Chronic Hepatitis B: Current Management and Future Directions
Abstract
1. Introduction
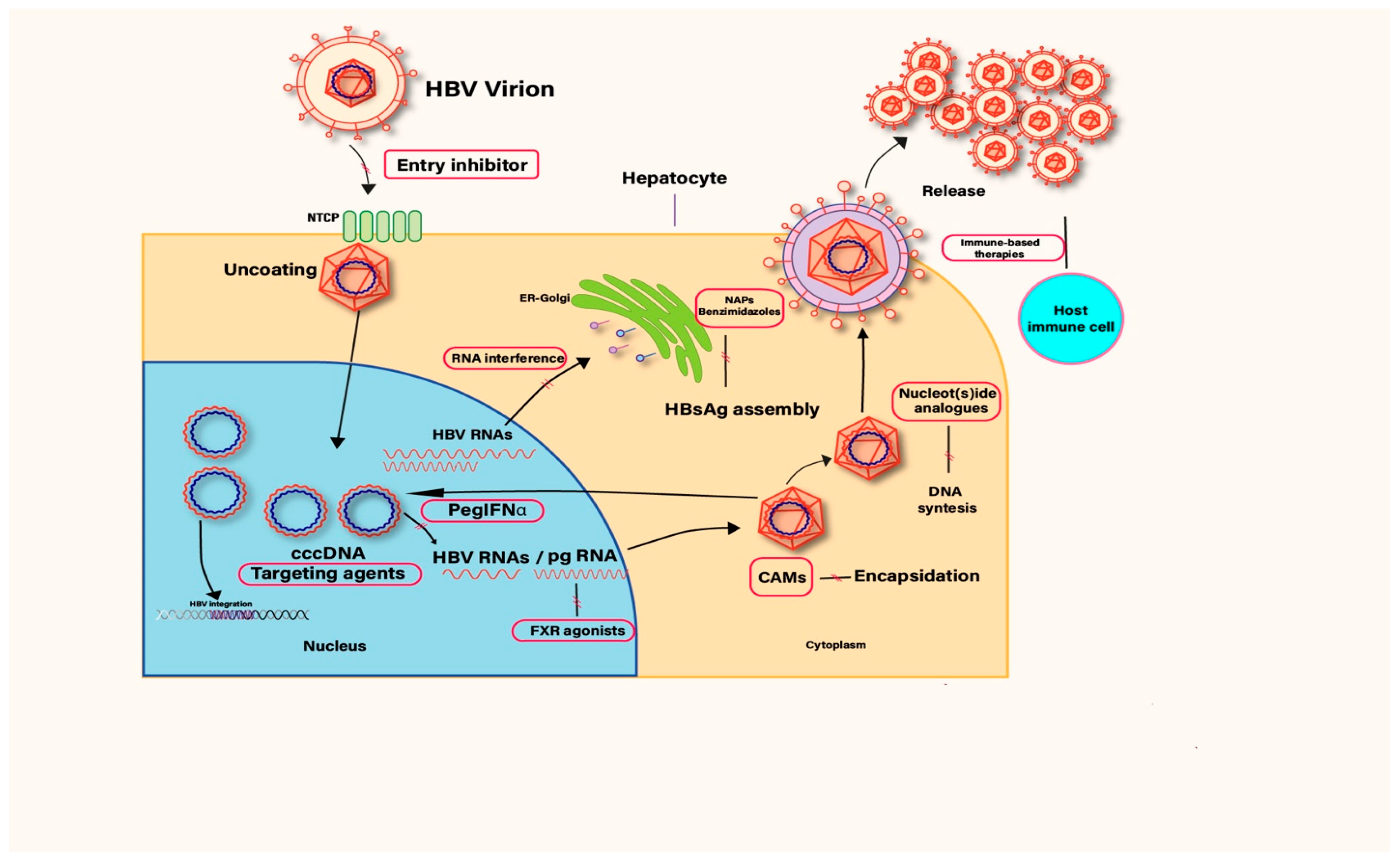
1.1. HBV Lifecycle
1.2. Goals of Treatment
1.3. Factors Predicting Response to Antiviral Treatment
2. Current Treatment Options for CHB
2.1. PegIFNα
2.2. Nucleot(s)ide Analogs
2.2.1. Lamivudine (LAM)
2.2.2. Telbivudine (LdT)
2.2.3. Entecavir (ETV)
2.2.4. Adefovir Dipivoxil (ADV)
2.2.5. Tenofovir Disoproxil Fumarate (TDF)
2.3. New Antivirals in Use
2.3.1. Tenofovir Alafenamide (TAF)
2.3.2. Besifovir
3. Emerging Therapies: What Is on the Horizon?
3.1. Novel Direct-Acting-Antivirals (DAAs)
3.1.1. Targeting cccDNA
- 1)
- Nitazoxanide: As an antiparasitic drug, nitazoxanide has inhibited degradation of a structural maintenance of chromosomes complex, which blocks HBV RNA transcription. In one pilot study, after 48 weeks, HBV DNA became undetectable in almost 90% of the treatment-naïve participants with CHB [25,26]. It requires further large-scale studies for its approval for antiviral indication.
- 2)
- DNA cleavage enzymes: Zinc-finger-like nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR)-associated system 9 (Cas9) proteins are among the DNA cleavage enzymes targeting cccDNA [9]. Currently in preclinical phases, studies have shown the CRISPR-Cas9 system being effective in HBV quasi-species [27]. However, safe and efficient delivery to infected hepatocytes in humans—without inducing host immune reactions to the gene-editing components and preventing off-target effects and genotoxicity of such molecules in general—remains to be an obstacle to translational studies [28].
- 3)
- APOBEC3 (A3) enzymes: Apolipoprotein B mRNA editing catalytic polypeptide-like (APOBEC) deaminases can degrade cccDNA without damaging hepatocytes [29]. Certain interferons (alpha, beta, lambda) can stimulate APOBEC3 activity, promoting cccDNA destruction [30]. Off-target mutagenesis induced by APOBEC3 enzymes in the host genome, along with variable expression of these enzymes—which can be suppressed by factors such as hypoxia-inducible factor 1 alpha (HIF1α), upregulated in chronic liver disease and impairing APOBEC3-mediated antiviral effects—are the main barriers to therapeutic use [31,32].
3.1.2. Targeting Anti-Apoptosis Proteins
3.1.3. Capsid Assembly Modulators
- Core protein allosteric modulator-I or heteroaryldihydropyrimidines (CpAM-I or HAPs): Cause aberrant, nonfunctional core proteins.
- 1)
- RO7049389: RO7049389 is an oral molecule studied in a multi-center randomized controlled (RCT) phase I study, where treatment with this molecule led to a decrease in HBV DNA and RNA, but there was no change in HBsAg levels. Post-treatment observation showed viral rebound to pretreatment levels [38,39].
- 2)
- 3)
- Bay41–4109: This is a molecule tested only in preclinical studies, and shown to reduce HBV replication and intracellular HBV RNA, HBV antigenemia, and cccDNA formation [42].
- 1)
- NVR 3–778: This molecule, in combination with Peg-IFNα, is shown in a phase 1b trial to induce greater suppression in viremia and HBV RNA; however, HBsAg and HBeAg did not change significantly within 28 days of treatment [43].
- 2)
- Bersacapavir (JNJ6379): Bersacapavir is a CAM that induced defective capsid formation, reducing cccDNA [44]. In a double-blind study, it resulted in a decline in viremia; however, viral loads returned to baseline after stopping therapy [45]. It is now being tested in combination with NAs and siRNA agents.
- 3)
- Vebicorvir (ABI-H0731): Vebicorvir monotherapy showed strong initial viral suppression but relapse after discontinuation; combination regimens are under investigation. In a 24-week phase II trial, a combination of the novel core inhibitor vebicorvir with ETV in treatment-naïve patients with HBeAg positive CHB infection demonstrated greater HBV DNA reduction and increased ALT normalization rates over ETV alone. However, most of the patient population were of Asian descent, and findings cannot be generalized to a global population [46].
3.1.4. RNA Interference
- 1)
- ARC-520: In a phase II clinical trial, ARC-520 reduced HBsAg in chimpanzees and patients who were HBeAg-positive, but the effect was not as much pronounced in HBeAg-negative ones [48]. Due to dose-dependent and delayed hypersensitivity reactions observed in animal models caused by EX1 excipient of ARC-520, its clinical development is discontinued [49].
- 2)
- JNJ-3989: In a phase II study, JNJ-3989 is shown to reduce HBsAg, and a sustained HBsAg reduction was observed in more than 50% of the patients after cessation of treatment. When combined with TDF or ETV, it induced sustained HBV-RNA and HBeAg reduction; however, it has not achieved HBsAg sero-clearance off-treatment [50].
- 3)
- Imdusiran (AB-729): Imdusiran is a small interfering RNA (si-RNA) therapeutic that resulted in HBsAg decline without rebound rise post-treatment. When given as a single dose to HBeAg-negative patients with low viremia, it also led to a significant drop in HBsAg and undetectable HBV RNA levels in all patients up to 36 weeks. It was active against NA- and CAM-resistant HBV isolates, and combination with standard-of-care agents was additive. Current data do not meet regulatory requirements for market approval yet [51,52].
- 4)
- Bepirovirsen (GSK3228836): It is an antisense oligonucleotide molecule that targets HBV mRNAs and acts to decrease viral protein levels. In a phase 2b trial, Bepirovirsen was given to both NA-naïve patients and individuals who were already on NA treatment, resulting in sustained HBsAg and HBV DNA loss in a small proportion of participants (10%) [53]. Bepirovirsen received a US-FDA Fast Track designation as of 2024 [54].
3.1.5. Host-Targeted Therapies
- 1)
- Viral Entry Inhibitors: Entry inhibitors act in various ways, mainly by interfering with peptides involved in HBV entry; however, they do not affect cccDNA. Thus, their utility would be in combination regimes with molecules that target cccDNA formation or degradation.
- Bulevirtide (Myrcludex): Sodium-taurocholate co-transporting polypeptide (NTCP) was identified as a host entry factor for HBV. Bulevirtide inhibits NTCP receptor binding, blocking HBV entry [55,56]. It showed HBV DNA decline in HBeAg-negative CHB patients but minimal effect on HBsAg [57]; hence, studies by and large focus on hepatitis D virus (HDV) co-infection, and it is now approved for chronic HDV infection in Europe [58].
- Monoclonal antibodies: Neutralizing antibodies can inhibit HBV entry and target viral envelope antigens recognizing various HBsAg epitopes, stimulating adaptive immunity. Current studies are in phase I or II [59].
- Cyclosporine: CysA derivatives without immunosuppressant activity can prevent HBV attachment to NTCP [60]; however, they have not advanced to human trials or regulatory review yet.
- 2)
- HBsAg release inhibitors:
- Nucleic acid polymers (NAPs): HBsAg both forms the surface of HBV virions and allows entry to hepatocytes via NTCP receptor. Blocking HBsAg release would prevent release of further enveloped viruses. DNA or RNA-based NAPs, which block release of HBsAg, are now being studied in trials for mono or combination therapy [61]. A phase 2 pilot study combined a NAP with either PEG-IFNα or TDF in HBeAg-negative treatment-naïve CHB patients, which resulted in HBsAg seroconversion in all patients [62]. Larger multicenter studies needed for the characterization of long-term safety—including possible HBsAg accumulation in hepatocytes causing ALT-flares—and to confirm HBsAg loss after treatment cessation [63].
- Benzimidazoles: BM601, a secretion inhibitor, inhibits transport of the HBV surface protein to the Golgi apparatus, lowering HBsAg and virion release without affecting HBeAg [64]; however, this effect has not been validated in animal or human models yet.
- 3)
- Farnesoid X receptor (FXR) agonists: Bile acid nuclear receptor FXR binds to cccDNA and enhances HBV transcription. Vonafexor is a FXR-agonist, which, in phase II trials with PegIFNα and entecavir, showed strong HBV DNA and HBsAg reductions in HBeAg-positive patients, with smaller effects in HBeAg-negative individuals [65]. The risk of hepatotoxicity via mitochondrial dysfunction, hepatocyte apoptosis—especially at higher doses or with chronic administration—and potential metabolic side effects—due to their central role in bile acid, lipid, and glucose metabolism—remain to be an obstacle for FXR agonists [66].
- 4)
- Cyclophilin inhibitors: Cyclophilins are host proteins that catalyze cis- to trans- conformation in protein folding and participate in cellular signaling and immunomodulation. There is substantial data that shows cyclophilin involvement in HIV and HCV infection [67]. Cyclophilins are also implicated in the HBV lifecycle. One molecule in this group, CRV341, is being studied in MASLD and HCC. In HBV transgenic mice, CRV431 reduced intrahepatic HBV DNA and moderately decreased serum HBsAg, with additive effects when combined with tenofovir analogs and no observed toxicity in these models [68].
3.2. Immune-Based Therapies
3.2.1. Activation of the Innate Immune System
- 1)
- Toll-like receptor (TLR) agonist: TLRs are expressed on macrophages and dendritic cells and recognize molecules from microorganisms. TLR-7 and 8 induce expression of genes involved in antiviral cytokine release. TLR-7 agonists studied are as follows: RO702053, JNJ-4964, and GS-9620 (Vesatolimod). Vesatolimod has shown to increase HBV-specific T cells but there was no significant drop in HBsAg levels [69]. TLR-8 agonist Selgantolimob was studied in HBeAg + CHB patients as a monotherapy, but the effect on HBsAg decline or HBeAg seroconversion was not satisfactory; however, when combined with TAF, there was a more pronounced reduction in HBsAg [70]. Combination regimens are being investigated, with the rationale that reducing antigen load with direct-acting antivirals may enhance the efficacy of immunomodulatory agents; however, sustained HBsAg loss has not been achieved yet to bring these molecules close to active clinical use.
- 2)
- Retinoic acid inducible gene-1 (RIG-1) agonist: RIG-1 is an intracytoplasmic dsRNA sensor, which plays a role in immune response to CHB. After activation, it induces cytokine production via intracellular pathways, especially IFN-l production, which is known to inhibit HBV replication directly and to activate innate and adaptive immunity [71]. A RIG-1 agonist, Inarigivir, has been studied in HBeAg-positive and -negative patients, as monotherapy, followed by switching to TDF. The study showed a reduction in HBV DNA and RNA; however, it was found to have significant hepatotoxic side effects. Therefore, the study was terminated [47].
- 3)
- Programmed death-1 (PD1) inhibition: The immune checkpoint receptor (ICR) blockade has opened a new era in cancer treatment. As immune response to HBV plays a major role in the development of CHB, the same pathway gained attraction in recent investigations. One of them showed programmed death-1 (PD1) being correlated with viremia and HBeAg and decreased with HBeAg seroconversion. In this context, PD1 inhibitor nivolumab was studied; however, it has only shown minimal decline in HBsAg [72].
3.2.2. Activation of the Adaptive Immune System
- 1)
- The IFN system: Despite PegIFNα being a second-line agent in CHB treatment due to its side effect profile, it is the only approved drug with finite duration. PegIFNα is now being studied in combination with other antiviral agents. IFN-λ has similarities with IFN-a but has fewer side effects due to its more restricted expression in epithelial and immune cells. Moreover, it was evaluated in a study where it showed earlier decline in viral load and similar HBeAg seroconversion when compared to PegIFNα [73]. However, based on post-treatment seroconversion, virologic suppression, and biochemical response rates, PegIFNα2a demonstrated greater overall efficacy. Nonetheless, findings suggest a potential role for IFN-λ, especially when combined with NAs, in supporting immune-mediated control of HBV, which may lead to the suppression of cccDNA activity [74].
- 2)
- Therapeutic vaccination: This approach seeks to retrain the immune system to recognize and attack HBV. Among these are GS-4774, ABX-203 (HeberNasvac), BRII-179, TG 1050, VTP-300, and TherVacB. Despite inducing strong immune response in unaffected individuals, HBV vaccines failed to show a benefit in HBV-infected patients until now.
- GS-4774, containing HBsAg and HBcAg, did not show superiority in HBsAg decline when compared to patients treated with NAs-only [75].
- ABX-203 (HeberNasvac), containing HBsAg and HBcAg, resulted in equally suppressed HBV DNA levels when compared with PegIFNα alone but did not result in loss of HBsAg [76].
- BRII-179, containing HBsAg, induced cellular and humoral immune response but did not result in a significant change in HBsAg levels [77].
- TG1050, an adenovirus-based vaccine, expressing HBsAg, HBcAg, and HBV polymerase, resulted in minor decrease in HBsAg [78].
- VTP300, an immunotherapeutic vaccine, has demonstrated to lower HBsAg levels both as monotherapy and in combination with Nivolumab or Imdusiran, with the combination resulting in sustained HBsAg declines [79].
- TherVacB, a modified vaccinia virus Ankara (MVA)-vector boosted heterologous vaccine, elicited HBV-specific antibody and T cell responses in wild type and HBV-carrier mice [80]. The first clinical trial with TherVacB started in 2024.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cohen, E.B.; Regev, A.; Garg, A.; Di Bisceglie, A.M.; Lewis, J.H.; Vierling, J.M.; Hey-Hadavi, J.; Steplewski, K.; Fettiplace, A.; Chen, C.L.; et al. Consensus Guidelines: Best Practices for the Prevention, Detection and Management of Hepatitis B Virus Reactivation in Clinical Trials with Immunosuppressive/Immunomodulatory Therapy. Drug Saf. 2024, 47, 321–332. [Google Scholar] [CrossRef]
- Boonstra, A.; Sari, G. HBV cccDNA: The Molecular Reservoir of Hepatitis B Persistence and Challenges to Achieve Viral Eradication. Biomolecules 2025, 15, 62. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Prevention, Care and the Treatment of Persons with Chronic Hepatitis B Infection; World Health Organization: Geneva, Switzerland, 2024.
- Mendenhall, M.A.; Hong, X.; Hu, J. Hepatitis B Virus Capsid: The Core in Productive Entry and Covalently Closed Circular DNA Formation. Viruses 2023, 15, 642. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, Z.; Huang, L.; Qiu, Z.; Xie, Y.; Jiang, S.; Feng, B. Achieving Chronic Hepatitis B Functional Cure: Factors and Potential Mechanisms. Virus Res. 2025, 351, 199507. [Google Scholar] [CrossRef]
- Ghany, M.G.; Buti, M.; Lampertico, P.; Lee, H.M.; on behalf of the 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference Faculty. Guidance on Treatment Endpoints and Study Design for Clinical Trials Aiming to Achieve Cure in Chronic Hepatitis B and D: Report from the 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference. Hepatology 2023, 78, 1654–1673. [Google Scholar] [CrossRef]
- Lin, C.-L.; Kao, J.-H. Hepatitis B Virus Genotypes and Variants. Cold Spring Harb. Perspect. Med. 2015, 5, a021436. [Google Scholar] [CrossRef]
- Ghany, M.G. Current Treatment Guidelines of Chronic Hepatitis B: The Role of Nucleos(t)Ide Analogues and Peginterferon. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 299–309. [Google Scholar] [CrossRef]
- Ozaras, R.; Tahan, V. (Eds.) Viral Hepatitis: Chronic Hepatitis B; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Choi, H.S.J.; Van Campenhout, M.J.H.; Van Vuuren, A.J.; Krassenburg, L.A.P.; Sonneveld, M.J.; De Knegt, R.J.; Hansen, B.E.; Janssen, H.L.A. Ultra-Long-Term Follow-up of Interferon Alfa Treatment for HBeAg-Positive Chronic Hepatitis B Virus Infection. Clin. Gastroenterol. Hepatol. 2021, 19, 1933–1940.e1. [Google Scholar] [CrossRef] [PubMed]
- Bacaksız, F.; Gökcan, H.; Akdoğan, M.; Gökçe, D.T.; Arı, D.; Gökbulut, V.; Ergün, Y.; Öztürk, Ö.; Kacar, S. Role of Hepatosteatosis in HBsAg Seroconversion in HBeAg-negative Chronic Hepatitis B Patients. Int. J. Clin. Pract. 2021, 75, e14899. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Littlejohn, M.; Yuen, L.; Jackson, K.; Mason, H.; Bayliss, J.; Rosenberg, G.; Gaggar, A.; Kitrinos, K.; Subramanian, M.; et al. HBeAg Levels at Week 24 Predict Response to 8 Years of Tenofovir in HBeAg-Positive Chronic Hepatitis B Patients. Aliment. Pharmacol. Ther. 2018, 47, 114–122. [Google Scholar] [CrossRef]
- Koumbi, L. Current and Future Antiviral Drug Therapies of Hepatitis B Chronic Infection. World J. Hepatol. 2015, 7, 1030. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.-D.; Wu, W.-F. Effect of Entecavir in the Treatment of Patients with Hepatitis B Virus-Related Compensated and Decompensated Cirrhosis. Exp. Ther. Med. 2017, 14, 3908–3914. [Google Scholar] [CrossRef][Green Version]
- Van Bömmel, F.; Stein, K.; Heyne, R.; Petersen, J.; Buggisch, P.; Berg, C.; Zeuzem, S.; Stallmach, A.; Sprinzl, M.; Schott, E.; et al. A Multicenter Randomized-Controlled Trial of Nucleos(t)Ide Analogue Cessation in HBeAg-Negative Chronic Hepatitis B. J. Hepatol. 2023, 78, 926–936. [Google Scholar] [CrossRef]
- Cornberg, M.; Sandmann, L.; Jaroszewicz, J.; Kennedy, P.; Lampertico, P.; Lemoine, M.; Lens, S.; Testoni, B.; Lai-Hung Wong, G.; Russo, F.P. EASL Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2025, 83, 502–583. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Bzowej, N.H.; Chang, K.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. AASLD Guidelines for Treatment of Chronic Hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef]
- Kang, L.; Pan, J.; Wu, J.; Hu, J.; Sun, Q.; Tang, J. Anti-HBV Drugs: Progress, Unmet Needs, and New Hope. Viruses 2015, 7, 4960–4977. [Google Scholar] [CrossRef]
- Shimizu, M.; Furusyo, N.; Ikezaki, H.; Ogawa, E.; Hayashi, T.; Ihara, T.; Harada, Y.; Toyoda, K.; Murata, M.; Hayashi, J. Predictors of Kidney Tubular Dysfunction Induced by Adefovir Treatment for Chronic Hepatitis B. World J. Gastroenterol. 2015, 21, 2116–2123. [Google Scholar] [CrossRef]
- Tacke, F.; Kroy, D.C. Treatment for Hepatitis B in Patients with Drug Resistance. Ann. Transl. Med. 2016, 4, 334. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, J.H.; Yim, H.J.; Seo, Y.S.; Yim, S.Y.; Lee, Y.-S.; Jung, Y.K.; Yeon, J.E.; Um, S.H.; Byun, K.S. Noninferiority Outcomes of Besifovir Compared to Tenofovir Alafenamide in Treatment-Naïve Patients with Chronic Hepatitis B. Gut Liver 2024, 18, 305–315. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Ahn, S.H.; Lee, K.S.; Um, S.H.; Cho, M.; Yoon, S.K.; Lee, J.-W.; Park, N.H.; Kweon, Y.-O.; Sohn, J.H.; et al. Two-Year Treatment Outcome of Chronic Hepatitis B Infection Treated with Besifovir vs. Entecavir: Results from a Multicentre Study. J. Hepatol. 2015, 62, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M.B.; Lucifora, J.; Mason, W.S.; Sureau, C.; Beck, J.; Levrero, M.; Kann, M.; Knolle, P.A.; Benkirane, M.; Durantel, D.; et al. Towards an HBV Cure: State-of-the-Art and Unresolved Questions—Report of the ANRS Workshop on HBV Cure. Gut 2015, 64, 1314–1326. [Google Scholar] [CrossRef]
- Tu, T.; McQuaid, T.J.; Jacobson, I.M. HBV-Induced Carcinogenesis: Mechanisms, Correlation with Viral Suppression, and Implications for Treatment. Liver Int. 2025, 45, e16202. [Google Scholar] [CrossRef] [PubMed]
- Sekiba, K.; Otsuka, M.; Ohno, M.; Yamagami, M.; Kishikawa, T.; Suzuki, T.; Ishibashi, R.; Seimiya, T.; Tanaka, E.; Koike, K. Inhibition of HBV Transcription From cccDNA With Nitazoxanide by Targeting the HBx–DDB1 Interaction. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 297–312. [Google Scholar] [CrossRef]
- Rossignol, J.; Bréchot, C. A Pilot Clinical Trial of Nitazoxanide in the Treatment of Chronic Hepatitis B. Hepatol. Commun. 2019, 3, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Zarifyan, D.; Goptar, I.; Chulanov, V. Orthologous CRISPR/Cas9 Systems for Specific and Efficient Degradation of Covalently Closed Circular DNA of Hepatitis B Virus. Cell. Mol. Life Sci. 2019, 76, 1779–1794. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Hashem, M.A.; Kohara, M.; Tsukiyama-Kohara, K. In Vivo Delivery Tools for Clustered Regularly Interspaced Short Palindromic Repeat/Associated Protein 9-Mediated Inhibition of Hepatitis B Virus Infection: An Update. Front. Microbiol. 2022, 13, 953218. [Google Scholar] [CrossRef]
- Revill, P.; Locarnini, S. Antiviral Strategies to Eliminate Hepatitis B Virus Covalently Closed Circular DNA (cccDNA). Curr. Opin. Pharmacol. 2016, 30, 144–150. [Google Scholar] [CrossRef]
- Bockmann, J.-H.; Stadler, D.; Xia, Y.; Ko, C.; Wettengel, J.M.; Schulze Zur Wiesch, J.; Dandri, M.; Protzer, U. Comparative Analysis of the Antiviral Effects Mediated by Type I and III Interferons in Hepatitis B Virus–Infected Hepatocytes. J. Infect. Dis. 2019, 220, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Ponomareva, N.; Bayurova, E.; Zakirova, N.; Kondrashova, A.; Goptar, I.; Nikiforova, A.; Sudina, A.; et al. Transient and Tunable CRISPRa Regulation of APOBEC/AID Genes for Targeting Hepatitis B Virus. Mol. Ther. Nucleic Acids 2023, 32, 478–493. [Google Scholar] [CrossRef]
- Riedl, T.; Faure-Dupuy, S.; Rolland, M.; Schuehle, S.; Hizir, Z.; Calderazzo, S.; Zhuang, X.; Wettengel, J.; Lopez, M.A.; Barnault, R.; et al. Hypoxia-Inducible Factor 1 Alpha–Mediated RelB/APOBEC3B Down-regulation Allows Hepatitis B Virus Persistence. Hepatology 2021, 74, 1766–1781. [Google Scholar] [CrossRef]
- Morrish, E.; Mackiewicz, L.; Silke, N.; Pellegrini, M.; Silke, J.; Brumatti, G.; Ebert, G. Combinatorial Treatment of Birinapant and Zosuquidar Enhances Effective Control of HBV Replication In Vivo. Viruses 2020, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Ebert, G.; Allison, C.; Preston, S.; Cooney, J.; Toe, J.G.; Stutz, M.D.; Ojaimi, S.; Baschuk, N.; Nachbur, U.; Torresi, J.; et al. Eliminating Hepatitis B by Antagonizing Cellular Inhibitors of Apoptosis. Proc. Natl. Acad. Sci. USA 2015, 112, 5803–5808. [Google Scholar] [CrossRef]
- Guo, F.; Zhao, Q.; Sheraz, M.; Cheng, J.; Qi, Y.; Su, Q.; Cuconati, A.; Wei, L.; Du, Y.; Li, W.; et al. HBV Core Protein Allosteric Modulators Differentially Alter cccDNA Biosynthesis from de Novo Infection and Intracellular Amplification Pathways. PLoS Pathog. 2017, 13, e1006658. [Google Scholar] [CrossRef]
- Lahlali, T.; Berke, J.M.; Vergauwen, K.; Foca, A.; Vandyck, K.; Pauwels, F.; Zoulim, F.; Durantel, D. Novel Potent Capsid Assembly Modulators Regulate Multiple Steps of the Hepatitis B Virus Life Cycle. Antimicrob. Agents Chemother. 2018, 62, e00835-18. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, F.; Tong, X.; Hoffmann, D.; Zuo, J.; Lu, M. Treatment of Chronic Hepatitis B Virus Infection Using Small Molecule Modulators of Nucleocapsid Assembly: Recent Advances and Perspectives. ACS Infect. Dis. 2019, 5, 713–724. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Zhou, X.; Gane, E.; Schwabe, C.; Tanwandee, T.; Feng, S.; Jin, Y.; Triyatni, M.; Lemenuel-Diot, A.; Cosson, V.; et al. Safety, Pharmacokinetics, and Antiviral Activity of RO7049389, a Core Protein Allosteric Modulator, in Patients with Chronic Hepatitis B Virus Infection: A Multicentre, Randomised, Placebo-Controlled, Phase 1 Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gane, E.; Schwabe, C.; Zhu, M.; Triyatni, M.; Zhou, J.; Bo, Q.; Jin, Y. A Five-in-One First-in-Human Study To Assess Safety, Tolerability, and Pharmacokinetics of RO7049389, an Inhibitor of Hepatitis B Virus Capsid Assembly, after Single and Multiple Ascending Doses in Healthy Participants. Antimicrob. Agents Chemother. 2020, 64, e01323-20. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F.; Zhu, X.; Chen, Y.; Chen, H.; Li, X.; Wu, M.; Li, C.; Liu, J.; Zhang, Y.; et al. Antiviral Activity and Pharmacokinetics of the Hepatitis B Virus (HBV) Capsid Assembly Modulator GLS4 in Patients With Chronic HBV Infection. Clin. Infect. Dis. 2021, 73, 175–182. [Google Scholar] [CrossRef]
- Zhao, N.; Jia, B.; Zhao, H.; Xu, J.; Sheng, X.; Luo, L.; Huang, Z.; Wang, X.; Ren, Q.; Zhang, Y.; et al. A First-in-Human Trial of GLS4, a Novel Inhibitor of Hepatitis B Virus Capsid Assembly, Following Single- and Multiple-Ascending-Oral-Dose Studies with or without Ritonavir in Healthy Adult Volunteers. Antimicrob. Agents Chemother. 2019, 64, e01686-19. [Google Scholar] [CrossRef]
- Rat, V.; Seigneuret, F.; Burlaud-Gaillard, J.; Lemoine, R.; Hourioux, C.; Zoulim, F.; Testoni, B.; Meunier, J.-C.; Tauber, C.; Roingeard, P.; et al. BAY 41-4109-Mediated Aggregation of Assembled and Misassembled HBV Capsids in Cells Revealed by Electron Microscopy. Antivir. Res. 2019, 169, 104557. [Google Scholar] [CrossRef]
- Yuen, M.F.; Gane, E.J.; Kim, D.J.; Weilert, F.; Yuen Chan, H.L.; Lalezari, J.; Hwang, S.G.; Nguyen, T.; Flores, O.; Hartman, G.; et al. Antiviral Activity, Safety, and Pharmacokinetics of Capsid Assembly Modulator NVR 3-778 in Patients with Chronic HBV Infection. Gastroenterology 2019, 156, 1392–1403.e7. [Google Scholar] [CrossRef]
- Vandenbossche, J.; Yogaratnam, J.; Hillewaert, V.; Rasschaert, F.; Talloen, W.; Biewenga, J.; Snoeys, J.; Kakuda, T.N.; Palmer, M.; Nangosyah, J.; et al. Drug-Drug Interactions with the Hepatitis B Virus Capsid Assembly Modulator JNJ-56136379 (Bersacapavir). Clin. Pharmacol. Drug Dev. 2022, 11, 1419–1429. [Google Scholar] [CrossRef]
- Zoulim, F.; Lenz, O.; Vandenbossche, J.J.; Talloen, W.; Verbinnen, T.; Moscalu, I.; Streinu-Cercel, A.; Bourgeois, S.; Buti, M.; Crespo, J.; et al. JNJ-56136379, an HBV Capsid Assembly Modulator, Is Well-Tolerated and Has Antiviral Activity in a Phase 1 Study of Patients with Chronic Infection. Gastroenterology 2020, 159, 521–533.e9. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, M.S.; Agarwal, K.; Ma, X.; Nguyen, T.T.; Schiff, E.R.; Hann, H.-W.L.; Dieterich, D.T.; Nahass, R.G.; Park, J.S.; Chan, S.; et al. Safety and Efficacy of Vebicorvir Administered with Entecavir in Treatment-Naïve Patients with Chronic Hepatitis B Virus Infection. J. Hepatol. 2022, 77, 1265–1275. [Google Scholar] [CrossRef]
- Phillips, S.; Jagatia, R.; Chokshi, S. Novel Therapeutic Strategies for Chronic Hepatitis B. Virulence 2022, 13, 1111–1132. [Google Scholar] [CrossRef] [PubMed]
- Wooddell, C.I.; Yuen, M.-F.; Chan, H.L.-Y.; Gish, R.G.; Locarnini, S.A.; Chavez, D.; Ferrari, C.; Given, B.D.; Hamilton, J.; Kanner, S.B.; et al. RNAi-Based Treatment of Chronically Infected Patients and Chimpanzees Reveals That Integrated Hepatitis B Virus DNA Is a Source of HBsAg. Sci. Transl. Med. 2017, 9, eaan0241. [Google Scholar] [CrossRef]
- Wooddell, C.I.; Mak, L.Y.; Seto, W.-K.; Given, B.D.; Yuen, M.-F. Virological Insights from ARC-520 siRNA and Entecavir Treated Chronically HBV-Infected Patients and Chimpanzees. Microorganisms 2025, 13, 1787. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.-F.; Locarnini, S.; Lim, T.H.; Strasser, S.I.; Sievert, W.; Cheng, W.; Thompson, A.J.; Given, B.D.; Schluep, T.; Hamilton, J.; et al. Combination Treatments Including the Small-Interfering RNA JNJ-3989 Induce Rapid and Sometimes Prolonged Viral Responses in Patients with CHB. J. Hepatol. 2022, 77, 1287–1298. [Google Scholar] [CrossRef]
- Thi, E.P.; Ye, X.; Snead, N.M.; Lee, A.C.H.; Micolochick Steuer, H.M.; Ardzinski, A.; Graves, I.E.; Espiritu, C.; Cuconati, A.; Abbott, C.; et al. Control of Hepatitis B Virus with Imdusiran, a Small Interfering RNA Therapeutic. ACS Infect. Dis. 2024, 10, 3640–3649. [Google Scholar] [CrossRef]
- Dusheiko, G.; Agarwal, K.; Maini, M.K. New Approaches to Chronic Hepatitis B. N. Engl. J. Med. 2023, 388, 55–69. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Lim, S.-G.; Plesniak, R.; Tsuji, K.; Janssen, H.L.A.; Pojoga, C.; Gadano, A.; Popescu, C.P.; Stepanova, T.; Asselah, T.; et al. Efficacy and Safety of Bepirovirsen in Chronic Hepatitis B Infection. N. Engl. J. Med. 2022, 387, 1957–1968. [Google Scholar] [CrossRef]
- GSK Receives US FDA Fast Track Designation for Bepirovirsen in Chronic Hepatitis B. Available online: https://www.gsk.com/media/o22eqbgg/gsk-receives-us-fda-fast-track-designation-for-bepirovirsen-press-release.pdf (accessed on 20 August 2025).
- Fourati, S.; Pawlotsky, J.-M. Recent Advances in Understanding and Diagnosing Hepatitis B Virus Infection. F1000Research 2016, 5, 2243. [Google Scholar] [CrossRef]
- Seeger, C.; Mason, W.S. Molecular Biology of Hepatitis B Virus Infection. Virology 2015, 479–480, 672–686. [Google Scholar] [CrossRef]
- Bogomolov, P.; Alexandrov, A.; Voronkova, N.; Macievich, M.; Kokina, K.; Petrachenkova, M.; Lehr, T.; Lempp, F.A.; Wedemeyer, H.; Haag, M.; et al. Treatment of Chronic Hepatitis D with the Entry Inhibitor Myrcludex B: First Results of a Phase Ib/IIa Study. J. Hepatol. 2016, 65, 490–498. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Aleman, S.; Brunetto, M.; Blank, A.; Andreone, P.; Bogomolov, P.; Chulanov, V.; Mamonova, N.; Geyvandova, N.; Morozov, V.; et al. Bulevirtide Monotherapy in Patients with Chronic HDV: Efficacy and Safety Results through Week 96 from a Phase III Randomized Trial. J. Hepatol. 2024, 81, 621–629. [Google Scholar] [CrossRef]
- Beretta, M.; Mouquet, H. Advances in Human Monoclonal Antibody Therapy for HBV Infection. Curr. Opin. Virol. 2022, 53, 101205. [Google Scholar] [CrossRef]
- Watashi, K.; Sluder, A.; Daito, T.; Matsunaga, S.; Ryo, A.; Nagamori, S.; Iwamoto, M.; Nakajima, S.; Tsukuda, S.; Borroto-Esoda, K.; et al. Cyclosporin A and Its Analogs Inhibit Hepatitis B Virus Entry Into Cultured Hepatocytes Through Targeting a Membrane Transporter, Sodium Taurocholate Cotransporting Polypeptide (NTCP). Hepatology 2014, 59, 1726–1737. [Google Scholar] [CrossRef]
- Bazinet, M.; Pântea, V.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Albrecht, J.; Schmid, P.; Le Gal, F.; Gordien, E.; Krawczyk, A.; et al. Safety and Efficacy of REP 2139 and Pegylated Interferon Alfa-2a for Treatment-Naive Patients with Chronic Hepatitis B Virus and Hepatitis D Virus Co-Infection (REP 301 and REP 301-LTF): A Non-Randomised, Open-Label, Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2017, 2, 877–889. [Google Scholar] [CrossRef]
- Bazinet, M.; Pântea, V.; Placinta, G.; Moscalu, I.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Iarovoi, L.; Smesnoi, V.; Musteata, T.; et al. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)Ide Therapy. Gastroenterology 2020, 158, 2180–2194. [Google Scholar] [CrossRef]
- Mak, L.-Y.; Hui, R.W.-H.; Seto, W.-K.; Yuen, M.-F. Novel Drug Development in Chronic Hepatitis B Infection: Capsid Assembly Modulators and Nucleic Acid Polymers. Clin. Liver Dis. 2023, 27, 877–893. [Google Scholar] [CrossRef]
- Xu, Y.-B.; Yang, L.; Wang, G.-F.; Tong, X.-K.; Wang, Y.-J.; Yu, Y.; Jing, J.-F.; Feng, C.-L.; He, P.-L.; Lu, W.; et al. Benzimidazole Derivative, BM601, a Novel Inhibitor of Hepatitis B Virus and HBsAg Secretion. Antivir. Res. 2014, 107, 6–15. [Google Scholar] [CrossRef]
- Gopalakrishna, H.; Ghany, M.G. Perspective on Emerging Therapies to Achieve Functional Cure of Chronic Hepatitis B. Curr. Hepatol. Rep. 2024, 23, 241–252. [Google Scholar] [CrossRef]
- Zhang, T.; Feng, S.; Li, J.; Wu, Z.; Deng, Q.; Yang, W.; Li, J.; Pan, G. Farnesoid X Receptor (FXR) Agonists Induce Hepatocellular Apoptosis and Impair Hepatic Functions via FXR/SHP Pathway. Arch. Toxicol. 2022, 96, 1829–1843. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, A.; Persaud, M.; Simons, L.M.; Bulnes-Ramos, A.; Buffone, C.; Martinez-Lopez, A.; Scoca, V.; Di Nunzio, F.; Hiatt, J.; Marson, A.; et al. Cyclophilin A Prevents HIV-1 Restriction in Lymphocytes by Blocking Human TRIM5α Binding to the Viral Core. Cell Rep. 2020, 30, 3766–3777.e6. [Google Scholar] [CrossRef] [PubMed]
- Gallay, P.; Ure, D.; Bobardt, M.; Chatterji, U.; Ou, J.; Trepanier, D.; Foster, R. The Cyclophilin Inhibitor CRV431 Inhibits Liver HBV DNA and HBsAg in Transgenic Mice. PLoS ONE 2019, 14, e0217433. [Google Scholar] [CrossRef]
- Agarwal, K.; Ahn, S.H.; Elkhashab, M.; Lau, A.H.; Gaggar, A.; Bulusu, A.; Tian, X.; Cathcart, A.L.; Woo, J.; Subramanian, G.M.; et al. Safety and Efficacy of Vesatolimod (GS-9620) in Patients with Chronic Hepatitis B Who Are Not Currently on Antiviral Treatment. J. Viral Hepat. 2018, 25, 1331–1340. [Google Scholar] [CrossRef]
- Gane, E.J.; Dunbar, P.R.; Brooks, A.E.; Zhang, F.; Chen, D.; Wallin, J.J.; Van Buuren, N.; Arora, P.; Fletcher, S.P.; Tan, S.K.; et al. Safety and Efficacy of the Oral TLR8 Agonist Selgantolimod in Individuals with Chronic Hepatitis B under Viral Suppression. J. Hepatol. 2023, 78, 513–523. [Google Scholar] [CrossRef]
- Phillips, S.; Mistry, S.; Riva, A.; Cooksley, H.; Hadzhiolova-Lebeau, T.; Plavova, S.; Katzarov, K.; Simonova, M.; Zeuzem, S.; Woffendin, C.; et al. Peg-Interferon Lambda Treatment Induces Robust Innate and Adaptive Immunity in Chronic Hepatitis B Patients. Front. Immunol. 2017, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 Blockade with Nivolumab with and without Therapeutic Vaccination for Virally Suppressed Chronic Hepatitis B: A Pilot Study. J. Hepatol. 2019, 71, 900–907. [Google Scholar] [CrossRef]
- Chan, H.L.Y.; Ahn, S.H.; Chang, T.-T.; Peng, C.-Y.; Wong, D.; Coffin, C.S.; Lim, S.G.; Chen, P.-J.; Janssen, H.L.A.; Marcellin, P.; et al. Peginterferon Lambda for the Treatment of HBeAg-Positive Chronic Hepatitis B: A Randomized Phase 2b Study (LIRA-B). J. Hepatol. 2016, 64, 1011–1019. [Google Scholar] [CrossRef]
- Chronopoulou, S.; Tsochantaridis, I. Interferon Lambda: The Next Frontier in Antiviral Therapy? Pharmaceuticals 2025, 18, 785. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; Pan, C.Q.; Han, S.-H.B.; Trinh, H.N.; Fessel, W.J.; Rodell, T.; Massetto, B.; Lin, L.; Gaggar, A.; Subramanian, G.M.; et al. Randomized Phase II Study of GS-4774 as a Therapeutic Vaccine in Virally Suppressed Patients with Chronic Hepatitis B. J. Hepatol. 2016, 65, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Al Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Guillen, G.; Penton, E.; Tuero, A.; Yoshida, O.; Hiasa, Y.; Onji, M. Treatment of Chronic Hepatitis B Naïve Patients with a Therapeutic Vaccine Containing HBs and HBc Antigens (a Randomized, Open and Treatment Controlled Phase III Clinical Trial). PLoS ONE 2018, 13, e0201236. [Google Scholar] [CrossRef]
- Ma, H.; Lim, T.H.; Leerapun, A.; Weltman, M.; Jia, J.; Lim, Y.; Tangkijvanich, P.; Sukeepaisarnjaroen, W.; Ji, Y.; Le Bert, N.; et al. Therapeutic Vaccine BRII-179 Restores HBV-Specific Immune Responses in Patients with Chronic HBV in a Phase Ib/IIa Study. JHEP Rep. 2021, 3, 100361. [Google Scholar] [CrossRef]
- Zoulim, F.; Fournier, C.; Habersetzer, F.; Sprinzl, M.; Pol, S.; Coffin, C.S.; Leroy, V.; Ma, M.; Wedemeyer, H.; Lohse, A.W.; et al. Safety and Immunogenicity of the Therapeutic Vaccine TG1050 in Chronic Hepatitis B Patients: A Phase 1b Placebo-Controlled Trial. Hum. Vaccines Immunother. 2020, 16, 388–399. [Google Scholar] [CrossRef]
- Tak, W.Y.; Chuang, W.-L.; Chen, C.-Y.; Tseng, K.-C.; Lim, Y.-S.; Lo, G.-H.; Heo, J.; Agarwal, K.; Bussey, L.; Teoh, S.L.; et al. Phase Ib/IIa Randomized Study of Heterologous ChAdOx1-HBV/MVA-HBV Therapeutic Vaccination (VTP-300) as Monotherapy and Combined with Low-Dose Nivolumab in Virally-Suppressed Patients with CHB. J. Hepatol. 2024, 81, 949–959. [Google Scholar] [CrossRef]
- Su, J.; Brunner, L.; Ates Oz, E.; Sacherl, J.; Frank, G.; Kerth, H.A.; Thiele, F.; Wiegand, M.; Mogler, C.; Aguilar, J.C.; et al. Activation of CD4 T Cells during Prime Immunization Determines the Success of a Therapeutic Hepatitis B Vaccine in HBV-Carrier Mouse Models. J. Hepatol. 2023, 78, 717–730. [Google Scholar] [CrossRef]
| Response Type | Definition |
|---|---|
| Biochemical response | ALT returns to normal range |
| Serologic response | Loss of HBsAg with anti-HBs development, or loss of HBeAg with anti-HBe development in HBeAg-positive patients |
| Virologic response to Peg-IFN | HBV DNA <2000 IU/mL; if maintained ≥12 months after treatment completion, termed sustained virologic response |
| Virologic response to nucleos(t)ide analogues (NAs) | Undetectable HBV DNA |
| Partial virologic response (to NAs) | ≥1 log10 IU/mL HBV DNA decline, but still detectable after ~24 weeks of NA therapy |
| Complete response | Sustained virologic suppression with HBsAg seroconversion |
| Sustained off-treatment response | No relapse observed during follow-up after therapy is stopped |
| Histologic response | ≥2-point reduction in necroinflammatory score without fibrosis worsening, or ≥1 stage improvement in fibrosis by METAVIR |
| Primary non-response (to NAs) | HBV DNA decline <1 log10 IU/mL after 12 weeks of therapy |
| Virologic breakthrough | ≥1 log10 IU/mL increase in HBV DNA from lowest level while on therapy |
| Viral relapse | HBV DNA >2000 IU/mL after stopping therapy in a patient who had prior virologic suppression |
| Clinical relapse | Viral relapse accompanied by ALT elevation > 2 × ULN |
| Drug | Pregnancy Category | Major Side Effects | Suggested Monitoring |
|---|---|---|---|
| Pegylated IFN-α2a | C | Flu-like illness, mood/psychiatric changes, blood count suppression, autoimmune phenomena | CBC * and TSH ** every 3 months; clinical surveillance for neuropsychiatric, autoimmune, or infectious complications |
| Lamivudine | C | Risk of pancreatitis, lactic acidosis | Amylase if symptomatic; lactate when clinically indicated |
| Telbivudine | B | CK *** elevation, muscle toxicity, neuropathy; lactic acidosis | CK if symptoms develop; lactate if clinically indicated |
| Entecavir | C | Lactic acidosis (rare) | Lactate if clinically indicated |
| Adefovir | C | Nephrotoxicity (acute renal failure, Fanconi-like syndrome), tubular dysfunction, lactic acidosis | Baseline CrCl ****; periodic monitoring of renal function, phosphorus, urine glucose/protein (especially in high-risk patients); bone mineral density (BMD) if fracture/osteoporosis risk; lactate if clinical concern |
| Tenofovir | B | Renal injury (nephropathy, Fanconi syndrome), bone loss/osteomalacia, lactic acidosis | Baseline and periodic CrCl; phosphorus and urine markers yearly if risk present; consider baseline BMD in high-risk groups; lactate if clinically indicated |
| Agent/Class | Representative | Trial/Registry | Phase | Primary Endpoints/Outcome Notes | Cost Estimate * | Readiness |
|---|---|---|---|---|---|---|
| Interferons | PegIFNα2a (Pegasys ®) | NCT00487747 | Approved | HBeAg seroconversion, HBsAg decline, ALT normalization | ~$1336/dose (US list price) | Market (Approved) |
| NAs (Nucleos(t)ide analogs) | Lamivudine | Historic pivotal | Approved | HBV DNA suppression < LOD ** at Wk *** 48; effective but resistance issues | ~$16/day (generic US) | Market (Approved) |
| Telbivudine | Historic trials | Approved | HBV DNA suppression; HBeAg seroconversion; resistance limits use | ~$20–30/day | Market (Approved; supplanted) | |
| Entecavir | Pivotal | Approved | HBV DNA < LOD at Wk 48; high potency, low resistance | ~$2–3/day (generic) | Market (Approved) | |
| Adefovir dipivoxil | Historic | Approved | HBV DNA suppression at Wk 48; supplanted by newer agents due to potency/safety | ~$35–40/day | Market (Older) | |
| Tenofovir disoproxil fumarate (TDF) | Pivotal | Approved | HBV DNA < 29 IU/mL at Wk 48 (noninferiority design); high potency, long term safety concerns | ~$3–4/day (generic) | Market (Approved) | |
| Tenofovir alafenamide (TAF, Vemlidy ®) | Phase 3 pivotal | Approved | HBV DNA < 29 IU/mL at Wk 48, ALT normalization; safer kidney/bone profile | ~$19–20/day | Market (Approved; generics emerging) | |
| Besifovir | NCT01937806 (Phase 3 registry) | Phase III/Korea approval | Virological response (HBV DNA < threshold at Wk 48); non-inferior to TDF | Projected ~$15–20/day | Near (regional approval in Korea, not FDA/EU ****) | |
| Novel DAAs | Nitazoxanide | Small Phase 2 | Phase II/pilot | HBV DNA decline, HBsAg change; weak pilot data shows antiviral signal | <$10/day (generic antiparasitic) | Far (investigational for HBV) |
| DNA cleavage (CRISPR, TALEN, etc.) | Preclinical | Preclinical | cccDNA cleavage; antigen reduction. Promising, but delivery issues | N/A | Far | |
| APOBEC3-based approaches | Preclinical | Preclinical | cccDNA deamination; promising in vitro/in vivo | N/A | Far | |
| Capsid assembly modulators (CAMs) | ABI-H0731 (Vebicorvir), JNJ-56136379 (Bersacapavir), RO7049389, GLS4, BAY41-4109, NVR-3-778 | NCT04820686 (ABI combos), NCT04667104 (JNJ combos) | Phase I–IIb/some combo studies | HBsAg/HBV DNA reduction at Wk 24/48; relapse issues | Projected ~$50–100/day | Medium (Phase II; combo trials progressing) |
| Apoptosis/cIAP inhibitors | Birinapant, ABT-869, etc. | Early exploratory | Phase I | Safety; exploratory antiviral endpoints; enhances efficacy of ETV | Projected ~$50–200/day | Far (early) |
| RNA interference (RNAi) | JNJ-3989 (JNJ-73763989), AB-729 (Imdusiran) | NCT04980482 | Phase II | HBsAg mean log decline at Wk 12–24, safety. Strong HBsAg reduction when added to standard of care | Projected siRNA pricing ~$500–1000+/month | Medium (promising durability signals in Phase II) |
| GSK3228836 (Bepirovirsen) | B-Clear Ph2b (NCT04449029) | Phase II → III initiated | HBsAg reduction/loss; FDA fast track | Projected ~$1000–2000+/month | Medium → Near (Phase III in progress) | |
| Viral entry inhibitors | Bulevirtide (Hepcludex ®) | EMA ***** HDV trials | Approved (HDV)/HBV investigational | HDV RNA decline, ALT normalization | ~$46k/year list price | Near (EU approval in 2020; FDA pending) |
| Monoclonal antibodies | Anti-HBs mAbs | Multiple Phase I-II | Phase I–II | Safety; HBsAg neutralization/decline. Studies in combo for finite therapy | Projected biologic pricing: Hundreds–thousands per dose | Far → Medium |
| Cyclophilin inhibitors | Cyclosporine | Early repurposing studies | Early repurposing studies | Safety, viral load endpoints in small cohorts; mixed data | Generic cyclosporine inexpensive, not priced as antiviral. | Far |
| CRV431 | Phase I registry | Phase I | Safety; viral load endpoints; pharmacokinetics | Projected pricing ~$50-200/day | Far | |
| HBsAg release inhibitors (NAPs) | REP-2139 | Phase II combo | Phase II | HBsAg decline/loss; anti-HBs seroconversion. Promising combo with PegIFN2α | Projected ~$500–1000+/month (complex biologic/nucleic acid therapy) | Medium (small promising trials, safety/logistics considered) |
| FXR agonists | Vonafexor | Phase II | Phase II | HBV DNA/HBsAg change; more effective in HBeAg+ | Projected small molecule pricing~$300–600/month | Medium |
| Innate immune activators | TLR agonists (GS-9620-Vesatolimod, Selgantolimod, RO702053, JNJ4964) | Various NCTs | Phase I–II | Safety; immune activation; HBsAg/HBV DNA changes. Long term sustained efficacy is not proven | Projected ~$200–500/dose | Medium → Far (modest single-agent efficacy) |
| RIG-I agonist (Inarigivir) | Early NCTs | Early/development holds | Safety; HBsAg/HBV DNA endpoints; hepatotoxicity issues | Projected small-molecule pricing ~$50–200/day | Far/uncertain | |
| Adaptive immunity activators | PD-1 inhibitor (Nivolumab) | Small NCTs | Phase I | Safety (hepatic flares), immune response markers | Oncology biologic pricing $10k–20k/dose, HBV use experimental | Far/experimental |
| IFN-λ (pegylated) | Phase II | Phase II | HBsAg decline/loss, ALT normalization; better tolerated than PegIFNα2a | Projected PegIFN pricing ~$1000–3000/course | Medium | |
| Therapeutic vaccines | GS-4774, ABX-203 (HeberNasvac), BRII-179, TG1050, VTP-300, TherVacB | Multiple/Representative NCTs per vaccine | Phase I–II (some regional Phase II/III for HeberNasvac) | Safety; immune biomarker responses; HBsAg changes/conversion; modest single-agent efficacy, combination approaches favored | Projected vaccine program pricing $50–1000+/course | Far → Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ertugrul, H.; Ekiz, E.; Islak Mutcali, S.; Tahan, V.; Daglilar, E. Chronic Hepatitis B: Current Management and Future Directions. Diseases 2025, 13, 311. https://doi.org/10.3390/diseases13100311
Ertugrul H, Ekiz E, Islak Mutcali S, Tahan V, Daglilar E. Chronic Hepatitis B: Current Management and Future Directions. Diseases. 2025; 13(10):311. https://doi.org/10.3390/diseases13100311
Chicago/Turabian StyleErtugrul, Hamza, Esra Ekiz, Sibel Islak Mutcali, Veysel Tahan, and Ebubekir Daglilar. 2025. "Chronic Hepatitis B: Current Management and Future Directions" Diseases 13, no. 10: 311. https://doi.org/10.3390/diseases13100311
APA StyleErtugrul, H., Ekiz, E., Islak Mutcali, S., Tahan, V., & Daglilar, E. (2025). Chronic Hepatitis B: Current Management and Future Directions. Diseases, 13(10), 311. https://doi.org/10.3390/diseases13100311







