, cardiovascular disease, and mortality in South America: A PURE substudy. Eur. Heart J. 2022, 43, 2841–2851. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.N.; Khodor, S.; Ali, A.; Nusairat, L.; Mahmood, A.; Nahhas, G.; Dabbous, S.; Spears, J.; Jafri, S.; Werns, S. National Trends of Tobacco, Alcohol, and Drug Use in Patients Admitted with Acute Myocardial Infarction. Cardiovasc. Revascularization Med. 2021, 26, 26–31. [Google Scholar] [CrossRef]
- Dukić, M.; Radonjić, T.; Jovanović, I.; Zdravković, M.; Todorović, Z.; Kraišnik, N.; Aranđelović, B.; Mandić, O.; Popadić, V.; Nikolić, N.; et al. Alcohol, Inflammation, and Microbiota in Alcoholic Liver Disease. Int. J. Mol. Sci. 2023, 24, 3735. [Google Scholar] [CrossRef]
- Xue, R.; Li, Q.; Geng, Y.; Wang, H.; Wang, F.; Zhang, S. Abdominal obesity and risk of CVD: A dose-response meta-analysis of thirty-one prospective studies. Br. J. Nutr. 2021, 126, 1420–1430. [Google Scholar] [CrossRef]
- Moltrer, M.; Pala, L.; Cosentino, C.; Mannucci, E.; Rotella, C.M.; Cresci, B. Body mass index (BMI), waist circumference (WC), waist-to-height ratio (WHtR) e waist body mass index (wBMI): Which is better? Endocrine 2022, 76, 578–583. [Google Scholar] [CrossRef]
- Ke, J.F.; Wang, J.W.; Lu, J.X.; Zhang, Z.H.; Liu, Y.; Li, L.X. Waist-to-height ratio has a stronger association with cardiovascular risks than waist circumference, waist-hip ratio and body mass index in type 2 diabetes. Diabetes Res. Clin. Pract. 2022, 183, 109151. [Google Scholar] [CrossRef] [PubMed]
- Marketou, M.E.; Buechler, N.S.; Fragkiadakis, K.; Plevritaki, A.; Zervakis, S.; Maragkoudakis, S.; Tsiavos, A.; Simantirakis, E.; Kochiadakis, G. Visceral fat and cardiometabolic future in children and adolescents: A critical update. Pediatr. Res. 2023, 94, 1639–1647. [Google Scholar] [CrossRef]
- Sucato, V.; Coppola, G.; Manno, G.; Vadalà, G.; Novo, G.; Corrado, E.; Galassi, A.R. Coronary Artery Disease in South Asian Patients: Cardiovascular Risk Factors, Pathogenesis and Treatments. Curr. Probl. Cardiol. 2023, 48, 101228. [Google Scholar] [CrossRef]
- Butovskaya, M.; Sorokowska, A.; Karwowski, M.; Sabiniewicz, A.; Fedenok, J.; Dronova, D.; Negasheva, M.; Selivanova, E.; Sorokowski, P. Waist-to-hip ratio, body-mass index, age and number of children in seven traditional societies. Sci. Rep. 2017, 7, 1622. [Google Scholar] [CrossRef]
- Poobalan, A.; Aucott, L. Obesity Among Young Adults in Developing Countries: A Systematic Overview. Curr. Obes. Rep. 2016, 5, 2–13. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio as a Risk Marker for Metabolic Syndrome and Cardiovascular Disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Gregory, C.O.; Dai, J.; Ramirez-Zea, M.; Stein, A.D. Occupation is more important than rural or urban residence in explaining the prevalence of metabolic and cardiovascular disease risk in Guatemalan adults. J. Nutr. 2007, 137, 1314–1319. [Google Scholar] [CrossRef]
- Ahn, N.; Kim, K. High-density lipoprotein cholesterol (HDL-C) in cardiovascular disease: Effect of exercise training. Integr. Med. Res. 2016, 5, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Lazo-Porras, M.; Bernabe-Ortiz, A.; Málaga, G.; Gilman, R.H.; Acuña-Villaorduña, A.; Cardenas-Montero, D.; Smeeth, L.; Miranda, J.J. Low HDL cholesterol as a cardiovascular risk factor in rural, urban, and rural-urban migrants: PERU MIGRANT cohort study. Atherosclerosis 2016, 246, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kalas, M.A.; Chavez, L.; Leon, M.; Taweesedt, P.T.; Surani, S. Abnormal liver enzymes: A review for clinicians. World J. Hepatol. 2021, 13, 1688–1698. [Google Scholar] [CrossRef]
- Singh, G.P.; Mitra, Y.; Singh, J.; Padda, A. Prevalence of health problems among the regular alcohol users (chronic alcoholics) in urban and rural area of district Amritsar: Punjab: India. Public health Rev. Int. J. Public Health Res. 2019, 6, 35–40. [Google Scholar] [CrossRef][Green Version]
- Gedam, S.R.; Ajab, D.; Patil, P.S.; Sharma, A.; Kumar, K.; Babar, V. Psychiatric Comorbidity, Severity of Dependence and Liver Enzymes Dysfunction among Alcohol Dependent Individuals: A Cross-sectional Study from Central Rural India. J. Clin. Diagn. Res. 2019, 13, VC01–VC05. [Google Scholar] [CrossRef]
- Giurranna, E.; Nencini, F.; Bettiol, A.; Borghi, S.; Argento, F.R.; Emmi, G.; Silvestri, E.; Taddei, N.; Fiorillo, C.; Becatti, M. Dietary Antioxidants and Natural Compounds in Preventing Thrombosis and Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 11457. [Google Scholar] [CrossRef]
- Seubsman, S.A.; Kelly, M.; Yuthapornpinit, P.; Sleigh, A. Cultural resistance to fast-food consumption? A study of youth in North Eastern Thailand. Int. J. Consum. Stud. 2009, 33, 669–675. [Google Scholar] [CrossRef]
- Cherian, G. Chapter 16—Eggs and Health: Nutrient Sources and Supplement Carriers. In Complementary and Alternative Therapies and the Aging Population; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 333–346. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Wu, J. Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gowri, B.S.; Lakshmi, A.J.; Prakash, J. Retention of nutrients in green leafy vegetables on dehydration. J. Food Sci. Technol. 2013, 50, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.W. Sprouts and Microgreens-Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, M.; Park, J.; Kim, M. Evaluation of the thiamine, riboflavin, and niacin contents in fermented soybean processed foods in various Korean provinces. J. Korean Soc. Food Sci. Nutr. 2022, 51, 688–697. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Life 2023, 13, 655. [Google Scholar] [CrossRef]
- Trevanich, S.; Sribuathong, S.; Bundidamorn, D. The Potential Health Benefits of Traditional Thai-Fermented Foods and Beverages. In Functional Properties of Traditional Foods, 1st ed.; Kristbergsson, K., Ötles, S., Eds.; Springer: Boston, MA, USA, 2016; pp. 39–73. [Google Scholar] [CrossRef]
- Kuria, A.; Tian, H.; Li, M.; Wang, Y.; Aaseth, J.O.; Zang, J.; Cao, Y. Selenium status in the body and cardiovascular disease: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 3616–3625. [Google Scholar] [CrossRef]
- Giacconi, R.; Chiodi, L.; Boccoli, G.; Costarelli, L.; Piacenza, F.; Provinciali, M.; Malavolta, M. Reduced levels of plasma selenium are associated with increased inflammation and cardiovascular disease in an Italian elderly population. Exp. Gerontol. 2021, 145, 111219. [Google Scholar] [CrossRef]
- Lotto, V.; Choi, S.W.; Friso, S. Vitamin B6: A challenging link between nutrition and inflammation in CVD. Br. J. Nutr. 2011, 106, 183–195. [Google Scholar] [CrossRef]
- Minović, I.; Kieneker, L.M.; Gansevoort, R.T.; Eggersdorfer, M.; Touw, D.J.; Voerman, A.J.; Connelly, M.A.; Boer, R.A.; Hak, E.; Bos, J.; et al. Vitamin B6, Inflammation, and Cardiovascular Outcome in a Population-Based Cohort: The Prevention of Renal and Vascular End-Stage Disease (PREVEND) Study. Nutrients 2020, 12, 2711. [Google Scholar] [CrossRef] [PubMed]
- Hernofialdi; Rini, E.A.; Machmud, R. The effect of vitamin c supplementation on intercellular adhesion molecule-1 (ICAM-1) concentration on male adolescent obesity in Padang. Int. J. Pediatr. Endocrinol. 2013, 2013, P86. [Google Scholar] [CrossRef]
- Zheng, R.; Wanglaoji, G.; Ye, J.; Chan, K.I.; Li, C.; Zhong, Z. Anti-inflammatory effects of natural products from vitamin C-rich fruits: A comprehensive review. Food Front. 2024, 5, 2383–2422. [Google Scholar] [CrossRef]
- Satheannoppakao, W.; Kasemsup, R.; Inthawong, R.; Chariyalertsak, S.; Sangthong, R.; Taneepanichskul, S.; Putwatana, P.; Kessomboon, P.; Aekplakorn, W. Sodium intake and socio-demographic determinants of the non-compliance with daily sodium intake recommendations: Thai NHES IV. J. Med. Assoc. Thai 2013, 96, S161–S170. [Google Scholar]
- O’Donnell, M.; Mente, A.; Yusuf, S. Evidence relating sodium intake to blood pressure and CVD. Curr. Cardiol. Rep. 2014, 16, 529. [Google Scholar] [CrossRef]
- Sabir, S.; Hongsibsong, S.; Chuljerm, H.; Parklak, W.; Ounjaijean, S.; Fakfum, P.; Kausar, S.; Kulprachakarn, K. Assessment of urinary oxidative stress biomarkers associated with fine particulate matter (PM2.5) exposure in Chiang Mai, Thailand. PeerJ 2025, 13, e19047. [Google Scholar] [CrossRef]


| Characteristics | Rural (n = 23) | Peri-Urban (n = 24) | Total (n = 47) | p-Value |
|---|---|---|---|---|
| Sex, N (%) | 0.036 * | |||
| Male | 9 (39.1%) | 3 (12.5%) | 12 (25.5%) | |
| Female | 14 (60.9%) | 21 (87.5%) | 35 (74.5%) | |
| Age (years), mean ± SD | 55.4 ± 5.8 | 60.0 ± 9.4 | 57.7 ± 8.1 | 0.053 |
| Alcohol consumption, N (%) | 0.077 | |||
| Never drank | 9 (39.1%) | 15 (65.2%) | 24 (52.2%) | |
| Drank | 14 (60.9%) | 8 (34.8%) | 22 (47.8%) | |
| No answer | - | 1 | ||
| Smoking status, N (%) | 0.012 * | |||
| Never smoked | 9 (39.1%) | 18 (78.3%) | 27 (58.7%) | |
| Current smoker | 8 (34.8%) | 1 (4.4%) | 9 (19.6%) | |
| Former smoker | 6 (26.1%) | 4 (17.4%) | 10 (21.7%) | |
| Chronic diseases, N (%) | ||||
| Diabetes mellitus | 5 (21.7%) | 4 (16.7%) | 9(19.2%) | 0.724 |
| Hypertension | 20 (87.0%) | 21 (87.5%) | 41 (87.2%) | 1.000 |
| Hyperlipidemia | 12 (52.2%) | 17 (70.8%) | 29 (61.7%) | 0.188 |
| Stroke | 0 | 0 | 0 | - |
| Heart disease | 0 | 0 | 0 | - |
| Other diseases (e.g., GERD, allergy/asthma, herniated disk, thyroid disorders) | 1 (4.4%) | 4 (16.7%) | 5 (10.6%) | 0.348 |
| Physical Examination | Rural (n = 23) | Peri-Urban (n = 24) | p-Value |
|---|---|---|---|
| Body height (cm) | 155.4 ± 7.1 | 155.5 ± 5.5 | 0.972 |
| Body weight (kg) | 61.5 ± 12.1 | 60.7 ± 12.7 | 0.826 |
| Body mass index, BMI (kg/m2) | 25.4 ± 4.8 | 25.0 ± 4.8 | 0.787 |
| Waist circumference, WC (cm) | 87.4 ± 12.5 | 84.7 ± 11.0 | 0.446 |
| Male | 87.4 ± 9.2 | 92.3 ± 5.8 | 0.409 |
| Female | 87.4 ± 14.6 | 83.5 ± 11.2 | 0.389 |
| Waist-to-hip ratio, WHR | 0.90 ± 0.07 | 0.85 ± 0.07 | 0.010 * |
| Male | 0.90 ± 0.05 | 0.90 ± 0.03 | 0.916 |
| Female | 0.90 ± 0.07 | 0.80 ± 0.06 | 0.043 * |
| Systolic blood pressure, SBP (mmHg) | 134.3 ± 12.6 | 128.9 ± 14.5 | 0.182 |
| Diastolic blood pressure, DBP (mmHg) | 83.2 ± 8.6 | 81.3 ± 11.0 | 0.500 |
| Heart rate (bpm) | 76.9 ± 10.8 | 80.0 ± 15.1 | 0.602 |
| Blood Chemistry Parameters | Rural (n = 23) | Peri-Urban (n = 24) | p-Value |
|---|---|---|---|
| Fasting blood glucose, FBG (mg/dL) | 108.9 ± 40.9 | 111.1 ± 33.7 | 0.277 |
| Lipid profiles | |||
| Total cholesterol, TC (mg/dL) | 197.9 ± 31.1 | 192.8 ± 37.8 | 0.618 |
| Triglyceride, TG (mg/dL) | 137.0 ± 55.5 | 107.8 ± 45.3 | 0.057 |
| High-density lipoprotein cholesterol, HDL-C (mg/dL) | 42.6 ± 12.4 | 48.6 ± 11.4 | 0.025 * |
| Low-density lipoprotein cholesterol, LDL-C (mg/dL) | 144.9 ± 40.6 | 133.4 ± 45.3 | 0.367 |
| Liver function tests | |||
| Alanine aminotransferase, ALT (U/L) | 18.2 ± 12.1 | 12.3 ± 14.4 | 0.007 ** |
| Aspartate aminotransferase, AST (U/L) | 30.3 ± 18.2 | 26.6 ± 31.2 | 0.008 ** |
| Alkaline phosphatase, ALP (U/L) | 116.4 ± 28.9 | 89.8 ± 26.3 | 0.002 ** |
| Kidney function tests | |||
| Creatinine (mg/dL) | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.133 |
| Blood urea nitrogen, BUN (mg/dL) | 12.8 ± 4.6 | 12.5 ± 2.8 | 0.730 |
| Serum electrolytes | |||
| Calcium, Ca (mg/dL) | 9.1 ± 0.4 | 9.1 ± 0.5 | 0.571 |
| Sodium, Na (mmol/L) | 141.6 ± 8.4 | 142.8 ± 1.7 | 0.383 |
| Potassium, K (mmol/L) | 4.1 ± 0.4 | 4.0 ± 0.5 | 0.300 |
| Chloride, Cl (mmol/L) | 106.3 ± 2.7 | 105.9 ± 2.5 | 0.548 |
| Carbon dioxide, CO2 (mmol/L) | 24.6 ± 2.1 | 25.4 ± 2.4 | 0.217 |
| Macronutrients | Rural (n = 23) | Peri-Urban (n = 24) | p-Value ns |
|---|---|---|---|
| Carbohydrate (%) | 60.1 (49.9–72.1) | 55.9 (53.2–67.3) | 0.338 |
| Protein (%) | 15.9 (12.8–22.1) | 16.8 (14.1–23.4) | 0.848 |
| Fat (%) | 20.2 (8.4–27.3) | 22.8 (17.3–28.9) | 0.194 |
| Variables | Rural (n = 23) | Peri-Urban (n = 24) | p-Value |
|---|---|---|---|
| Energy (kcal) | 1794.2 (1514.1–2271.3) | 1576.6 (1468.2–2322.9) | 0.702 |
| Carbohydrates (g) | 275.5 (201.2–366.1) | 269.5 (210.7–364.6) | 0.686 |
| Sugars (g) | 19.0 (12.8–33.4) | 39.3 (22.7–58.1) | 0.004 ** |
| Proteins (g) | 72.6 (57.6–111.5) | 76.6 (64.1–93.5) | 0.898 |
| Animal protein (g) | 39.9 (24.8–84.1) | 31.3 (27.6–56.7) | 0.444 |
| Vegetable protein (g) | 29.1 (22.6–34.4) | 22.8 (16.3–29.7) | 0.043 * |
| Fats (g) | 45.1 (16.3–58.3) | 41.6 (31.0–68.6) | 0.670 |
| Total saturated fatty acids (g) | 11.0 (3.4–17.5) | 13.5 (7.7–20.5) | 0.115 |
| Cholesterol (mg) | 275.8 (182.6–481.6) | 169.2 (83.2–266.4) | 0.023 * |
| Calcium (mg) | 483.3 (311.6–908.1) | 566.2 (251.4–874.3) | 0.718 |
| Phosphorus (mg) | 734.6 (609.4–132.4) | 731.6 (613.5–907.1) | 0.407 |
| Iron (mg) | 15.0 (11.2–19.6) | 12.8 (9.5–18.1) | 0.317 |
| Potassium (mg) | 1744.7 (1354.8–2665.5) | 1760.9 (1367.9–2131.6) | 0.766 |
| Sodium (mg) | 3465.6 (2854.5–4156.4) | 3248.6 (2670.4–4280.7) | 0.831 |
| Magnesium (mg) | 62.6 (22.0–104.5) | 50.8 (27.5–111.8) | 0.101 |
| Copper (mg) | 1.2 (0.8–1.4) | 0.9 (0.6–1.2) | 0.898 |
| Selenium (μg) | 63.3 (28.9–95.3) | 25.4 (6.0–48.1) | 0.011 * |
| Zinc (mg) | 7.9 (5.3–8.0) | 6.7 (4.5–8.9) | 0.686 |
| Vitamin A (μg RAE) | 429.4 (308.1–1006.2) | 234.1 (88.7–348.2) | 0.005 ** |
| Retinol (μg) | 161.1 (9.2–345.6) | 23.3 (4.6–162.0) | 0.160 |
| β-carotene (μg) | 1598.9 (853.8–3426.8) | 744.3 (287.5–1883.5) | 0.015 * |
| Vitamin B1 (mg) | 1.0 (0.7–3.2) | 1.2 (0.8–1.8) | 0.831 |
| Vitamin B2 (mg) | 1.4 (1.1–1.8) | 1.1 (0.7–1.5) | 0.089 |
| Vitamin B6 (mg) | 0.7 (0.4–1.4) | 0.4 (0.2–0.8) | 0.033 * |
| Vitamin B12 (mg) | 1.2 (0.01–3.4) | 0.7 (0.001–2.4) | 0.265 |
| Niacin (mg) | 20.0 (16.6–29.0) | 17.4 (14.3–21.0) | 0.050 * |
| Vitamin C (mg) | 96.3 (51.5–136.8) | 47.5 (33.6–57.4) | 0.006 ** |
| Vitamin E (mg) | 0.5 (0.2–1.6) | 1.0 (0.3–1.8) | 0.523 |
| Dietary fiber (g) | 15.5 (10.1–20.1) | 12.4 (8.7–17.3) | 0.142 |
| Lifestyle Behaviors | ICAM-1 a | VCAM-1 a | IL-6 a | |||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| Alcohol consumption | 0.019 | 0.912 | −0.081 | 0.912 | −0.313 | 0.105 |
| Smoking status | −0.210 | 0.206 | −0.009 | 0.957 | 0.369 | 0.063 |
| Nutrient intakes a | ||||||
| Sugars | 0.574 | <0.001 1 | 0.533 | <0.001 4 | 0.374 | 0.045 |
| Cholesterol | −0.157 | 0.340 | −0.085 | 0.605 | 0.276 | 0.147 |
| Selenium | −0.473 | 0.002 7 | −0.485 | 0.002 6 | −0.172 | 0.372 |
| Vitamin A | −0.348 | 0.030 | −0.362 | 0.024 | −0.381 | 0.041 |
| β-carotene | −0.235 | 0.150 | −0.192 | 0.242 | −0.424 | 0.022 |
| Vitamin B6 | −0.372 | 0.020 | −0.552 | <0.001 3 | −0.232 | 0.226 |
| Niacin | −0.265 | 0.103 | −0.334 | 0.038 | −0.327 | 0.084 |
| Vitamin C | −0.497 | 0.001 5 | −0.553 | <0.001 2 | −0.163 | 0.397 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parklak, W.; Kulprachakarn, K.; Kawichai, S.; Fakfum, P.; Jiraya, P.; Kijkuokool, P.; Khiaolaongam, W.; Chuljerm, H. Association Between Dietary Patterns and Lifestyle Habits with Vascular Inflammatory Responses in Individuals with Hypertension Living in PM2.5-Polluted Areas: A Cross-Sectional Pilot Study in Chiang Mai Province, Thailand. Diseases 2025, 13, 258. https://doi.org/10.3390/diseases13080258
Parklak W, Kulprachakarn K, Kawichai S, Fakfum P, Jiraya P, Kijkuokool P, Khiaolaongam W, Chuljerm H. Association Between Dietary Patterns and Lifestyle Habits with Vascular Inflammatory Responses in Individuals with Hypertension Living in PM2.5-Polluted Areas: A Cross-Sectional Pilot Study in Chiang Mai Province, Thailand. Diseases. 2025; 13(8):258. https://doi.org/10.3390/diseases13080258
Chicago/Turabian StyleParklak, Wason, Kanokwan Kulprachakarn, Sawaeng Kawichai, Puriwat Fakfum, Putita Jiraya, Praporn Kijkuokool, Wiritphon Khiaolaongam, and Hataichanok Chuljerm. 2025. "Association Between Dietary Patterns and Lifestyle Habits with Vascular Inflammatory Responses in Individuals with Hypertension Living in PM2.5-Polluted Areas: A Cross-Sectional Pilot Study in Chiang Mai Province, Thailand" Diseases 13, no. 8: 258. https://doi.org/10.3390/diseases13080258
APA StyleParklak, W., Kulprachakarn, K., Kawichai, S., Fakfum, P., Jiraya, P., Kijkuokool, P., Khiaolaongam, W., & Chuljerm, H. (2025). Association Between Dietary Patterns and Lifestyle Habits with Vascular Inflammatory Responses in Individuals with Hypertension Living in PM2.5-Polluted Areas: A Cross-Sectional Pilot Study in Chiang Mai Province, Thailand. Diseases, 13(8), 258. https://doi.org/10.3390/diseases13080258








