Spatial and Temporal Dynamics of Chikungunya Incidence in Brazil and the Impact of Social Vulnerability: A Population-Based and Ecological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Area
2.2. Population, Case Definition, and Eligibility Criteria
2.3. Variables and Data Sources
2.4. Data Processing and Analysis
2.4.1. Descriptive Analysis
2.4.2. Temporal Trend Analysis
2.4.3. Spatial Analysis
2.4.4. Global Spatial Regression Analysis
2.4.5. Resources and Software
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Postigo-Hidalgo, I.; Jo, W.K.; Pedroso, C.; Brites, C.; Drexler, J.F. Introduction of chikungunya virus in coastal northeast Brazil. Lancet Microbe 2023, 4, e764. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, M.F.S.; Silva, A.P.d.S.C.; Lacerda, H.R. A spatial analysis of co-circulating dengue and chikungunya virus infections during an epidemic in a region of Northeastern Brazil. Spat. Spatiotemporal Epidemiol. 2023, 46, 100589. [Google Scholar] [CrossRef]
- Costa, L.B.; Barreto, F.K.d.A.; Barreto, M.C.A.; dos Santos, T.H.P.; Andrade, M.d.M.O.d.; Farias, L.A.B.G.; de Freitas, A.R.R.; Martinez, M.J.; Cavalcanti, L.P.d.G. Epidemiology and Economic Burden of Chikungunya: A Systematic Literature Review. Trop. Med. Infect. Dis. 2023, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Bettis, A.A.; Jackson, M.L.; Yoon, I.-K.; Breugelmans, J.G.; Goios, A.; Gubler, D.J.; Powers, A.M. The global epidemiology of chikungunya from 1999 to 2020: A systematic literature review to inform the development and introduction of vaccines. PLoS Negl. Trop. Dis. 2022, 16, e0010069. [Google Scholar] [CrossRef] [PubMed]
- Grabenstein, J.D.; Tomar, A.S. Global geotemporal distribution of chikungunya disease, 2011–2022. Travel. Med. Infect. Dis. 2023, 54, 102603. [Google Scholar] [CrossRef]
- de Souza, W.M.; de Lima, S.T.; Mello, L.M.S.; Candido, D.S.; Buss, L.; Whittaker, C.; Claro, I.M.; Chandradeva, N.; Granja, F.; de Jesus, R.; et al. Spatiotemporal dynamics and recurrence of chikungunya virus in Brazil: An epidemiological study. Lancet Microbe 2023, 4, e319–e329. [Google Scholar] [CrossRef] [PubMed]
- Carabali, M.; Harper, S.; Neto, A.S.L.; Sousa, G.d.S.d.; Caprara, A.; Restrepo, B.N.; Kaufman, J.S. Spatiotemporal distribution and socioeconomic disparities of dengue, chikungunya and Zika in two Latin American cities from 2007 to 2017. Trop. Med. Int. Health 2021, 26, 301–315. [Google Scholar] [CrossRef] [PubMed]
- da Silva Queiroz, E.R.; de Andrade Medronho, R. Spatial analysis of the incidence of Dengue, Zika and Chikungunya and socioeconomic determinants in the city of Rio de Janeiro, Brazil. Epidemiol. Infect. 2021, 149, e188. [Google Scholar] [CrossRef]
- Araujo, D.d.C.; dos Santos, A.D.; Lima, S.V.M.A.; Vaez, A.C.; Cunha, J.O.; de Araujo, K.C.G.M. Determining the association between dengue and social inequality factors in north-eastern Brazil: A spatial modelling. Geospat. Health 2020, 15, 854. [Google Scholar] [CrossRef]
- de Margarette Oliveira de Andrade, M.; de Almeida Barreto, F.K.; Coelho, T.M.S.; Praça Pinto, G.; Queiroz, I.T.; Nogueira, C.T.; Freitas, A.R.R.; Ferreira, M.J.M.; Alencar, C.H.; de Góes Cavalcanti, L.P.; et al. Chikungunya in Brazil: An epidemic of high cost for private healthcare, 2017. Trop. Med. Int. Health 2022, 27, 925–933. [Google Scholar] [CrossRef]
- Bolado, I.M.; Gómez, M.S.; Villa-Blanco, I.; Zarrabeitia, L.R.; Aurrecoechea, E.; Jimeno, T.R.; Calvo, J. SAT0468 Infectious spondylodiscitis in a spanish regional hospital between 2000 and 2018: 66 cases. BMJ 2019, 1, 1323. [Google Scholar]
- Bartholomeeusen, K.; Daniel, M.; LaBeaud, D.A.; Gasque, P.; Peeling, R.W.; Stephenson, K.E.; Ng, L.F.P.; Ariën, K.K. Chikungunya fever. Nat. Rev. Dis. Primers 2023, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Hladish, T.J.; Pearson, C.A.; Toh, K.B.; Rojas, D.P.; Manrique-Saide, P.; Vazquez-Prokopec, G.M. Designing effective control of dengue with combined interventions. Proc. Natl. Acad. Sci. USA 2020, 117, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.R.; Enk, M.J.; Guimarães, R.J.d.P.S.e. Spatial analysis studies of endemic diseases for health surveillance: Application of scan statistics for surveillance of tuberculosis among residents of a metropolitan municipality aged 60 years and above. Cienc. Saude Coletiva 2021, 26, 5149–5156. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde (BR), Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis. Chikungunya: Manejo Clínico; Ministério da Saúde: Brasília, Brazil, 2017. [Google Scholar]
- Anselin, L. Spatial Regression Analysis in R A Workbook. Urbana 2005, 51, 61801. [Google Scholar]
- Moran, P.A.P. A Test for the Serial Independence of Residuals. Biometrika 1950, 37, 178. [Google Scholar] [CrossRef]
- Mourad, O.; Makhani, L.; Chen, L.H. Chikungunya: An Emerging Public Health Concern. Curr. Infect. Dis. Rep. 2022, 24, 217–228. [Google Scholar] [CrossRef]
- Silva, N.M.D.; Teixeira, R.A.G.; Cardoso, C.G.; Siqueira, J.B.; Coelho, G.E.; Oliveira, E.S.F.D. Vigilância de chikungunya no Brasil: Desafios no contexto da Saúde Pública. Epidemiol. Serv. Saude 2018, 27, e2017127. [Google Scholar] [CrossRef]
- Ministério da Saúde (BR). Boletim Epidemiológico; Secretaria de Vigilância em Saúde, Brasília Ministério da Saúde: Brasília, Brazil, 2017. [Google Scholar]
- Yakob, L. Predictable Chikungunya Infection Dynamics in Brazil. Viruses 2022, 14, 1889. [Google Scholar] [CrossRef]
- De Araújo Neto, F.J.; Pinto, A.L.L.; De Oliveira, A.M.C.; De Souza, F.R.C.; Pinto Pedrosa, R.M.; Faria Paiva, L.G.; Linhares Oliveira, M.M.; Melo Cunha, M.; Júnior Aguiar, O.P.; Primo Satiro, R.; et al. O perfil epidemiológico das arboviroses no brasil de 2017 a 2022: Uma análise do impacto da pandemia de COVID-19. Braz. J. Implantol. Health Sci. 2023, 5, 6423–6434. [Google Scholar] [CrossRef]
- Lisboa, T.R.; Serafim, I.B.M.; Serafim, J.C.M.; Serafim, J.C.M.; Ramos, A.C.; Nascimento, R.M.; Roner, M.N.B. Relationship between the incidence of arbovirus cases and the pandemic of COVID-19. Rev. Interdiscip. Cienc. Apl. 2022, 6, 31–36. [Google Scholar]
- Santana, L.S.; Braga, J.U. Spatial diffusion of Zika fever epidemics in the Municipality of Salvador-Bahia, Brazil, in 2015-2016: Does Zika fever have the same spread pattern as Dengue and Chikungunya fever epidemics? Rev. Soc. Bras. Med. Trop. 2020, 53, e20190563. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.D.; Branco, M.D.; Vasconcelos, V.V.; Queiroz, R.C.; Araujo, A.S.; Câmara, A.P.; Fushita, A.T.; Silva, M.D.; Silva, A.A.; Santos, A.M. Autoregressive spatial modeling of possible cases of dengue, chikungunya, and Zika in the capital of Northeastern Brazil. Rev. Soc. Bras. Med. Trop. 2021, 54, e0223-2021. [Google Scholar] [CrossRef] [PubMed]
- Anjos, R.S.; Nóbrega, R.S.; Ferreira, H.D.; Lacerda, A.P.; Sousa-Neves, N.D. Exploring local and global regression models to estimate the spatial variability of Zika and Chikungunya cases in Recife, Brazil. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200027. [Google Scholar] [CrossRef] [PubMed]
- Kazazian, L.; Lima Neto, A.S.; Sousa, G.S.; Nascimento, O.J.; Castro, M.C. Spatiotemporal transmission dynamics of co-circulating dengue, Zika, and chikungunya viruses in Fortaleza, Brazil: 2011–2017. PLoS Negl. Trop. Dis. 2020, 14, e0008760. [Google Scholar] [CrossRef] [PubMed]
- Aguiar-Santos, M.; Mendes, L.G.; Passos, D.F.; Santos, T.G.; Lins, R.H.; Monte, A.C. Spatial analysis of Chikungunya fever incidence and the associated socioeconomic, demographic, and vector infestation factors in municipalities of Pernambuco, Brazil, 2015–2021. Rev. Bras. Epidemiol. 2023, 26, e230018. [Google Scholar] [CrossRef]
- Costa, S.D.; Branco, M.D.; Aquino Junior, J.; Rodrigues, Z.M.; Queiroz, R.C.; Araujo, A.S.; Câmara, A.P.; Santos, P.S.; Pereira, E.D.; Silva, M.D.; et al. Spatial analysis of probable cases of dengue fever, chikungunya fever and zika virus infections in Maranhao State, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e62. [Google Scholar] [CrossRef]
| Period | Chikungunya Incidence | ||
|---|---|---|---|
| APC (CI 95%) | p-Value | Trend | |
| 2017 | 1.90 (0.96–2.48) | <0.001 | Increasing |
| 2018–2019 | −0.47 (−1.12–0.93) | 0.354 | Stationary |
| 2020–2021 | −0.93 (−1.18–0.98) | <0.001 | Decreasing |
| 2022–2023 | −0.10 (−0.30–0.11) | 0.247 | Stationary |
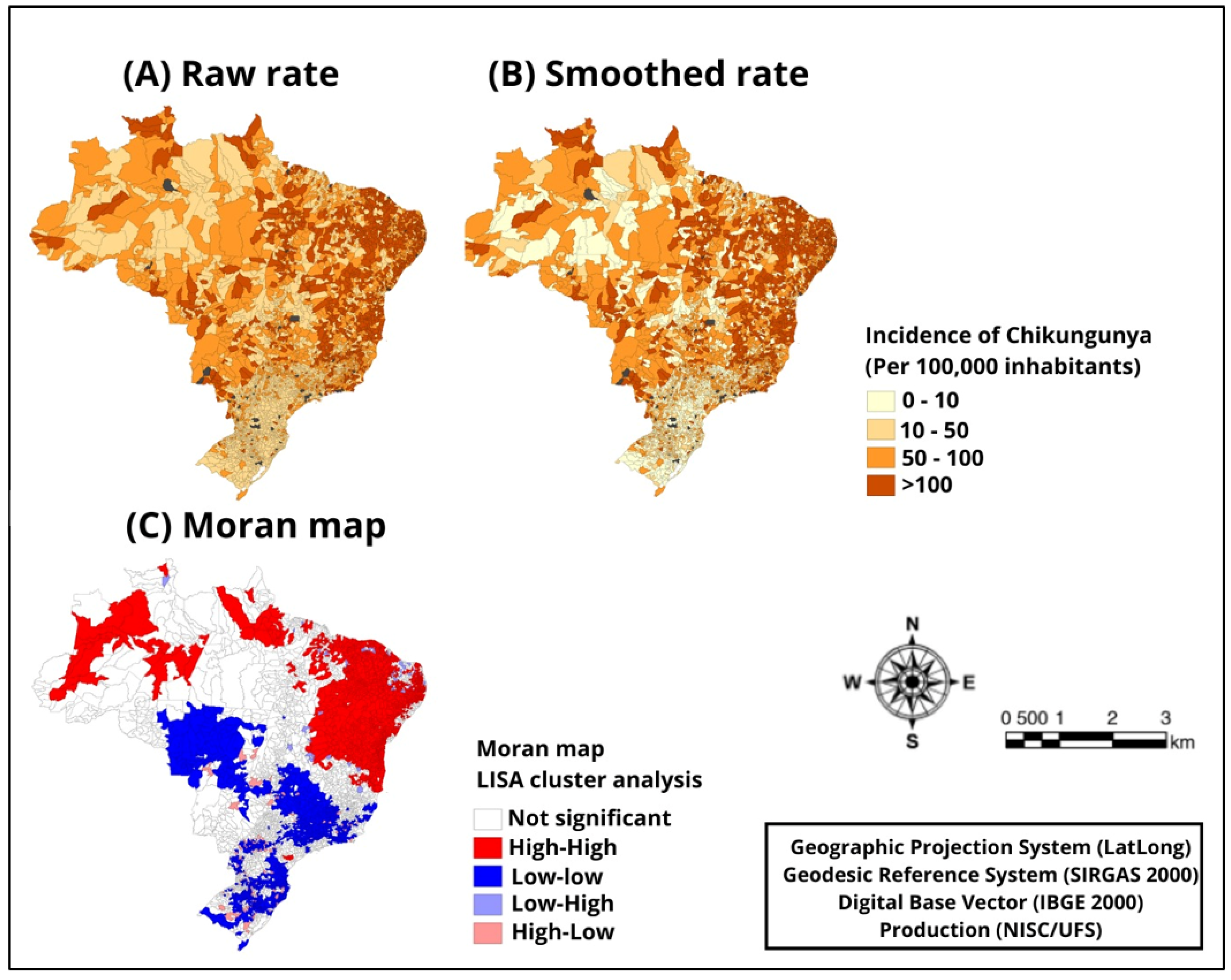
| Variable | Cases | Global Moran’s Index | p |
|---|---|---|---|
| 2017 | 127.390 | 0.78 | 0.001 |
| 2018 | 50.860 | 0.65 | 0.001 |
| 2019 | 52.689 | 0.63 | 0.001 |
| 2020 | 29.339 | −0.63 | 0.001 |
| 2021 | 41.817 | 0.67 | 0.001 |
| 2022 | 120.932 | 0.58 | 0.002 |
| 2023 | 64.748 | 0.57 | 0.001 |
| 2017–2023 | 487.775 | 0.80 | 0.001 |
| Social Vulnerability Indicators | Chikungunya | |
|---|---|---|
| Rho | p-Value | |
| Social vulnerability index | 0.34 | <0.01 |
| Social vulnerability index—human capital dimension | 0.34 | <0.01 |
| Social vulnerability index—income and work dimension | 0.34 | <0.01 |
| Percentage of people in households with inadequate water supply and sanitation | 0.35 | <0.01 |
| Literacy rate of the population aged 15 years and older | 0.36 | <0.01 |
| Percentage of people aged 15 to 24 years who do not study, do not work, and have a per capita household income equal to or less than half the minimum wage (as of 2010). | 0.28 | <0.01 |
| HDI longevity | −0.31 | <0.01 |
| HDI income | −0.33 | <0.01 |
| Per capita income | −0.33 | <0.01 |
| Vulnerable population aged 15 to 24 years | 0.45 | <0.01 |
| Population in vulnerable households with elderly people | 0.45 | <0.01 |
| Percentage of the population in households with a density >2 | 0.35 | <0.01 |
| Literacy rate—18 years or older | 0.38 | <0.01 |
| Per capita income of those vulnerable to poverty | −0.33 | <0.01 |
| Social Vulnerability Indicators | Collinearity Statistics | |||
|---|---|---|---|---|
| Tolerance | VIF | t | p | |
| Social vulnerability index | 0.066 | 15.175 | −2.659 | 0.08 |
| Social vulnerability index—human capital dimension | 0.078 | 12.839 | −2.799 | 0.05 |
| Social vulnerability index—income and work dimension | 0.107 | 9.336 | 4.366 | <0.001 |
| Percentage of people in households with inadequate water supply and sanitation | 0.427 | 2.342 | 0.984 | 0.32 |
| Literacy rate of the population aged 15 years and older | 0.001 | 1845.373 | −2.045 | 0.04 |
| Percentage of people aged 15 to 24 years who do not study, do not work, and have a per capita household income equal to or less than half the minimum wage (as of 2010). | 0.244 | 4.093 | 3.490 | <0.001 |
| HDI longevity | 0.184 | 5.446 | 2.180 | 0.02 |
| HDI income | 0.035 | 28.749 | 2.899 | 0.04 |
| Per capita income | 0.078 | 12.809 | −2.633 | 0.08 |
| Vulnerable population aged 15 to 24 years | 0.038 | 26.248 | −3.606 | <0.001 |
| Population in vulnerable households with elderly people | 0.038 | 26.429 | 4.619 | <0.001 |
| Percentage of the population in households with a density >2 | 0.027 | 3.885 | 0.970 | 0.33 |
| Literacy rate–18 years or older | 0.001 | 1908.591 | 2.576 | 0.01 |
| Per capita income of those vulnerable to poverty | 0.107 | 9.353 | 0.725 | 0.46 |
| Social Vulnerability Indicators | OLS Model | Spatial Lag Model | Erro Spatial Model | |||
|---|---|---|---|---|---|---|
| Coefficient | p | Coefficient | p | Coefficient | p | |
| Social vulnerability indicators | −2.69 | <0.001 | −1.28 | 0.003 | −1.04 | 0.15 |
| Social vulnerability index—human capital dimension | 0.16 | 0.78 | 0.14 | 0.760 | 0.25 | 0.64 |
| Social vulnerability index—income and work dimension | 3.95 | <0.001 | 1.93 | <0.001 | 1.02 | 0.003 |
| Percentage of people in households with inadequate water supply and sanitation | 0.16 | <0.001 | 0.80 | <0.001 | 0.95 | 0.007 |
| Percentage of people aged 15 to 24 years who do not study, do not work, and have a per capita household income equal to or less than half the minimum wage (as of 2010). | 0.60 | <0.001 | 0.16 | 0.003 | −0.05 | 0.38 |
| HDI longevity | −2.60 | <0.001 | −0.76 | 0.221 | 0.46 | 0.94 |
| Per capita income | 0.60 | 0.002 | 0.94 | <0.001 | 0.14 | <0.001 |
| Percentage of the population in households with a density >2 | 0.79 | 0.003 | 0.58 | 0.004 | 0.12 | 0.006 |
| Per capita income of those vulnerable to poverty | 0.21 | 0.37 | 0.21 | 0.900 | −0.002 | 0.89 |
| Model Evaluation Criteria | OLS Model | Spatial Lag Model | Erro Spatial Model | |||
| Determination coefficient (p-value) | 0.58 (p = 0.002) | 0.78 (<0.001) | 0.22 (p = 0.008) | |||
| Log likelihood | 5.1 | 11.6 | 2.2 | |||
| Akaike criterion | 49.6 | 26.2 | 78.5 | |||
| Schwarz criterion | 119.1 | 108.5 | 200.6 | |||
| Moran index (p-value) | 0.38 (p = 0.001) | −0.014 (p = 0.44) | 0.12 (p = 0.002) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus Santos, T.; de Araújo, K.C.G.M.; Góes, M.A.d.O.; Bezerra-Santos, M.; Ribeiro, C.J.N.; dos Santos, A.D.; Camargo, E.L.S.; Souza, R.C.S.; Mendes, I.A.C.; Sousa, A.F.L.d.; et al. Spatial and Temporal Dynamics of Chikungunya Incidence in Brazil and the Impact of Social Vulnerability: A Population-Based and Ecological Study. Diseases 2024, 12, 135. https://doi.org/10.3390/diseases12070135
de Jesus Santos T, de Araújo KCGM, Góes MAdO, Bezerra-Santos M, Ribeiro CJN, dos Santos AD, Camargo ELS, Souza RCS, Mendes IAC, Sousa AFLd, et al. Spatial and Temporal Dynamics of Chikungunya Incidence in Brazil and the Impact of Social Vulnerability: A Population-Based and Ecological Study. Diseases. 2024; 12(7):135. https://doi.org/10.3390/diseases12070135
Chicago/Turabian Stylede Jesus Santos, Thiago, Karina Conceição Gomes Machado de Araújo, Marco Aurélio de Oliveira Góes, Marcio Bezerra-Santos, Caíque Jordan Nunes Ribeiro, Allan Dantas dos Santos, Emerson Lucas Silva Camargo, Regina Claudia Silva Souza, Isabel Amélia Costa Mendes, Alvaro Francisco Lopes de Sousa, and et al. 2024. "Spatial and Temporal Dynamics of Chikungunya Incidence in Brazil and the Impact of Social Vulnerability: A Population-Based and Ecological Study" Diseases 12, no. 7: 135. https://doi.org/10.3390/diseases12070135
APA Stylede Jesus Santos, T., de Araújo, K. C. G. M., Góes, M. A. d. O., Bezerra-Santos, M., Ribeiro, C. J. N., dos Santos, A. D., Camargo, E. L. S., Souza, R. C. S., Mendes, I. A. C., Sousa, A. F. L. d., & da Conceição Araújo, D. (2024). Spatial and Temporal Dynamics of Chikungunya Incidence in Brazil and the Impact of Social Vulnerability: A Population-Based and Ecological Study. Diseases, 12(7), 135. https://doi.org/10.3390/diseases12070135











