Abstract
Background and aim: Candida infection is a significant cause of morbidity and mortality in neonatal intensive care units (NICU) globally. We aimed to conduct a systematic review to investigate the prevalence of candida among causative organisms of neonatal sepsis and identify the distribution of candida species infecting Saudi neonates. Methods: We comprehensively searched Web of Science, Scopus, PubMed, and Cochrane Library from their inception till November 2023. After screening titles, abstracts, and full texts, we ultimately included 21 eligible studies. The designs of the included studies were randomized clinical trials, cohorts, case–control, and case reports; the methodological quality was appraised using the Cochrane risk of bias assessment tool, NIH tool for observational studies, and Murad tool for assessing case reports. Results: Our systematic review and meta-analysis pooled data reported in 21 studies in the Saudi populations, which provided data on different types of candidal infections in 2346 neonates. The pooled data of ten retrospective studies enrolling 1823 neonates revealed that candida species resembled 4.2% of the causative organisms of neonatal sepsis among Saudi neonates (95%CI [2.5%; 5.9%], p = 0.000). Additionally, out of a total of 402 candida species that were identified among the included studies, C. albicans prevailed mostly among Saudi neonates, followed by C. parapsilosis, NS candida, and C. tropicalis (50.25%, 21.40%, 12.44%, and 9.45%, respectively). Conclusions: We found that candida species prevailed in 4.2% of 1823 cases of neonatal sepsis; the most common candida species was C. albicans. We could not pool data regarding risk factors or susceptibility of candida species to different treatment modalities due to insufficient data, requiring future large-scale, high-quality studies to be conducted.
1. Introduction
Candida infection is a significant cause of morbidity and mortality in neonatal intensive care units (NICU) globally [1,2]. Vulnerable premature and low birth weight infants are at exceptionally high risk, with reported candidiasis incidence rates ranging from 7 to 20% in developing regions [3,4,5]. Candida commonly colonizes the skin, mucosal surfaces like the oral cavities, gastrointestinal tracts, and vagina [1,6]. However, certain situations like reduced immune response, microflora disruption, and exposure to medical devices allow Candida overgrowth and potential dissemination [7,8]. Invasive candidiasis is defined as candida infections involving normally sterile blood, tissues, bones, and organs [1], while non-invasive, superficial Candida infections may present as oral thrush or diaper dermatitis [9,10]. Beyond mortality threats, both invasive and superficial neonatal candidiasis correlate to worsened neurodevelopment among survivors [11].
Globally, candida remains the third most prevalent cause of neonatal late-onset sepsis, behind Staphylococcus and Klebsiella bacteria [11,12]. In developing regions, reported incidence rates of combined invasive and superficial candidiasis infections in neonatal intensive care units range widely from around 3% to over 25% [2,4,13]. Premature rupture of membranes, very low birth weight, use of broad-spectrum antibiotics and steroids, fungal colonization, surgery, endotracheal intubation, underlying gastrointestinal disorders, and central vascular access heighten vulnerability [14]. Despite antifungal treatment, attributable mortality remains high between 10 and 40% [15]. Beyond death, neurodevelopment impairment among survivors presents an equally alarming longer-term burden [16].
Saudi Arabia is a critical focal area for tackling neonatal candidiasis, given the estimated higher national incidence rates than in Western Europe and North America [17]. Local single-center reports suggest between 5 and 20% of neonatal sepsis cases show confirmed Candida infections depending on geographical area [18,19]. However, substantial gaps exist regarding the large-scale epidemiological synthesis of neonatal candidiasis prevalence across Saudi Arabia using consolidated national data.
Therefore, this systematic review and meta-analysis aim to thoroughly investigate the existing literature on neonatal candidiasis in Saudi Arabia. We aim to assess the overall prevalence of candida among other causative organisms of neonatal sepsis, identify the common candida species infecting neonates, and identify primary risk factors, standard treatment regimens, and attributable mortality rate on a national level.
2. Method
Our systematic review and meta-analysis (SRMA) was crafted following the most recent Cochrane Handbook guidelines [20] and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [21].
3. Literature Search
We conducted a comprehensive search of databases including Scopus, Web Of Science, PubMed, Google Scholar, and the Cochrane Library from their inception until November 2023 using the following keywords: ((((Neonates OR Neonate OR neonatal OR infant OR fetal OR newborn OR Premature OR Preterm OR Fetal OR postmature OR dysmature OR dysmaturity OR (small for gestational age) OR (low birth weight) OR LBW OR (intrauterine growth restriction) OR IUGR)) AND (candida OR candidiasis OR candida OR candidemia OR monilia OR monilial OR moniliasis OR thrush)) AND (Saudi OR “Saudi Arabia” OR “Kingdom of Saudi Arabia” OR KSA OR SA OR Riyadh OR Medina OR Mekka)). The search results were compiled using EndNote software (Version 20.2.1), duplicates were removed, and the remaining studies were transferred to an Excel sheet for further screening.
4. Inclusion and Exclusion Criteria
We included any English-language study in our SRMA that met the following criteria: population—neonates from Saudi Arabia aged less than one month; exposure—any superficial or deep candidal infection; control—not specified; outcome—any safety and efficacy outcomes; study designs—randomized clinical trials (RCTs), cohorts, case–control, case series, or case reports. Studies were excluded if they defined their neonatal population with an age range that exceeded one month, combined neonates and infants into one group, studied a mixed population of Saudi and non-Saudi subjects, were not written in English, or were animal studies.
5. Data Extraction
We collected data about study ID (last name of first author–publication year), title, study design, initiation date, termination date, study duration, hospital name, Saudi city, primary neonatal pathology/outcome and its definition by the authors, events of candidal infection, total study population, sex, candida species, birth weight, mode of delivery, and conclusion.
6. Assessment of Risk of Bias
Our SRMA incorporated RCTs, cohorts, cross-sectional studies, and case reports. The RCTs were evaluated methodologically using the first version of the Cochrane Risk of Bias (ROB) tool [22]. This tool comprises seven domains: selection, performance, detection, attrition, and reporting biases. Each domain was assessed as having a high, low, or unclear risk of bias. The methodological quality of the cohorts and cross-sectional studies was assessed using the NIH tool [23], which consists of 14 questions, each scored as zero, 0.5, or one. The overall study quality was classified as poor, fair, or good based on the total score (0–7 points, 7.5–10.5 points, or 11–14 points, respectively). Case reports were evaluated using the Murad et al. quality appraisal tool [24], which includes four main domains of selection, ascertainment, causality, and reporting, investigated through eight questions. Each study was assigned a score of eight, denoting it as poor, fair, or good quality.
7. Data Synthesis
We analyzed the available categorical outcomes as pooled proportions with 95% CIs; the random effect model was applied. We investigated the statistical heterogeneity between studies using the I2 statistics Chi-squared test, with p < 0.1 considered heterogeneous and I2 ≥ 50% suggestive of high heterogeneity. We conducted statistical analyses using Comprehensive Meta-Analysis Software (Version 3) (CMA, Englewood, NJ, USA).
8. Result
8.1. Literature Review
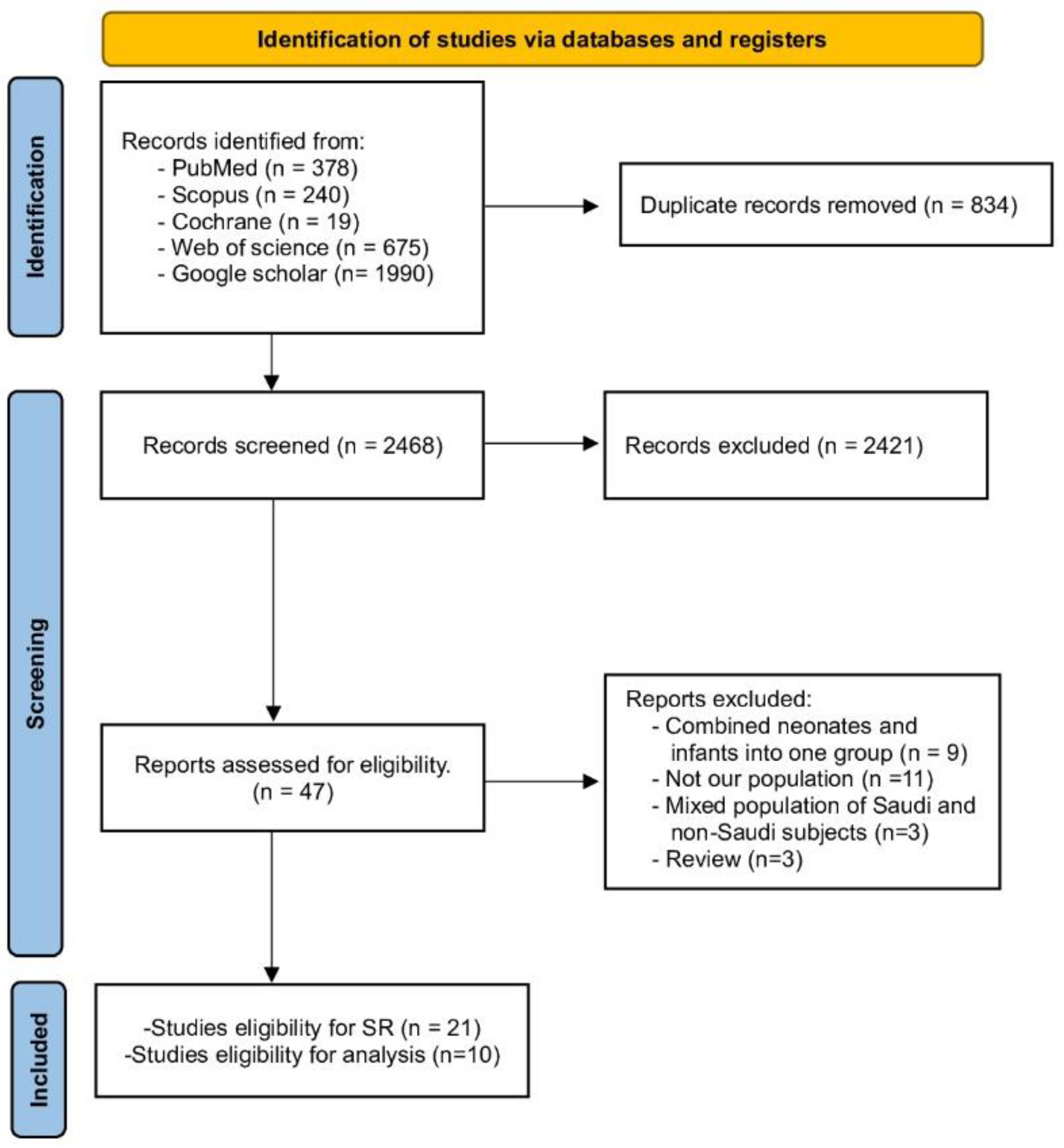
A thorough search was conducted across various databases, including WOS, PubMed, Scopus, Cochrane Library, and Google Scholar. This search yielded 3302 records in total. After removing duplicates, 2468 studies remained. These were screened based on their title and abstracts. Of these, 47 studies were further scrutinized by examining their full texts for relevance. Ultimately, 21 studies were selected for our Systematic Review. Out of them, only ten studies were included in the analysis. Further details can be found in Figure 1 (PRISMA).
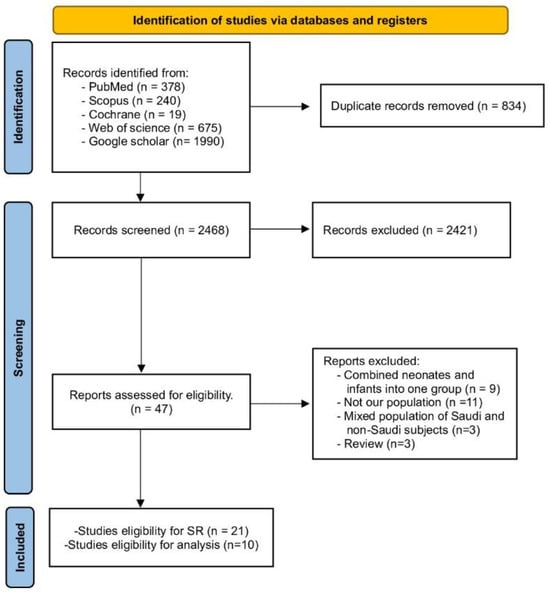
Figure 1.
PRISMA flow chart.
8.2. Characteristics of the Studies
This SRMA incorporated data from 21 studies [18,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] conducted in Saudi Arabia. These studies, which spanned various cities, including Aseer, Jeddah, Medina, Qatif, Tabuk, and Taif, provided data on different types of candidal infections in 2346 neonates. The majority of these studies, 14 in total, were carried out in the city of Riyadh. The studies had an average duration of 3.8 years, ranging from 1 year to 7.25 years. Approximately 50% of the population studied were male neonates (Figure 2). Additional details regarding the characteristics of the eligible studies and populations are presented in Table 1.
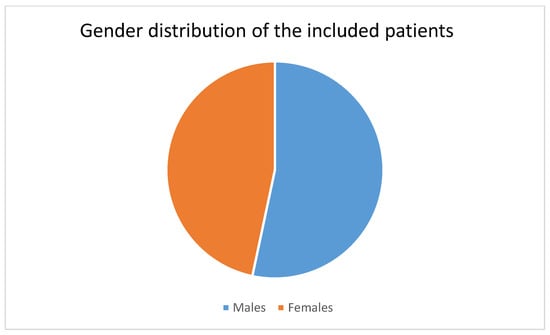
Figure 2.
Distribution of males and females in the included patients.

Table 1.
Summary and baseline characteristics of the included studies.
8.3. Assessment of Studies Quality
This SRMA included two Randomized Controlled Trials (RCTs) [26,29], 15 cohort studies [18,25,27,28,30,31,32,35,36,37,38,39,40,41,42], one case–control study [43], and three case reports [33,34,44]. The Murad tool assessed the case reports, which indicated either poor [34,44] or fair methodological quality [33]. The RCTs were evaluated to have an unclear risk of bias. The 15 cohort studies and one case-control study were judged to be fair or good quality. Supplementary Tables S1–S3 provide more details.
8.4. Prevalence of Candida among Causative Organisms of Neonatal Sepsis
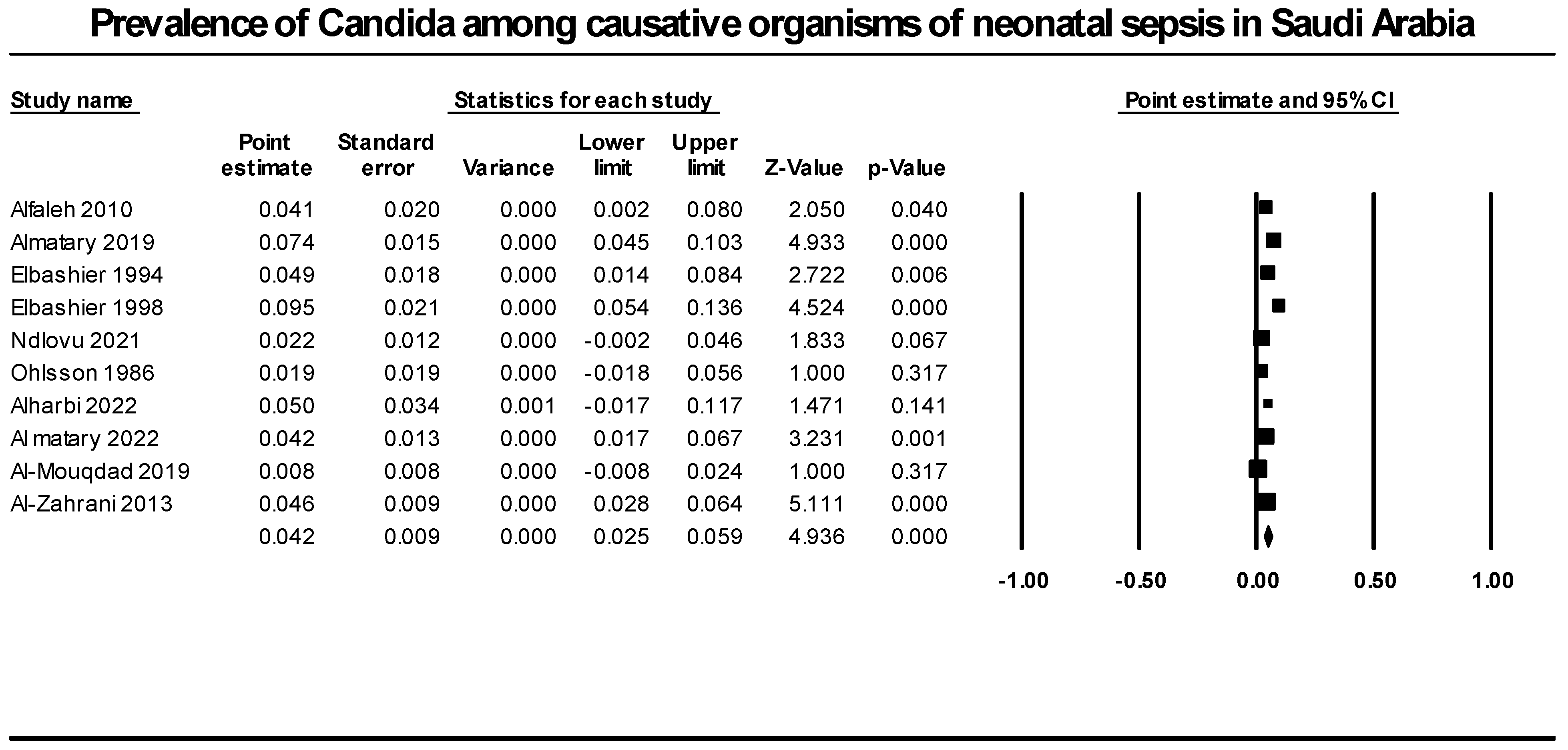
We synthesized data from ten retrospective studies that included 1823 neonatal infection cases to determine the prevalence of candida as a cause of neonatal sepsis. Our analysis revealed that candida species prevalence accounted for 4.2% of all neonatal sepsis causative agents (95% CI [2.5%; 5.9%], p = 0.000). We detected significant heterogeneity that could not be resolved (p = 0.0001, I2 = 72.1%) (Figure 3).
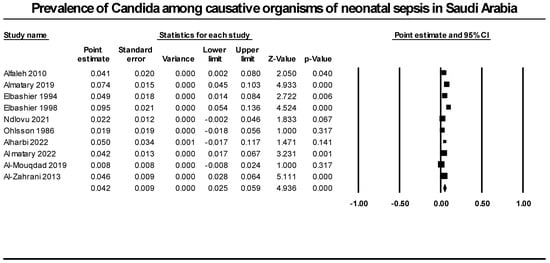
Figure 3.
Prevalence of candida among causative organisms of neonatal sepsis in Saudi Arabia. Included studies (Alfaleh 2010 [37], Almatary 2019 [19], Elbashier 1994 [30], Elbashier 1998 [31], Ndlovu 2021 [28], Ohlsson 1986 [29], Alharbi 2022 [18], Al matary 2022 [40], Al-Mouqdad 2019 [39], Al-Zahrani 2013 [37]).
8.5. Distribution of Candida Species among Saudi Neonates in Different Cities
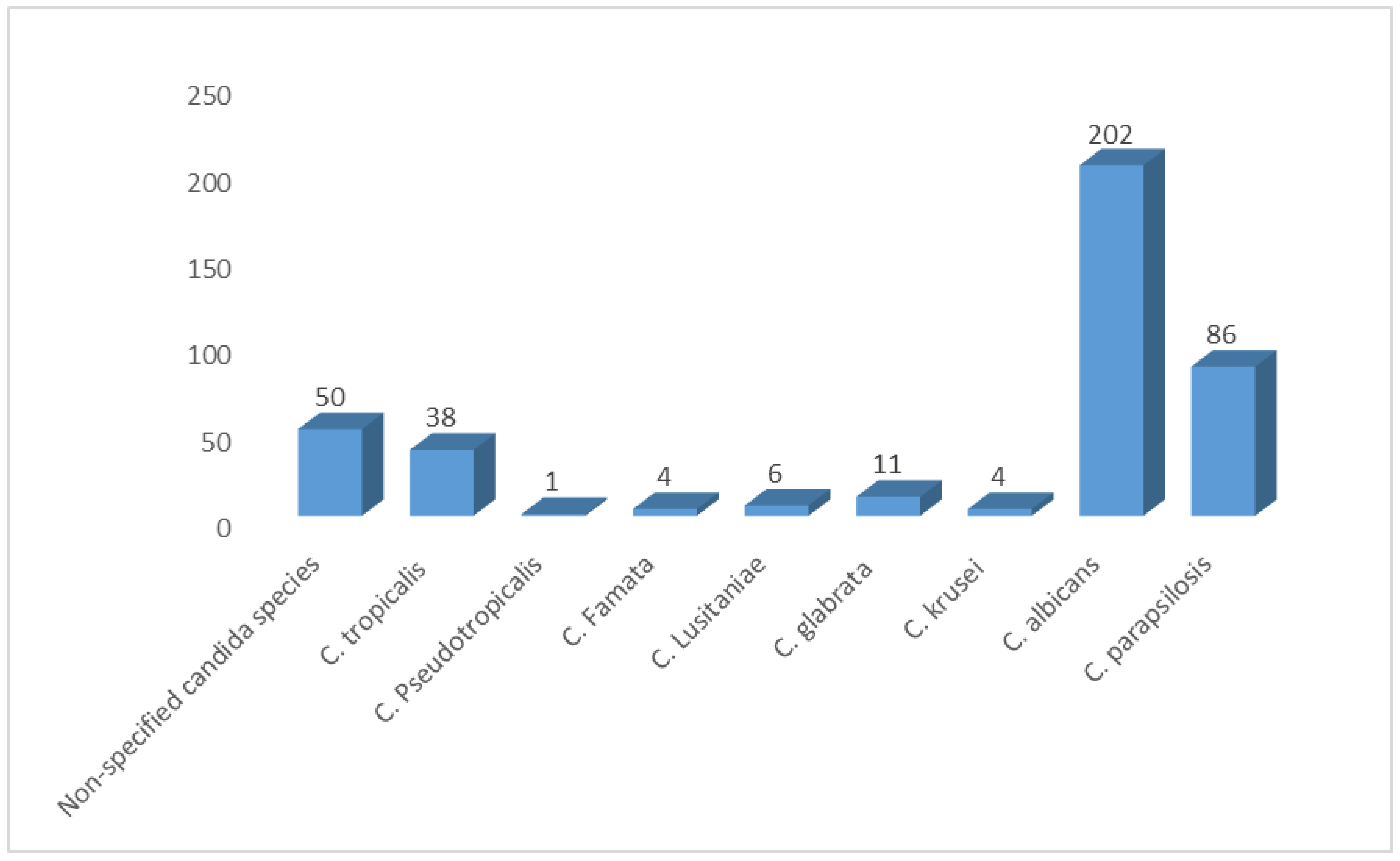
We pooled data from 21 studies and identified 402 yeasts isolated from neonates. Our SRMA showed that C. albicans was the most prevalent Candida species, representing 50.25% of all isolates. C. parapsilosis and C. tropicalis followed, representing 21.40% and 9.45%, respectively. Non-specified candida species (NS candida) represented 12.44% of the identified isolates. Moreover, we found a low prevalence of some species, such as C. glabrata, C. lusitaniae, C. krusei, C. famata, and C. pseudotropicalis (2.73%, 1.5%, 0.99%, 0.99%, 0.25%, respectively) (Figure 4).
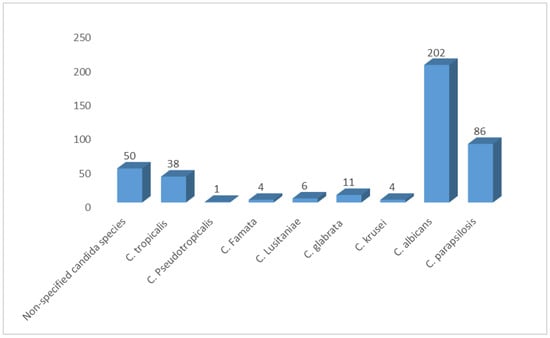
Figure 4.
Distribution of Candida species among Saudi neonates in Saudi Arabia.
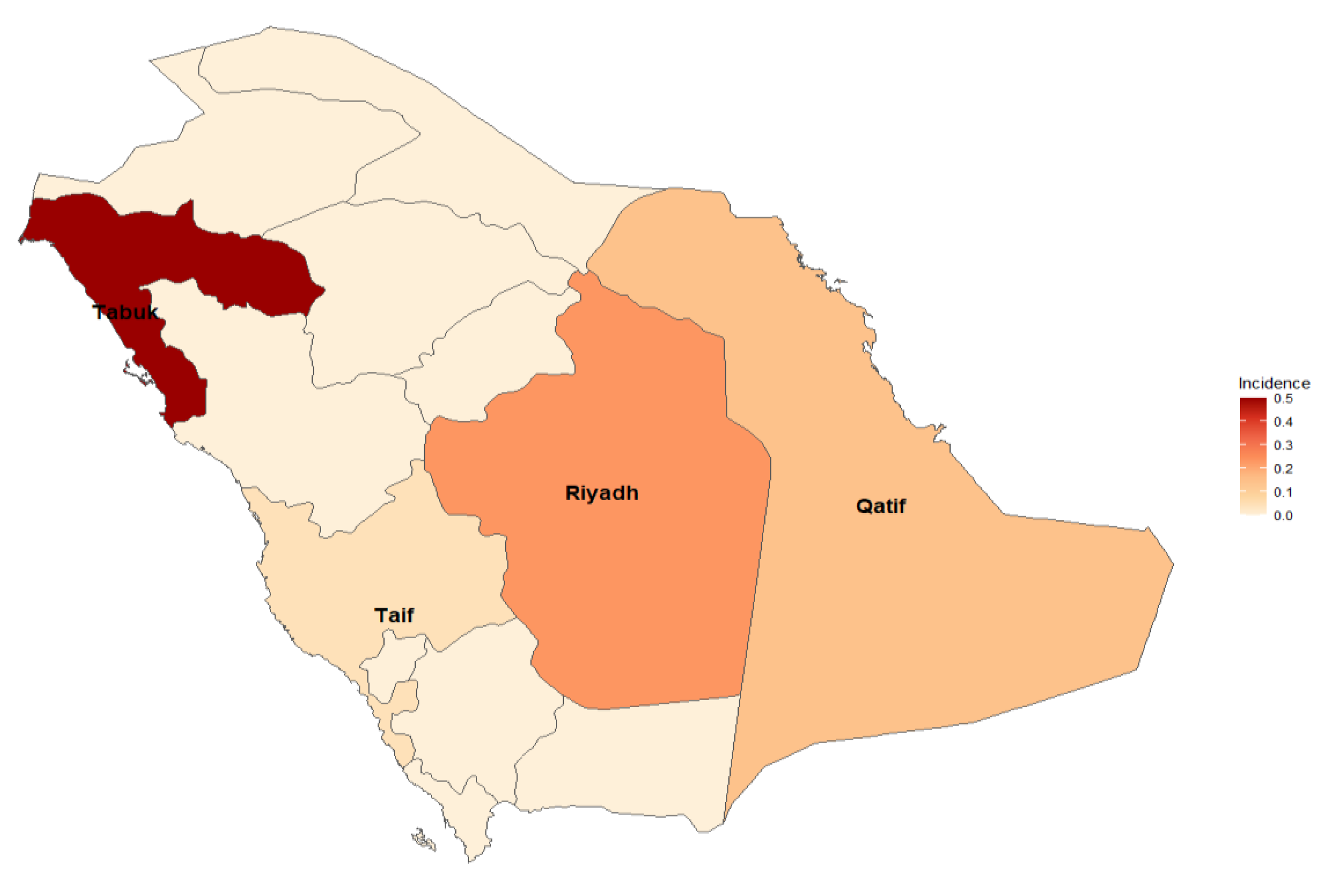
Nevertheless, neonatal Candida infections were observed in various cities. Notable findings include Qatif, where approximately 7.58% of neonates were affected, and Riyadh, with an incidence of approximately 4.66% of neonates. Tabuk reported a lower incidence of approximately 2.17, while Taif had a slightly higher incidence of approximately 4.55%. These findings emphasize the importance of monitoring and managing Candida infections in neonates across different regions (Figure 5).
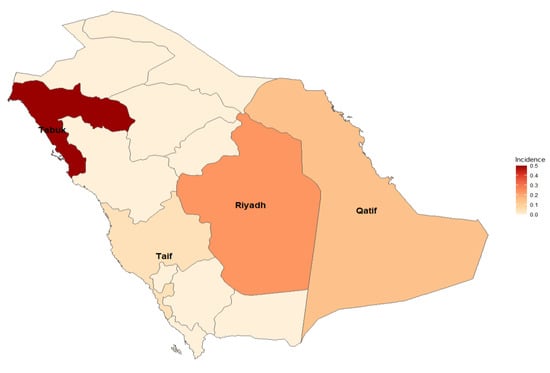
Figure 5.
Different regions in Saudi Arabia with higher incidences of Candida infections.
8.6. Invasive Candidiasis
Four studies reported data on 245 Saudi neonates from Aseer, Medina, and Riyadh with invasive candidiasis. Al-Jasser and colleagues investigated the distribution of candida species across different age groups and found 17 cases of invasive candidiasis in NICU over six years. C. albicans (13 cases) was the predominant candida species in the study population [41]. Another study conducted in Aseer Central Hospital compared the safety and efficacy of amphotericin B and caspofungin for treating 32 cases of neonatal invasive candidiasis, of which C. albicans was isolated from 24 cases. The results indicated that caspofungin was superior, achieving a favorable response in 86.7% of patients, compared to only 41.7% in the amphotericin B group (p = 0.04). Moreover, the caspofungin group had a significantly lower incidence of adverse events than the amphotericin B group [26]. Almoosa et al. identified 66 cases of invasive candidiasis among King Fahad Medical City neonates over five years. The most common candida species were C. albicans, C. tropicalis, C. parapsiolosis, C. lusitaniae, and C. famata (29 cases, 11 cases, 11 cases, four cases, three cases, respectively) [35]. Eisi et al. conducted a retrospective study and found 130 cases of neonatal candidiasis in the Madinah Maternity and Children Hospital NICU over seven years. The study classified neonates based on deep tissue involvement. Deep tissue invasion was found in 16 cases. It was significantly associated with a higher risk of cerebral palsy, heart failure, and more extended hospital stay than the non-deep tissue invasion group [32].
8.7. Other Forms of Candidal Infection/Involvement in the Saudi Neonates
The prevalence and impact of candidal infection in Saudi neonates is a topic of interest for several studies. Mersal et al. compared nystatin and fluconazole as prophylactic agents for candidal colonization in preterm or low birth weight infants. They randomly assigned 57 neonates to either group and found no difference in safety outcomes or invasive candidal infection rates. Nystatin was also more cost-effective than fluconazole [29].
Al-Hussaini et al. examined the candida species and their antifungal susceptibility in 100 neonates. They reported a colonization rate of 51%, with higher rates in preterm neonates. The most common sites of colonization were the perianal area and oral cavity; the most common species was C. albicans (58.8%). The isolated candida species showed moderate sensitivity to fluconazole, itraconazole, and ketoconazole (68.6%, 80%, and 64.7%, respectively) while showing low sensitivity to amphotericin B (33%) [36].
Other studies explored the role of candida in neonatal conjunctivitis and ventilator-associated pneumonia (VAP). Faraz found that candida accounted for 3.37% of neonatal conjunctivitis cases [25], while Afify et al. found that candida accounted for 9% of neonatal VAP cases. They suggested that candida infection was a significant risk factor for VAP in neonates [42].
Some case reports illustrated the challenges of diagnosing and treating different candidal infections in Saudi neonates. Abuhajj and his team highlighted a case of congenital cutaneous candidiasis, which can often be mistaken for benign or bacterial eruptions. They described a neonate with a congenital maculopapular eruption that spanned the torso, diaper area, extremities, palms, and soles. The neonate exhibited leukocytosis (>30 k) and bilateral lung opacifications. Initial treatment with empirical antibiotics exacerbated the condition. However, a significant improvement was observed just three days after initiating intravenous amphotericin B and topical miconazole, pending the results of a skin-scraping culture. After two weeks of treatment, the neonate’s skin returned to normal, and the child was discharged in stable condition [43]. Azhar et al. reported a case involving a female neonate who was admitted to the hospital with symptoms including fever (38.1 °C), leukocytosis, tachycardia (168 bpm), and hypotension. Initially treated as a septic shock with IV fluids, empirical ampicillin, and cefotaxime, subsequent chest X-rays revealed a sizeable cardiac shadow. This was attributed to vegetation in both the left and right ventricles and a thickened pericardium, as detected by echocardiography. Following the administration of intravenous fluconazole and amphotericin B, the size of the vegetation decreased until it completely disappeared, as confirmed by an echocardiography after 40 days. The patient was discharged following clinical improvements [33].
Al-Arishi documented a case of a male neonate with a severely dysplastic right kidney and a moderately hydronephrotic left kidney. A left cutaneous pyelostomy was performed on the fourth day of life due to declining renal function. Urine analysis from the pyelostomy catheter revealed numerous yeast cells, and a complete blood count and blood samples confirmed the presence of candidemia. Treatment with Ambisome rendered the blood completely sterile after just five days of initiation [34].
9. Discussion
Our study included 21 studies, encompassing a total of 2346 neonates. The consolidated data from ten retrospective studies, which enrolled 1823 neonates, indicated that candida species accounted for 4.2% of the causative organisms of neonatal sepsis among Saudi neonates. Out of the 402 candida species identified in the included studies, C. albicans was the most prevalent among Saudi neonates, followed by C. parapsilosis, NS candida, and C. tropicalis. Furthermore, a literature review revealed that four studies reported 245 neonates diagnosed with invasive candidiasis, with candida accounting for 3.37% and 9% of organisms causing neonatal conjunctivitis and Ventilator-Associated Pneumonia (VAP), respectively.
A previous multicenter study conducted in eight Arab countries over 19 years (1990–2009) enrolled data on sepsis from 2308 neonates [44]. However, they found inadequate data regarding the prevalence of candida among other microbiological causes of sepsis. They concluded that Candida species had been emerging in multiple Arab countries, including Egypt, Bahrain, UAE, and Kuwait; a study conducted in Kuwait stated that candida represented 14% of the causes of neonatal sepsis [45]. Additionally, a study by Pillay and colleagues, conducted over three years and enrolling 681 cases of neonatal sepsis, reported results consistent with ours, with fungal isolates accounting for 4.5% of cases of neonatal sepsis and the majority of cases being bacterial (over 95%) [46].
We found that C. albicans, C. parapsilosis, and C. tropicalis were the most common yeast isolated from 402 neonates (50.25%, 21.40%, and 9.45%, respectively). Moreover, we found a low prevalence of some species such as C. glabrata, C. lusitaniae, C. krusei, C. famata, and C. pseudotropicalis (2.73%, 1.5%, 0.99%, 0.99%, 0.25%, respectively). Non-specified candida species (NS candida) represented 12.44% of the identified isolates. This was consistent with what was reported by an 11-year, multinational, retrospective study with participants from 23 different European countries. They found that among 422 neonates with candidal infections, C. albicans, C. parapsilosis, and C. tropicalis were the most common species, prevailing in 60.1%, 27.7%, and 2.8%, respectively [47]. Another study by Cook enrolled 127 neonates from eight countries and found that the most common isolated candida species were C. albicans, C.parapsilosis, and C. auris (35%, 30%, and 14%, respectively) [48].
However, a three-year study conducted in South Africa found that C. parapsilosis was the most prevalent, followed by C. albicans and NS candida (45.2%, 29%, and 25.8%, respectively) [46]. These discrepancies could be attributed to the different demographics, narrower time scale of the study, and a small sample of 31 candida species. Our study pooled data over twenty years and enrolled over 400 candida species among neonates.
We could not pool and analyze data regarding possible risk factors for neonatal candidiasis or susceptibility of candida species to different treatment modalities. However, some included studies provided data regarding significant risk factors for different candidal infections. Eisi and colleagues found that gestational age of less than 32 weeks and candidal infection of central venous catheters were significantly associated with deep candidal infections [32]. Additionally, Afify et al. found that the nosocomial infection by candida was a risk factor for developing ventilator-associated pneumonia (VAP), in addition to other factors, including prolonged NICU admission, invasive maneuvers, hypothermia, high CRP, and hypoalbuminemia [42]. The literature review provided additional insights about other possible risk factors for candidal infection in neonates. A multicenter study by Xia and colleagues found that over 90% of neonates diagnosed with invasive candidiasis were administered an extensive course of broad-spectrum antibiotics.
Moreover, about 70% and 60% of the neonates were either low birth weight or had central venous catheters, respectively [49]. Manzoni et al. confirmed that a central venous catheter posed a significant risk of developing candidal infection [50]. The influence of low birth weight, central venous catheter, and prior extensive antibiotic course and their significant relation to candidal infection in neonates was confirmed by Benjamin Jr. [51].
Regarding treatment modalities for neonatal candidiasis, Mersal et al. compared nystatin and fluconazole as prophylactic agents for candidal colonization in preterm or low birth weight infants. They randomly allocated 57 neonates to either group and found no difference in safety outcomes or rates of invasive candidal infection. Nystatin also proved to be more cost-effective than fluconazole [29].
A study by HSU analyzed 342 episodes of candidiasis in neonates and children and reported that 97.1% received antifungal treatment, but 2.9% were untreated due to patient demise before proceeding to treatment. Antifungal therapy commenced, on average, 1.81 days after obtaining diagnostic cultures, notably delayed in neonates compared to children (2.1 vs. 1.7 days). The mean duration of antifungal therapy was 18.5 days. Among the 332 treated episodes, 45.5% experienced regimen modifications, primarily due to poor initial response (66.9%), suspected resistance (23.8%), or undocumented reasons (9.3%). Initial prescriptions favored fluconazole (62.3%), followed by amphotericin B (24.7%) and caspofungin (4.5%), while final regimens were fluconazole/voriconazole (39.5%), amphotericin B (29.2%) and echinocandin (28.9%). Catheter removal within three days occurred in only 32.2% of cases. Neonates faced prolonged fungemia, higher treatment failure rates (31.0% vs. 19.7%), and increased sepsis-related mortality (28.3% vs. 17.5%) and in-hospital mortality (42.7% vs. 25.4%) post-invasive candidiasis compared to children. Susceptibility studies on 295 isolates revealed a 14.6% fluconazole-resistant or susceptible-dose-dependent candida rate, with no significant differences between neonates and non-neonatal pediatric cases [52].
We conducted the first systematic review and meta-analysis (SRMA) to evaluate the incidence of candidal infections in neonates from Saudi Arabia. We amalgamated data from 2346 neonates across 21 studies. These studies were carried out in diverse Saudi cities such as Riyadh, Jeddah, Medina, Qatif, Tabuk, and Taif, bolsters our findings’ applicability to the entire Saudi population. We confined our data inclusion to neonates, which were either explicitly defined as less than one month old or as neonates without an age specification. We excluded any study that defined neonates as older than one month or included a mixed-age group. Nonetheless, our study had certain limitations. The data we pooled spanned a considerable period from 1989 to 2020, which could lead to inconsistencies concerning the diagnostic laboratory methods and identification of various candida species. Most of the included studies were conducted in Riyadh City, which might limit the applicability of our findings to the entire Saudi population. Our data included three case reports and several studies over two decades. Despite these limitations, we endeavored to compile all accessible evidence to provide a comprehensive overview of the prevalence of candidal infections among Saudi neonates. However, we could not pool and analyze data about potential risk factors for neonatal candidiasis or the susceptibility of different candida species to various treatment modalities. We recommend conducting comprehensive future studies to enhance our understanding of Candida species prevalence among neonates in Saudi Arabia. Additionally, exploring antifungal resistance in greater detail for the identified Candida species would be valuable.
10. Conclusions
In conclusion, our study evaluated the epidemiology of Candida species among neonates in Saudi Arabia. We found that candida species were present in 4.2% of 1823 neonatal sepsis cases, based on data from ten retrospective studies conducted between 1980 and 2020 in Aseer, Jeddah, Medina, Riyadh, Qatif, Tabuk, and Taif. Among the 402 candida isolates identified, C. albicans was the most common, followed by C. parapsilosis and C. tropicalis. Unfortunately, due to insufficient data, we could not pool information regarding risk factors or the susceptibility of different candida species to various treatment modalities. We advocate for future large-scale, high-quality studies to provide detailed insights for pediatricians and primary care physicians regarding neonatal candidal infections in Saudi neonates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diseases12070154/s1, Table S1: The Cochrane Collaboration’s tool for assessing risk of bias. Table S2: NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Table S3: Assessment Tool for Case Report Studies.
Author Contributions
Conceptualization, data curation, formal analysis, methodology, resources, software, validation, visualization, writing—original draft, writing—review, and editing were performed by A.M.; A.M. and M.A. were involved in investigation and methodology, contributing equally to these areas. M.A. was specifically responsible for general project administration, resources, and supervision, ensuring the project’s overall direction and management. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primer 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, G.; Lovero, G.; Giglio, O.; Barbuti, G.; Montagna, O.; Laforgia, N. Candidemia in the neonatal intensive care unit: A retrospective, observational survey and analysis of literature data. BioMed Res. Int. 2017, 2017, 7901763. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.G.; Benjamin, D.K. Neonatal candidiasis: Diagnosis, prevention, and treatment. J. Infect. 2014, 69, S19–S22. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.K.; Stoll, B.J.; Fanaroff, A.A.; McDonald, S.A.; Oh, W.; Higgins, R.D. Neonatal candidiasis among extremely low birth weight infants: Risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006, 117, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Chakrabarti, A. Strategies to reduce mortality in adult and neonatal candidemia in developing countries. J. Fungi 2017, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- R, A.N.; Rafiq, N.B. Candidiasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK560624/ (accessed on 17 December 2023).
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis—Opportunism versus pathogenicity: A review of the virulence traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Brizuela, M.; Raja, A. Oral Candidiasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK545282/ (accessed on 17 December 2023).
- Benitez Ojeda, A.B.; Mendez, M.D. Diaper Dermatitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK559067/ (accessed on 17 December 2023).
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD neonatal research network. Pediatrics 2002, 110, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Juyal, D.; Sharma, M.; Pal, S.; Rathaur, V.; Sharma, N. Emergence of non-albicans candida species in neonatal candidemia. N. Am. J. Med. Sci. 2013, 5, 541–545. [Google Scholar] [CrossRef]
- Kelly, M.S.; Benjamin, D.K.; Smith, P.B. The epidemiology and diagnosis of invasive candidiasis among premature infants. Clin. Perinatol. 2015, 42, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Gudlaugsson, O.; Gillespie, S.; Lee, K.; Berg, J.V.; Hu, J.; Messer, S.; Herwaldt, L.; Pfaller, M.; Diekema, D. Attributable mortality of nosocomial candidemia. Revisit. Clin. Infect. Dis. 2003, 37, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Adams-Chapman, I.; Bann, C.M.; Das, A.; Goldberg, R.N.; Stoll, B.J.; Walsh, M.C. Neurodevelopmental outcome of extremely low birth weight infants with candida infection. J. Pediatr. 2013, 967, 961–967.e3. [Google Scholar] [CrossRef]
- Osman, M.; Al Bikai, A.; Rafei, R.; Mallat, H.; Dabboussi, F.; Hamze, M. Update on invasive fungal infections in the Middle Eastern and North African region. Braz. J. Microbiol. 2020, 51, 1771–1789. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.S. Common Bacterial Isolates Associated with Neonatal Sepsis and Their Antimicrobial Profile: A Retrospective Study at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. Cureus 2022, 14, e21107. [Google Scholar] [CrossRef] [PubMed]
- Al-Matary, A.; Heena, H.; AlSarheed, A.S.; Ouda, W.; AlShahrani, D.A.; Wani, T.A. Characteristics of neonatal Sepsis at a tertiary care hospital in Saudi Arabia. J. Infect. Public Health 2019, 12, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions [Internet]. Available online: https://training.cochrane.org/handbook (accessed on 17 December 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 17 December 2023).
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid.-Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Faraz, A.; Farhan, M.; Ali, M. Microbial etiology of neonatal conjunctivitis in a general hospital in Saudi Arabia. Al-Shifa J. Ophthalmol. 2019, 15, 30. [Google Scholar]
- Wahab Mohamed, W.A.; Ismail, M. A Randomized, Double-Blind, Prospective Study of Caspofungin vs. Amphotericin B for the Treatment of Invasive Candidiasis in Newborn Infants. J. Trop. Pediatr. 2012, 58, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Bailey, T.; Takieddine, F. Changing Etiology and Outcome of Neonatal Septicemia in Riyadh, Saudi Arabia. Acta Paediatr. 1986, 75, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, B. Nosocomial Infections in Neonatal Intensive Care Unit of a Public Healthcare Facility in Saudi Arabia. 2021. Available online: https://uir.unisa.ac.za/handle/10500/27907 (accessed on 16 December 2023).
- Mersal, A.; Alzahrani, I.; Azzouz, M.; Alsubhi, A.; Alsawaigh, H.; Albshri, N.; Bajammal, M.; Avand, G.; Almahbosh, A. Oral nystatin versus intravenous fluconazole as neonatal antifungal prophylaxis: Non-inferiority trial. J. Clin. Neonatol. 2013, 2, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Elbashier, A.M.; Abusrair, H.; Owa, J.A. Aetiology of neonatal septicaemia in Qatif, Saudi Arabia. Early Child Dev. Care 1994, 98, 31–38. [Google Scholar] [CrossRef]
- Elbashier, A.M.; Malik, A.G.; Khot, A.P. Blood Stream Infections: Micro-Organisms, Risk Factors and Mortality Rate in Qatif Central Hospital. Ann. Saudi Med. 1998, 18, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Eisi, H.; Ibraheem, S.; Hisham, T.; Al-Harbi, A.; Saidy, K.; Ali, I.; Nour, I.; Nasef, N. Risk factors and outcomes of deep tissue Candida invasion in neonates with invasive candidiasis. Mycoses 2022, 65, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A. Successful management of fungal pericarditis and endocarditis in a neonate: A case report. J. Saudi Heart Assoc. 2012, 24, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Arishi, H.A.; Alaiyan, S.A.; Dj, M. Liposomalamphotericin B in neonates with invasive Candidiasis. Am. J. Perinatol. 1997, 14, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Almoosa, Z.; Ahmed, G.Y.; Omran, A.; AlSarheed, A.; Alturki, A.; Alaqeel, A.; Alshehri, M.; Alfawaz, T.; Alshahrani, D. Invasive Candidiasis in pediatric patients at King Fahad Medical City in Central Saudi Arabia: A 5-year retrospective study. Saudi Med. J. 2017, 38, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Alhussaini, M.S. Incidence of Candida species colonization in neonatal intensive care unit at Riyadh Hospital, Saudi Arabia. Med. J. Indones. 2016, 25, 171–181. [Google Scholar] [CrossRef]
- AlFaleh, K.M. Incidence of Late Onset Neonatal Sepsis in Very Low Birth Weight Infants in a Tertiary Hospital An ongoing challenge. Sultan Qaboos Univ. Med. J. 2010, 10, 227–230. [Google Scholar] [PubMed]
- Al-Zahrani, A.K.; Eed, E.M.; Alsulaimani, A.A.; Abbadi, S.H. Healthcare Associated Infection in the Neonatal Intensive Care Unit of King Abdl Aziz Specialist Hospital, Taif, KSA. Adv. Infect. Dis. 2013, 3, 300–305. [Google Scholar] [CrossRef][Green Version]
- Al-Mouqdad, M.M.; Egunsola, O.; Ali, S.; Asfour, S.S. A Neonatal Unit Experience with Empiric Antibiotics for Late-onset Neonatal Sepsis: A Retrospective Study. Pediatr. Qual. Saf. 2019, 4, e239. [Google Scholar] [CrossRef] [PubMed]
- Al-Matary, A.; Al Sulaiman, M.; Al-Otaiby, S.; Qaraqei, M.; Al-Matary, M. Association between the timing of antibiotics administration and outcome of neonatal sepsis. J. Infect. Public Health 2022, 15, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Al-Jasser, A.M. Distribution of Candida species among bloodstream isolates. Saudi Med. J. 2004, 25, 566–569. [Google Scholar] [PubMed]
- Afify, M.; AI-Zahrani, S.; Nouh, M.A. Risk factors for the development of ventilator—Associated pneumonia in critically-Ill neonates. Life Sci. J. 2012, 9, 302–307. [Google Scholar]
- Abuhajj, R.; Abdoun, M.; Syeda, B.; Abdulla, R.; Al Zahrani, A. Congenital Candidiasis in a Preterm Neonate: Raising Awareness of a Rare and Unpredictable Disease. Dr. Sulaiman Al Habib Med. J. 2021, 3, 144–146. [Google Scholar] [CrossRef]
- Tosson, A.M.S.; Speer, C.P. Microbial pathogens causative of neonatal sepsis in Arabic countries. J. Matern. Fetal Neonatal Med. 2011, 24, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, H.H.; Udo, E.E.; Rajaram, U. Neonatal septicemia in al-jahra hospital, kuwait: Etiologic agents and antibiotic sensitivity patterns. Med. Princ. Pract. 2001, 10, 145–150. [Google Scholar] [CrossRef]
- Pillay, D.; Naidoo, L.; Swe Swe-Han, K.; Mahabeer, Y. Neonatal sepsis in a tertiary unit in South Africa. BMC Infect. Dis. 2021, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Pana, Z.-D.; Oletto, A.; Lundin, R.; Castagnola, E.; Lehrnbecher, T.; Groll, A.H.; Roilides, E. Etiology and Outcome of Candidemia in Neonates and Children in Europe: An 11-year Multinational Retrospective Study. Pediatr. Infect. Dis. J. 2020, 39, 114–120. [Google Scholar] [CrossRef]
- Cook, A.; Ferreras-Antolin, L.; Adhisivam, B.; Ballot, D.; Berkley, J.A.; Bernaschi, P.; Carvalheiro, C.G.; Chaikittisuk, N.; Chen, Y.; Chibabhai, V.; et al. Neonatal invasive candidiasis in low- and middle-income countries: Data from the NeoOBS study. Med. Mycol. 2023, 61, myad010. [Google Scholar] [CrossRef]
- Xia, H.; Wu, H.; Xia, S.; Zhu, X.; Chen, C.; Qiu, G.; Zhou, W.; Shi, Y.; Ma, L.; Sun, J.; et al. Invasive Candidiasis in preterm neonates in China: A retrospective study from 11 NICUS during 2009–2011. Pediatr. Infect. Dis. J. 2014, 33, 106–109. [Google Scholar] [CrossRef]
- Manzoni, P.; Farina, D.; Leonessa, M.; d’Oulx, E.A.; Galletto, P.; Mostert, M.; Miniero, R.; Gomirato, G. Risk factors for progression to invasive fungal infection in preterm neonates with fungal colonization. Pediatrics 2006, 118, 2359–2364. [Google Scholar] [CrossRef]
- Benjamin, D.K.; Stoll, B.J.; Gantz, M.G.; Walsh, M.C.; Sánchez, P.J.; Das, A.; Shankaran, S.; Higgins, R.D.; Auten, K.J.; Miller, N.A.; et al. Neonatal Candidiasis: Epidemiology, Risk Factors, and Clinical Judgment. Pediatrics 2010, 126, e865–e873. [Google Scholar] [CrossRef]
- Hsu, J.-F.; Lai, M.-Y.; Lee, C.-W.; Chu, S.-M.; Wu, I.-H.; Huang, H.-R.; Lee, I.-T.; Chiang, M.-C.; Fu, R.-H.; Tsai, M.-H. Comparison of the incidence, clinical features and outcomes of invasive candidiasis in children and neonates. BMC Infect. Dis. 2018, 18, 194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).