Global Epidemiology of Smoking and Liver Cancer from 1990 to 2021
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Definition
2.3. Statistical Analysis
3. Results
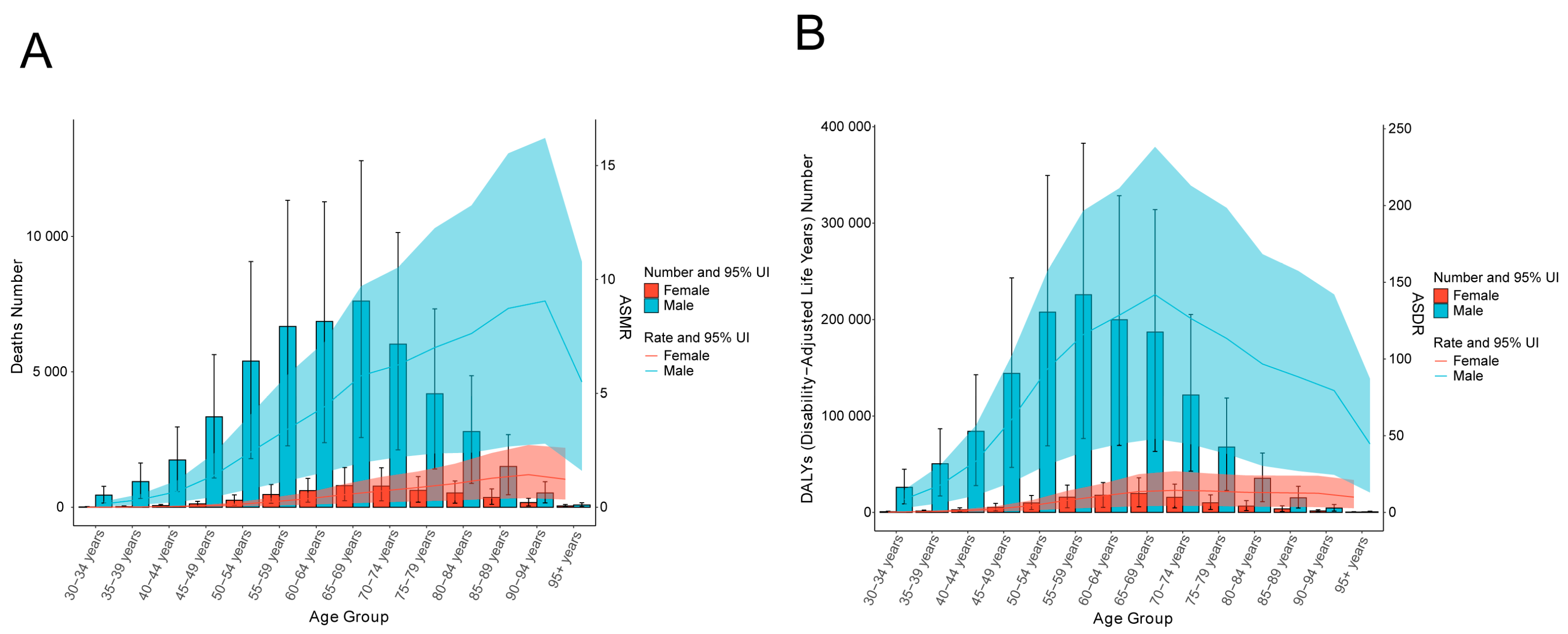
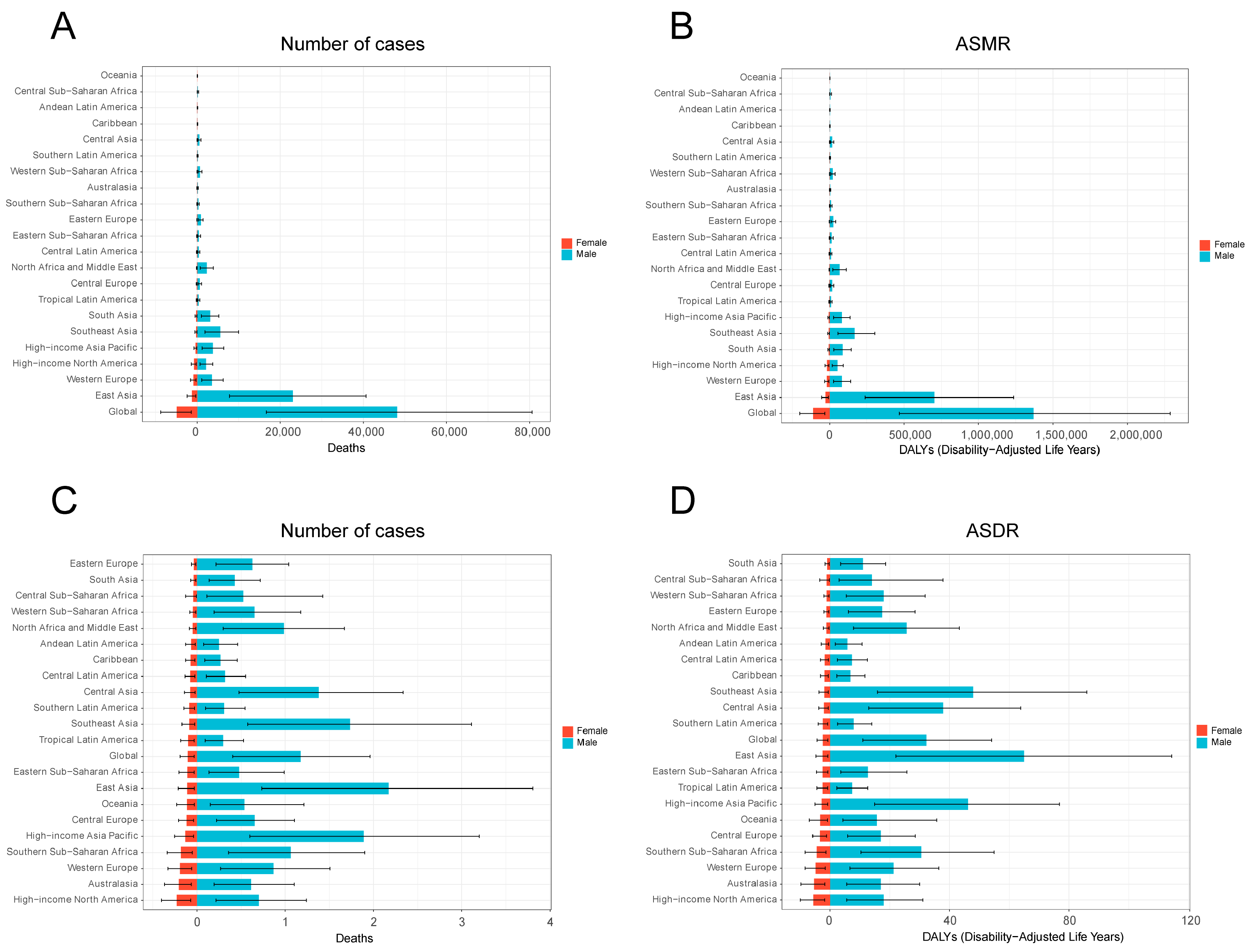
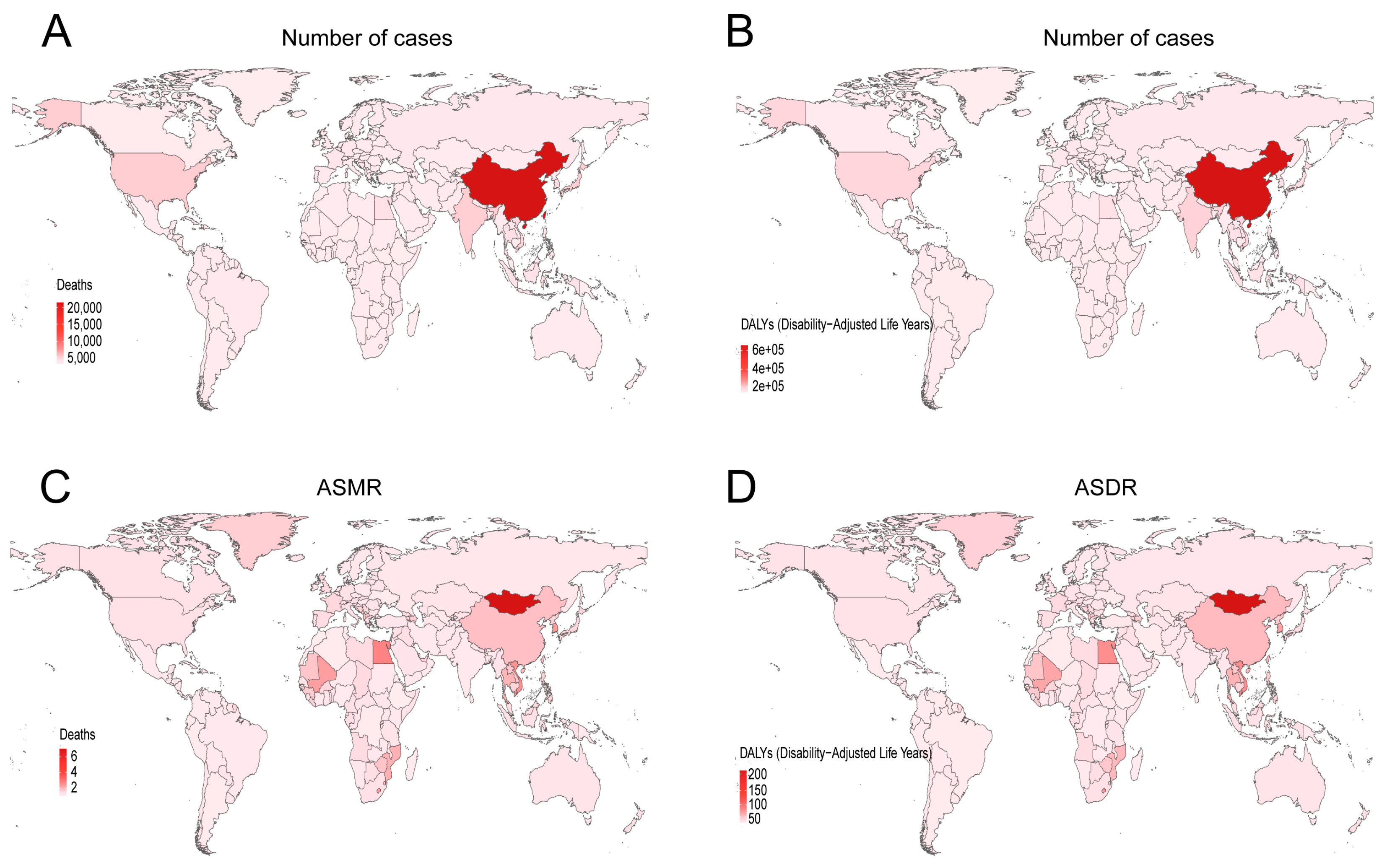
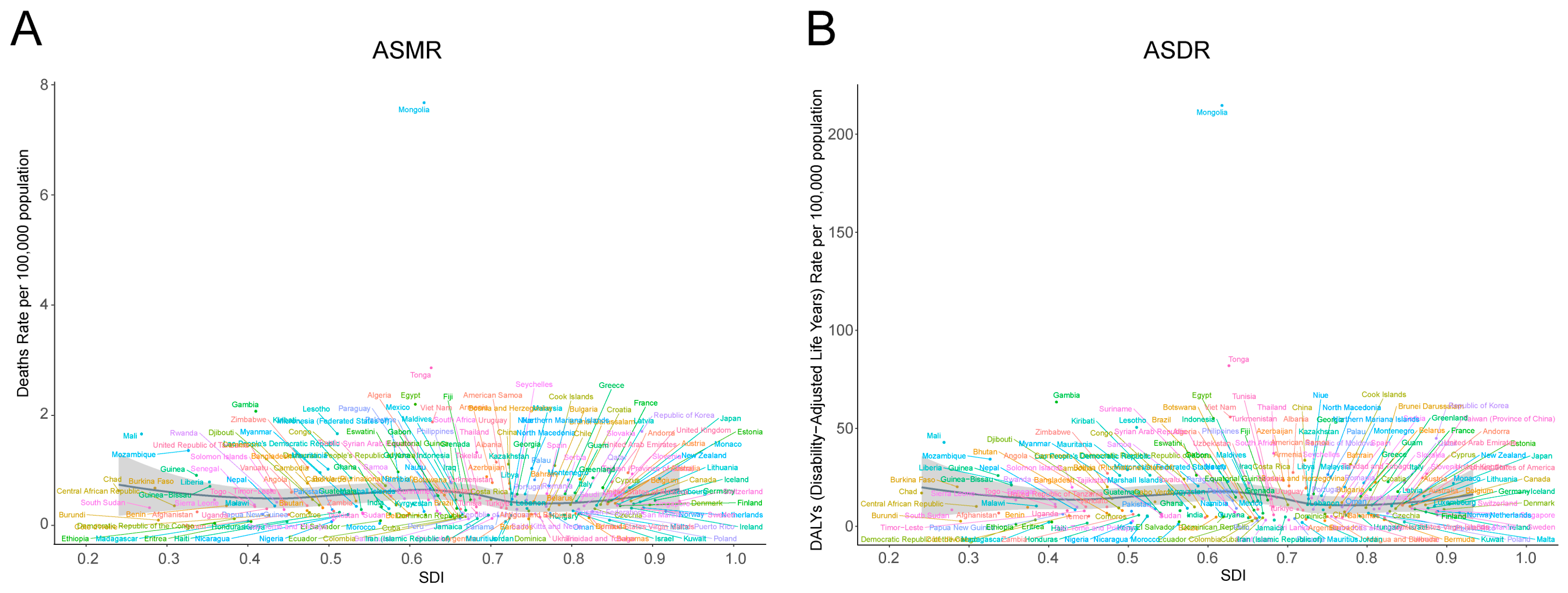
3.1. Global Burden of Smoking-Related Liver Cancer in 2021
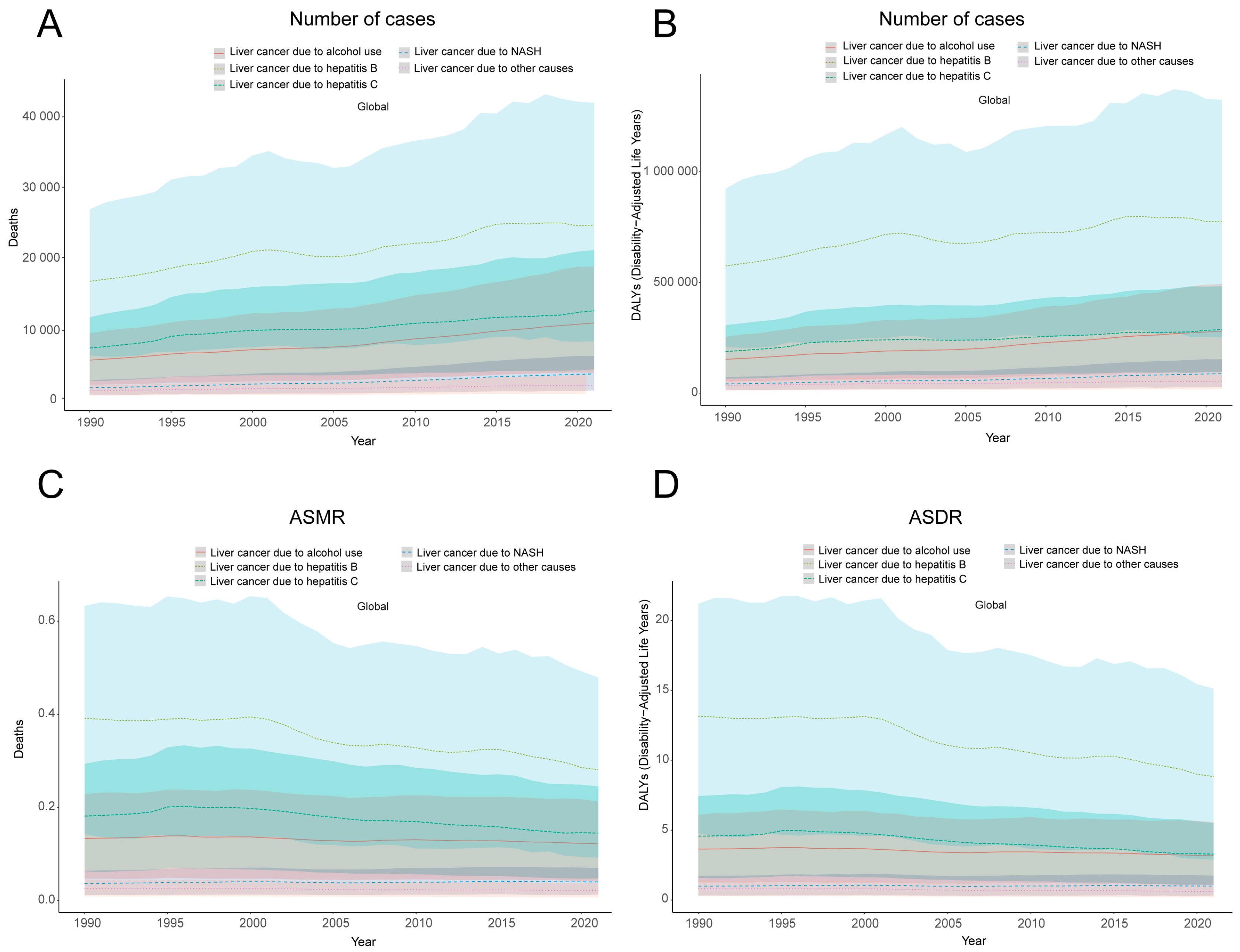
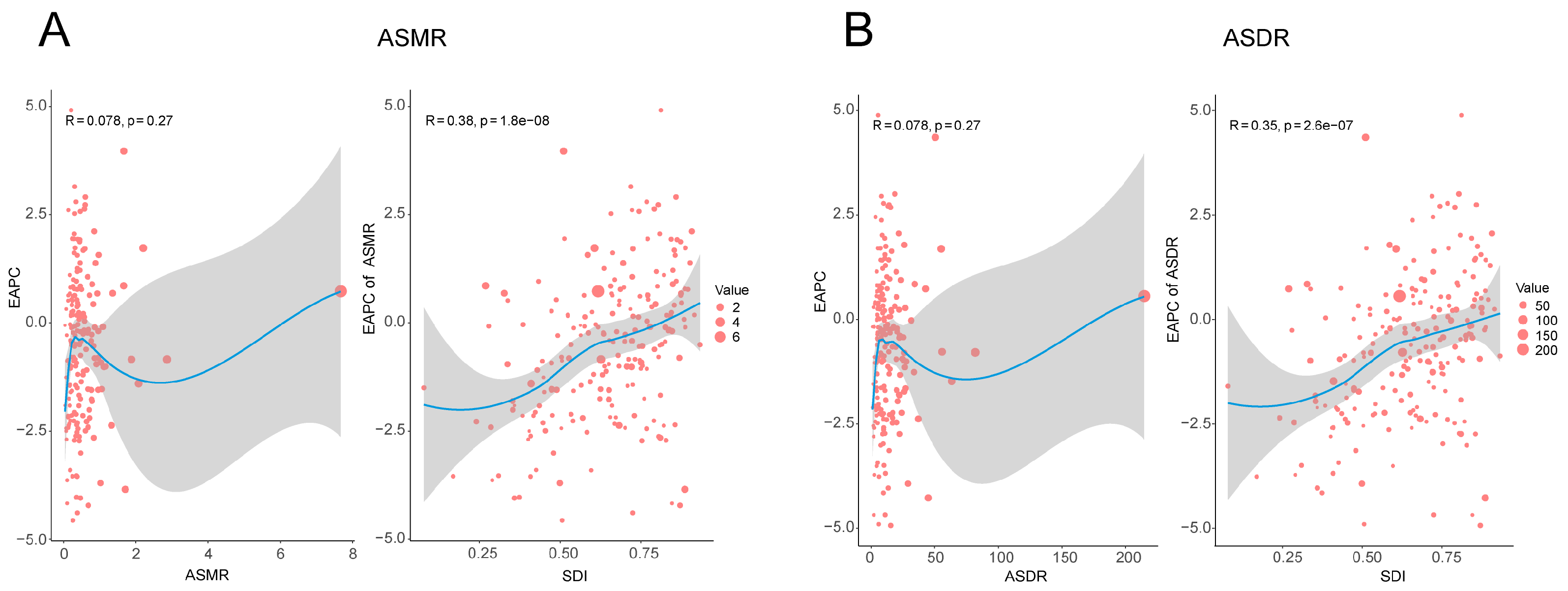
3.2. Temporal Trend of Smoking-Related Liver Cancer Burden from 1990 to 2021
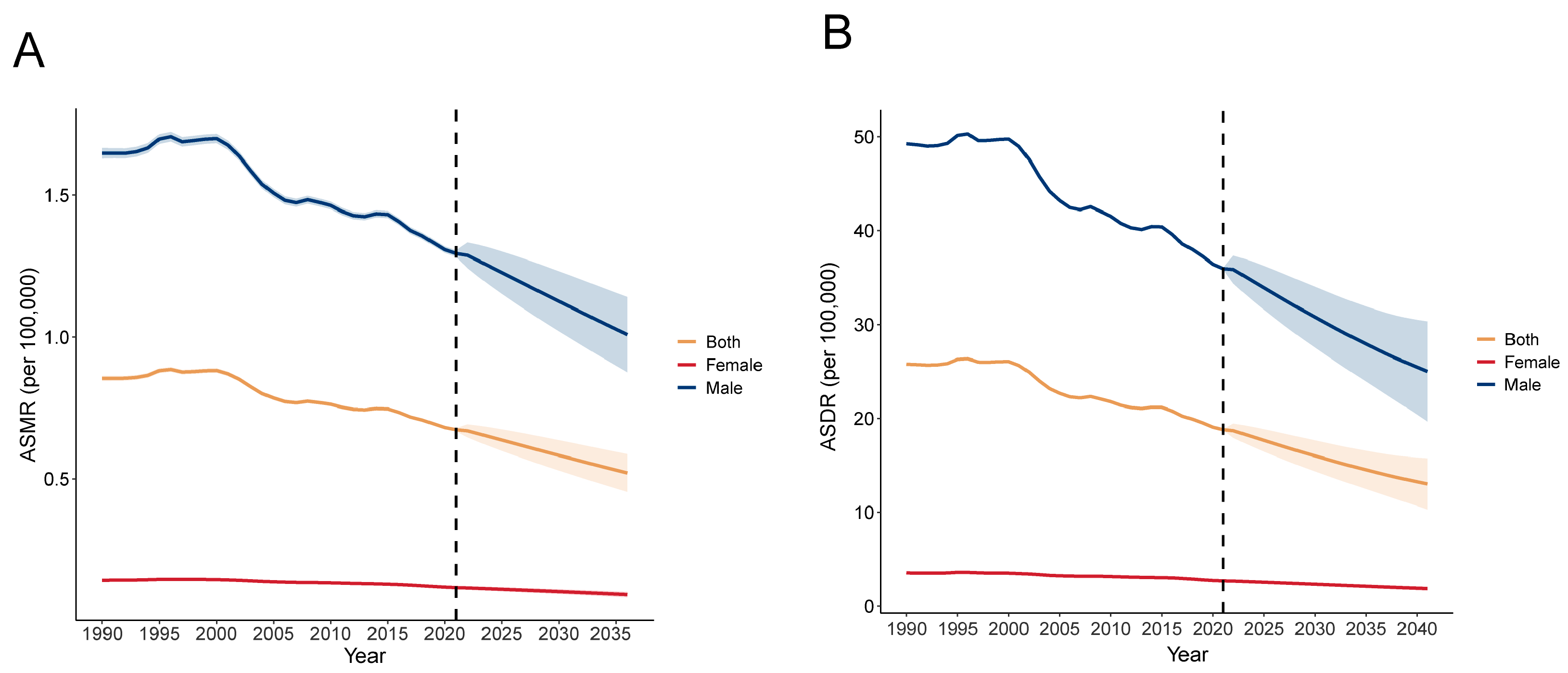
3.3. Future Trends of Global Liver Cancer Burden Related to Smoking from 2022 to 2040
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Possenti, I.; Martini, A.; Bagnardi, V.; Specchia, C.; Garavello, W.; Odone, A.; Smits, L.J.M.; Gallus, S.; Lugo, A. Association between cigarette smoking and nasopharyngeal cancer risk: A meta-analysis. Rhinology 2025, 63, 13–21. [Google Scholar] [CrossRef]
- Rota, M.; Possenti, I.; Valsassina, V.; Santucci, C.; Bagnardi, V.; Corrao, G.; Bosetti, C.; Specchia, C.; Gallus, S.; Lugo, A. Dose-response association between cigarette smoking and gastric cancer risk: A systematic review and meta-analysis. Gastric Cancer 2024, 27, 197–209. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.; Ding, Y.; Meng, X.; Zhang, C.; Sun, X. Effect of smoking-related features and 731 immune cell phenotypes on esophageal cancer: A two-sample and mediated Mendelian randomized study. Front. Immunol. 2024, 15, 1336817. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Liu, J.; Rao, Z.; Wang, M.; Shen, H.; Zeng, S. Trends in liver cancer mortality in China from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e074348. [Google Scholar] [CrossRef] [PubMed]
- Moryson, W.; Stawinska-Witoszynska, B. Premature Mortality Due to Tobacco-Related Malignancies in Poland. Int. J. Gen. Med. 2021, 14, 2171–2182. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Z.; Lin, C.; Fan, H.; Zhang, X.; Wang, H.; Han, X.; Li, Y.; Mu, L.; Yu, S.; et al. Global epidemiology of early-onset liver cancer attributable to specific aetiologies and risk factors from 2010 to 2019. J. Glob. Health 2023, 13, 04167. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Helbling, D.; Schob, O.; Eltobgy, M.; Mohamed, H.; Schmidt, J.; Giryes, A.; Mehrabi, A.; Iype, S.; John, H.; et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J. Evid.-Based Med. 2017, 10, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Chaudhary, P.; Varshney, N.; Bin Razzak, K.S.; Verma, D.; Khan Zahra, T.R.; Janmeda, P.; Sharifi-Rad, J.; Dastan, S.D.; Mahmud, S.; et al. Tobacco Smoking and Liver Cancer Risk: Potential Avenues for Carcinogenesis. J. Oncol. 2021, 2021, 5905357. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Miloradovic, D.; Stojanovic, M.D.; Harrell, C.R.; Polosa, R.; Rust, S.; Volti, G.L.; Caruso, M.; Jakovljevic, V.; Djonov, V.; et al. Cigarette smoke attenuates mesenchymal stem cell-based suppression of immune cell-driven acute liver failure. Toxicol. Lett. 2023, 385, 12–20. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, J.; Yang, R.; Chen, S.; Jian, H.; Zeng, P. The Global, Regional, and National Burden of Tracheal, Bronchus, and Lung Cancer Caused by Smoking: An Analysis Based on the Global Burden of Disease Study 2021. Ann. Glob. Health 2024, 90, 77. [Google Scholar] [CrossRef]
- Han, S.; Zhao, S.; Zhong, R.; Li, P.; Pang, Y.; He, S.; Duan, J.; Gong, H.; Shi, J.; Liu, L.; et al. Global burden, trends, and disparities in kidney cancer attributable to smoking from 1990 to 2021. Front. Public Health 2024, 12, 1506542. [Google Scholar] [CrossRef]
- Liu, S.; Lv, Y.; Zhang, Y.; Suo, H.; Wang, F.; Gao, S. Global trends and burden of stroke attributable to particulate matter pollution from 1990 to 2019. Ecotoxicol. Environ. Saf. 2024, 274, 116205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kan, C.; Han, F.; Zhang, J.; Ding, C.; Guo, Z.; Huang, N.; Zhang, Y.; Hou, N.; Sun, X. Global, Regional, and National Epidemiology of Diabetes in Children From 1990 to 2019. JAMA Pediatr. 2023, 177, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA A Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.T.F. Forecasting the effects of smoking prevalence scenarios on years of life lost and life expectancy from 2022 to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Public Health 2024, 9, e729–e744. [Google Scholar] [CrossRef]
- Jokhadze, N.; Das, A.; Dizon, D.S. Global cancer statistics: A healthy population relies on population health. CA A Cancer J. Clin. 2024, 74, 224–226. [Google Scholar] [CrossRef]
- Yach, D. The origins, development, effects, and future of the WHO Framework Convention on Tobacco Control: A personal perspective. Lancet 2014, 383, 1771–1779. [Google Scholar] [CrossRef]
- Bialous, S.; Da Costa, E.S.V.L. Where next for the WHO Framework Convention on Tobacco Control? Tob. Control 2022, 31, 183–186. [Google Scholar] [CrossRef]
- Ji, Y.T.; Liu, S.W.; Zhang, Y.M.; Duan, H.Y.; Liu, X.M.; Feng, Z.W.; Li, J.J.; Lyu, Z.Y.; Huang, Y.B. Comparison of the latest cancer statistics, cancer epidemic trends and determinants between China and the United States. Zhonghua Zhong Liu Za Zhi 2024, 46, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Baecker, A.; Wu, M.; Zhou, J.Y.; Yang, J.; Han, R.Q.; Wang, P.H.; Jin, Z.Y.; Liu, A.M.; Gu, X.; et al. Interaction between tobacco smoking and hepatitis B virus infection on the risk of liver cancer in a Chinese population. Int. J. Cancer 2018, 142, 1560–1567. [Google Scholar] [CrossRef]
- Asia, G.B.D.; All Cancers, C. Temporal patterns of cancer burden in Asia, 1990-2019: A systematic examination for the Global Burden of Disease 2019 study. Lancet Reg. Health Southeast Asia 2024, 21, 100333. [Google Scholar] [CrossRef]
- Katanoda, K.; Jiang, Y.; Park, S.; Lim, M.K.; Qiao, Y.L.; Inoue, M. Tobacco control challenges in East Asia: Proposals for change in the world’s largest epidemic region. Tob. Control 2014, 23, 359–368. [Google Scholar] [CrossRef]
- King, B.A.; Ahluwalia, I.B.; Bacelar Gomes, A.; Fong, G.T. Combating the tobacco epidemic in North America: Challenges and opportunities. Tob. Control 2022, 31, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Bottorff, J.L.; Haines-Saah, R.; Kelly, M.T.; Oliffe, J.L.; Torchalla, I.; Poole, N.; Greaves, L.; Robinson, C.A.; Ensom, M.H.; Okoli, C.T.; et al. Gender, smoking and tobacco reduction and cessation: A scoping review. Int. J. Equity Health 2014, 13, 114. [Google Scholar] [CrossRef]
- Soma, T.; Nagata, M. Immunosenescence, Inflammaging, and Lung Senescence in Asthma in the Elderly. Biomolecules 2022, 12, 1456. [Google Scholar] [CrossRef]
- Fu, X.; Qin, P.; Li, F.; Zhu, H.; You, H.; Zhang, Y.; Xu, B.; Li, T.; Zhang, F.; Han, L.; et al. The inter-link of ageing, cancer and immunity: Findings from real-world retrospective study. Immun. Ageing I A 2023, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Park, E.; Kang, H.Y.; Oh, J.K. Combined effects of smoking and alcohol consumption on the risk of liver cancer according to metabolic syndrome: A nested case-control study in South Korea. Int. J. Cancer 2024, 155, 654–665. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xiao, W.; Fan, X. A review of MASLD-related hepatocellular carcinoma: Progress in pathogenesis, early detection, and therapeutic interventions. Front. Med. 2024, 11, 1410668. [Google Scholar] [CrossRef]
- Lyratzopoulos, G.; Abel, G.A.; Brown, C.H.; Rous, B.A.; Vernon, S.A.; Roland, M.; Greenberg, D.C. Socio-demographic inequalities in stage of cancer diagnosis: Evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann. Oncol. 2013, 24, 843–850. [Google Scholar] [CrossRef]
- Palipudi, K.M.; Gupta, P.C.; Sinha, D.N.; Andes, L.J.; Asma, S.; McAfee, T.; Group, G.C. Social determinants of health and tobacco use in thirteen low and middle income countries: Evidence from Global Adult Tobacco Survey. PLoS ONE 2012, 7, e33466. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Chrysavgis, L.; Vachliotis, I.D.; Chartampilas, E.; Cholongitas, E. Nonalcoholic fatty liver disease and hepatocellular carcinoma:Insights in epidemiology, pathogenesis, imaging, prevention and therapy. Semin. Cancer Biol. 2023, 93, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Paraje, G.; Flores Munoz, M.; Wu, D.C.; Jha, P. Reductions in smoking due to ratification of the Framework Convention for Tobacco Control in 171 countries. Nat. Med. 2024, 30, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Nargis, N.; Yong, H.H.; Driezen, P.; Mbulo, L.; Zhao, L.; Fong, G.T.; Thompson, M.E.; Borland, R.; Palipudi, K.M.; Giovino, G.A.; et al. Socioeconomic patterns of smoking cessation behavior in low and middle-income countries: Emerging evidence from the Global Adult Tobacco Surveys and International Tobacco Control Surveys. PLoS ONE 2019, 14, e0220223. [Google Scholar] [CrossRef] [PubMed]


| 1990 | 2021 | 1990–2021 | |||||
|---|---|---|---|---|---|---|---|
| Number of Death Cases (95% UI) | ASDR/100,000 (95% UI) | Number of Death Cases (95% UI) | ASDR/100,000 (95% UI) | EAPC (95% CI) | Trend | ||
| Global | 31,744.398 (11,068.228–51,199.905) | 0.769 (0.268–1.241) | 53,053.676 (18,267.574–88,110.999) | 0.609 (0.210–1.012) | −0.84 (−0.96– −0.73) | Down | |
| Gender | Male | 29,040.31 (10,240.20–46,715.17) | 1.49 (0.52–2.39) | 48,166.53 (16,663.66–80,568.20) | 1.17 (0.41–1.96) | −0.86 (−0.98– −0.75) | Down |
| Female | 2704.09 (868.17–4637.61) | 0.128 (0.04–0.22) | 4887.15 (1464.14–8775.50) | 0.11 (0.03–0.19) | −0.63 (–0.75– −0.52) | Down | |
| Age group | 30–34 | 514.76 (172.79–863.89) | 0.13 (0.04–2.84) | 463.74 (158.64–5855.98 ) | 0.08 (0.03–1.24) | −2.82 (−3.39– −2.25) | Down |
| 35–39 | 1160.10 (399.51–1910.09) | 0.33 (0.11–4.07) | 977.10 (331.27–9451.67) | 0.17 (0.06–2.12) | −2.60 (–2.93– −2.26) | Down | |
| 40–44 | 2000.17 (710.04–3274.25) | 0.70 (0.25–5.11) | 1806.27 (598.51–12,007.16) | 0.36 (0.12–3.03) | −2.35 (−2.60– −2.10) | Down | |
| 45–49 | 2607.94 (948.61–4257.31) | 1.12 (0.41–6.16) | 3462.42 (1128.39–12,343.67) | 0.73 (0.24–3.86) | −1.33 (−1.63– −1.03) | Down | |
| 50–54 | 3782.14 (1412.90–6045.76) | 1.78 (0.66−6.61) | 5656.14 (1878.91–14,152.70) | 1.27 (0.42−5.13) | −0.95 (−1.13– −0.78) | Down | |
| 55–59 | 4728.26 (1675.97–7546.34) | 2.55 (0.90–5.01) | 7148.62 (2425.84–47,974.11) | 1.81 (0.61–3.34) | −1.09 (−1.20– −0.99) | Down | |
| 60–64 | 5172.44 (1800.88–8206.44) | 3.22 (1.12–4.96) | 7475.55 (2601.19–5830.23) | 2.34 (0.81–6.66) | −1.08 (−1.25– −0.92) | Down | |
| 65–69 | 4672.00 (1651.33–7612.81) | 3.78 (1.34–4.31) | 8417.42 (2841.35–13,334.20) | 3.05 (1.03–7.29) | −0.98 (−1.13– −0.70) | Down | |
| 70–74 | 3351.73 (1146.18–5600.29) | 3.96 (1.35–6.34) | 6803.65 (2380.09–59,070.12) | 3.31 (1.16–3.60) | −0.85 (−1.03– −0.67) | Down | |
| 75–79 | 2178.10 (713.96–3645.89) | 3.54 (1.16–2.54) | 4816.61 (1612.02–25,435.19) | 3.65 (1.22–5.28) | −0.05 (−0.29– −0.20) | Down | |
| 80–84 | 1028.69 (330.22–1770.76) | 2.91 (0.68–0.93) | 3324.78 (1028.86–14,135.43) | 3.80 (1.17–6.44) | 1.10 (0.85– 1.35) | Up | |
| 85–89 | 430.49 (135.54–749.92) | 2.85 (0.90–0.22) | 1869.39 (568.23–20,495.48) | 4.09 (0.54–1.24) | 1.19 (1.04– 1.34) | Up | |
| 90–94 | 103.58 (31.51–184.89) | 2.42 (0.74–0.54) | 700.33 (213.57–794.46) | 3.91 (1.19–0.13) | 1.41 (1.30– 1.53) | Up | |
| 95+ | 14.00 (4.19–25.82) | 1.38 (0.41–1.14) | 131.67 (37.63–1671.88) | 2.42 (0.69–0.30) | 1.66 (1.53– 1.80) | Up | |
| Liver cancer subtypes | Liver cancer due to NASH | 1478.70 (481.08–2539.93) | 0.037 (0.01–0.06) | 3461.05 (6021.55–1111.96) | 0.04 (0.01–0.07) | 0.23 (0.14–0.31) | Up |
| Liver cancer due to alcohol use | 5414.39 (1910.61–9195.91) | 0.13 (0.05–0.23) | 10,700.49 (3659.14–18,701.10) | 0.12 (0.04–0.21) | –0.39 (–0.45–−0.33) | Down | |
| Liver cancer due to hepatitis B | 16,626.05 (6069.01–26,870.83) | 0.39 (0.14–0.63) | 24,612.92 (8093.26–42,003.77) | 0.28 (0.09–0.48 ) | −1.05 (−1.17– −0.93) | Down | |
| Liver cancer due to hepatitis C | 7132.23 (2477.06–11,518.32) | 0.18 (0.06–0.29) | 12,429.99 (4105.23–21,046.42) | 0.14 (0.05–0.24) | −1.11 (−1.30–−0.91) | Down | |
| Liver cancer due to other causes | 1093.04 (393.63–1877.90) | 0.03 (0.01–0.05) | 1849.23 (646.42–3235.51) | 0.02 (0.01–0.04) | −0.73 (−0.82–−0.65) | Down | |
| 1990 | 2021 | 1990–2021 | |||||
|---|---|---|---|---|---|---|---|
| Number of Death Cases (95% UI) | ASDR/100,000 (95% UI) | Number of Death Cases (95% UI) | ASDR/100,000 (95% UI) | EAPC (95% CI) | Trend | ||
| Global | 991,836.337 (348,071.515–1,595,195.380) | 23.191 (8.129–37.308) | 1,482,896.286 (504,999.552–2,478,905.619) | 16.903 (5.755–28.259) | −1.121(−1.241–−1.001) | Down | |
| Gender | Male | 923,139.30 (326,907.28–1,480,638.48) | 44.33 (15.67–71.22) | 1,370,991.56 (467666.46–2286527.40) | 32.341 (11.05–54.07) | −1.123 (−1.244–−1.003) | Down |
| Female | 68,697.04 (22,596.93–117,541.21) | 3.19 (1.05–5.46) | 111,904.73 (33,712.72–200,080.73) | 2.43 (0.73–4.34) | −0.86 (−0.96– −0.76) | Down | |
| Age group | 30–34 | 29,794.10 (9996.51–50,050.01) | 7.73 (2.59–12.99) | 26,889.20 (9195.48–46,104.60) | 4.45 (1.52–7.63) | −2.82 (−3.39– −2.25) | Down |
| 35–39 | 61,565.66 (21,211.41–101,339.99) | 17.48 (6.02–28.77) | 51,943.86 (17,618.11–88,977.87) | 9.26 (3.14–15.86) | −2.60 (−2.93– −2.26) | Down | |
| 40–44 | 96,291.25 (34,185.99–157,664.06) | 33.61 (11.93–55.03) | 86,967.04 (28,806.14–147,039.89) | 17.38 (5.76–29.39) | −2.35 (−2.60– −2.11) | Down | |
| 45–49 | 112,636.42 (40,962.00–183,920.08) | 48.51 (17.64–79.21) | 14,9559.58 (48,725.60–252,855.80) | 31.59 (10.29–53.40) | −1.33 (−1.63– −1.03) | Down | |
| 50–54 | 145,254.65 (54,230.96–232,028.88) | 68.33 (25.51–109.15) | 217,615.36 (72,308.57–364,295.63) | 48.91 (16.25–81.88) | −0.95 (−1.12– −0.77) | Down | |
| 55–59 | 159,579.74 (56,525.87–254,671.31) | 86.17 (30.52–137.51) | 241,731.52 (82,014.18–406,240.77) | 61.09 (20.72–102.66) | −1.09 (−1.20– −0.98) | Down | |
| 60–64 | 150,628.76 (52,439.19–239,231.89) | 93.79 (32.65–148.95) | 217,757.20 (75,803.12–359,553.51) | 68.04 (23.68–112.34) | −1.08 (−1.25– −0.92) | Down | |
| 65–69 | 114,668.30 (40,527.15–186,926.60) | 92.77 (32.79–151.22) | 206,699.43 (69,767.39–346,901.88) | 74.93 (25.29–125.76) | −0.91 (−1.12– −0.70) | Down | |
| 70–74 | 67,713.64 (23,158.94–113,274.60) | 79.98 (27.35–133.80) | 137,603.40 (48,070.90−234,461.89) | 66.85 (23.35–113.91) | −0.85 (−1.03– −0.67) | Down | |
| 75–79 | 35,249.11 (11,539.12–59,018.19) | 57.26 (18.75–95.88) | 77,885.62 (26,070.00–134,848.71) | 59.06 (19.77–102.25) | −0.06 (−0.30– 0.19) | Down | |
| 80–84 | 13,074.15 (4201.56–22,454.81) | 36.96 (11.88–63.47) | 42,136.62 (13,025.89–73,918.81) | 48.11 (14.87–84.40) | 1.09 (0.84– 1.34) | Up | |
| 85–89 | 4356.63 (1369.72–7580.15) | 28.83 (9.06–50.16) | 18,898.78 (5742.38–33,844.34) | 41.33 (10.56–74.02) | 1.19 (1.04– 1.33) | Up | |
| 90–94 | 908.15 (275.71–1618.18) | 21.19 (6.43–37.76) | 6139.53 (1873.62–11,164.60) | 34.32 (10.47–62.41) | 1.41 (1.30– 1.53) | Up | |
| 95+ | 115.78 (34.57–213.38) | 11.37 (3.40–20.96) | 1069.15 (305.54–2189.27) | 19.62 (5.61–40.17) | 1.59 (1.45– 1.72) | Up | |
| Liver cancer subtypes | Liver cancer due to NASH | 41,635.59 (13,881.89–71,836.25) | 1.00 (0.33–1.71) | 87,848.06 (28,384.64–153,226.93) | 1.00 (0.32–1.74) | 0.02 (–0.1– 0.07) | Up |
| Liver cancer due to alcohol use | 152,660.03 (53,320.11–254,128.01) | 3.64 (1.27–6.08) | 280,207.22 (94,450.64–493,221.35) | 3.17 (1.07–5.57) | −0.51 (−0.57– −0.44) | Down | |
| Liver cancer due to hepatitis B | 574,319.57 (208,112.37–923,069.70) | 13.15 (4.77–21.19) | 773,836.16 (252,193.88–1,325,255.78) | 8.83 (2.88–15.12) | −1.34 (−1.47– −1.21) | Down | |
| Liver cancer due to hepatitis C | 187,776.50 (65,141.13–306,686.18) | 4.57 (1.58–7.44) | 287,396.22 (95,249.47–482,855.80) | 3.29 (1.09–5.52) | −1.34 (−1.51– −1.17) | Down | |
| Liver cancer due to other causes | 35,444.63 (13,154.74–60,361.64) | 0.82 (0.30–1.39) | 53,608.62 (18,727.27–93,654.99) | 0.61 (0.21–1.06) | −1.02 (−1.11– −0.92) | Down | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ma, Y.; Duan, A.; Fan, X. Global Epidemiology of Smoking and Liver Cancer from 1990 to 2021. Diseases 2025, 13, 356. https://doi.org/10.3390/diseases13110356
Wang J, Ma Y, Duan A, Fan X. Global Epidemiology of Smoking and Liver Cancer from 1990 to 2021. Diseases. 2025; 13(11):356. https://doi.org/10.3390/diseases13110356
Chicago/Turabian StyleWang, Jinguo, Yang Ma, Aixu Duan, and Xiaoming Fan. 2025. "Global Epidemiology of Smoking and Liver Cancer from 1990 to 2021" Diseases 13, no. 11: 356. https://doi.org/10.3390/diseases13110356
APA StyleWang, J., Ma, Y., Duan, A., & Fan, X. (2025). Global Epidemiology of Smoking and Liver Cancer from 1990 to 2021. Diseases, 13(11), 356. https://doi.org/10.3390/diseases13110356






