Hepatocellular Carcinoma: A Comprehensive Review
Abstract
1. Introduction
2. Pathogenesis and Genetics
3. Risk Factors for HCC
3.1. Viral Infections
3.2. MASLD
3.3. Alcohol
3.4. Toxins
3.5. Smoking
4. Epidemiology
5. Clinical Features
6. Surveillance
7. Diagnosis
7.1. Imaging, Radiomics and AI
7.2. Pathology
7.3. Liquid Biopsy
8. Treatment
- Very early stage (stage 0): Single lesion < 2 cm, compensated liver disease, ECOG 0.
- Early stage (stage A): tumor within Milan criteria, ECOG 0, compensated liver disease.
- Intermediate or Stage B: Tumor outside of Milan criteria, compensated liver disease, ECOG 0.
- Advanced (stage C): vascular invasion or distal metastases, compensated cirrhosis, ECOG 1-2.
- Terminal: decompensated liver disease, ECOG 3-4, any size tumor.
9. Treatment Options
9.1. Ablation
9.2. Resection
9.3. Liver Transplantation
9.4. Trans-Arterial Therapies
9.5. Systemic Therapies
- Tyrosine kinase inhibitors: sorafenib, regorafenib, lenvatinib and cabozantinib.
- Monoclonal anti-angiogenic antibodies like bevacizumab and ramucirumab.
- Immune checkpoint inhibitors, including pembrolizumab, nivolumab, atezolizumab and durvalumab. The former two are programmed death 1 (PD1) inhibitors and the latter two are inhibitors of programmed death ligand 1 (PD-L1).
- Another category of ICIs includes cytotoxic T-lymphocyte-associated protein 4 inhibitors (CTLA4), including ipilimumab and tremelimumab.
9.6. Treatment Recommendations
9.7. Future Direction
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Kanwal, F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014, 60, 1767–1775. [Google Scholar] [CrossRef]
- Xu, J. Trends in Liver Cancer Mortality Among Adults Aged 25 and Over in the United States, 2000–2016. 2018. Available online: https://www-cdc-gov.wvu.idm.oclc.org/nchs/products/databriefs/db314.htm (accessed on 3 April 2025).
- Nzeako, U.C.; Goodman, Z.D.; Ishak, K.G. Hepatocellular carcinoma in cirrhotic and noncirrhotic livers. A clinico-histopathologic study of 804 North American patients. Am. J. Clin. Pathol. 1996, 105, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sandhu, S.; Lai, J.P.; Sandhu, D.S. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J. Hepatol. 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127 (Suppl. S1), S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Trinchet, J.C.; Bourcier, V.; Chaffaut, C.; Ait Ahmed, M.; Allam, S.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; De Ledinghen, V.; et al. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort). Hepatology 2015, 62, 737–750. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Splan, M.F.; Weiss, N.S.; McDonald, G.B.; Beretta, L.; Lee, S.P. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2007, 5, 938–945.e4. [Google Scholar] [CrossRef]
- Levrero, M.; Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 2016, 64 (Suppl. S1), S84–S101. [Google Scholar] [CrossRef]
- D’Souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: Trends, projections and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.A.; Patil, R.; Harrison, S.A. NAFLD-related hepatocellular carcinoma: The growing challenge. Hepatology 2023, 77, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.J.; Yang, H.I.; Wu, H.C.; Liu, J.; Wang, L.Y.; Lu, S.N.; Lee, M.H.; Jen, C.L.; You, S.L.; Santella, R.M.; et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int. J. Cancer 2017, 141, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Fracanzani, A.L.; Conte, D.; Fraquelli, M.; Taioli, E.; Mattioli, M.; Losco, A.; Fargion, S. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non-iron-related chronic liver disease. Hepatology 2001, 33, 647–651. [Google Scholar] [CrossRef]
- Perlmutter, D.H. Pathogenesis of chronic liver injury and hepatocellular carcinoma in alpha-1-antitrypsin deficiency. Pediatr. Res. 2006, 60, 233–238. [Google Scholar] [CrossRef]
- Shimazu, T.; Tsubono, Y.; Kuriyama, S.; Ohmori, K.; Koizumi, Y.; Nishino, Y.; Shibuya, D.; Tsuji, I. Coffee consumption and the risk of primary liver cancer: Pooled analysis of two prospective studies in Japan. Int. J. Cancer 2005, 116, 150–154. [Google Scholar] [CrossRef]
- Gallus, S.; Bertuzzi, M.; Tavani, A.; Bosetti, C.; Negri, E.; La Vecchia, C.; Lagiou, P.; Trichopoulos, D. Does coffee protect against hepatocellular carcinoma? Br. J. Cancer 2002, 87, 956–959. [Google Scholar] [CrossRef]
- Simon, T.G.; Duberg, A.S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef]
- Villanueva, A.; Longo, D.L. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Tanaka, M.; Miyajima, A. Liver regeneration and fibrosis after inflammation. Inflamm. Regen. 2016, 36, 19. [Google Scholar] [CrossRef]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Li, C.Y.; Lin, Y.Y.; Ho, M.C.; Chen, D.S.; Chen, P.J.; Yeh, S.H. Androgen Receptor Enhances Hepatic Telomerase Reverse Transcriptase Gene Transcription After Hepatitis B Virus Integration or Point Mutation in Promoter Region. Hepatology 2019, 69, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239.e4. [Google Scholar] [CrossRef]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Schulze, K.; Imbeaud, S.; Letouze, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef]
- Buch, S.; Stickel, F.; Trepo, E.; Way, M.; Herrmann, A.; Nischalke, H.D.; Brosch, M.; Rosendahl, J.; Berg, T.; Ridinger, M.; et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 2015, 47, 1443–1448. [Google Scholar] [CrossRef]
- Wang, B.; Huang, G.; Wang, D.; Li, A.; Xu, Z.; Dong, R.; Zhang, D.; Zhou, W. Null genotypes of GSTM1 and GSTT1 contribute to hepatocellular carcinoma risk: Evidence from an updated meta-analysis. J. Hepatol. 2010, 53, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Pessino, G.; Scotti, C.; Maggi, M.; Immuno-Hub, C. Hepatocellular Carcinoma: Old and Emerging Therapeutic Targets. Cancers 2024, 16, 901. [Google Scholar] [CrossRef]
- Hoshida, Y.; Nijman, S.M.; Kobayashi, M.; Chan, J.A.; Brunet, J.P.; Chiang, D.Y.; Villanueva, A.; Newell, P.; Ikeda, K.; Hashimoto, M.; et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009, 69, 7385–7392. [Google Scholar] [CrossRef]
- Wands, J. Hepatocellular carcinoma and sex. N. Engl. J. Med. 2007, 357, 1974–1976. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.E.M.; Cabibbo, G.; Craxi, A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses 2022, 14, 986. [Google Scholar] [CrossRef]
- Sze, K.M.; Ho, D.W.; Chiu, Y.T.; Tsui, Y.M.; Chan, L.K.; Lee, J.M.; Chok, K.S.; Chan, A.C.; Tang, C.N.; Tang, V.W.; et al. Hepatitis B Virus-Telomerase Reverse Transcriptase Promoter Integration Harnesses Host ELF4, Resulting in Telomerase Reverse Transcriptase Gene Transcription in Hepatocellular Carcinoma. Hepatology 2021, 73, 23–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Wen, X.; Zhang, S.; Yang, Y. Association of ACYP2 and MPHOSPH6 genetic polymorphisms with the risk of hepatocellular carcinoma in chronic hepatitis B virus carriers. Oncotarget 2017, 8, 86011–86019. [Google Scholar] [CrossRef]
- Axley, P.; Ahmed, Z.; Ravi, S.; Singal, A.K. Hepatitis C Virus and Hepatocellular Carcinoma: A Narrative Review. J. Clin. Transl. Hepatol. 2018, 6, 79–84. [Google Scholar] [CrossRef]
- Gajos-Michniewicz, A.; Czyz, M. WNT/β-catenin signaling in hepatocellular carcinoma: The aberrant activation, pathogenic roles, and therapeutic opportunities. Genes. Dis. 2024, 11, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef]
- Puigvehi, M.; Moctezuma-Velazquez, C.; Villanueva, A.; Llovet, J.M. The oncogenic role of hepatitis delta virus in hepatocellular carcinoma. JHEP Rep. 2019, 1, 120–130. [Google Scholar] [CrossRef]
- Talamantes, S.; Lisjak, M.; Gilglioni, E.H.; Llamoza-Torres, C.J.; Ramos-Molina, B.; Gurzov, E.N. Non-alcoholic fatty liver disease and diabetes mellitus as growing aetiologies of hepatocellular carcinoma. JHEP Rep. 2023, 5, 100811. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Kaseb, A.; Etzel, C.J.; El-Serag, H.; Spitz, M.R.; Chang, P.; Hale, K.S.; Liu, M.; Rashid, A.; Shama, M.; et al. Genetic variation in the PNPLA3 gene and hepatocellular carcinoma in USA: Risk and prognosis prediction. Mol. Carcinog. 2013, 52 (Suppl. S1), E139–E147. [Google Scholar] [CrossRef] [PubMed]
- Rich, N.E.; Hester, C.; Odewole, M.; Murphy, C.C.; Parikh, N.D.; Marrero, J.A.; Yopp, A.C.; Singal, A.G. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2019, 17, 551–559.e1. [Google Scholar] [CrossRef]
- Lin, C.-C.; Mo, L.-R.; Chang, C.-Y.; Perng, D.-S.; Hsu, C.-C.; Lo, G.-H.; Chen, Y.-S.; Yen, Y.-C.; Hu, J.-T.; Yu, M.-L.; et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J. Hepatol. 2013, 58, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, F.; Hussain, S.P.; Cerutti, P. Aflatoxin B1 induces the transversion of G-->T in codon 249 of the p53 tumor suppressor gene in human hepatocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 8586–8590. [Google Scholar] [CrossRef]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef]
- Boccia, S.; Miele, L.; Panic, N.; Turati, F.; Arzani, D.; Cefalo, C.; Amore, R.; Bulajic, M.; Pompili, M.; Rapaccini, G.; et al. The effect of CYP, GST, and SULT polymorphisms and their interaction with smoking on the risk of hepatocellular carcinoma. Biomed. Res. Int. 2015, 2015, 179867. [Google Scholar] [CrossRef]
- Chuang, S.C.; Lee, Y.C.; Hashibe, M.; Dai, M.; Zheng, T.; Boffetta, P. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: A meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 1261–1268. [Google Scholar] [CrossRef]
- Petrick, J.L.; Campbell, P.T.; Koshiol, J.; Thistle, J.E.; Andreotti, G.; Beane-Freeman, L.E.; Buring, J.E.; Chan, A.T.; Chong, D.Q.; Doody, M.M.; et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br. J. Cancer 2018, 118, 1005–1012. [Google Scholar] [CrossRef]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007, 317, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, T.; Roy, G.; Fujimoto, N.; Asahara, T.; Ito, A. Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemoprevention by leuprorelin. Jpn. J. Cancer Res. 2001, 92, 249–256. [Google Scholar] [CrossRef]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755.e3. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Saba, L.; Bosco, S.; Rossi, M.; Miles, K.A.; Di Miscio, R.; Lombardo, C.V.; Tamponi, E.; Piga, M.; Catalano, C. Hepatocellular carcinoma (HCC) in non-cirrhotic liver: Clinical, radiological and pathological findings. Eur. Radiol. 2014, 24, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, X.; Dai, J.; Ding, C.; Zhang, Z.; Lin, Z.; Hu, J.; Lu, M.; Wang, Z.; Qi, Y.; et al. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020, 11, 822. [Google Scholar] [CrossRef]
- Benson, A.B.; D’aNgelica, M.I.; Abbott, D.E.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; Chahal, P.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Arguedas, M.R.; Chen, V.K.; Eloubeidi, M.A.; Fallon, M.B. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: A cost-utility analysis. Am. J. Gastroenterol. 2003, 98, 679–690. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Singal, A.G.; Tayob, N.; Mehta, A.; Marrero, J.A.; El-Serag, H.; Jin, Q.; de Viteri, C.S.; Fobar, A.; Parikh, N.D. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology 2022, 75, 541–549. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Jin, Q.; Tayob, N.; Salem, E.; Luster, M.; Alsarraj, A.; Khaderi, S.; Singal, A.G.; Marrero, J.A.; Asrani, S.K.; et al. HES V2.0 outperforms GALAD for detection of HCC: A phase 3 biomarker study in the United States. Hepatology 2025, 81, 465–475. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, T.; Wang, H.; Ma, X. The Value of Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in the Diagnosis of Hepatocellular Carcinoma: A Meta-Analysis. J. Ultrasound Med. 2022, 41, 1537–1547. [Google Scholar] [CrossRef]
- Fraquelli, M.; Nadarevic, T.; Colli, A.; Manzotti, C.; Giljaca, V.; Miletic, D.; Stimac, D.; Casazza, G. Contrast-enhanced ultrasound for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst. Rev. 2022, 2022, CD013483. [Google Scholar] [CrossRef]
- Savsani, E.; Shaw, C.M.; Forsberg, F.; Wessner, C.E.; Lyshchik, A.; O’Kane, P.; Liu, J.B.; Balasubramanya, R.; Roth, C.G.; Naringrekar, H.; et al. Contrast-enhanced US Evaluation of Hepatocellular Carcinoma Response to Chemoembolization: A Prospective Multicenter Trial. Radiology 2023, 309, e230727. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Argemi, J.; Ronot, M.; Paradis, V.; Meyer, T.; Mazzaferro, V.; Jepsen, P.; Golfieri, R.; Galle, P.; Dawson, L.; et al. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef] [PubMed]
- Chatzipanagiotou, O.P.; Loukas, C.; Vailas, M.; Machairas, N.; Kykalos, S.; Charalampopoulos, G.; Filippiadis, D.; Felekouras, E.; Schizas, D. Artificial intelligence in hepatocellular carcinoma diagnosis: A comprehensive review of current literature. J. Gastroenterol. Hepatol. 2024, 39, 1994–2005. [Google Scholar] [CrossRef]
- Qi, L.; Zhu, Y.; Li, J.; Zhou, M.; Liu, B.; Chen, J.; Shen, J. CT radiomics-based biomarkers can predict response to immunotherapy in hepatocellular carcinoma. Sci. Rep. 2024, 14, 20027. [Google Scholar] [CrossRef]
- Yuan, G.; Song, Y.; Li, Q.; Hu, X.; Zang, M.; Dai, W.; Cheng, X.; Huang, W.; Yu, W.; Chen, M.; et al. Development and Validation of a Contrast-Enhanced CT-Based Radiomics Nomogram for Prediction of Therapeutic Efficacy of Anti-PD-1 Antibodies in Advanced HCC Patients. Front. Immunol. 2020, 11, 613946. [Google Scholar] [CrossRef]
- Dessie, E.Y.; Tu, S.J.; Chiang, H.S.; Tsai, J.J.P.; Chang, Y.S.; Chang, J.G.; Ng, K.L. Construction and Validation of a Prognostic Gene-Based Model for Overall Survival Prediction in Hepatocellular Carcinoma Using an Integrated Statistical and Bioinformatic Approach. Int. J. Mol. Sci. 2021, 22, 1632. [Google Scholar] [CrossRef]
- Chen, M.; Cao, J.; Hu, J.; Topatana, W.; Li, S.; Juengpanich, S.; Lin, J.; Tong, C.; Shen, J.; Zhang, B.; et al. Clinical-Radiomic Analysis for Pretreatment Prediction of Objective Response to First Transarterial Chemoembolization in Hepatocellular Carcinoma. Liver Cancer 2021, 10, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, K.; Krzyzinski, M.; Bartczak, T.; Korzeniowski, K.; Lamparski, K.; Wroblewski, T.; Grat, M.; Holowko, W.; Mech, K.; Lisowska, J.; et al. A novel radiomics approach for predicting TACE outcomes in hepatocellular carcinoma patients using deep learning for multi-organ segmentation. Sci. Rep. 2024, 14, 14779. [Google Scholar] [CrossRef]
- Miranda, J.; Horvat, N.; Fonseca, G.M.; Araujo-Filho, J.A.B.; Fernandes, M.C.; Charbel, C.; Chakraborty, J.; Coelho, F.F.; Nomura, C.H.; Herman, P. Current status and future perspectives of radiomics in hepatocellular carcinoma. World J. Gastroenterol. 2023, 29, 43–60. [Google Scholar] [CrossRef]
- Silva, M.A.; Hegab, B.; Hyde, C.; Guo, B.; Buckels, J.A.; Mirza, D.F. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: A systematic review and meta-analysis. Gut 2008, 57, 1592–1596. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhowmik, S.; Majumdar, S.; Goswami, A.; Chakraborty, J.; Gupta, S.; Aggarwal, S.; Ray, S.; Chatterjee, R.; Bhattacharyya, S.; et al. The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low alpha-fetoprotein. Int. J. Cancer 2020, 147, 2934–2947. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chen, X.; Yang, Z. Polymeric immunoglobulin receptor (PIGR) exerts oncogenic functions via activating ribosome pathway in hepatocellular carcinoma. Int. J. Med. Sci. 2021, 18, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Chamseddine, S.; Yavuz, B.G.; Mohamed, Y.I.; Lee, S.S.; Yao, J.C.; Hu, Z.I.; LaPelusa, M.; Xiao, L.; Sun, R.; Morris, J.S.; et al. Circulating Galectin-3: A Prognostic Biomarker in Hepatocellular Carcinoma. J. Immunother. Precis. Oncol. 2024, 7, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Prospective suRveillance for very Early hepatoCellular cARcinoma(PreCar) expert panel; Wang, H.; Hou, J.; Wang, C. Expert consensus on early screening strategies for liver cancer in China. Chin. J. Hepatol. 2021, 29, 515–522. [Google Scholar] [CrossRef]
- Clinic, C. Liquid Biopsy: What It Is & What Liquid Biopsy Tests Are FDA-Approved. Available online: https://my.clevelandclinic.org/health/diagnostics/23992-liquid-biopsy (accessed on 20 June 2025).
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef]
- Cha, C.; Fong, Y.; Jarnagin, W.R.; Blumgart, L.H.; DeMatteo, R.P. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J. Am. Coll. Surg. 2003, 197, 753–758. [Google Scholar] [CrossRef]
- Mlynarsky, L.; Menachem, Y.; Shibolet, O. Treatment of hepatocellular carcinoma: Steps forward but still a long way to go. World J. Hepatol. 2015, 7, 566–574. [Google Scholar] [CrossRef]
- Yao, F.Y.; Fidelman, N. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: Where do we stand with tumor down-staging? Hepatology 2016, 63, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Figueras, J.; Jaurrieta, E.; Valls, C.; Benasco, C.; Rafecas, A.; Xiol, X.; Fabregat, J.; Casanovas, T.; Torras, J.; Baliellas, C.; et al. Survival after liver transplantation in cirrhotic patients with and without hepatocellular carcinoma: A comparative study. Hepatology 1997, 25, 1485–1489. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Melero, I.; Yau, T.; Kang, Y.K.; Kim, T.Y.; Santoro, A.; Sangro, B.; Kudo, M.; Hou, M.M.; Matilla, A.; Tovoli, F.; et al. Nivolumab plus ipilimumab combination therapy in patients with advanced hepatocellular carcinoma previously treated with sorafenib: 5-year results from CheckMate 040. Ann. Oncol. 2024, 35, 537–548. [Google Scholar] [CrossRef]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Duurland, C.L.; Gunst, T.; Boer, H.C.D.; Bosch, M.; Telford, B.J.; Vos, R.M.; Xie, X.; Zang, M.; Wang, F.; Shao, Y.; et al. INT-1B3, an LNP formulated miR-193a-3p mimic, promotes anti-tumor immunity by enhancing T cell mediated immune responses via modulation of the tumor microenvironment and induction of immunogenic cell death. Oncotarget 2024, 15, 470–485. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J.; Zhou, H.; Chen, Y.; Zhi, Y.; Zhang, B.; Chen, L.; Chu, X.; Wang, R.; Zhang, C. PU.1/microRNA-142-3p targets ATG5/ATG16L1 to inactivate autophagy and sensitize hepatocellular carcinoma cells to sorafenib. Cell Death Dis. 2018, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Z.; Hou, N.; Li, Y.; Wang, X.; Wu, F.; Sun, R.; Han, J.; Sun, H.; Song, T.; et al. MicroRNA-214 targets Wnt3a to suppress liver cancer cell proliferation. Mol. Med. Rep. 2017, 16, 6920–6927. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liu, X.; Li, Z.; Wang, G.; Feng, Z.; Liu, Y.; Yang, H.; Tan, C.; Zhang, Z.; Li, K. LncRNA LINC00667 aggravates the progression of hepatocellular carcinoma by regulating androgen receptor expression as a miRNA-130a-3p sponge. Cell Death Discov. 2021, 7, 387. [Google Scholar] [CrossRef]
- Dai, H.; Tong, C.; Shi, D.; Chen, M.; Guo, Y.; Chen, D.; Han, X.; Wang, H.; Wang, Y.; Shen, P. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: A single-arm, open-label, phase II trial. Oncoimmunology 2020, 9, 1846926. [Google Scholar] [CrossRef]

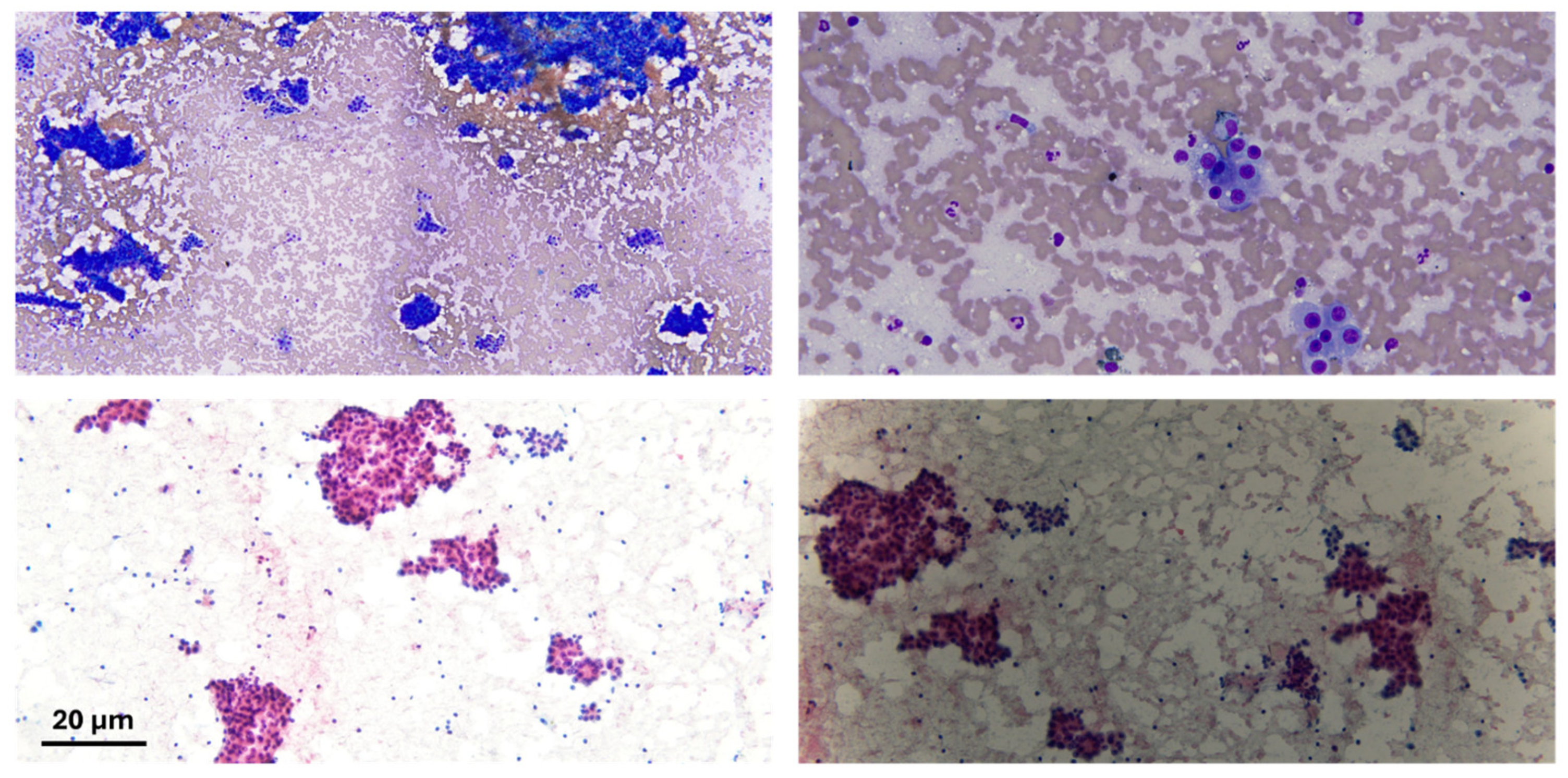
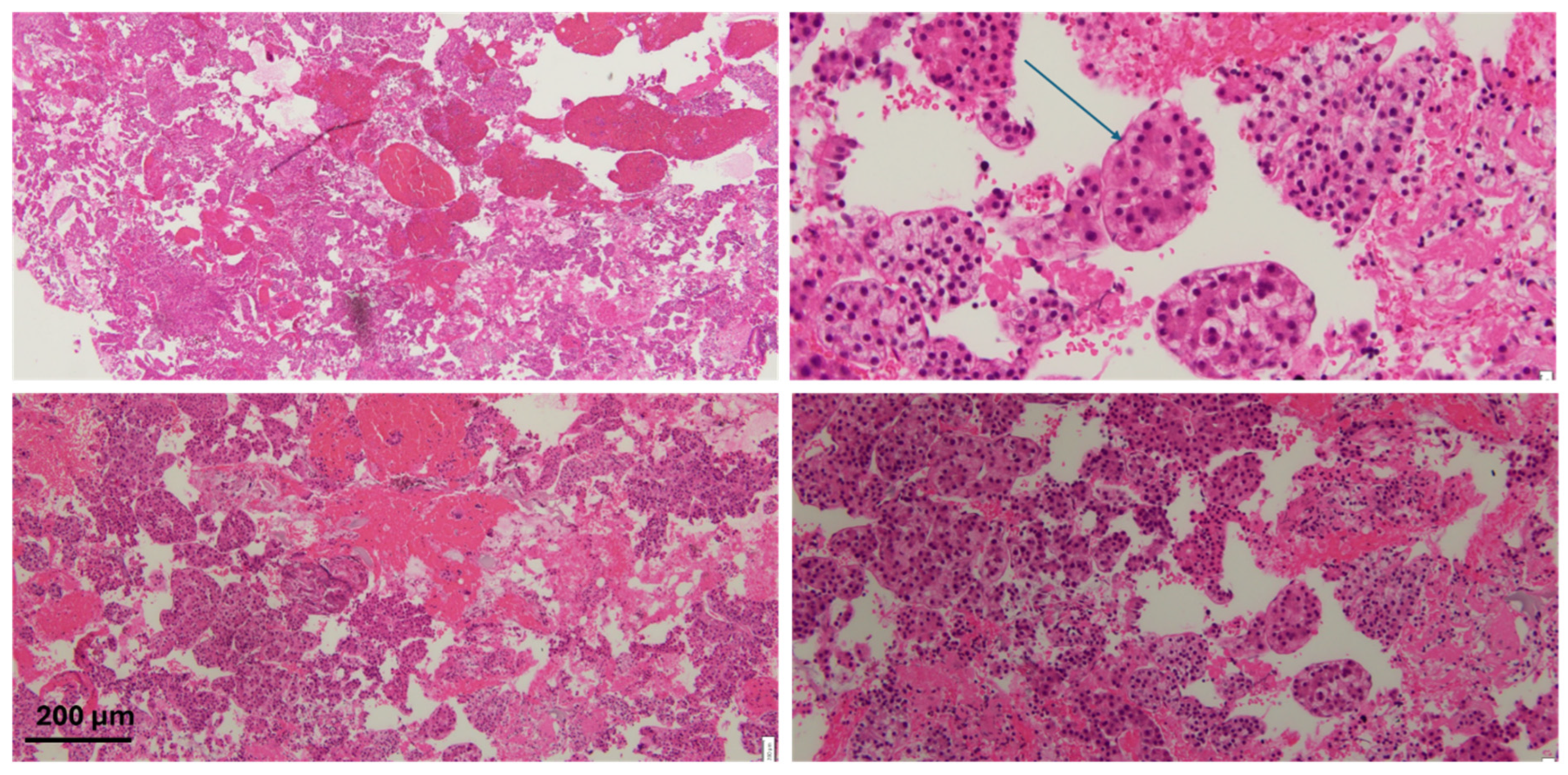

| Risk Factor | Category | Mechanism |
|---|---|---|
| Cirrhosis (any etiology) | Liver Disease | Regenerative nodules, chronic inflammation |
| Chronic Hepatitis B Virus (HBV) | Viral Infection | Integration into host genome, direct oncogenesis |
| Chronic Hepatitis C Virus (HCV) | Viral Infection | Chronic inflammation, cirrhosis, fibrosis |
| Alcohol Use Disorder | Lifestyle | Chronic liver injury, cirrhosis |
| Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) | Metabolic/Liver Disease | Lipotoxicity, inflammation, progression to cirrhosis |
| Aflatoxin B1 Exposure | Environmental | DNA adduct formation (e.g., p53 mutations) |
| Obesity | Metabolic | Associated with MASLD |
| Smoking | Lifestyle | Carcinogen exposure |
| Hemochromatosis | Genetic Disorder | Iron overload, oxidative stress |
| Primary Biliary Cholangitis | Autoimmune Liver Disease | Chronic inflammation, bile duct damage |
| α1-Antitrypsin Deficiency | Genetic Disorder | Abnormal ATT protein accumulation, cirrhosis |
| Male Sex | Demographic | Possibly hormonal; epidemiological association |
| Older Age | Demographic | Cumulative exposure to risk factors |
| Family History of HCC | Genetic/Environmental | May reflect shared genes or exposures |
| (A) | ||||
| APHE | Size (mm) | 0 Additional Features | 1 Additional Feature | ≥2 Additional Features |
| None | <20 | LR-3 | LR-3 | LR-4 |
| ≥20 | LR-3 | LR-4 | LR-4 | |
| Nonrim APHE | <10 | LR-3 | LR-4 | LR-4 |
| 10–19 | LR-3 | LR-4/LR-5 * | LR-5 | |
| ≥20 | LR-4 | LR-5 | LR-5 | |
| (B) | ||||
| APHE | Size (mm) | 0 Additional Features | 1 Additional Feature | ≥2 Additional Features |
| None | Any size | rLR-3 | rLR-4 | rLR-5 |
| Nonrim APHE | <20 | rLR-3 | rLR-5 | rLR-5 |
| ≥20 | rLR-4 | LR-5 | LR-5 | |
| Grade 0 | Full level of activity with no limitations |
| Grade 1 | Ability to perform activities of light/sedentary nature |
| Grade 2 | Capable of performing activities of daily living but not able to work, ambulatory for >50% of waking time |
| Grade 3 | Limited self-care, bed- or chair-bound for >50% of waking time |
| Grade 4 | Completely confined to bed or chair |
| Grade 5 | Pt deceased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, N.; Anwar, J.; Sulaiman, A.; Naumova, N.N.; Anwar, N. Hepatocellular Carcinoma: A Comprehensive Review. Diseases 2025, 13, 207. https://doi.org/10.3390/diseases13070207
Amin N, Anwar J, Sulaiman A, Naumova NN, Anwar N. Hepatocellular Carcinoma: A Comprehensive Review. Diseases. 2025; 13(7):207. https://doi.org/10.3390/diseases13070207
Chicago/Turabian StyleAmin, Nisar, Javaria Anwar, Abdullahi Sulaiman, Nadia Nikolaeva Naumova, and Nadeem Anwar. 2025. "Hepatocellular Carcinoma: A Comprehensive Review" Diseases 13, no. 7: 207. https://doi.org/10.3390/diseases13070207
APA StyleAmin, N., Anwar, J., Sulaiman, A., Naumova, N. N., & Anwar, N. (2025). Hepatocellular Carcinoma: A Comprehensive Review. Diseases, 13(7), 207. https://doi.org/10.3390/diseases13070207





