Abstract
Background: There is still debate whether ribavirin should be added to direct-acting antivirals (DAAs) for the management of treatment-experienced individuals with non-genotype-1 hepatitis C. This study compared the efficacy and safety of adding ribavirin to sofosbuvir-based combinations compared to sofosbuvir-based regimens alone in treating non-genotype 1 hepatitis C virus (HCV) in individuals who have been previously treated. Methods: We searched Cochrane CENTRAL, PubMed, SCOPUS, CINAHL and preprint databases from inception to September 2023 for randomized controlled trials (RCTs) that compared sofosbuvir-based regimens with ribavirin to sofosbuvir-based regimens alone in previously treated individuals with non-genotype 1 HCV infection. Data extraction and quality of study assessments were performed by two independent authors, and synthesis was performed using bias-adjusted models, heterogeneity using I2, and publication bias using funnel plots. Results: Eight RCTs compared sofosbuvir-based combinations with and without ribavirin were included. Overall, the addition of ribavirin to sofosbuvir, compared to sofosbuvir alone, did not show a benefit in achieving sustained virological response (SVR) (OR 0.91, 95% CI 0.26–3.17, I2 = 70.0%) with moderate certainty in Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) evidence. In subgroup analysis, there was no benefit of adding ribavirin to sofosbuvir in individuals with non-genotype 1 HCV. The additional ribavirin was associated with increased adverse events (OR 2.03, 95% CI 1.58–2.6, I2 = 8.0%) and treatment discontinuation (OR 1.81, 95% CI 0.78–4.28, I2 = 0.0%). Conclusions: The moderate certainty evidence suggests that adding ribavirin to sofosbuvir-based regimens may not confer benefit in achieving SVR in previously treated individuals with non-genotype 1 HCV but increases the odds of adverse events and treatment discontinuation. More evidence is needed on the effect of additional ribavirin in achieving SVR in individuals with decompensated cirrhosis. Registration: The protocol is registered on the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022368868).
1. Introduction
Hepatitis C virus (HCV) is a global epidemic that affects an estimated 58 million infected individuals worldwide, resulting in roughly 400,000 deaths each year, primarily from complications including liver cirrhosis and hepatocellular carcinoma [1]. Treatment rates for HCV remain low, with only 13% of people receiving treatment [1]. In the absence of a vaccine, the elimination of this virus will have to be achieved using behavior-based methods to stop transmission and cure existing cases using antiviral therapy. One of the most significant advances in HCV treatment has been the development of direct-acting antivirals (DAAs), which have high cure rates, with up to 95% of treated individuals achieving sustained virological response (SVR) within 12 weeks after treatments [1,2]. DAAs are classified into four classes based on their therapeutic target and mechanism of action [3], and major guideline bodies recommend first-line DAAs that are pan-genotypic (i.e., treat all HCV genotypes), such as sofosbuvir and velpatasvir [4,5,6]. Apart from their potency, DAAs have relatively few side effects when given orally over a short time [1].
Despite the availability of highly efficacious curative DAAs, a significant testing and treatment gap remains. In addition, there are still individuals who require treatment after either failure of DAA therapy or failure of interferon-based regimens, which were previously recommended [7]. Some of the treatment failures have been attributed to certain HCV genotypes, which do not respond well to treatment. For example, genotype 4 did not respond well to interferon-based therapies, while genotype 3 may not respond well to some DAAs [8,9]. HCV has eight common genotypes (genotypes 1 to 8), with genotype 1 being the most common globally and also the most researched, and the genotypes have varying distribution in different geographical regions [5,10,11]. Despite the availability of pangenotypic DAAs, there is still a need for research on optimal treatments for other types of HCV that are not genotype 1 [12].
There is still no consensus on the treatment options for retreating HCV [11]. Current retreatment options usually involve combining sofosbuvir with another class of DAA, extending the treatment duration [11,13], and some guidelines [4,13] recommend the addition of ribavirin to DAAs. Ribavirin, a guanosine analog, has been used as an adjunct to interferon-based therapies, and several trials have investigated its use as an add-on to DAAs in retreating HCV [14,15,16]. Ribavirin acts by directly inhibiting HCV viral replication and HCV RNA polymerase [14]. However, ribavirin is potentially teratogenic, may result in hemolytic anemia decreased lymphocyte counts, and has potential for possible carcinogenicity [17]. Because of its teratogenicity, the drug is contraindicated in women planning to be pregnant [18].
Randomized controlled trials (RCTs) of adding ribavirin to DAAs have produced conflicting results, especially in treatment-experienced participants. For example, two RCTs concluded that adding ribavirin to DAAs did not improve treatment outcomes and increased the risk of side effects in people who required retreatment for HCV [15,19]. However, another RCT demonstrated the benefit of achieving SVR when ribavirin was added to DAAs in retreating HCV [20]. Existing meta-analyses of RCTs have shown no benefit in adding ribavirin to DAAs when treating either treatment-naïve [16] or treatment-experienced participants with genotype 1 HCV [21,22]. However, an evidence gap remains regarding whether ribavirin has benefit when added to DAAs for the retreatment of non-genotype 1 HCV. Therefore, we conducted a systematic review and meta-analysis to assess the efficacy and safety of adding ribavirin to DAAs, particularly to sofosbuvir-based regimens, compared to DAAs alone in the retreatment of individuals with non-genotype 1 HCV.
2. Methods
2.1. Study Design
This research is a systematic review and meta-analysis and follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23] (Supplementary Document S1). The protocol for this study is registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022368868).
2.2. Data Sources
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Scopus, Cumulated Index to Nursing and Allied Health Literature (CINAHL), and the databases of preprints such as medRXIV for studies published from January 2010 to November 2022, with no language restrictions. The search was conducted during November 2022, using a search strategy (Supplementary Document S2) and updated during September 2023.
2.3. Search Methods
2.3.1. Search Terms for DAAs
“sofosbuvir” OR “sovaldi” OR “simeprevir” OR “olysio” OR “daclatasvir” OR “daklinza” OR “ledipasvir” OR “harvoni” OR “elbasvir” OR “grazoprevir” OR “zepatier” OR “velpatasvir” OR “epclusa” OR “ombitasvir” OR “paritaprevir” OR “dasabuvir” OR “viekira pak” OR voxilaprevir OR ritonavir OR 3D OR glecaprevir OR pibrentasvir OR mavyret OR “direct-acting agents” OR “direct acting antiviral” OR daa.
2.3.2. Search Terms for Hepatitis C
“Hepatitis c” OR HCV OR “chronic hepatitis C” OR “Acute Hepatitis C”.
2.3.3. Search Terms for Ribavirin
Ribavirin OR Rebetol OR Ribasphere OR RibaPak OR Copegus OR Virazole OR Moderiba OR “Tribavirin” OR “Vilona” “Viramide” OR “Virazide” OR “ICN-1229” OR “ICN 1229” OR “ICN1229” OR “Ribamide” OR “Ribamidil” OR “Ribamidyl” OR “RBV”.
2.3.4. Search Terms for Hepatitis C Non-Genotype 1
“Genotype 2” OR “genotype 3” OR “genotype 4” OR “genotype 5” OR “genotype 6” OR “GT2” OR “GT3” OR “GT4” OR “GT5” OR “GT6” OR “non-genotype 1” OR “non genotype 1” OR “non GT1”.
2.4. Procedure for Selection of Studies
The study records from the search were imported into EndNote for deduplication and, subsequently, uploaded to the Rayyan systematic review management website (https://www.rayyan.ai/) for screening using the title and abstracts. The full text of preliminarily included study records was retrieved and evaluated for eligibility by two independent investigators. In cases of disagreement, a third investigator was consulted to make the final decision.
2.5. Eligibility
Studies included were limited to original RCTs that compared the efficacy and safety of Sofosbuvir-based combinations with and without ribavirin in treating non-genotype 1 HCV in treatment-experienced participants [24]. Included studies should have specified the HCV genotype, reported the analyses of treatment-experienced individuals separately, used sofosbuvir across both arms and had full text available. We excluded observational studies, reviews, RCTs with only genotype 1 or treatment-naïve individuals, animal or lab studies, and RCTs that did not report the main outcome of SVR12. In studies with both genotype 1 and non-genotype 1, we extracted data only from non-genotype 1 participants. Moreover, data were extracted for groups of equal treatment duration.
2.6. Key Definitions
Retreatment is initiated in patients with treatment failure, defined as the failure to achieve SVR at the end of treatment, which can be due to non-response relapse or re-infection [25].
2.7. Outcomes
The primary efficacy outcome was SVR, which is defined as HCV-RNA levels below 15 IU/mL, measured 12 weeks after the end of treatment [26]. The primary safety outcome was treatment discontinuation. The secondary efficacy outcome was SVR at 24 weeks (SVR24) after treatment completion [26]. The secondary safety endpoints were developing any common adverse events, including fatigue, headache, dermatologic manifestations, and gastroenterological symptoms such as nausea, vomiting, and diarrhea [27], as well as serious adverse events.
2.8. Data Extraction
Data extraction was performed independently by two authors using Microsoft Excel. The extracted data included data on study design, date, location, sample size, HCV genotype, type of prior treatment, treatment regimen, the number of individuals with SVR at 12 and 24 weeks, side effects, serious side effects, and treatment discontinuation.
2.9. Assessing the Quality of Included Studies
The quality of the included studies was assessed using the Methodological Standard for Epidemiological Research (MASTER) scale, comprising seven standards subdivided into 36 safeguards [28].
2.10. Data Synthesis
Synthesis for Efficacy and Safety of DAAs With and Without Ribavirin
In this study, we recalculated the unadjusted odds ratio (OR) for each study to calculate pooled odds ratios (ORs) for SVR12 and other outcomes, along with their 95% confidence intervals (CIs), using the quality-effects model [29]. The quality-effects model uses random error variance in addition to variance due to systematic error as weights. The systematic error variance weights were derived from quality ranks, which were derived from the quality assessment, thereby adjusting for possible bias in the analysis phase [29]. The random-effects model assumes that the treatment effects obtained from an assumed superpopulation of studies (which do not exist) follow a normal distribution, thus giving spuriously precise Cls. Moreover, because of the increase in the mean squared error (MSE), the point estimate from the meta-analysis has a possibility of deviating away from the true value much more than with the quality-effects model [30,31]. Forest plots were used to display the pooled OR estimates and their CIs. Sensitivity analysis was conducted to re-analyze data without bias adjustment using the quality-effects model and the random-effects model [32]. We used Stata version 17 (College Station, TX, USA) with the metan package for the meta-analysis and reported exact p values. We used the I2 statistic, Cochran’s Q p-value, and the Galbraith plot to assess heterogeneity and I2 values of 25%, 50%, and 75% were interpreted as low, moderate, and high inconsistency categories, respectively [33]. We conducted subgroup analysis for studies with previous treatment regimens (either DAAs or interferon-based regimens) and for the cirrhosis status. We investigated publication bias using Doi plots and the Luis Furuya-Kanamori (LFK) index [34], in addition to funnel plots and Egger regression [35]. The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework was used to rate evidence quality and recommendation strength for the primary outcome of SVR12 [36].
2.11. Ethics
This systematic review utilized published data. Therefore, there was no need for ethical approval.
3. Results
3.1. Search Results
A total of 7206 studies were identified from all the searches. After the removal of duplicates and manual screening by title and abstract, 112 studies remained for full-text screening, of which 102 were excluded for reasons shown in Figure 1. Most studies excluded after full-text screening the full text were not RCTs (n = 53) or did not compare the drugs of interest (n = 23). Eight studies were chosen according to the inclusion criteria [19,37,38,39,40,41,42,43]. Two RCTs were excluded because they did not use sofosbuvir in DAAs combination [20,44].
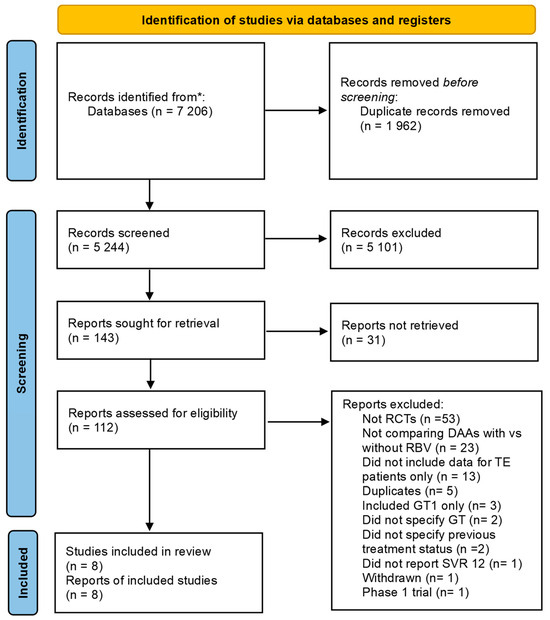
Figure 1.
PRISMA flow chart illustrates the screening, exclusion, and selection of the studies in the meta-analysis. Abbreviations: (*) Identified from multiple sources (CENTRAL, PubMed, Scopus, CINAHL, and the databases of preprints such as medRXIV), Randomized controlled trials (RCTs), Direct-acting Antiviral (DAA), Ribavirin (RBV), Treatment Experienced (TE), Genotype (GT), Sustained Viral Response (SVR).
3.2. Characteristics of Included Studies
The eight RCTs included [19,37,38,39,40,41,42,43] had a total of 772 participants from eight countries, with three studies from the United States of America, two from Egypt, and the remainder from multiple countries (Table 1). One study focused only on genotype 2 [37], three studies on genotype 3 [39,40,41], two studies on genotype 4 [38,43] and two studies included several genotypes [19,42]. All studies included some participants with compensated cirrhosis, and three of them included individuals with cirrhosis only [19,39,41]. Most studies excluded human immunodeficiency virus (HIV) and hepatitis B virus (HBV) co-infections [19,37,38,40,42,43], but two studies included HIV co-infected participants [39,41]. All studies included participants previously treated with interferons, while three studies also included individuals with prior DAA treatment [19,39,40] (Table 1).

Table 1.
Characteristics of included studies.
3.3. Assessment of the Quality of Included Studies
Most of the included studies achieved MASTER scale scores ranging from 29 to 31 out of 36, indicating an overall high quality of evidence (Supplementary Figure S1). One study did not meet the first safeguard as they excluded a participant after randomization [38]. Additionally, two studies did not adequately report how allocation concealment was conducted [37,39].
3.4. Efficacy of Adding Ribavirin to DAAs in Achieving SVR at 12 Weeks—GRADE Assessment
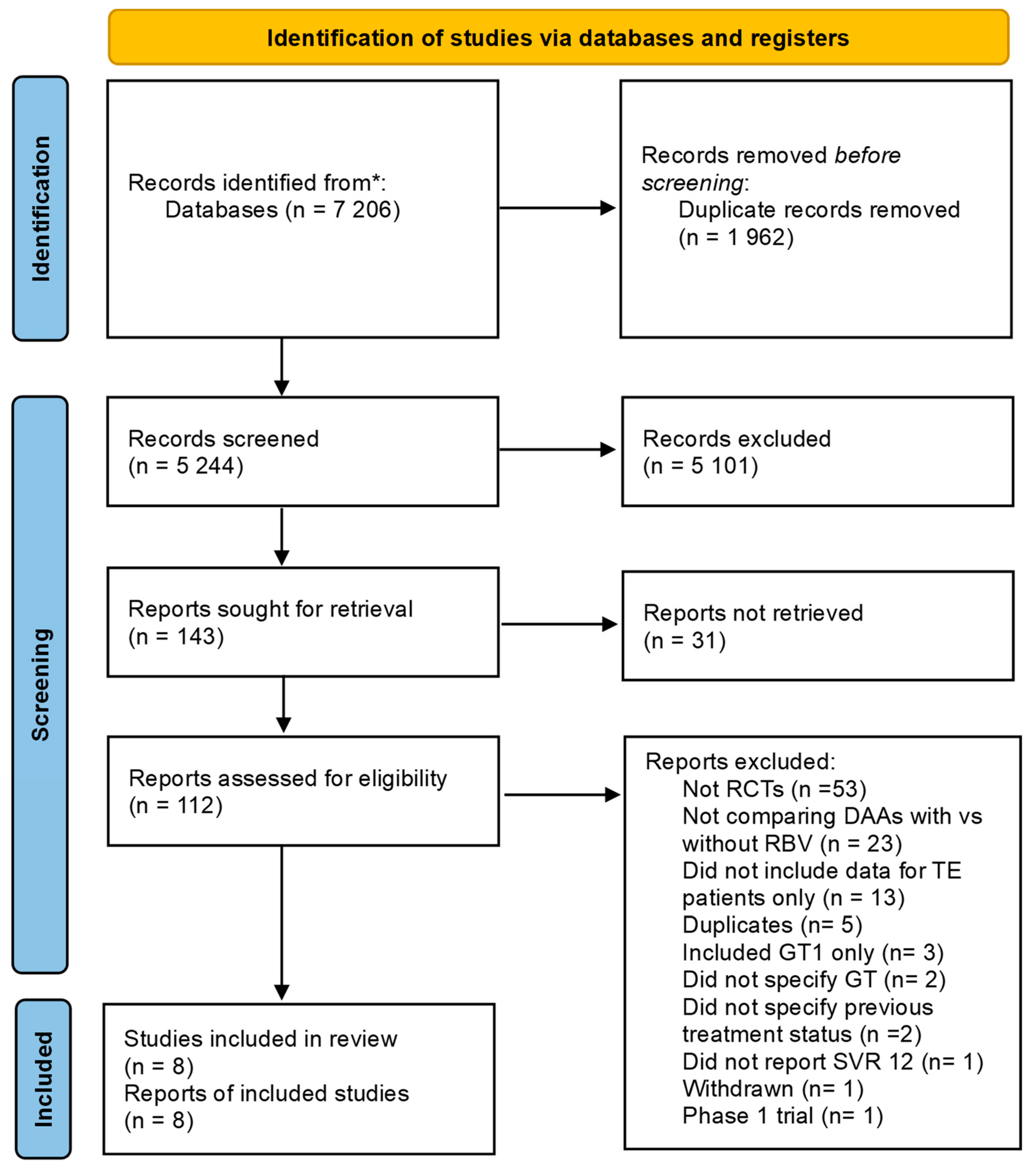
All eight included RCTs [19,37,38,39,40,41,42,43] with a total of 772 participants, of which 420 were on additional ribavirin, and 352 were on sofosbuvir-based regimens alone, reported data on SVR12. The effect of adding ribavirin to sofosbuvir-based regimens varied slightly across studies; one study showed a reduction in efficacy [40], six showed no effect [19,37,38,39,41,43], and one study reported an increase in efficacy [42]. The studies included were RCTs, which provide high-quality evidence, and therefore, the initial GRADE was set as high certainty, pending assessments of other GRADE domains.
3.5. Overall Effect Size and Consistency
In the overall synthesis, the addition of ribavirin to sofosbuvir-based regimens did not improve SVR achievement at 12 weeks (OR 0.91, 95%CI 0.26–3.17), with moderate statistical heterogeneity (I2 = 70.0%) (Figure 2). This effect was the same even when the random-effects model was used (Figure 2). Visual inspection of the forest plot showed some inconsistency, and measures of heterogeneity all suggested some statistical heterogeneity for this outcome (I2 = 70.0%, Cochran’s Q p value = 0.001, tau2 = 1.498). The Galbraith plot (Supplementary Figure S2) showed an effect near the null, represented by an almost horizontal line, and some heterogeneity shown by two studies outside the upper and lower CI. Therefore, the GRADE was downgraded by one level because of the lack of consistency.
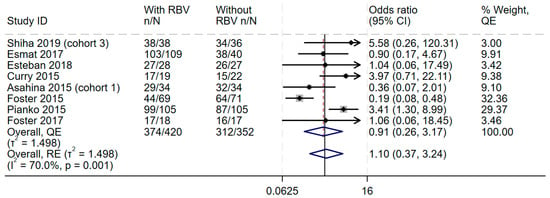
Figure 2.
Efficacy of adding ribavirin to sofosbuvir-based regimens compared to sofosbuvir-based regimens alone. The vertical dashed line at OR = 1 represents the line of no effect. The diamond at the bottom represents the pooled overall estimate from all studies, with its center indicating the overall OR and its width denoting the 95% CI [19,37,38,39,40,41,42,43]. Abbreviations: Confidence interval (CI), Direct-acting Antiviral (DAA), Genotype (GT), interferon (IFN), Quality Effects (QE), Random Effects (RE).
3.6. Directness, Study Quality, and Publication Bias
In terms of directness, the interventions specified for these participants and the outcome of SVR12 were similar to those of interest to the healthcare system and clinicians. Most of the RCTs had a high-quality rating, and the funnel and Doi plots (Supplementary Figure S3) suggested that there was no publication bias. Therefore, after the assessment of these domains, the GRADE rating remained as moderate certainty evidence for the primary outcome.
3.7. Final GRADE Rating for the Efficacy of Adding Ribavirin to DAAs to Achieve SVR12
The GRADE was downgraded by one level due to heterogeneity in the RCTs, and therefore, the GRADE rating for the evidence for the main outcome was of moderate certainty that adding ribavirin to sofosbuvir-based regimens does not improve efficacy in achieving SVR12.
3.8. Sensitivity Analysis
Similar results were observed in the leave-one-out sensitivity analysis (Supplementary Figure S4).
3.9. Subgroup Analysis
When the analysis was stratified by the genotypes, no additional benefit was observed when ribavirin was added for genotype 3 (OR 0.78, 95% CI 0.09–6.63, I2 = 83.2%) and genotype 4 (OR 1.39, 95% CI 0.31–6.31, I2 = 4.7%) (Supplementary Table S1). Only one study [37] provided data on participants with genotype 2, and in that study, the addition of ribavirin greatly reduced the efficacy observed (OR 0.36, 95% CI 0.07–2.01). Although subgroup analysis by cirrhosis status due to lack of subgrouped data within the studies, in one study of individuals with decompensated cirrhosis, ribavirin showed benefit when added to sofosbuvir-based regimens, with 85% SVR12 achievement rate with ribavirin compared to 50% SVR12 in individuals without ribavirin (OR 3.97, 95% CI 0.71–22.11) [19].
3.10. SVR at 24 Weeks
Only one RCT [38] assessed SVR24. The study suggested no benefit in achieving SVR24 when ribavirin was added to sofosbuvir-based regimens (OR 0.90, 95% CI 0.17–4.67).
3.11. Safety of Adding Ribavirin to DAAs
Any adverse events, serious adverse events and discontinuation of treatment.
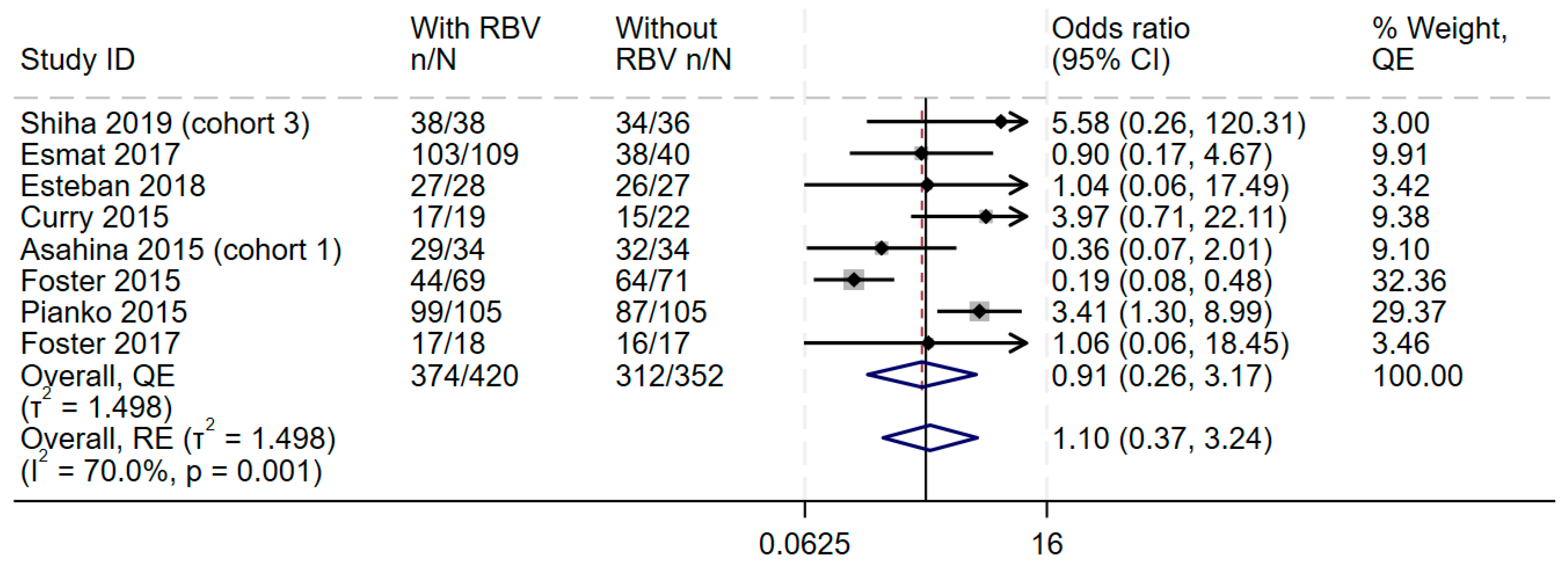
Data from all eight studies showed increase in the odds of adverse events in the additional ribavirin group, with little to no heterogeneity (OR 2.03, 95% CI 1.58–2.6, I2 = 8.0%) (Figure 3 and Supplementary Figures S6 and S7) and higher odds of discontinuation of treatment (OR 1.81, 95% CI 0.78–4.28, I 2 = 0.0%) (Supplementary Figures S8–S10). The synthesis also suggested no difference in the odds of serious adverse events (OR 1.13, 95% CI 0.61–2.06, I2 = 21.9%, n = 7 studies) (Supplementary Figures S11–S13).
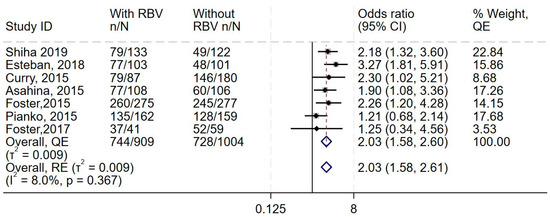
Figure 3.
Effect of adding ribavirin to sofosbuvir-based regimens on adverse events. The vertical dashed line at OR = 1 represents the line of no effect. The diamond at the bottom represents the pooled overall estimate from all studies, with its center indicating the overall OR and its width denoting the 95% CI [19,37,39,40,41,42,43].
This forest plot shows the overall analysis of the odds of adverse events in the treatment group (DAAs and ribavirin) compared to the control group (DAAs only), which was reported by all eight studies. The meta-analytic effect size using the Quality effects and Random effects models are (OR 2.03) and (OR 2.03), respectively.
4. Discussion
In this meta-analysis of eight RCTs, we found that adding ribavirin to sofosbuvir-based regimens did not improve efficacy in achieving SVR12 in adults previously treated for non-genotype 1 HCV. Additionally, the findings confirm that adding ribavirin to sofosbuvir increases the odds of adverse events and treatment discontinuation.
Our synthesis shows that there is no difference in efficacy when ribavirin is added to sofosbuvir-based regimens, compared to sofosbuvir-based regimens alone with an overall odds ratio of 0.91 of achieving SVR12, with moderate certainty GRADE evidence. These findings are similar to those of other meta-analyses that found no benefit in achieving SVR12 with the addition of ribavirin to DAA regimens in other sub-populations [2,45,46,47,48,49]. For example, in HCV genotype 1, regardless of prior treatment history, two meta-analyses have shown that adding ribavirin to DAAs did not improve the achievement of SVR, with overall risk ratios of one in both analyses [46,48]. Additionally, we found that ribavirin also did not add to the efficacy of sofosbuvir-based regimens when SVR was assessed at 24 weeks, although this result came from one study. Notably, some guidelines [4,50] still recommend the use of ribavirin in retreating HCV, perhaps due to a lack of up-to-date syntheses in non-genotype 1 treatment-experienced populations [11], which this study now provides.
While we restricted our analyses to RCTs that had HCV genotypes determined in each participant, our findings, together with those of existing meta-analyses [45,46,47,48,49,51], suggest that given the availability of pangenotypic DAAs such as sofosbuvir and velpatasvir [11] there may be no need to genotype HCV infections before starting treatment [51]. A recent RCT, which we excluded because of a lack of genotype reporting, also found no benefit in adding ribavirin to DAAs when retreating HCV in Egypt [15]. Notably, the findings from our included studies showed a high degree of efficacy, with treatment success of around 90% in both ribavirin and non-ribavirin trial arms, again confirming the high efficacy of DAAs.
The current meta-analysis confirmed that ribavirin was associated with an increase in the odds of individuals discontinuing treatment. Previous meta-analyses [2,45,46,47,48,49,52] have not reported on treatment discontinuation, which is a key factor influencing outcomes, especially in individuals who need retreatment. However, other meta-analyses have found ribavirin to be associated with a higher risk of adverse events [2,47,52], which aligns with our findings. Notably, ribavirin cannot be used in both male and female individuals who are planning to have children because of its high teratogenicity [18].
The strengths of this study include the inclusion of studies where genotyping was performed, clear eligibility criteria, a comprehensive search of the literature and rigorous analysis, and the use of GRADE evidence certainty, making the results of this study easier to translate to clinical practice and practice guidelines. A limitation of this study is the moderate heterogeneity observed in the synthesis of the primary outcome (SVR12), which we investigated using subgroup analysis. Another limitation is that we did not investigate the appropriate DAA combinations for retreating HCV.
5. Conclusions
The moderate certainty evidence suggests that adding ribavirin to sofosbuvir-based regimens may not confer benefit in achieving SVR in previously treated individuals with non-genotype 1 HCV but increases the odds of adverse events and treatment discontinuation. More evidence is needed on the effect of additional ribavirin in achieving SVR in individuals with decompensated cirrhosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diseases13050138/s1.
Author Contributions
S.H.—Conceptualization, Investigation, Formal analysis, Validation, Visualization, Data Curation, Resources, Software, Methodology, Writing—Original Draft, Writing—Review & Editing, Funding acquisition, Supervision, Project administration. A.A.A.-R.—Conceptualization, Investigation, Formal analysis, Validation, Visualization, Data Curation, Resources, Software, Methodology, Writing—Original Draft, Writing—Review & Editing. K.J.—Methodology, Investigation, Validation, Visualization, Resources, Software, Writing—Original Draft, Writing—Review & Editing, Funding acquisition. M.A.A.-T.—Conceptualization, Investigation, Validation, Visualization, Data Curation, Resources, Software, Methodology, Writing—Original Draft, Writing—Review & Editing. M.E.—Conceptualization, Investigation, Validation, Visualization, Data Curation, Resources, Software, Methodology, Writing—Original Draft, Writing—Review & Editing. M.K.A.-H.—Conceptualization, Investigation, Validation, Visualization, Data Curation, Resources, Software, Methodology, Writing—Original Draft, Writing—Review & Editing. W.A.-F.—Conceptualization, Investigation, Validation, Visualization, Data Curation, Resources, Software, Funding acquisition, Methodology, Writing—Original Draft, Writing—Review & Editing. Y.A.K.—Conceptualization, Investigation, Validation, Visualization, Data Curation, Resources, Software, Methodology, Writing—Original Draft, Writing—Review & Editing. Y.D.—Conceptualization, Investigation, Validation, Visualization, Data Curation, Resources, Software, Methodology, Writing—Original Draft, Writing—Review & Editing. A.-N.E.—Methodology, Investigation, Writing—Review & Editing, Supervision. T.C.—Conceptualization, Investigation, Formal analysis, Validation, Visualization, Data curation, Resources, Software, Methodology, Writing—Original Draft, Writing—Review & Editing, Funding acquisition, Supervision, Project administration, Guarantor of Review. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Publication funding provided by the University of Washington Tacoma.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available in the published RCTs.
Acknowledgments
We would like to express our deepest appreciation to the department of population medicine in Qatar University for their continuous help throughout the research process.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DAA | Direct-acting Antiviral |
| HCV | Hepatitis C Virus |
| Hb | Hemoglobin |
| RR | Risk Ratio |
| RCT | Randomized Controlled Trial |
| SVR | Sustained Virological Response |
References
- World Health Organization. WHO Publishes Updated Guidance on Hepatitis C Infection—With New Recommendations on Treatment of Adolescents and Children, Simplified Service Delivery and Diagnostics; WHO: Geneva, Switzerland, 2022.
- Xue, W.; Liu, K.; Qiu, K.; Shen, Y.; Pan, Z.; Hu, P.; Peng, M.; Chen, M.; Ren, H. A systematic review with meta-analysis: Is ribavirin necessary in sofosbuvir-based direct-acting antiviral therapies for patients with HCV recurrence after liver transplantation? Int. J. Infect. Dis. 2019, 83, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pockros, P.J. Direct-Acting Antivirals for the Treatment of Hepatitis C Virus Infection. Available online: https://www.uptodate.com/contents/direct-acting-antivirals-for-the-treatment-of-hepatitis-c-virus-infection (accessed on 24 March 2025).
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H.; European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Zein, N.N. Clinical significance of Hepatitis C virus genotypes. Clin. Microbiol. Rev. 2000, 13, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Aronsohn, A.; Price, J.; Re, V.L.; the American Association for the Study of Liver Diseases–Infectious Diseases Society of America HCV Guidance Panel. Hepatitis C guidance 2023 update: American association for the study of liver diseases—Infectious diseases society of America recommendations for testing, managing, and treating hepatitis C virus infection. Clin. Infect. Dis. 2023, ciad319. [Google Scholar] [CrossRef]
- Webster, G.; Barnes, E.; Brown, D.; Dusheiko, G. HCV genotypes—Role in pathogenesis of disease and response to therapy. Best Pract. Res. Clin. Gastroenterol. 2000, 14, 229–240. [Google Scholar] [CrossRef]
- Pabjan, P.; Brzdęk, M.; Chrapek, M.; Dziedzic, K.; Dobrowolska, K.; Paluch, K.; Garbat, A.; Błoniarczyk, P.; Reczko, K.; Stępień, P.; et al. Are there still difficult-to-treat patients with chronic hepatitis C in the era of direct-acting antivirals? Viruses 2022, 14, 96. [Google Scholar] [CrossRef]
- Derbala, M.; Amer, A.; Bener, A.; Lopez, A.C.; Omar, M.; El Ghannam, M. Pegylated interferon-alpha 2b-ribavirin combination in egyptian patients with genotype 4 chronic hepatitis. J. Viral Hepat. 2005, 12, 380–385. [Google Scholar] [CrossRef]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucl. Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.S.; Muir, A.J. Management of Chronic Hepatitis C Virus Infection: Antiviral Retreatment Following Relapse in Adults. Available online: https://www.uptodate.com/contents/management-of-chronic-hepatitis-c-virus-infection-antiviral-retreatment-following-relapse-in-adults?search=Management%20of%20chronic%20hepatitis%20C%20virus%20infection%3A%20Antiviral%20retreatment%20following%20relapse%20in%20adults&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1 (accessed on 24 March 2025).
- Chan, A.; Patel, K.; Naggie, S. Genotype 3 infection: The last stand of hepatitis C virus. Drugs 2017, 77, 131–144. [Google Scholar] [CrossRef]
- HCV Guidance; American Association for the Study of Liver Disease. Retreatment of Persons in Whom Prior Therapy Failed. Available online: https://www.hcvguidelines.org/treatment-experienced (accessed on 24 March 2025).
- Te, H.S.; Randall, G.; Jensen, D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol. Hepatol. N. 2007, 3, 218–225. [Google Scholar]
- El-Kassas, M.; Emadeldeen, M.; Hassany, M.; Esmat, G.; Gomaa, A.A.; El-Raey, F.; Congly, S.E.; Liu, H.; Lee, S.S. A randomized-controlled trial of SOF/VEL/VOX with or without ribavirin for retreatment of chronic hepatitis C. J. Hepatol. 2023, 79, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, M.; Hezode, C.; Gerstoft, J.; Vierling, J.M.; Mallolas, J.; Pol, S.; Kugelmas, M.; Murillo, A.; Weis, N.; Nahass, R.; et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus Co-infection (C-WORTHY): A randomised, open-label phase 2 trial. Lancet 2015, 385, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Mooney, K.; Melvin, M.; Douglas, T. Ribavirin: The need for exposure precautions. Clin. J. Oncol. Nurs. 2014, 18, E93–E96. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Sustiva (Efavirenz) Prescribing Information (NDA 021511, s023). 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021511s023lbl.pdf (accessed on 1 April 2025).
- Curry, M.P.; O’Leary, J.G.; Bzowej, N.; Muir, A.J.; Korenblat, K.M.; Fenkel, J.M.; Reddy, K.R.; Lawitz, E.; Flamm, S.L.; Schiano, T.; et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 2015, 373, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Kwo, P.; Gane, E.J.; Peng, C.Y.; Pearlman, B.; Vierling, J.M.; Serfaty, L.; Buti, M.; Shafran, S.; Stryszak, P.; Lin, L.; et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology 2017, 152, 164–175.e4. [Google Scholar] [CrossRef]
- Bansal, S.; Singal, A.K.; McGuire, B.M.; Anand, B.S. Impact of all oral anti-hepatitis C virus therapy: A meta-analysis. World J. Hepatol. 2015, 7, 806–813. [Google Scholar] [CrossRef]
- Suwanthawornkul, T.; Anothaisintawee, T.; Sobhonslidsuk, A.; Thakkinstian, A.; Teerawattananon, Y. Efficacy of second generation direct-acting antiviral agents for treatment naïve hepatitis C genotype 1: A systematic review and network meta-analysis. PLoS ONE 2015, 10, e0145953. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Li, H.C.; Lo, S.Y. Hepatitis C virus: Virology, diagnosis and treatment. World J. Hepatol. 2015, 7, 1377–1389. [Google Scholar] [CrossRef]
- Salmon, D.; Trimoulet, P.; Gilbert, C.; Solas, C.; Lafourcade, E.; Chas, J.; Piroth, L.; Lacombe, K.; Katlama, C.; Peytavin, G.; et al. Factors associated with DAA virological treatment failure and resistance-associated substitutions description in HIV/HCV coinfected patients. World J. Hepatol. 2018, 10, 856–866. [Google Scholar] [CrossRef]
- Burgess, S.V.; Hussaini, T.; Yoshida, E.M. Concordance of sustained virologic response at weeks 4, 12 and 24 post-treatment of hepatitis C in the era of new oral direct-acting antivirals: A concise review. Ann. Hepatol. 2016, 15, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Chang, T.S.; Lu, S.N.; Su, H.J.; Chang, S.Z.; Hsu, C.W.; Chen, M.Y. An investigation of the side effects, patient feedback, and physiological changes associated with direct-acting antiviral therapy for hepatitis C. Int. J. Environ. Res. Public Health 2019, 16, 4981. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.C.; Glass, K.; Clark, J.; Ritskes-Hoitinga, M.; Munn, Z.; Tugwell, P.; Doi, S.A.R. The methodological standards for epidemiological research (MASTER) scale demonstrated a unified framework for bias assessment. J. Clin. Epidemiol. 2021, 134, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.A.; Thalib, L. A quality-effects model for meta-analysis. Epidemiology 2008, 19, 94–100. [Google Scholar] [CrossRef]
- Doi, S.A.R.; Furuya-Kanamori, L.; Thalib, L.; Barendregt, J.J. Meta-analysis in evidence-based healthcare: A paradigm shift away from random effects is overdue. Int. J. Evid.-Based Health 2017, 15, 152–160. [Google Scholar] [CrossRef]
- Stone, J.C.; Glass, K.; Munn, Z.; Tugwell, P.; Doi, S.A.R. Comparison of bias adjustment methods in meta-analysis suggests that quality effects modeling may have less limitations than other approaches. J. Clin. Epidemiol. 2020, 117, 36–45. [Google Scholar] [CrossRef]
- Hedeker, D.; Gibbons, R.D. A random-effects ordinal regression model for multilevel analysis. Biometrics 1994, 50, 933–944. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S.; Ben Van Den, A. Cochrane handbook for systematic reviews of interventions. Int. Coach. Psychol. Rev. 2020, 15, 123–125. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid.-Based. Health 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Asahina, Y.; Itoh, Y.; Ueno, Y.; Matsuzaki, Y.; Takikawa, Y.; Yatsuhashi, H.; Genda, T.; Ikeda, F.; Matsuda, T.; Dvory-Sobol, H. Ledipasvir-sofosbuvir for treating japanese patients with chronic hepatitis C virus genotype 2 infection. Liver Int. 2018, 38, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Esmat, G.; Elbaz, T.; El Raziky, M.; Gomaa, A.; Abouelkhair, M.; Gamal El Deen, H.; Sabry, A.; Ashour, M.; Allam, N.; Abdel-Hamid, M.; et al. Effectiveness of ravidasvir plus sofosbuvir in interferon-naïve and treated patients with chronic hepatitis C genotype-4. J. Hepatol. 2017, 68, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Pineda, J.A.; Calleja, J.L.; Casado, M.; Rodríguez, M.; Turnes, J.; Morano Amado, L.E.; Morillas, R.M.; Forns, X.; Pascasio Acevedo, J.M.; et al. Efficacy of sofosbuvir and velpatasvir, with and without ribavirin, in patients with hepatitis C virus genotype 3 infection and cirrhosis. Gastroenterology 2018, 155, 1120–1127.e4. [Google Scholar] [CrossRef]
- Foster, G.R.; Afdhal, N.; Roberts, S.K.; Bräu, N.; Gane, E.J.; Pianko, S.; Lawitz, E.; Thompson, A.; Shiffman, M.L.; Cooper, C.; et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N. Engl. J. Med. 2015, 373, 2608–2617. [Google Scholar] [CrossRef]
- Foster, G.R.; Agarwal, K.; Cramp, M.E.; Moreea, S.; Barclay, S.; Collier, J.; Brown, A.S.; Ryder, S.D.; Ustianowski, A.; Forton, D.M.; et al. Elbasvir/grazoprevir and sofosbuvir for hepatitis C virus genotype 3 infection with compensated cirrhosis: A randomized trial. Hepatology 2018, 67, 2113–2126. [Google Scholar] [CrossRef]
- Pianko, S.; Flamm, S.L.; Shiffman, M.L.; Kumar, S.; Strasser, S.I.; Dore, G.J.; McNally, J.; Brainard, D.M.; Han, L.; Doehle, B.; et al. Sofosbuvir plus velpatasvir combination therapy for treatment-experienced patients with genotype 1 or 3 hepatitis C virus infection: A randomized trial. Ann. Intern. Med. 2015, 163, 809–817. [Google Scholar] [CrossRef]
- Shiha, G.; Esmat, G.; Hassany, M.; Soliman, R.; Elbasiony, M.; Fouad, R.; Elsharkawy, A.; Hammad, R.; Abdel-Razek, W.; Zakareya, T.; et al. Ledipasvir/sofosbuvir with or without ribavirin for 8 or 12 weeks for the treatment of HCV genotype 4 infection: Results from a randomised phase III study in egypt. Gut 2019, 68, 721–728. [Google Scholar] [CrossRef]
- Lawitz, E.; Yoshida, E.M.; Buti, M.; Vierling, J.M.; Almasio, P.L.; Bruno, S.; Ruane, P.J.; Hassanein, T.I.; Lalezari, J.P.; Mullhaupt, B.; et al. Safety and efficacy of the fixed-dose combination regimen of MK-3682/grazoprevir/MK-8408 with or without ribavirin in non-cirrhotic or cirrhotic patients with chronic HCV GT1, 2 or 3 infection (part B of C-CREST-1 &2). Hepatology 2016, 64, 60A. [Google Scholar]
- Elshafie, S.; Trivedi-Kapoor, R.; Ebell, M. Safety and efficacy of sofosbuvir-based medication regimens with and without ribavirin in hepatitis C patients: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2022, 47, 1149–1158. [Google Scholar] [CrossRef]
- Ren, X.D.; Fu, X.; He, Y.Q.; Li, C.Y.; Guo, M.; Qiao, M. Safety and efficacy of sofosbuvir-velpatasvir: A meta-analysis. Medicine 2022, 101, e31183. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Abushouk, A.I.; Attia, A.; Gadelkarim, M.; Gabr, M.; Negida, A.; Abdel-Daim, M.M. Safety and Efficacy of Sofosbuvir plus Velpatasvir with or without Ribavirin for Chronic Hepatitis C Virus Infection: A systematic review and meta-analysis. J. Infect. Public Health 2018, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Abushouk, A.I.; Menshawy, A.; Mohamed, A.; Negida, A.; Loutfy, S.A.; Abdel-Daim, M.M. Safety and efficacy of ombitasvir/paritaprevir/ritonavir and dasabuvir with or without ribavirin for treatment of hepatitis C virus genotype 1: A systematic review and meta-analysis. Clin. Drug Investig. 2017, 37, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; Tan, P.; Huang, J.W.; Yuan, K.F. Ledipasvir + sofosbuvir for liver transplant recipients with recurrent hepatitis C: A systematic review and meta-analysis. Transplant. Proc. 2017, 49, 1855–1863. [Google Scholar] [CrossRef]
- American Association for the Study of Liver Diseases-Infectious Diseases Society of America. (n.d.). HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. (Updated 24 October 2022). Available online: https://www.hcvguidelines.org/treatment-experienced (accessed on 1 April 2025).
- Chopra, S.; Arora, S. Patient Evaluation and Selection for Antiviral Therapy for Chronic Hepatitis C Virus Infection. Available online: https://www.uptodate.com/contents/patient-evaluation-and-selection-for-antiviral-therapy-for-chronic-hepatitis-c-virus-infection (accessed on 24 March 2025).
- Loo, J.H.; Xu, W.X.F.; Low, J.T.; Tay, W.X.; Ang, L.S.; Tam, Y.C.; Thurairajah, P.H.; Kumar, R.; Wong, Y.J. Efficacy and safety of sofosbuvir/velpatasvir with or without ribavirin in hepatitis C genotype 3 compensated cirrhosis: A meta-analysis. World J. Hepatol. 2022, 14, 1248–1257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).