STRILL: Phase I Trial Evaluating Stereotactic Body Radiotherapy (SBRT) Dose Escalation for Re-Irradiation of Inoperable Peripheral Lung Lesions
Abstract
1. Introduction
2. Material and Methods
- Inoperable primary non-small cell lung cancer or other metastatic primaries with lung metastases, already treated with radical dose RT;
- Peripheral lesion (>2 cm from the tracheo-bronchial tree);
- Inoperable local recurrence (defined as a tumor recurrence overlapping the 50% isodose field) confirmed by documented radiographic findings and/or pathological biopsies within the thoracic area;
- Patients had previously received curative intent RT of more than 50 Gy for conventionally fractionated RT or a biologically equivalent dose of more than 75 Gy for SBRT;
- No active distant metastasis or controlled distant metastasis at the time of re-irradiation;
- Eighteen years of age or older.
3. Results
3.1. Toxicity
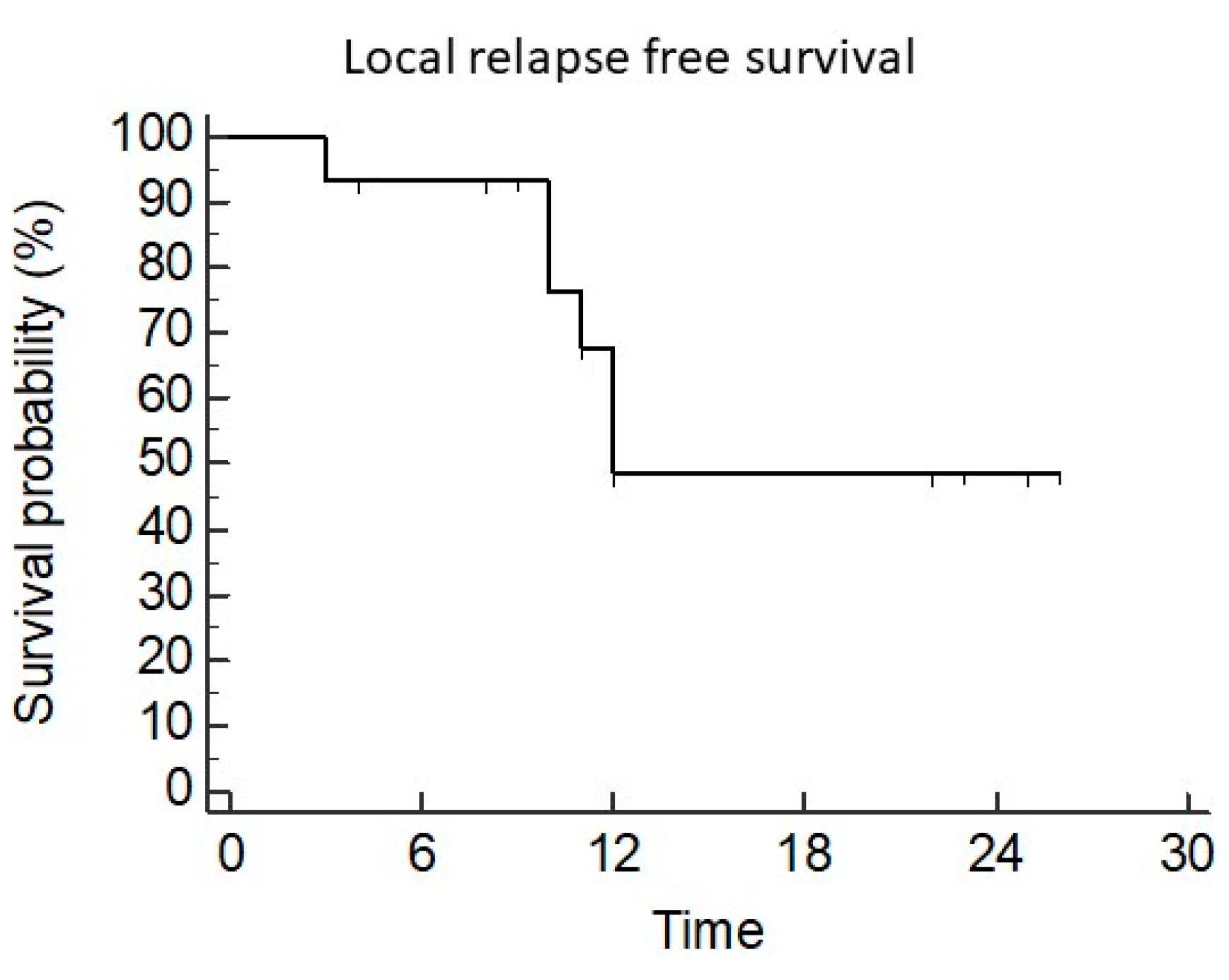
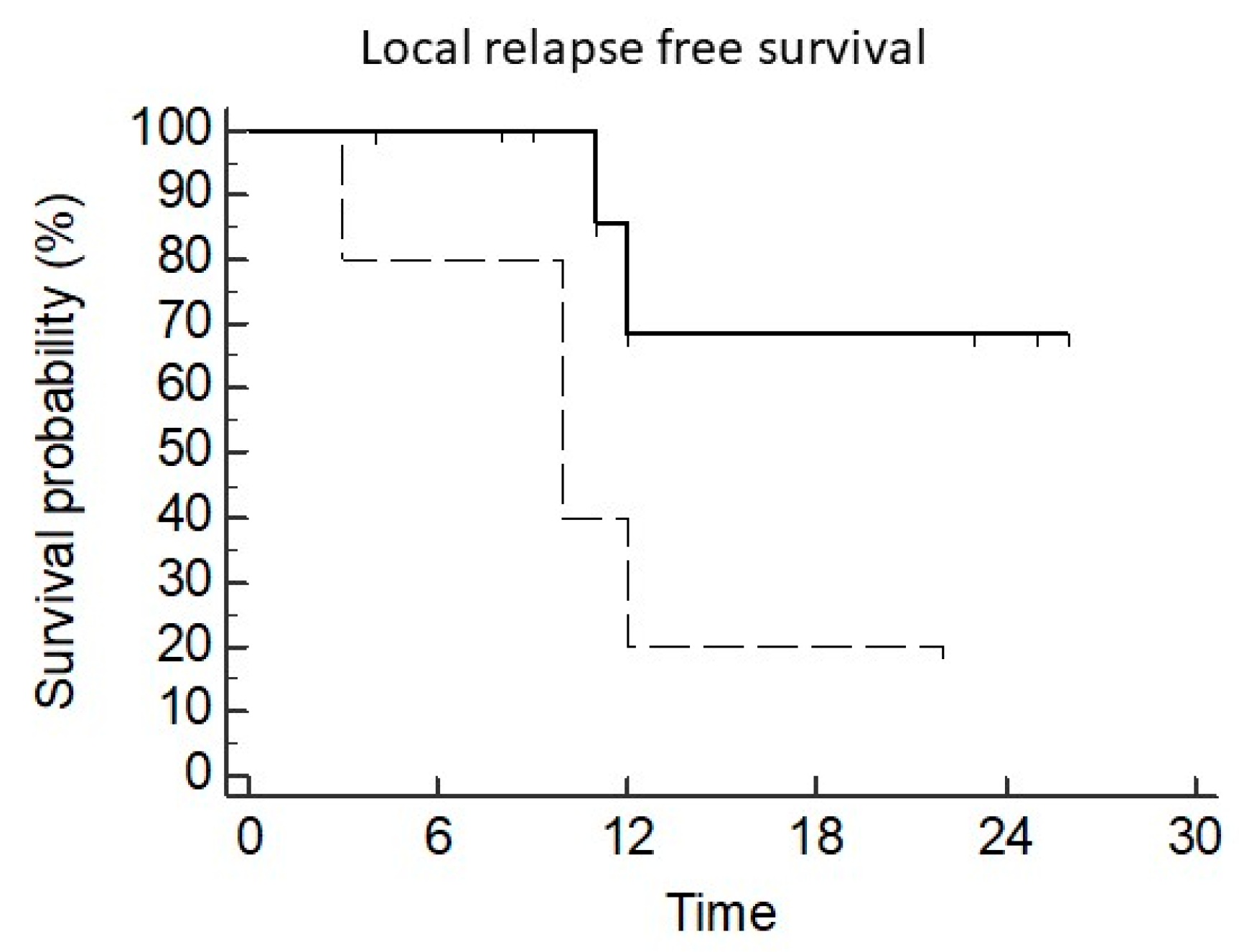
3.2. Local Control
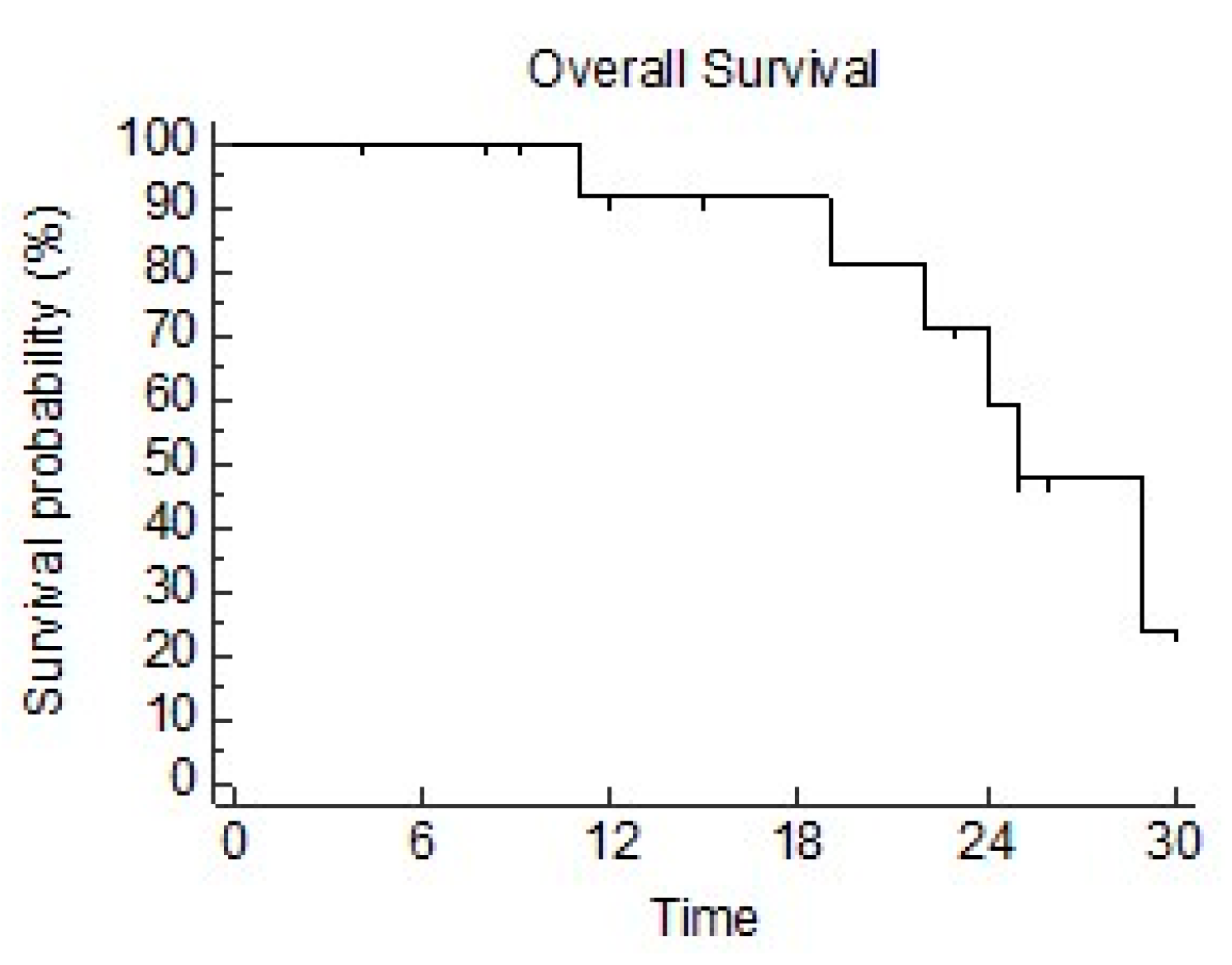
3.3. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamaji, M.; Chen, F.; Matsuo, Y.; Ueki, N.; Hiraoka, M.; Date, H. Treatment and prognosis of isolated local relapse after stereotactic body radiotherapy for clinical stage I non-small-cell lung cancer: Importance of salvage surgery. J. Thorac. Oncol. 2015, 10, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Antonoff, M.B.; Correa, A.M.; Sepesi, B.; Nguyen, Q.-N.; Walsh, G.L.; Swisher, S.G.; Vaporciyan, A.A.; Mehran, R.J.; Hofstetter, W.L.; Rice, D.C. Salvage pulmonary resection after stereotactic body radiotherapy: A feasible and safe option for local failure in selected patients. J. Thorac. Cardiovasc. Surg. 2017, 154, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-L.; Jiang, G.-L.; Qian, H.; Wang, L.-J.; Yang, H.-J.; Fu, X.-L.; Zhao, S. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: A prospective phase I-II clinical trial. Int. J. Radiat. Oncol. 2003, 57, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Fukuda, H.; Matsui, K.; Hirashima, T.; Hosono, M.; Takada, Y.; Inoue, Y. Non-small-cell lung cancer: Reirradiation for loco-regional relapse previously treated with radiation therapy. Int. J. Clin. Oncol. 2005, 10, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Balter, P.A.; Rebueno, N.; Sharp, H.J.; Liao, Z.; Komaki, R.; Chang, J.Y. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, J.M.; Kuremsky, J.G.; Blackstock, A.W.; Munley, M.T.; Kearns, W.T.; Hinson, W.H.; Lovato, J.F.; Miller, A.A.; Petty, W.J.; Urbanic, J.J. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother. Oncol. 2014, 110, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.W.; Videtic, G.M.; Djemil, T.; Stephans, K.L. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung, S.B.R.T. Int. J. Radiat. Oncol. 2014, 90, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Horne, Z.D.; Dohopolski, M.J.; Clump, D.A.; Burton, S.A.; Heron, D.E. Thoracic reirradiation with SBRT for residual/recurrent and new primary NSCLC within or immediately adjacent to a prior high-dose radiation field. Pr. Radiat. Oncol. 2018, 8, e117–e123. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lin, R.; Li, D.; Nie, L.; Warren, K.E. Time-to-Event Bayesian Optimal Interval Design to Accelerate Phase I TrialsTITE-BOIN to Accelerate Phase I Trials. Clin. Cancer Res. 2018, 24, 4921–4930. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Brooks, E.D.; Komaki, R.; Liao, Z.; Jeter, M.; McAleer, M.; Chang, J.Y. Long-term outcomes of salvage stereotactic ablative radiotherapy for isolated lung ecurrence of non small cell lung cancer: A phase II clinical trial. J. Thorac. Oncol. 2017, 12, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Maddalo, M.; D’Angelo, E.; Fiorica, F.; Argenone, A.; Scricciolo, M.; Cozzi, S.; Massaccesi, M. Thoracic re-irradiation with 3D-conformal or more advanced techniques: A systematic review of treatment safety by the Re-irradiation Study Group of the Italian Association of Radiation Oncology, A.I.R.O. Crit. Rev. Oncol./Hematol. 2021, 167, 103500. [Google Scholar] [CrossRef] [PubMed]
- Rulach, R.; Ball, D.; Chua, K.L.; Dahele, M.; De Ruysscher, D.; Franks, K.; Gomez, D.; Guckenberger, M.; Hanna, G.G.; Louie, A.V.; et al. An International Expert Survey on the Indications and Practice of Radical Thoracic Reirradiation for Non-Small Cell Lung Cancer. Adv. Radiat. Oncol. 2021, 6, 100653. [Google Scholar] [CrossRef] [PubMed]
- Janopaul-Naylor, J.R.; Cao, Y.; McCall, N.S.; Switchenko, J.M.; Tian, S.; Chen, H.; Higgins, K.A. Definitive intensity modulated proton re-irradiation for lung cancer in the immunotherapy era. Front. Oncol. 2023, 12, 1074675. [Google Scholar] [CrossRef] [PubMed]
- Grambozov, B.; Stana, M.; Kaiser, B.; Karner, J.; Gerum, S.; Ruznic, E.; Zehentmayr, F. High dose thoracic re-irradiation chemo-immunotherapy for centrally recurrent, N.S.C.L.C. Cancers 2022, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.D.; Sun, B.; Feng, L.; Verma, V.; Zhao, L.; Gomez, D.R.; Liao, Z.; Jeter, M.; O’reilly, M.; Welsh, J.W.; et al. Association of long-term outcomes and survival with multidisciplinary salvage treatment for local and regional recurrence after stereotactic ablative radiotherapy for early stage lung cancer. JAMA Netw. Open 2018, 1, e181390. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Faivre-Finn, C.; Le Pechoux, C.; Peeters, S.; Belderbos, J. High-dose re-irradiation following radical radiotherapy for non-small-cell lung cancer. Lancet Oncol. 2014, 15, e620–e624. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; Chen, Y.; Liu, X.; Zaorsky, N.G.; Mani, K.; Niu, Z.-M.; Zheng, B.-Y.; Zeng, H.-Y.; Yan, Y.-Y.; Li, Y.-J.; et al. Reirradiation with stereotactic body radiotherapy for primary or secondary lung malignancies: Tumor control probability and safety analyses. Radiother. Oncol. 2023, 187, 109817. [Google Scholar] [CrossRef] [PubMed]
- Viani, G.A.; Arruda, C.V.; De Fendi, L.I. Effectiveness and safety of reirradiation with stereotactic ablative radiotherapy of lung cancer after a first course of thoracic radiation: A meta-analysis. Am. J. Clin. Oncol. 2020, 43, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, D.Y.; Wu, H.G.; Lee, J.H.; Kim, H.J. Treatment outcomes of re-irradiation using stereotactic ablative radiotherapy to lung: A propensity score matching analysis. Radiat. Oncol. 2021, 16, 222. [Google Scholar] [CrossRef] [PubMed]
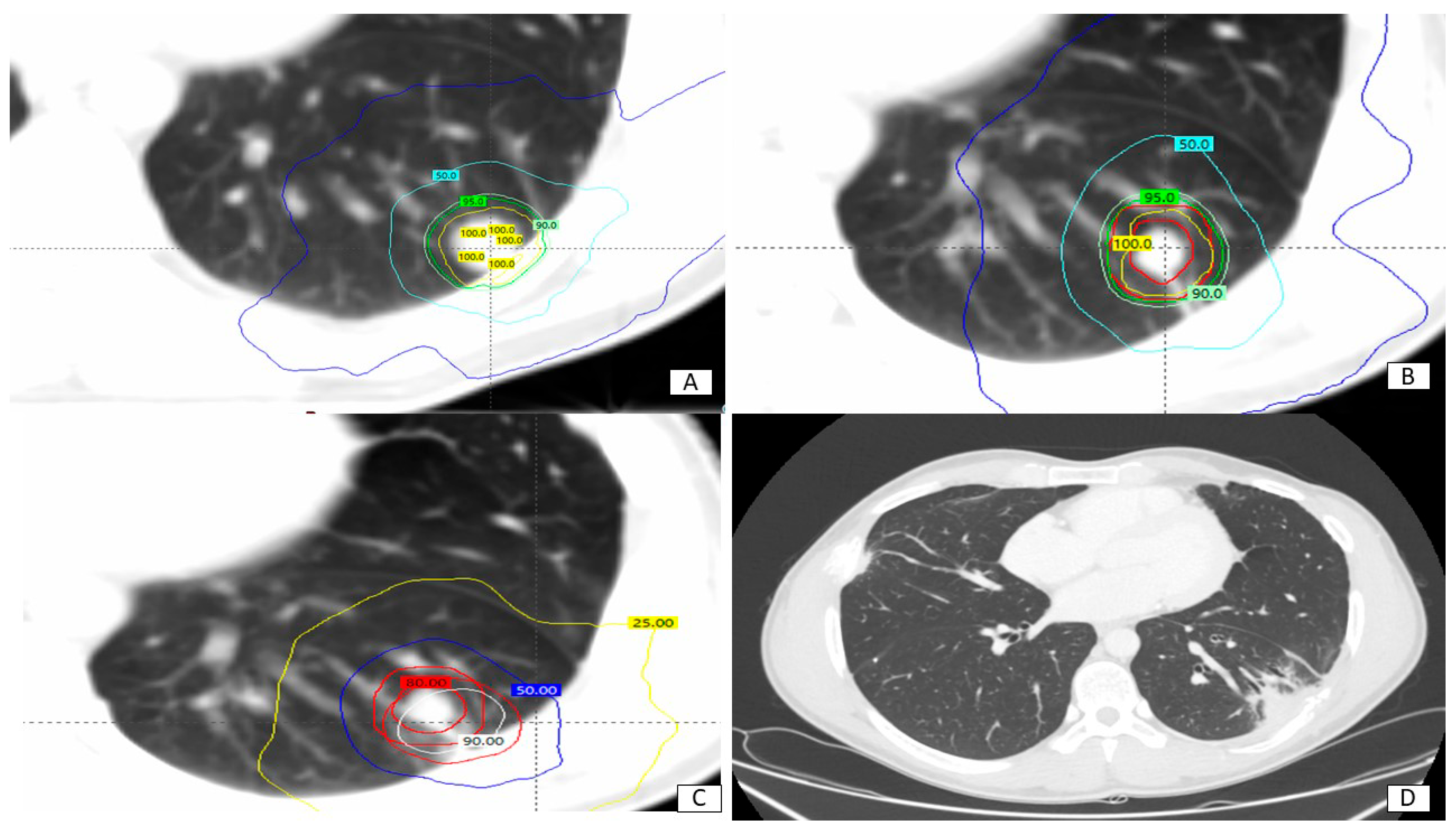
| Features | Patients (n) | Percentage |
|---|---|---|
| SEX Male Female | 11 4 | 73% 27% |
| SMOKING HISTORY Never Former Current | 2 9 4 | 13% 60% 27% |
| COMORBIDITIES 0 1 >1 | 3 11 1 | 20% 73% 7% |
| PULMONARY COMORBIDITIES No BPCO | 13 2 | 87% 13% |
| CARDIAC COMORBIDITIES No Hypertension Atrial fibrillation Heart failure Acute coronary Syndrome | 5 8 3 1 1 | 33% 53% 20% 7% 7% |
| PRIMARY CANCER SITE Lung Larynx Rectum Sarcoma Unknown | 10 2 1 1 1 | 66% 13% 7% 7% 7% |
| PRIMARY CANCER HISTOLOGY Adenocarcinoma Squamocellular carcinoma Synovial sarcoma Epithelial NOS | 8 5 1 1 | 53% 33% 7% 7% |
| PREVIOUS CANCER TREATMENTS Surgery Systemic Therapy | 6 8 | 40% 53% |
| FIRST RT ON TARGET LESION TYPE SBRT Hypofractionated RT Conventional RT | 12 2 1 | 80% 13% 7% |
| OVERLAP In site 50% | 6 9 | 40% 60% |
| PS AT SBRT 0 1 | 9 6 | 60% 40% |
| SBRT TOTAL DOSE IN 5 FRACTIONS 30 Gy 40 Gy 50 Gy | 5 5 5 | 33% 33% 33% |
| Volume | First RT Course Dose (Gy) Median; Range; IQ Range | Re-SBRT Dose (Gy) Median; Range; IQ Range | Sum in EQD2 (Gy) Median; Range; IQ Range |
|---|---|---|---|
| PTV Dmax | 54.6; 51.12–68.92; 6.855 | 42.5; 31.6–53.17; 18.21 | 148.7; 104.3–199.7; 47.75 |
| PTV Dmean | 40.2; 18.28–54.18; 16.345 | 40; 29.86–50.45; 20 | 120; 75.4–171.30; 34.9 |
| Bronchus Dmax | 2.73; 0.13–57.71; 31.385 | 8.05; 0.13–20.11; 12.965 | 9.2; 0.2–101.2; 76.6 |
| Esophagus Dmean | 8.16; 5.14–57.29; 13.46 | 7.6; 5.6–12.02; 3.49 | 18.2; 11–71.7; 33.6 |
| Spinal cord Dmax | 8.62; 0.25–29.14; 13.215 | 5.59; 0.32–14.05; 4.16 | 13; 3.1–66.4; 14.85 |
| Large vessels Dmax | 9.09; 0.4–58.26; 32.885 | 4.33; 0.16–18.23; 8.43 | 10.1; 0.2–107.1; 58.1 |
| Lung Dmean | 2.91; 1.11–12.50; 4.355 | 2.24; 0.92–5.21; 1.155 | 3.9; 1.3–10.5; 5.7 |
| Heart Dmax | 7.74; 0.2–58.1; 21.93 | 7.46; 0.17–47.15; 14.62 | 15.99; 0.2–151.2; 69.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschini, D.; Loi, M.; Marzo, A.M.; Dominici, L.; Spoto, R.; Bertolini, A.; Lo Faro, L.; La Fauci, F.; Marini, B.; Di Cristina, L.; et al. STRILL: Phase I Trial Evaluating Stereotactic Body Radiotherapy (SBRT) Dose Escalation for Re-Irradiation of Inoperable Peripheral Lung Lesions. Diseases 2024, 12, 153. https://doi.org/10.3390/diseases12070153
Franceschini D, Loi M, Marzo AM, Dominici L, Spoto R, Bertolini A, Lo Faro L, La Fauci F, Marini B, Di Cristina L, et al. STRILL: Phase I Trial Evaluating Stereotactic Body Radiotherapy (SBRT) Dose Escalation for Re-Irradiation of Inoperable Peripheral Lung Lesions. Diseases. 2024; 12(7):153. https://doi.org/10.3390/diseases12070153
Chicago/Turabian StyleFranceschini, Davide, Mauro Loi, Antonio Marco Marzo, Luca Dominici, Ruggero Spoto, Anna Bertolini, Lorenzo Lo Faro, Francesco La Fauci, Beatrice Marini, Luciana Di Cristina, and et al. 2024. "STRILL: Phase I Trial Evaluating Stereotactic Body Radiotherapy (SBRT) Dose Escalation for Re-Irradiation of Inoperable Peripheral Lung Lesions" Diseases 12, no. 7: 153. https://doi.org/10.3390/diseases12070153
APA StyleFranceschini, D., Loi, M., Marzo, A. M., Dominici, L., Spoto, R., Bertolini, A., Lo Faro, L., La Fauci, F., Marini, B., Di Cristina, L., & Scorsetti, M. (2024). STRILL: Phase I Trial Evaluating Stereotactic Body Radiotherapy (SBRT) Dose Escalation for Re-Irradiation of Inoperable Peripheral Lung Lesions. Diseases, 12(7), 153. https://doi.org/10.3390/diseases12070153






