Recent Developments in Rare Ovarian Carcinosarcoma: Literature Review and Case Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Identification and Ethical Considerations
2.2. Literature Review
2.3. Data Extraction and Synthesis
2.4. Diagnostic Protocol and Pathological Review
2.5. Treatment Algorithm and Follow-Up
3. Results
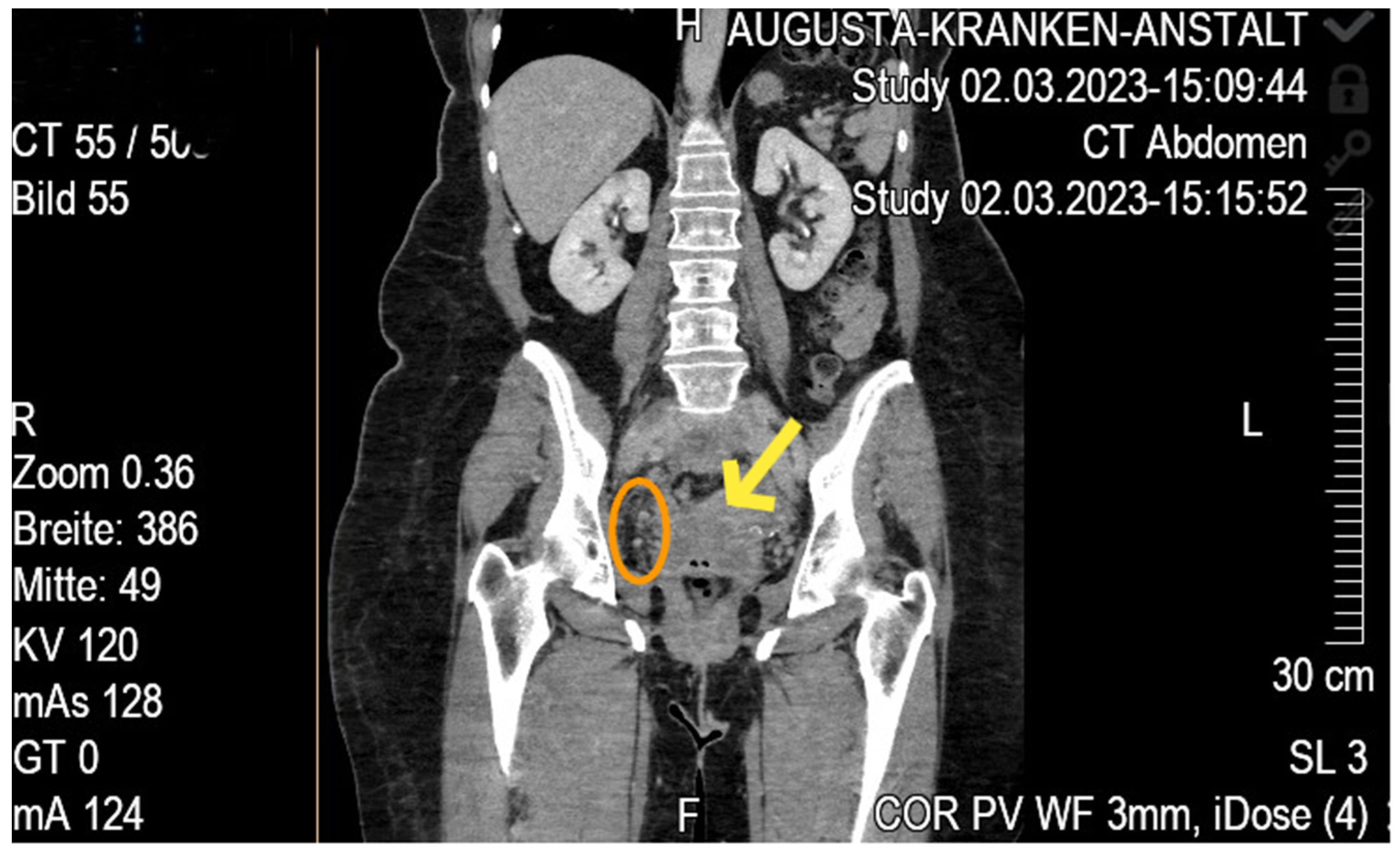
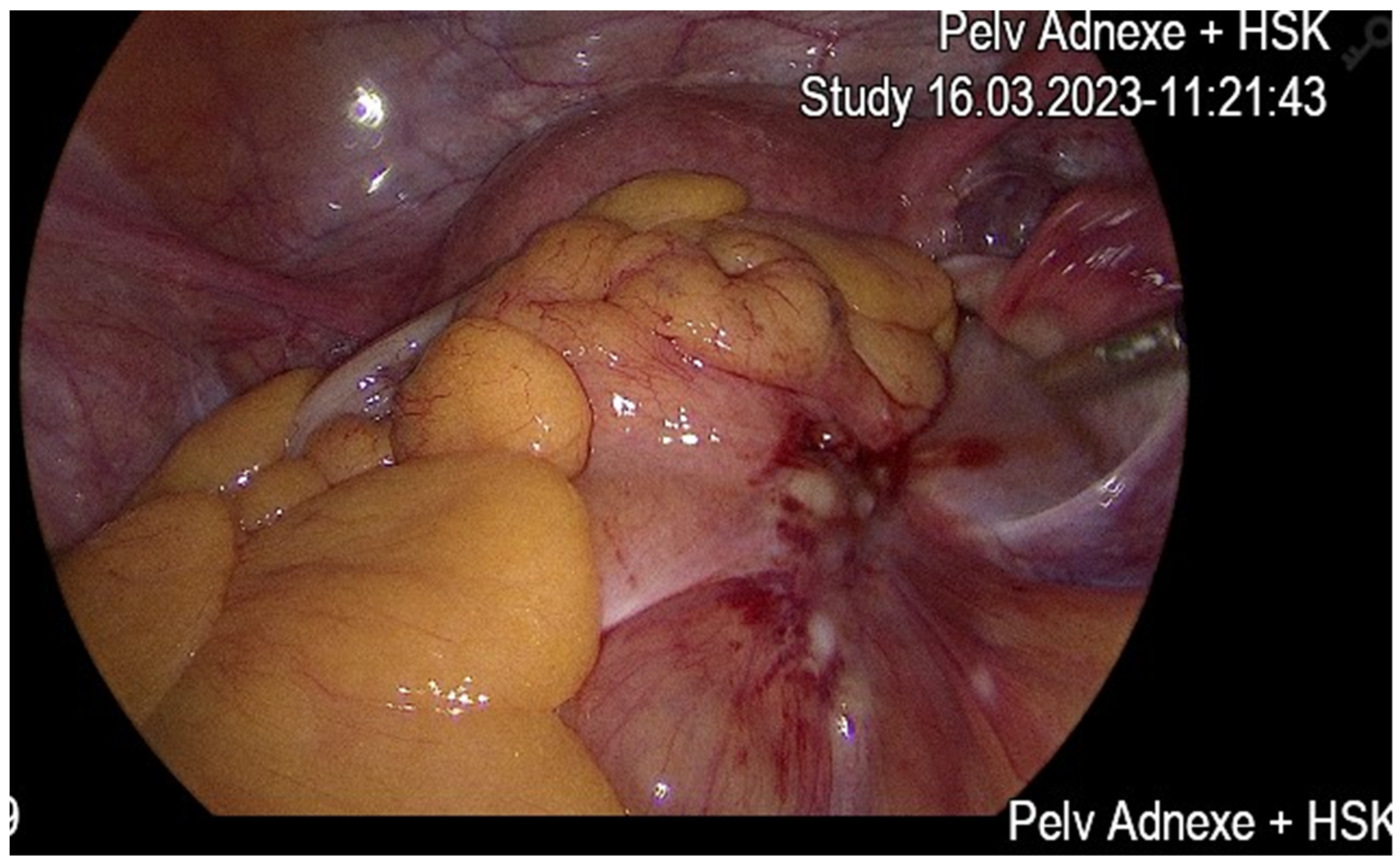
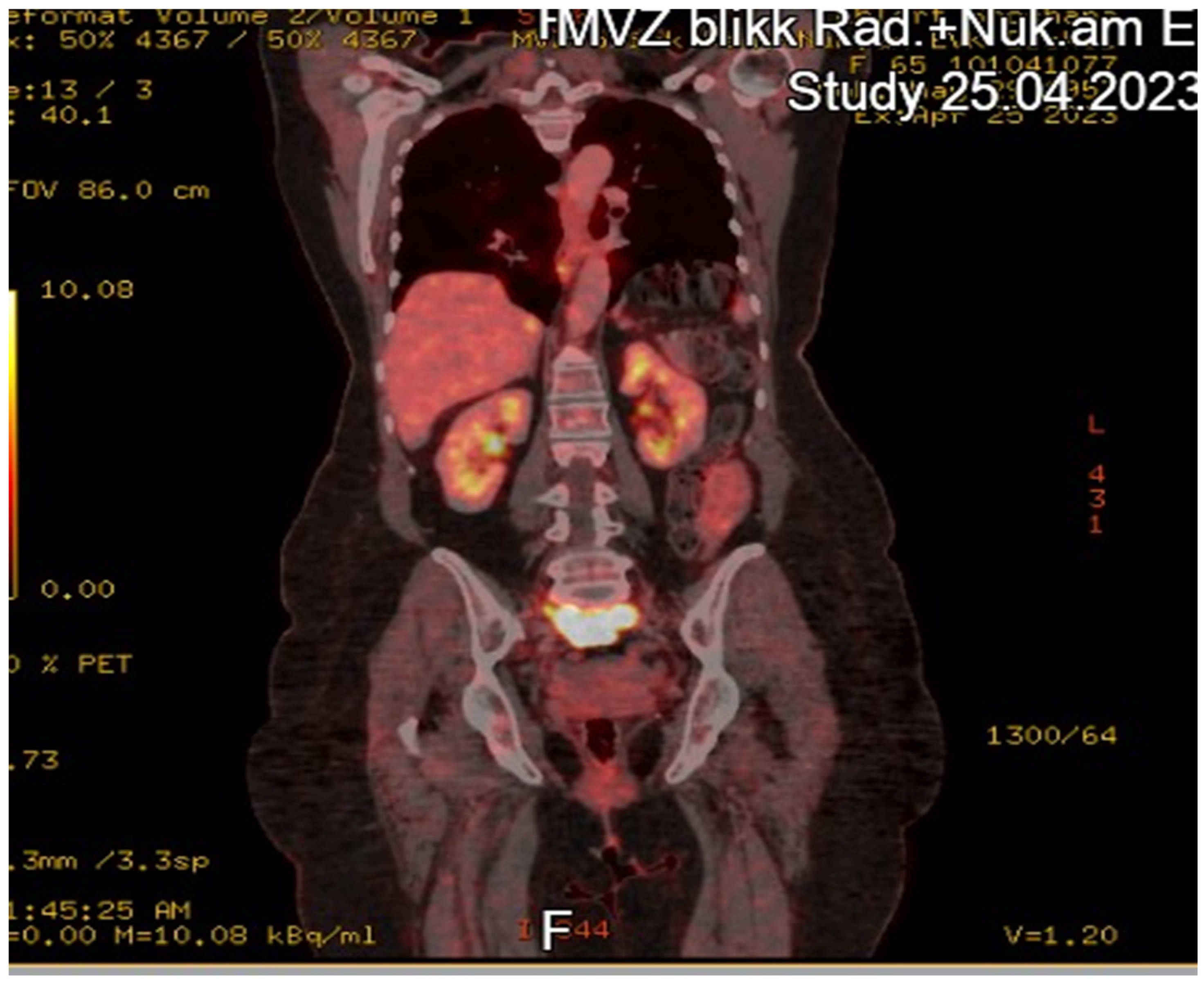
Case Report
4. Discussion
4.1. Analysis of Findings
4.2. Study Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.; Sharpe, J.C.; A’Hern, R.P.; Fisher, C.; Blake, P.; Shepherd, J.; Gore, M.E. Carcinosarcoma of the ovary: Incidence, prognosis, treatment and survival of patients. Ann. Oncol. 1995, 6, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Rauh-Hain, J.A.; Diver, E.J.; Clemmer, J.T.; Bradford, L.S.; Clark, R.M.; Growdon, W.B.; Goodman, A.K.; Boruta DM2nd Schorge, J.O.; del Carmen, M.G. Carcinosarcoma of the ovary compared to papillary serous ovarian carcinoma: A SEER analysis. Gynecol. Oncol. 2013, 131, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Choi, S.; Boussios, S. Frontiers of Ovarian Carcinosarcoma. Curr. Treat. Options Oncol. 2023, 24, 1667–1682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, E.; Stewart, M.; Rye, T.; Al-Nafussi, A.; Williams, A.R.; Bradburn, M.; Smyth, J.; Gabra, H. Carcinosarcoma of the ovary: 19 years of prospective data from a single center. Cancer 2004, 100, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Tate Thigpen, J.; Blessing, J.A.; DeGeest, K.; Look, K.Y.; Homesley, H.D. Gynecologic Oncology Group. Cisplatin as initial chemotherapy in ovarian carcinosarcomas: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 93, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, M.; Stasenko, M.; Uppal, S.; Erba, J.; Reynolds, R.K.; McLean, K. Comparison of first-line chemotherapy regimens for ovarian carcinosarcoma: A single institution case series and review of the literature. BMC Cancer 2018, 18, 172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ariyoshi, K.; Kawauchi, S.; Kaku, T.; Nakano, H.; Tsuneyoshi, M. Prognostic factors in ovarian carcinosarcoma: A clinicopathological and immunohistochemical analysis of 23 cases. Histopathology 2000, 37, 427–436. [Google Scholar] [CrossRef] [PubMed]
- George, E.M.; Herzog, T.J.; Neugut, A.I.; Lu, Y.S.; Burke, W.M.; Lewin, S.N.; Hershman, D.L.; Wright, J.D. Carcinosarcoma of the ovary: Natural history, patterns of treatment, and outcome. Gynecol. Oncol. 2013, 131, 42–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markman, M.; Brady, M.F.; Spirtos, N.M.; Hanjani, P.; Rubin, S.C. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: A Gynecologic Oncology Group Study. J. Clin. Oncol. 1998, 16, 2620–2624. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tang, C.; Liu, P.; Hao, H. Carcinosarcoma of the ovary: A case report and literature review. Front. Oncol. 2023, 13, 1278300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuo, K.; Ross, M.S.; Bush, S.H.; Yunokawa, M.; Blake, E.A.; Takano, T.; Ueda, Y.; Baba, T.; Satoh, S.; Shida, M.; et al. Tumor characteristics and survival outcomes of women with tamoxifen-related uterine carcinosarcoma. Gynecol. Oncol. 2017, 144, 329–335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damia, G.; Broggini, M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cicin, I.; Saip, P.; Eralp, Y.; Selam, M.; Topuz, S.; Ozluk, Y.; Aydin, Y.; Topuz, E. Ovarian carcinosarcomas: Clinicopathological prognostic factors and evaluation of chemotherapy regimens containing platinum. Gynecol. Oncol. 2008, 108, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Tsiouris, A.K.; Chatziantoniou, A.A.; Kanellos, F.S.; Tatsi, K. Ovarian carcinosarcoma: Current developments and future perspectives. Crit. Rev. Oncol. Hematol. 2019, 134, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.P.; d’Ablaing, G.; Brady, L.W.; Blessing, J.A.; Hreshchyshyn, M.M. A clinical and pathologic study of 30 cases of malignant mixed mullerian epithelial and mesenchymal ovarian tumors: A Gynecologic Oncology Group study. Gynecol. Oncol. 1984, 18, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Recent concepts of ovarian carcinogenesis: Type I and type II. Biomed. Res. Int. 2014, 2014, 934261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- del Carmen, M.G.; Birrer, M.; Schorge, J.O. Carcinosarcoma of the ovary: A review of the literature. Gynecol. Oncol. 2012, 125, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Harlow, B.L.; Weiss, N.S. Re: “Familial ovarian cancer: A population-based case-control study”. Am. J. Epidemiol. 1989, 130, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Nagao, S.; Kogiku, A.; Suzuki, K.; Shibutani, T.; Yamamoto, K.; Jimi, T.; Kitai, M.; Shiozaki, T.; Matsuoka, K.; Yamaguchi, S. A phase II study of the combination chemotherapy of bevacizumab and gemcitabine in women with platinum-resistant recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Ovarian Res. 2020, 13, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mano, M.S.; Rosa, D.D.; Azambuja, E.; Ismael, G.; Braga, S.; D’Hondt, V.; Piccart, M.; Awada, A. Current management of ovarian carcinosarcoma. Int. J. Gynecol. Cancer 2007, 17, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Fan Liang, F.; Shi, Y.; Chen, Y.; Tao, X.; Ding, J. Clinicopathological and molecular features of tubo-ovarian carcinosarcomas: A series of 51 cases. Front. Oncol. 2024, 14, 1427154. [Google Scholar] [CrossRef] [PubMed]
- St Laurent, J.D.; Abel, M.K.; Liu, J.; Quade, B.J.; Davis, M.R. Successful treatment of stage IVB ovarian carcinosarcoma with PARP inhibitor: A case report. Gynecol. Oncol. Rep. 2024, 45, 10808925. [Google Scholar] [CrossRef]
- Wang, W.; Ji, X.; Yang, H.; Wang, X. Ovarian carcinosarcoma with lung metastasis characterized by persistent fever: A case report and literature review. Medicine 2024, 103, e11521086. [Google Scholar] [CrossRef] [PubMed]
- Sumino, M.; Hashimoto, H.; Sato, N.; Dejima, M.; Sasajima, Y.; Sugita, M.; Morikawa, T. Ovarian carcinosarcoma of heterologous type occurring in an endometriotic cyst with 3-year recurrence-free survival: A case report and literature review. Jpn. J. Clin. Oncol. 2024, 14, 21–26. [Google Scholar] [CrossRef]
- Siala, R.; Mseddi, M.A.; Yaakoubi, C.; Kassar, A.Z.; Guizeni, R.; Slima, M.B. Sigmoido-ovarian fistula complicating ovarian carcinosarcoma: A case report. Int. J. Surg. Case Rep. 2024, 107, 11305719. [Google Scholar] [CrossRef] [PubMed]
- Elemian, S.; Jumean, S.; Paige, A.; Yaghi, S.; Shaaban, H.S. Initial presentation of ovarian carcinosarcoma with non-islet cell tumor hypoglycemia: A case report. Cureus 2024, 16, e11363008. [Google Scholar] [CrossRef]
- Yoriki, K.; Izumi, Y.; Tarumi, Y.; Okimura, H.; Maeda, E.; Mori, T. A case of ovarian carcinosarcoma with germline BRCA2 pathogenic variant. J. Obstet. Gynaecol. Res. 2024, 50, 16134. [Google Scholar] [CrossRef]
- Ha, H.I.; Kim, J.H.; Lim, J.; Song, Y.J.; Won, Y.J.; Lim, M.C. Incidence and treatment outcomes of ovarian carcinosarcoma from the national cancer registry of Korea. J. Gynecol. Oncol. 2024, 35, e31. [Google Scholar] [CrossRef]
- Tew, W.P.; Lacchetti, C.; Ellis, A.; Maxian, K.; Banerjee, S.; Bookman, M.; Jones, M.B.; Lee, J.M.; Lheureux, S.; Liu, J.F.; et al. PARP Inhibitors in the Management of Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3468–3493. [Google Scholar] [CrossRef]
- Hunt, J.T.; Chambers, L.M.; Yao, M.; Joehlin-Price, A.; Debernardo, R.; Rose, P.G. Lenvatinib plus pembrolizumab in patients with advanced or recurrent uterine carcinosarcoma. Gynecol. Oncol. Rep. 2021, 37, 100840. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, X.; Zou, Y. Primitive ovarian carcinosarcoma: A clinical and radiological analysis of five cases. J. Ovarian Res. 2020, 13, 129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McFarlane, I.; Porter, J.M.; Brownsell, E.; Ghaoui, N.; Connolly, K.C.; Herrington, C.S.; Hollis, R.L. Ovarian carcinosarcoma is highly aggressive compared to other ovarian cancer histotypes. Front. Oncol. 2024, 14, 1399979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sangha, R.; Jamal, R.; Spratlin, J.; Kuruvilla, J.; Sehn, L.H.; Beauchamp, E.; Weickert, M.; Berthiaume, L.G.; Mackey, J.R. A first-in-human phase I trial of daily oral zelenirstat, a N-myristoyltransferase inhibitor, in patients with advanced solid tumors and relapsed/refractory B-cell lymphomas. Invest. New Drugs. 2024, 42, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.L.; Liu, J.Y.; Qu, L.X.; La, H.; Wang, H.L.; Chen, X.X.; Wang, N.; Wei, Z.Z. Expression of Cyclin D1 gene in ovarian cancer and effect of silencing its expression on ovarian cancer cells based on the Oncomine database. Bioengineered 2021, 12, 9290–9300. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Design/Sample | Key Focus | Main Findings |
|---|---|---|---|
| Fan Liang et al. [21] | Retrospective analysis of 51 tubo-ovarian carcinosarcomas | Clinicopathological and molecular profiles | 65% advanced stage; suboptimal debulking → poor outcomes; 64% HRD+ |
| St Laurent et al. [22] | Single case report, stage IVB OCS + BRCA1 mutation | Successful PARP inhibitor therapy | Complete response on olaparib; underscores role of BRCA-driven OCS |
| Wang et al. [23] | Single case report, stage IVB OCS with lung metastases | Persistent fever as initial presentation | Diagnosed postoperatively; patient succumbed to disease progression |
| Sumino et al. [24] | Single case report, T1C OCS in an endometriotic cyst | Rare, early-stage OCS, heterologous type | 3-year recurrence-free survival after docetaxel/carboplatin |
| Siala et al. [25] | Single case report with complicated OCS (sigmoido-ovarian fistula) | Surgical management of GI involvement | Radical resection with Hartmann procedure; advanced disease on follow-up |
| Elemian et al. [26] | Single case report, advanced OCS with paraneoplastic hypoglycemia (NICTH) | Unusual endocrine manifestation | Despite chemo, patient died of multiorgan failure shortly after Dx |
| Yoriki et al. [27] | Single case report, germline BRCA2-associated OCS | Feasibility of maintenance PARP inhibitor | Stage IIIB OCS; ongoing remission at 25 months on PARP inhibitor |
| Ha et al. [28] | National registry (Korea): 458 OCS cases (1999–2018) | Epidemiology, incidence, and survival outcomes | 1.5% of epithelial ovarian cancers; median OS 39 mo; 5-year OS ~42.5% |
| Study | No. of Cases | Stage Distribution | Median PFS (Months) | Median OS (Months) | Key Clinicopathological Findings |
|---|---|---|---|---|---|
| Fan Liang et al. [21] | 51 | 65% advanced (≥III) | 27 | 40 |
|
| Ha et al. [28] | 458 | Localized 14%, Regional 21%, Distant 65% | Not specified | 39 |
|
| Reference | HRD/BRCA Status | Therapeutic Intervention | Outcome |
|---|---|---|---|
| Fan Liang et al. [21] | 64% HRD+; 0% MSI, low TMB | Standard chemo (platinum-based) | HRD positivity suggests possible PARPi benefit |
| St Laurent et al. [22] | Germline BRCA1 mutation | Olaparib maintenance | Stage IVB → Clinical remission |
| Sumino et al. [24] | Not reported | Docetaxel/carboplatin | 3-year recurrence-free survival (early-stage) |
| Elemian et al. [26] | Not specified; no testing described | Carboplatin/paclitaxel | Rapid deterioration; patient death |
| Yoriki et al. [27] | Germline BRCA2 mutation; HRD+ likely | PARPi (maintenance) | Ongoing remission at 25 months |
| Ha et al. [28] | No mention of HRD or BRCA specifically | Not detailed at molecular level | 5-year OS ~42.5%; national dataset |
| Study | Patient Age | Stage | Unique Presentation/Complication | Management | Outcome |
|---|---|---|---|---|---|
| St Laurent et al. [22] | 36 | IVB | BRCA1 mutation, small STIC lesion, LN spread | CRS + Carboplatin/Taxane + PARPi | No recurrence at 3+ years |
| Wang et al. [23] | 61 | IVB | Persistent fever, lung metastases | Cytoreductive surgery + chemo | Rapid progression, patient died |
| Sumino et al. [24] | 41 | T1C3 | OCS arising in endometriotic cyst | Secondary surgery + docetaxel/CBDCA | Recurrence-free at 40 months |
| Siala et al. [25] | 67 | T2b (est.) | Sigmoido-ovarian fistula + pelvic peritonitis | Emergent en-bloc resection (Hartmann) | Disease progression, scheduled for chemo |
| Elemian et al. [26] | 55 | Metastatic | NICTH (paraneoplastic hypoglycemia) | Glucose infusion + chemo | Deceased (multiorgan failure) |
| Yoriki et al. [27] | 43 | IIIB | Germline BRCA2, concurrent breast DCIS | CRS + platinum + PARPi maintenance | Alive, no recurrence at 25 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nienhaus, A.; Bernad, E. Recent Developments in Rare Ovarian Carcinosarcoma: Literature Review and Case Report. Diseases 2025, 13, 163. https://doi.org/10.3390/diseases13060163
Nienhaus A, Bernad E. Recent Developments in Rare Ovarian Carcinosarcoma: Literature Review and Case Report. Diseases. 2025; 13(6):163. https://doi.org/10.3390/diseases13060163
Chicago/Turabian StyleNienhaus, Alexandra, and Elena Bernad. 2025. "Recent Developments in Rare Ovarian Carcinosarcoma: Literature Review and Case Report" Diseases 13, no. 6: 163. https://doi.org/10.3390/diseases13060163
APA StyleNienhaus, A., & Bernad, E. (2025). Recent Developments in Rare Ovarian Carcinosarcoma: Literature Review and Case Report. Diseases, 13(6), 163. https://doi.org/10.3390/diseases13060163







