Abstract
In this study, we aimed to determine the utility of cytoreductive nephrectomy (CN) in real-world clinical practice and investigate whether CN contributes to improved oncological outcomes in patients with metastatic renal cell carcinoma (mRCC). This retrospective multicenter cohort study enrolled patients with mRCC who received systemic therapy at six institutions between May 2005 and May 2023. The patients were divided into those who did not undergo CN (Group I) and those who underwent CN (Group II). The primary endpoints were oncological outcomes, including cancer-specific survival (CSS) and progression-free survival (PFS). Altogether, 137 patients with mRCC were included in this study. The median CSS was 14 months in Group I and 32 months in Group II (p < 0.001). Additionally, the median PFS in Groups I and II was 5 and 13 months, respectively (p = 0.006). A multivariate analysis showed that CN was an independent prognostic factor for CSS and PFS. Hence, CN is a potential treatment modality that can improve oncological outcomes in patients with mRCC.
1. Introduction
The incidence rate of renal cell carcinoma (RCC) is 17.1 per 10,000 individuals, whereas the mortality rate is 3.6 per 10,000 individuals [1]. Metastatic RCC (mRCC) accounts for 13% of all RCC cases, with a 5-year relative survival rate of 14% [1]. Although mRCC has a significantly lower incidence rate than localized or regional RCC, it has a worse prognosis [1,2]. The therapeutic landscape for mRCC is currently undergoing rapid evolution with the approval of molecular targeted therapies (MTTs), immune checkpoint inhibitors (ICIs), and combinations of MTTs and ICIs [3,4,5,6,7,8,9].
Nevertheless, the role of cytoreductive nephrectomy (CN) in mRCC remains unclear. CN in patients with mRCC reportedly results in metastatic regression, the prevention of tumor growth, the suppression of new metastases, and a reduction in clinical symptoms by decreasing the release of cytokines and growth factors [10,11,12,13]. In addition, CN is also suggested to reduce paraneoplastic syndromes and improve quality of life (QOL) [14,15]. Two prospective randomized controlled trials in the early 2000s demonstrated that CN with interferon (IFN) significantly improved oncological outcomes compared with IFN alone in patients with mRCC having good performance status [11,16]. Another two prospective randomized trials conducted during the era when MTT was the standard of care for mRCC provided insights into the need for and importance of CN in improving oncological outcomes of patients with mRCC [17,18]. The Clinical Trial to Assess the Importance of Nephrectomy (CARMENA) trial has demonstrated that sunitinib monotherapy was not inferior to CN, with subsequent sunitinib in patients with mRCC who had intermediate or poor prognostic factors according to the Memorial Sloan Kettering Cancer Center (MSKCC) criteria [17]. However, a recent subgroup analysis of the CARMENA trial indicated that overall survival (OS) tended to be longer in patients treated with CN plus sunitinib than in those treated with sunitinib alone; furthermore, patients with mRCC having at least one International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk factor may benefit from undergoing upfront CN [19]. The Canadian Uro-Oncology Group jointly conducted the Immediate Surgery or Surgery After Sunitinib Malate in Treating Patients with Metastatic Kidney Cancer (SURTIME) trial, assessing the role of immediate or deferred CN in patients with mRCC who were administered sunitinib, and suggested that the median OS was significantly greater in the deferred CN group than in the immediate CN group [18]. A more recent study reported that the median OS in patients with mRCC was similar between the upfront and deferred CN plus systemic therapy groups, although the upfront CN with systemic therapy group exhibited a significantly better median OS [20]. However, with the combination therapy of TT and ICI as the mainstay of mRCC treatment, there remain numerous unsolved questions regarding the necessity of CN, its timing, and the background of patients who truly need the treatment when the main focus is on improving oncological outcomes.
Various studies have examined the significance of CN in improving the oncological outcomes of patients with mRCC [10,11,12,13,14,15,16,17,18,19]. Notably, a consensus has been reached regarding the significance of CN over the past 20 years [15]. However, significant changes have occurred in the systemic therapy of mRCC, including ICI and MTT, and re-evaluating the significance of CN and the timing of its implementation in current clinical practice may be necessary. Therefore, in this study, we aimed to investigate whether CN contributes to improved oncological outcomes in patients with mRCC and whether the combination of CN and systemic therapy could improve oncological outcomes.
2. Materials and Methods
2.1. Patient Selection
This retrospective multicenter cohort study included patients with mRCC who received systemic therapy with MTT, ICI, a combination of ICIs, or a combination of MTT and ICI at six institutions between May 2005 and May 2023. The enrolled patients were divided into those who did not undergo CN (Group I) and those who underwent CN (Group II). The following clinical characteristics of the enrolled patients were collected: age, sex, body mass index, Eastern Cooperative Oncology Group performance status (ECOG-PS) [21], primary IMDC risk stratification, tumor–node–metastasis classification according to the American Joint Committee on Cancer (AJCC) staging manual [22], treatment regimen, primary tumor histology, metastatic site, and number of metastases.
This study was approved by the Institutional Review Board of Gifu University (approval number: 2022-125). Owing to the retrospective design of the study, written informed consent was not obtained from the enrolled patients. This is because, in accordance with the Japanese Ethics Committee regulations and ethical guidelines, written consent is not required for retrospective or observational studies using existing or other data in which the results have already been published. The details of this study, which are available only in Japanese, can be found at https://www.med.gifu-u.ac.jp/visitors/disclosure/docs/2022-125.pdf (accessed on 14 August 2023).
2.2. Schedule of Systemic Therapy for mRCC
The MTT dose for mRCC was determined based on the strategy used by each treating institution [3,23].
For the ICI combination, 3 mg/kg nivolumab (NIVO) and 1 mg/kg ipilimumab (IPI) were administered intravenously at 3-week intervals to patients with mRCC until September 2018. In October 2018, NIVO was initiated at 240 mg for induction therapy, followed by maintenance therapy every 2 weeks.
For the combination of avelumab (AVE) and axitinib (AXI), AVE was administered intravenously every 2 weeks, whereas AXI was administered orally twice daily [5,24]. Regarding the pembrolizumab (PEM) + AXI regimen, PEM was administered intravenously at a single dose of 200 or 400 mg, whereas AXI was administered orally at 5 mg twice daily [6,13]. When the treatment regimen comprised NIVO and cabozantinib (CABO), NIVO was administered intravenously at a dose of 240 or 480 mg, whereas CABO was administered orally at 40 mg once daily [7,13]. For those receiving PEM and lenvatinib (LEN), PEM was administered intravenously at a dose of 200 or 400 mg, whereas LEN was administered orally at 20 mg once daily [8,13,24]. ICI therapy was discontinued after 2 years in patients treated with ICI + MTT.
Drug treatment method and dosage were determined at each institution, and whether, when, and how to perform CN were also based on the strategy of each institution. Upfront CN referred to CN performed before the initiation of systemic therapy in patients with mRCC who were diagnosed with metastatic disease [13,25]. Deferred CN was defined as a CN performed in patients with mRCC who received systemic therapy. Systemic treatment with or without CN was continued until disease progression was confirmed based on radiological evaluation or the development of tolerable treatment-related adverse events (TRAEs). Radiological progression was defined as the presence of one or more of the following findings: (1) new bone metastases identified on bone scans, computed tomography (CT), or magnetic resonance imaging (MRI); (2) regional soft tissue recurrence or metastasis based on the Response Evaluation Criteria in Solid Tumors guidelines version 1.1 [26]; and (3) at least one new visceral or soft tissue metastasis on CT [2].
2.3. Patient Evaluation
Patients were evaluated at baseline before undergoing systemic therapy based on their complete history; physical examination; and chest, abdominal, and pelvic CT and MRI findings. The AJCC staging manual was used to determine the tumor stage [22]. Patient outcomes were evaluated using CT or MRI performed at 1–3-month intervals until radiological disease progression or treatment discontinuation owing to TRAEs.
2.4. Statistical Analysis
The primary endpoints were oncological outcomes, including cancer-specific survival (CSS) and progression-free survival (PFS). The JMP 14 software (SAS Institute Inc., Cary, NC, USA) was used for data analysis. The Mann–Whitney U test or Kruskal–Wallis test was used to compare continuous variables between the two groups, whereas the Pearson’s chi-square test or Fisher’s exact test was used to compare changes in each category. The follow-up period was defined as the time from the initial diagnosis of mRCC to the last follow-up examination or date of death. CSS and PFS were determined from the time of initial diagnosis of mRCC to all-cause mortality and disease progression, respectively. Oncological outcomes were analyzed using the Kaplan–Meier method, and log-rank tests were used to examine differences in clinical variables. In the multivariate analysis, the Cox proportional hazards model was used to investigate the clinical factors that influenced oncological outcomes. Statistical significance was defined as a two-sided p-value < 0.05.
3. Results
3.1. Patient Characteristics
Among the 137 patients who were enrolled in this study, 13 were excluded because of selecting the best supportive care (7 patients), missing data (4 patients), receiving CN alone (1 patient), or undergoing renal artery embolization only (one patient). Finally, 124 patients with mRCC were included in the analysis.
Data on pretreatment characteristics of the enrolled patients are presented in Table 1. The patients’ median age was 68 years (interquartile range (IQR), 62–74 years), and the follow-up period was 19 months (IQR, 8–41 months). The follow-up period for patients receiving ICI + ICI, ICI + MTT, MTT alone, and interferon was 17 months (IQR, 8–27.5 months), 9.5 months (IQR, 7–14 months), 21.5 months (IQR, 9.3–55 months), and 49 months (IQR, 28–80 months), respectively. Based on the patients’ history, 57.3% of the patients with mRCC were administered MTT or IFN as the treatment regimen. The male sex was more common than the female sex among the enrolled patients. Additionally, patients in Group I were older and had more cases of clinical ≥ T3 and liver metastases than those in Group II. Only two cases were classified as having a favorable risk; however, this could be because the ECOG-PS relies on the subjective judgment of the primary physician, potentially leading to a possible bias. The median time from surgery to systemic therapy was 1 month (IQR, 0–4 months) in patients who underwent upfront CN, whereas the median time from systemic therapy to CN was 3.5 months (IQR, 1–10 months) in patients who underwent deferred CN. Of the patients classified in Group 2, 18 (25.7%) showed disease progression after the initiation of treatment for mRCC, with a median of 35 months (IQR, 2–5 months). No patient died of any cause during the period between surgery and systemic therapy or between systemic therapy and surgery.

Table 1.
Patient characteristics.
The clinical variables for enrolled patients with good performance status (ECOG-PS ≤ 1) are presented in Table 2.

Table 2.
Demographic data of enrolled patients with good performance status.
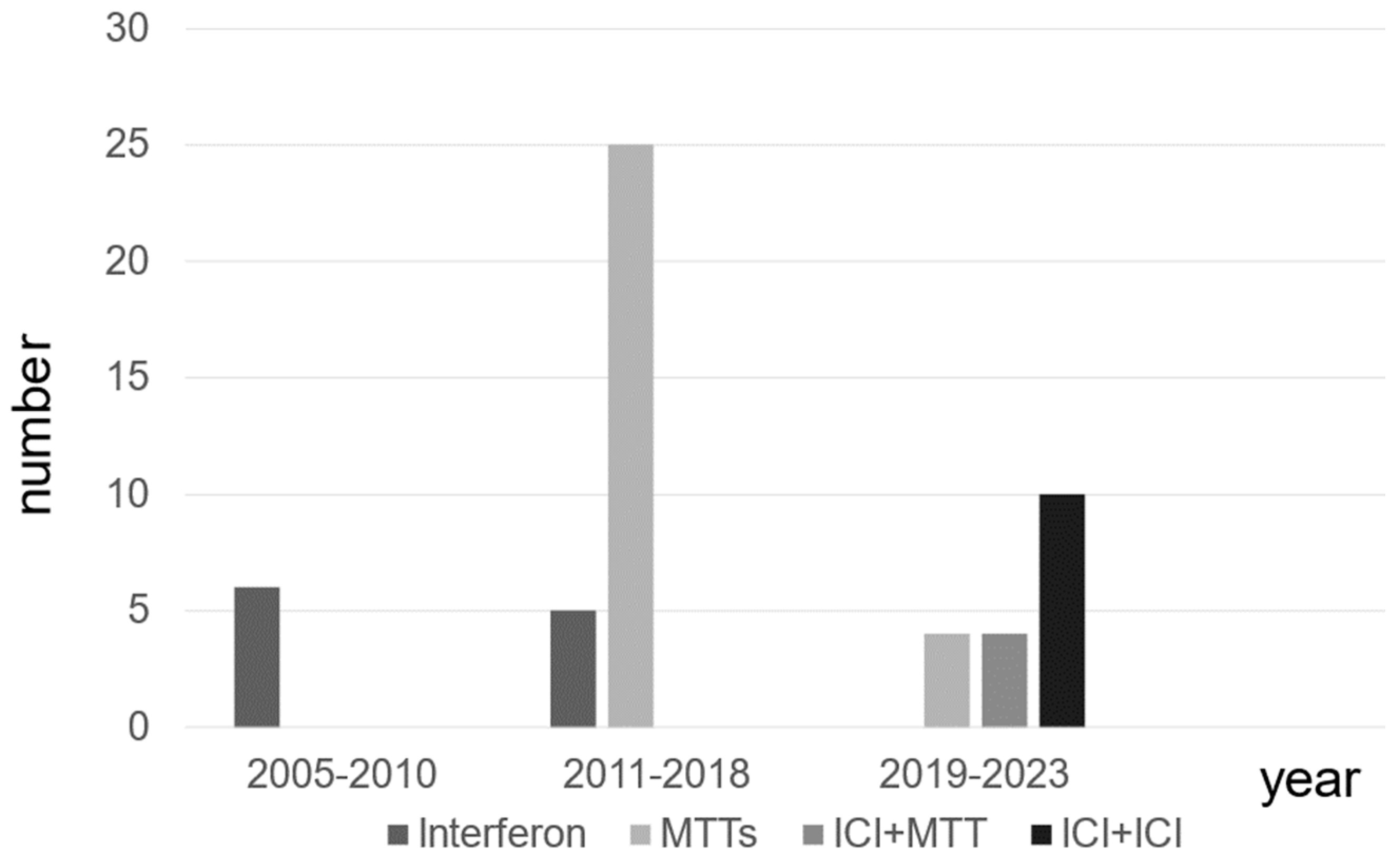
The types of systemic treatment for patients with mRCC at different time periods are presented. The use of MTT has increased since 2011, followed by a shift to combination therapy, including ICI, after 2019 in patients with mRCC (Figure 1).
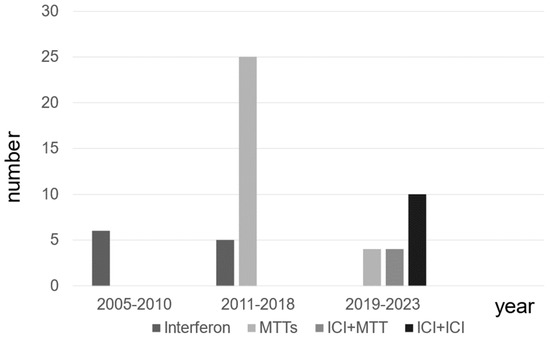
Figure 1.
The distribution of systemic therapies for patients with metastatic renal cell carcinoma.
3.2. Oncological Outcomes
At the end of the follow-up period, 66 (54.8%) patients died from RCC, and 2 (15%) died from other causes (details unknown). The median CSS and PFS of the enrolled patients were 19 (interquartile range (IQR): 8–41) and 8 (IQR: 3–17) months, respectively.
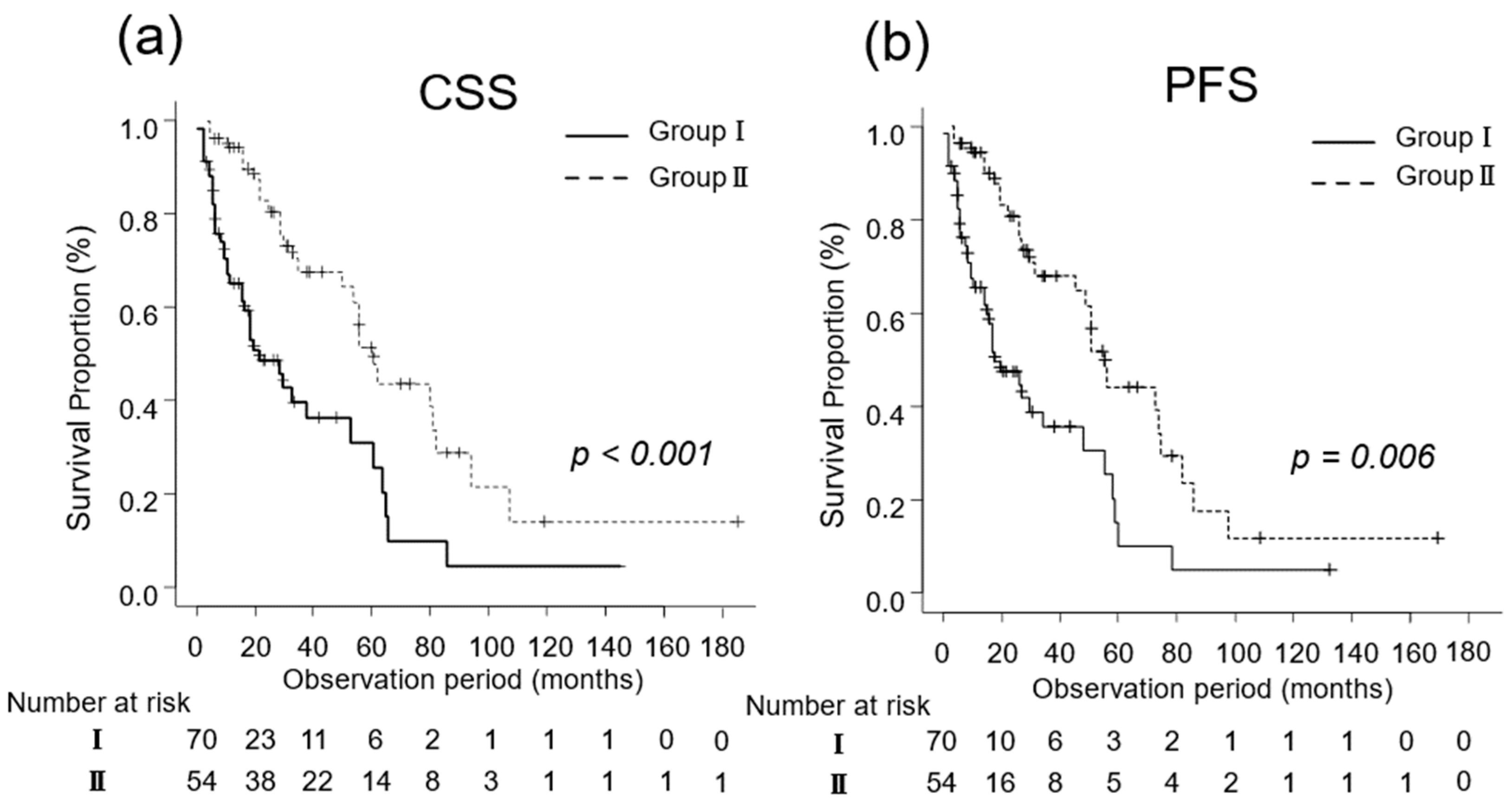
The median CSS was 14 (IQR, 6–27) and 32 (IQR, 18–60) months in Groups I (n = 70) and II (n = 54), respectively (p < 0.001; Figure 2a). Additionally, the median PFS in Groups I and II was 5 (IQR, 2–14) and 13 (IQR, 8–28) months, respectively (p = 0.006; Figure 2b).
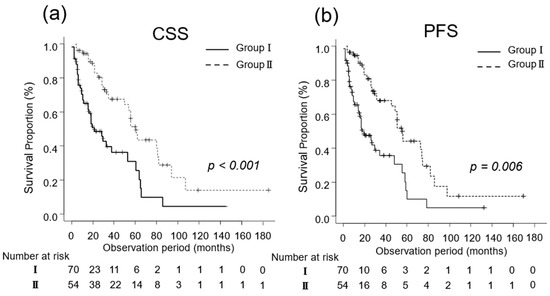
Figure 2.
Kaplan–Meier estimates of cancer-specific survival (CSS) and progression-free survival (PFS) in patients with metastatic renal cell carcinoma who did not undergo cytoreductive nephrectomy (Group I) and in those who underwent cytoreductive nephrectomy (Group II). (a) The median CSS is 14 months in Group I and 32 months in Group II (p < 0.001). (b) The 3-year PFS rate is 30.4% and 42.8% in Groups I and II, respectively (p = 0.006).
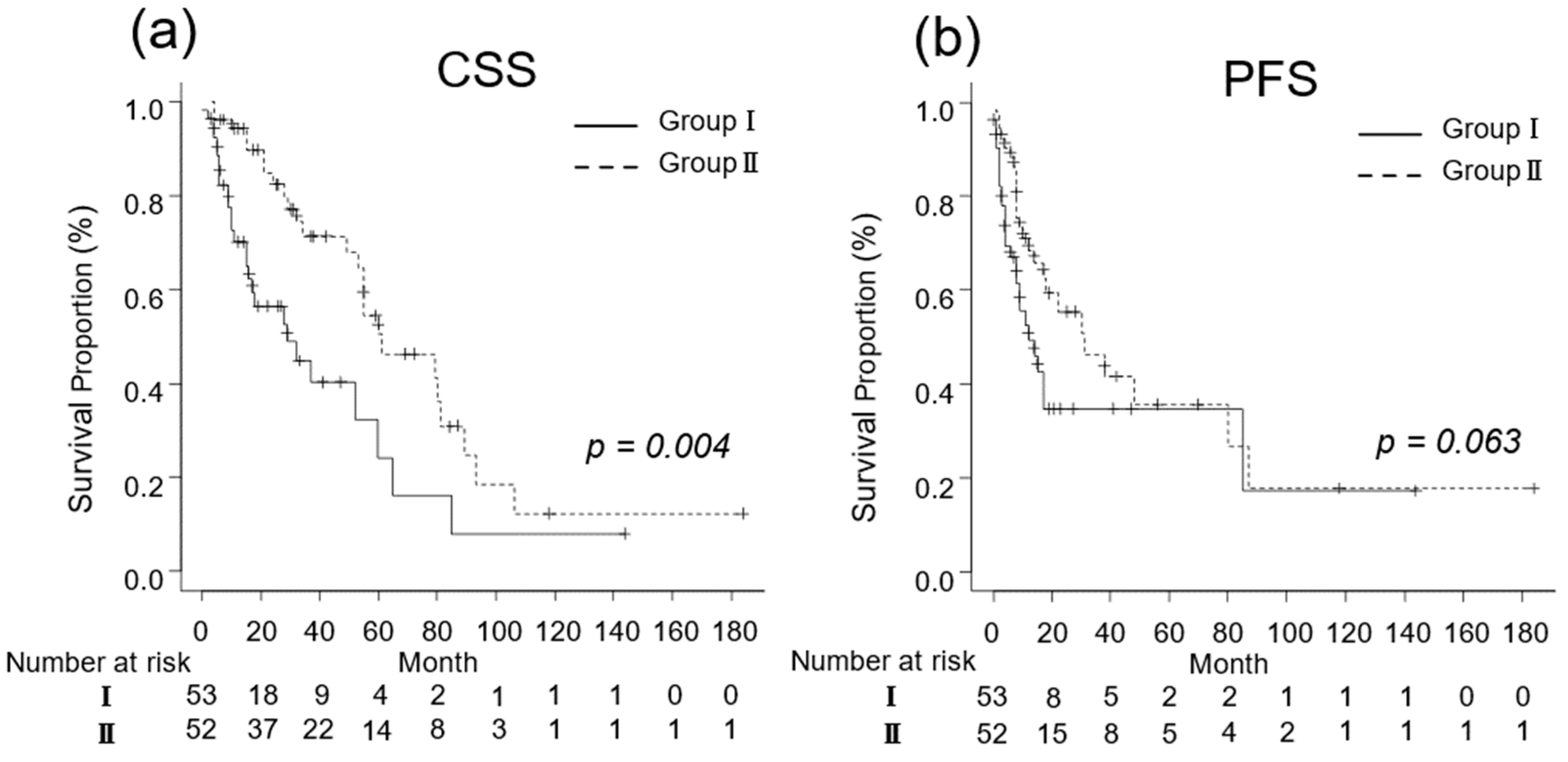
For patients with a good performance status (ECOG-PS ≤ 1), the oncological outcomes are presented in Figure 2. The median CSS was 14 (IQR, 6–29) and 32 (IQR, 17–60) months in Groups I (n = 53) and II (n = 52), respectively (p = 0.004; Figure 2a). The median PFS for Groups I and II was 7 (IQR, 3–14) and 13 (IQR, 8–26) months, respectively (p = 0.063; Figure 2b).
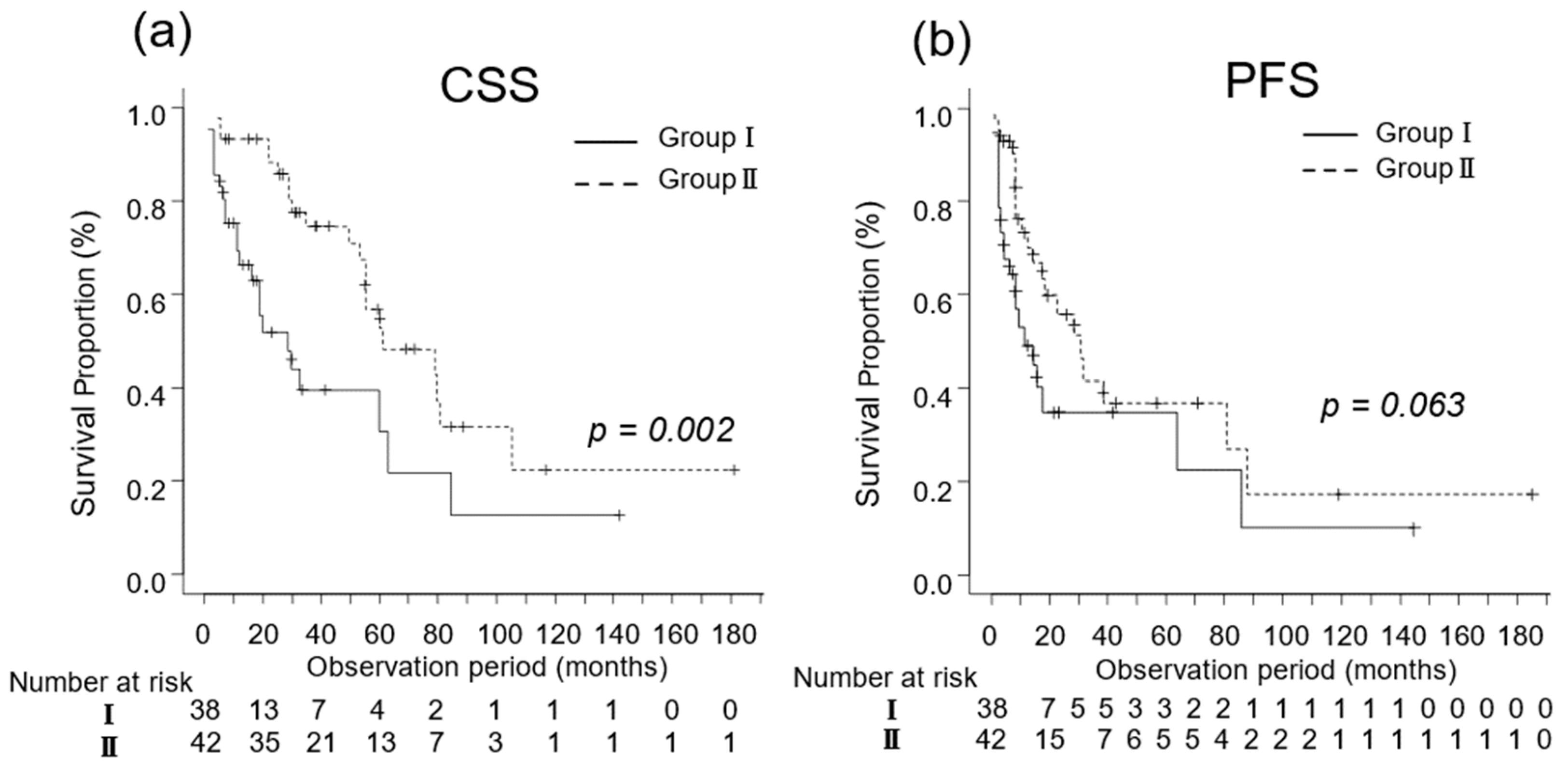
We investigated the oncological outcomes of patients with mRCC who underwent CN and in those who did not based on their IMDC risk classification. In patients with favorable-intermediate risk, the median CSS was 15 (IQR, 6–29) and 40 (IQR, 25–61) months in Groups I (n = 38) and II (n = 42), respectively (p = 0.002; Figure 3a). The median PFS was 14 (IQR, 6–63) and 30 (IQR, 14–87) months in Groups I (n = 38) and II (n = 42), respectively (p = 0.061; Figure 3b).
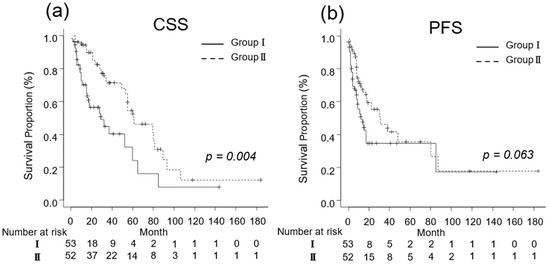
Figure 3.
Kaplan–Meier estimates of cancer-specific survival (CSS) and progression-free survival (PFS) in metastatic renal cell carcinoma patients with Eastern Cooperative Oncology Group performance status ≤ 1 who did not undergo cytoreductive nephrectomy (Group I) and in those who underwent cytoreductive nephrectomy (Group II). (a) The median CSS is 14 and 32 months in Groups I and II, respectively (p = 0.004; Figure 2a). (b) The 3-year PFS rate is 34.8% and 46.2% in Groups I and II, respectively (p = 0.063).
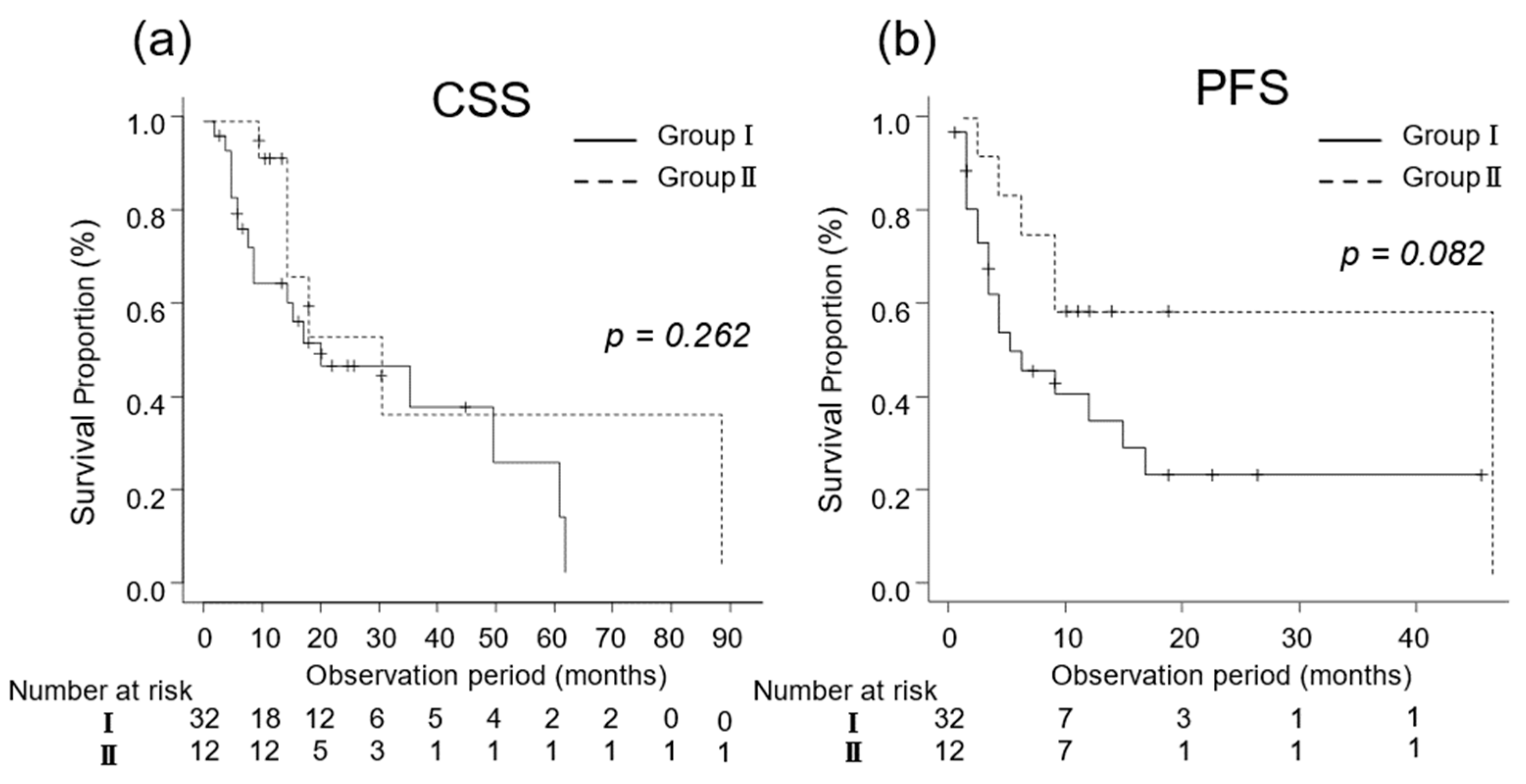
In patients at poor risk, the median CSS was 12 (IQR, 6–22) and 15 (IQR, 12–22) months in Groups I (n = 32) and II (n = 12), respectively (p = 0.262; Figure 4a). The median PFS was 5 (IQR, 3–15) and 48 (IQR, 4–NA) months in Groups I (n = 32) and II (n = 12), respectively (p = 0.082; Figure 4b).
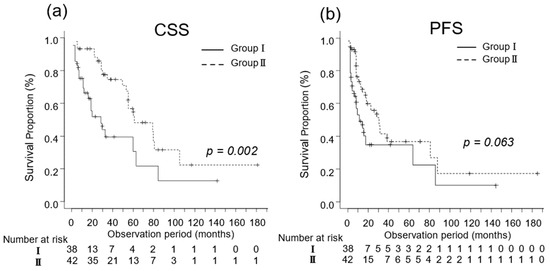
Figure 4.
Kaplan–Meier estimates of cancer-specific survival (CSS) and progression-free survival (PFS) in patients with favorable-intermediate risk metastatic renal cell carcinoma who did not undergo cytoreductive nephrectomy (Group I) and in those who underwent cytoreductive nephrectomy (Group II) based on their International Metastatic Renal Cell Carcinoma Database Consortium risk classification. (a) The median CSS is 15 and 40 months in Groups I and II, respectively (p = 0.002; Figure 3a). (b) The 3-year PFS rate is 36.2% in Group I and 42.7% in Group II (p = 0.061).
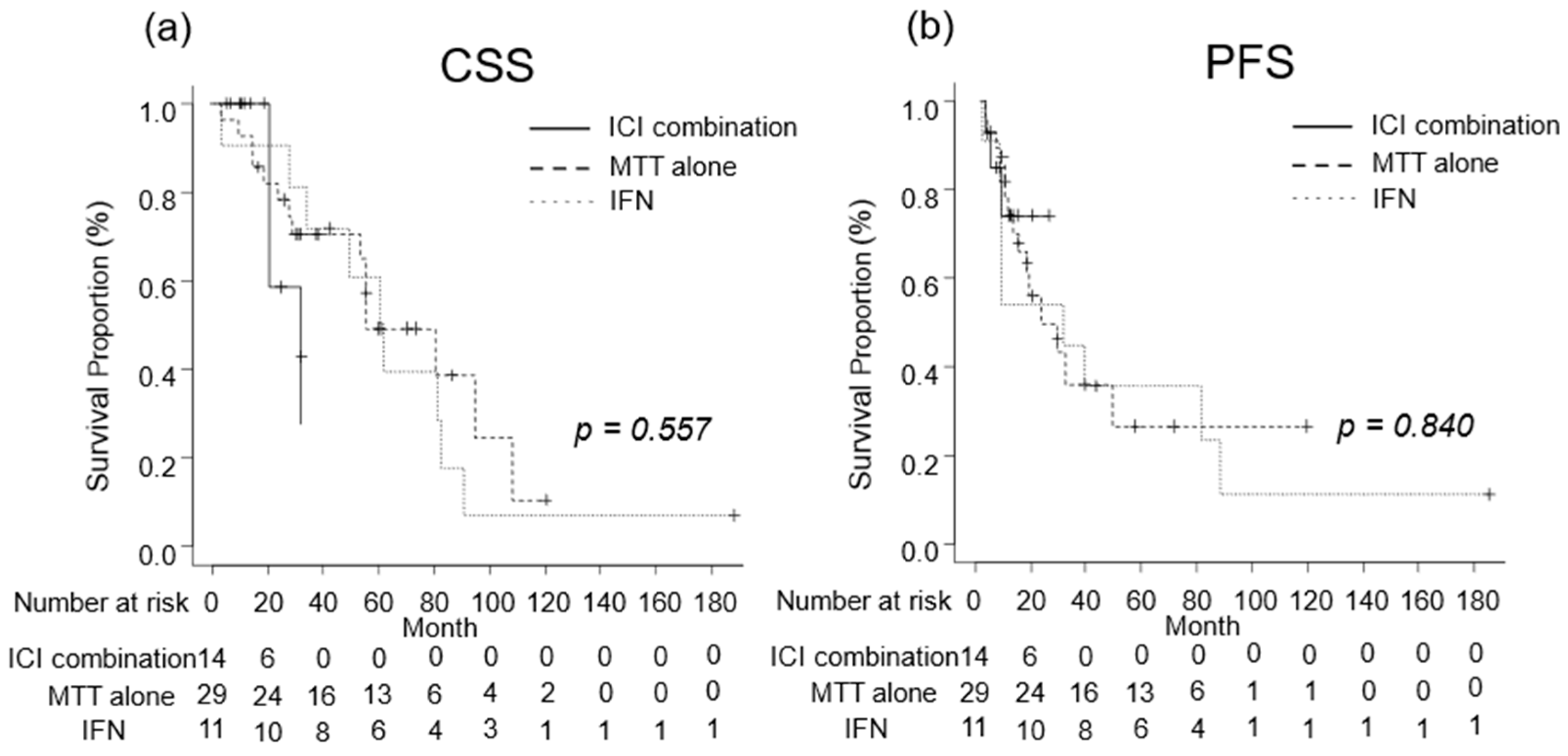
We investigated the oncological outcomes of patients with mRCC who received combination therapy including ICI, MTT alone, or IFN as systemic therapy. The median CSS was 32 (IQR, 21-NA) months in patients with mRCC receiving combination therapies including ICI, 79 (IQR, 53–106) months in those receiving MTT alone, and 61 (IQR, 28–81) months in those receiving IFN (Figure 5a). The median PFS was not reached (IQR, 8–NA months) in patients with mRCC who received combination therapies including ICI, 28 (IQR, 12-NA) months for those who received MTT alone, and 30 (IQR, 7–87) months in those who received IFN (Figure 5b). No significant differences in CSS or PFS were observed between the treatment regimens (Figure 6).
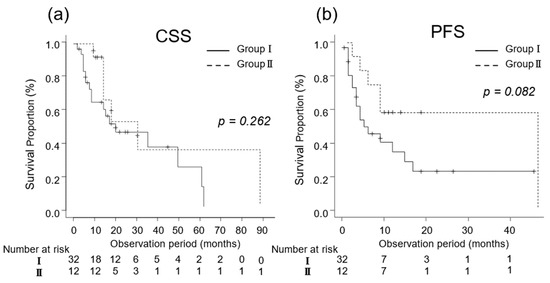
Figure 5.
Kaplan–Meier estimates of cancer-specific survival (CSS) and progression-free survival (PFS) in patients with poor risk metastatic renal cell carcinoma who did not undergo cytoreductive nephrectomy (Group I) and in those who underwent cytoreductive nephrectomy (Group II) based on their International Metastatic Renal Cell Carcinoma Database Consortium risk classification. (a) The median CSS is 12 and 15 months in Groups I and II, respectively (p = 0.262; Figure 4a). (b) The 3-year PFS rate is 23.1% in Group I and 58.3% in Group II (p = 0.082).
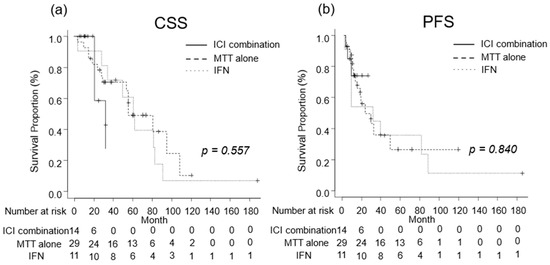
Figure 6.
Oncological outcomes by treatment regimen were evaluated using the Kaplan–Meier curves. (a) The median cancer-specific survival was 32 months in patients treated with combination therapy including immune checkpoint inhibitor (ICI), 79 months in those treated with molecular targeted therapy (MTT) alone, and 61 months in those treated with Interferon (IFN). (b) The median progression-free survival in patients who received combination therapy including ICI, MTT alone, and IFN alone were not reached, 28 months, and 30 months, respectively.
The multivariate analysis results indicated that CN was an independent prognostic factor for CSS and PFS (Table 3). Additionally, the examination of a multivariate Cox proportional hazard model with time-dependent covariates showed a hazard ratio of 13.24 (95% confidence interval (CI) 6.59–26.58, p < 0.001) for Group I compared to Group II. These results suggest that CN may contribute to the improvement of CSS.

Table 3.
Multivariate analysis according to oncological outcomes.
4. Discussion
The randomized controlled trial CARMENA demonstrated that sunitinib monotherapy resulted in noninferior OS compared to CN followed by sunitinib in moderate- and poor-risk patients with mRCC, based on the Memorial Sloan Kettering Cancer Center prognostic model [17]. Although the CARMENA study is a prospective study, interpreting the accuracy of the conclusions is difficult because of the following points. First, the study was discontinued because of the inability to accumulate the planned number of patients; second, the enrollment period was 8 years, which is relatively long. Third, only 20% of the centers were able to enroll more than eight cases (more than one case per year); fourth, the number of patients who could be followed up for >2 years was <100 in each group. Lastly, the abovementioned results suggest that the skills of each institution varied considerably, potentially resulting in biases despite the prospective design of the study. Notably, a secondary analysis of the CARMENA trial confirmed that patients who underwent secondary CN had a significantly longer OS than those who did not subsequently undergo CN (median OS: 48.5 and 15.7 months; hazard ratio (HR), 0.34; 95% CI, 0.22–0.54) [19], suggesting that some patients with mRCC benefit from CN treatment. High-intensity CN has been reported to prolong OS in stage IV upper urothelial carcinoma [27], and the usefulness of local therapy for metastatic tumors, as well as mRCC, is of interest in other carcinomas. In the present study, the OS of patients with mRCC who underwent CN was significantly prolonged regardless of the systemic therapy regimen. Furthermore, our results suggest that CN may improve oncological outcomes in patients with mRCC who have good ECOG-PS scores and no other organ metastases, such as brain or liver metastases, which are poor prognostic factors. Previous retrospective analyses of various datasets, including the National Cancer Database and IMDC, have consistently demonstrated that CN confers a survival benefit in patients with mRCCs with clear cell components [28,29]. Therefore, younger patients with mRCC who had fewer IMDC risk factors, no metastases with poor prognosis (such as bone, brain, or liver), and good performance status had a greater potential benefit from CN [13,29].
More than two decades ago, CN was recognized as a potential treatment method to improve oncological outcomes in patients with mRCC, based on the results of randomized controlled trials in which IFN and interleukin were administered as systemic therapy [11,15]. These well-selected patients may benefit from CN in the era of ICI, partly owing to the immune effects of CN [30]. Primary RCC is reportedly associated with the release of cytokines that promote inflammation and inhibit T cells, potentially inhibiting systemic antitumor immune responses [31]. Furthermore, primary renal tumor removal may be beneficial for patients with mRCC eliminating “immune sinks” and reducing the levels of immunosuppressive cytokines, thereby allowing for a more potent antitumor immune response [32]. The benefits of CN can be observed in six categories: regression of metastatic sites; decreased release of cytokines and growth factors that promote metastases; prevention of tumor growth and new metastases; relief of symptoms, such as hematuria and pain; decreased paraneoplastic syndromes; and improved QOL [10,11,12,13,14,15]. In a recent study, a subpopulation of CD133+/CD24+ cells was identified in clear cell RCC specimens and demonstrated self-renewal and clonogenic pluripotency [33]. Therefore, CN may inhibit tumor growth and metastasis by blocking not only surface proteins but also cancer stem cell signaling-specific pathways. In addition, prolonged OS has been reported in patients with mRCC who underwent multimodal treatment with CN, ICIs, and/or MTTs [13,29,34]. Similarly, CN has been reported to significantly prolong OS in patients with mRCC having various subtypes, such as sarcomatoid differentiation [35]. Thus, nephrectomy may be a potential treatment modality to provide a more effective immune response to mRCC lesions that have been or will be treated with ICIs [13]. Furthermore, the prognosis of patients with mRCC was statistically significantly prolonged after receiving the recent combination therapy including ICI [3,4,5,6,7,8,9]. In this study, we found no significant difference in oncological outcomes because the number of patients who underwent CN after receiving combination therapy including ICI was small and the observation period was short. Future studies in patients with mRCC in accordance with the current treatment strategy are warranted.
One of the main reservations regarding upfront CN for mRCC is the need to consider the risk that 15–30% of patients will not receive subsequent systemic therapy; many of such patients were unable to receive treatment owing to rapid disease progression or postoperative complications [10]. Therefore, a discussion on whether upfront CN should be considered in all patients is necessary.
The first consideration in systemic therapy for mRCC is the establishment of a method to identify patients with biologically aggressive diseases who have a poor prognosis [25]. The phase II study results of 66 patients who received sunitinib before scheduled CN revealed that 27% of the patients had disease progression before or at the time of surgery [36]. In another phase II trial involving 104 patients who received preoperative treatment with pazopanib before planned CN, 16% exhibited disease progression and were unable to receive surgery [37]. Those patients had a shorter OS than those who achieved clinical response (median OS 3.9 months vs. 24.0 months, HR = 3.92; 95% CI 1.78–8.63) [37,38]. Thus, identifying patients who are unlikely to benefit from CN by providing upfront systemic therapy may be possible [25]. However, deferred CN seems to be a treatment worth considering in the era of combination treatment with ICI + MTT, due to the fact that <10% of patients progress after their treatments [6,7,8]. Although some patients may show progression after treatment with ICI + MTT, the establishment of criteria for selecting patients who will benefit from ICI + MTT combination therapy is important.
Controversies remain on whether a combination with modern systemic therapy can improve survival in patients with mRCC and whether CN should be used as an upfront or deferred therapy [38]. A real-world multicenter analysis revealed no correlation between upfront and deferred CN and OS in patients with mRCC who underwent CN and received systemic therapy, although the OS of patients who underwent upfront CN was significantly higher than that of patients who received systemic therapy alone [21]. These results are similar to those of a retrospective study that observed no difference in OS between deferred and upfront CN, although patients with intermediate risk in the MSKCC classification benefited from upfront CN [39]. The SURTIME trial suggested that deferred CN, in which patients initiated systemic treatment with sunitinib and were offered CN if the disease did not progress, might be superior to upfront CN followed by sunitinib therapy for patients with mRCC [18]. In the intention-to-treat population, the 28-week PFS rates were 42% and 43% in the upfront and deferred CN groups, respectively (p = 0.61) [18]. At the time of analysis, 70% of patients in the upfront CN group and 57.1% in the deferred CN group had died, with cancer progression being the primary cause in 86% and 89% of patients in the upfront and deferred CN groups, respectively [18]. Additionally, patients receiving sunitinib before CN had a longer median OS than those undergoing upfront CN (32.4 vs. 15.0 months; HR, 0.57; 95% CI, 0.34–0.95) [19].
Although the ideal timing for delaying surgery remains unknown, deferred CN may be clinically beneficial in patients who respond to systemic therapy [19]. According to studies using the National Cancer Database, the OS of patients with mRCC who underwent deferred CN was at least equivalent to, if not longer than, that of patients who underwent upfront CN [28,40]. In the present study, although a comparison between upfront CN and deferred CN was not conducted, patients who underwent CN, particularly those with relatively good conditions, such as ECOG-PS ≤ 1 and IMDC favorable-intermediate risk, exhibited better OS and PFS compared with those who did not. Under such conditions, CN may improve oncological outcomes and serve as an effective treatment option. Additionally, considering factors such as QOL and medical costs, performing CN in such patients may be meaningful. Treatment with ICI often allows for the preservation of a relatively good QOL, although MTT therapy may cause challenging side effects, making sustaining treatment difficult and often requiring dose adjustments or interruptions [41]. Therefore, in patients who respond well to systemic therapy, implementing a deferred CN to provide drug-free intervals may improve the QOL. Cases in which deferred CN performed after systemic treatment resulted in no cancer progression, without the need to restart drug therapy, have been documented [42]. While reports on achieving long-term survival after discontinuing drug therapy after deferred CN are still relatively scarce, as similar cases continue to emerge in the future, it may be possible to offer the best treatment for many patients with mRCC. Furthermore, in the treatment of mRCC, the prolonged use of expensive medications poses a significant cost issue for both the patients and society, especially with the increasing prevalence of combination therapies involving ICI + ICI and ICI + MTT [43]. Considering its implication for cost, CN may offer potential improvements. Moreover, despite significant differences in patient backgrounds, choice of regimen in combination with systemic therapy, and timing of CN observed in the multivariate analysis, cytoreductive surgery may have potential benefits in improving oncological outcomes in mRCC patients. In the contemporary era of immunotherapy, multiple ongoing clinical trials are specifically examining the role of CN [44]. If the results of these prospective trials demonstrate the utility of CN, it may lead to the provision of better healthcare in various aspects, such as patients’ oncological outcomes, QOL, and economic costs. Further prospective study results are required to confirm these findings.
This study had some limitations. First, this was a retrospective multicenter, non-randomized study that may have involved a potential inherent bias. In particular, MTTs have been used as the first-line treatment for mRCC for approximately 10 years. In this study, the number of patients treated with combination ICI therapies was smaller than that of patients treated with MTT alone, and oncologic outcomes could not be investigated in detail. Therefore, the true efficacy of ICI + ICI and ICI + MTT in patients with mRCC could not be verified because many of them were treated with MTTs in this study. Second, the number of enrolled patients in this study was relatively small, thereby potentially leading to biased interpretations of the obtained results. The number of patients enrolled in the study was 137, which was very small; therefore, additional cases and long-term follow-up are necessary to determine the accuracy of the results of this study. However, CN has been suggested to be beneficial in some patients in the MTT era. Therefore, a larger number of patients treated with ICI could possibly yield similar results, although the limited sample size remains a limitation. Third, tumor progression, site of metastasis, and patient condition might have a significant impact on the failure to select a CN in patients with mRCC. In this study, a renal tumor biopsy was not performed in most patients in Group I, which may be because of the relatively high number of cases with poor ECOG-PS and the fact that the histological type was predicted based on imaging findings alone rather than based on a biopsy. Fourth, we observed a few cases in which diagnosis was not possible despite performing renal tumor biopsy. Approximately 70% of the patients, mainly in Group I, did not undergo pathological diagnosis by biopsy at the initiation of systemic treatment. In addition to the fact that imaging examinations such as CT and MRI could have predicted the histological type [45], we consider that biopsy was not performed based on the clinical judgment that treatment should be started as soon as possible because of the expected rapid progression of the disease. Empirical therapy might have been administered in many such cases; therefore, the oncological outcomes of Groups I and II could not be accurately evaluated based on the differences in histological types. Fifth, brain metastases were observed only in Group I patients. Liver and bone metastases were observed more frequently in Group I patients than in Group II patients, although without significant difference. Hence, patients in Group I may have additional potentially poor prognostic factors, which may have a negative impact on oncological outcomes. Sixth, this study could not investigate the oncological outcomes according to the timing of surgery (upfront or deferred CN) or the choice of systemic treatment. Upfront CN is generally performed in patients with good performance status and a relatively small number of metastases, whereas deferred CN is limited to patients who respond well to systemic therapy and have good PS at the time of the procedure. Therefore, we cannot eliminate the possibility that this difference influenced the results of the present study. Although the number of patients who underwent CN in this study was significantly smaller than that of patients who did not undergo CN, we evaluated the usefulness of CN in patients who underwent upfront or deferred CN. Seventh, patients who did not receive systemic therapy after CN or those who did not receive CN after systemic therapy because of disease progression or death were not included in this study. An analysis method such as the landmark analysis or time-varying treatment status analysis should have been employed for this study. Finally, the enrollment period of patients in this study was relatively long. Because significant changes in the treatment and surgical procedures for mRCC were observed during this period, the oncological outcome results may have been biased depending on the presence or absence of CN.
5. Conclusions
This retrospective multicenter study evaluated the efficacy of CN in patients with mRCC. Patients in Group II had a significantly longer CSS and PFS than those in Group I. Furthermore, the multivariate analysis results revealed that CN was an independent prognostic factor affecting oncological outcomes in patients with mRCC. CN may improve oncological outcomes in patients who receive systemic therapy before and after surgery. Standardizing the systemic treatment regimens and conducting a prospective multicenter study with a short enrollment period may be necessary to examine the efficacy of CN for mRCC.
Author Contributions
Conceptualization, N.K., K.I., and T.K.; methodology, N.K. and K.I.; investigation, N.K.; resources, N.K., K.I., Y.K., K.T., M.N., T.I. (Takashi Ishida), S.Y., F.S., M.K., S.T., K.K., D.K., M.T., Y.T., T.I. (Takayasu Ito), and K.N.; data curation, N.K. and T.K.; supervision, Y.T. and T.I. (Takayasu Ito); writing—original draft preparation, N.K.; writing—review and editing, T.K.; and funding acquisition, K.I. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (20K09535 and 22K16816).
Institutional Review Board Statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the institutional review boards of all the participating institutions (approval number: 2022-125).
Informed Consent Statement
The need for informed consent was waived because this was a retrospective cohort study. Opt-outs were performed on the Gifu University website. The study information can be accessed at https://www.med.gifu-u.ac.jp/visitors/disclosure/docs/2022-125.pdf (accessed on 14 August 2023).
Data Availability Statement
Data and material are provided in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CN | cytoreductive nephrectomy |
| CSS | cause-specific survival |
| ECOG-PS | Eastern Cooperative Oncology Group performance status |
| ICIs | immune checkpoint inhibitors |
| IFN | interferon |
| IMDC | International Metastatic Renal Cell Carcinoma Database Consortium |
| mRCC | metastatic renal cell carcinoma |
| MTTs | molecular targeted therapy |
| OS | overall survival |
| PFS | progression-free survival |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Claeys, T.; Lumen, N.; Kumps, C.; Praet, M.; De Meerleer, G.; Rottey, S.; Ost, P.; Devisschere, P.; Villeirs, G.; Fonteyne, V.; et al. The independent oncological role for cytoreductive nephrectomy in metastatic renal cell carcinoma: Prognostic features in the era of targeted therapies. Urol. Oncol. 2017, 35, 152.e13–152.e22. [Google Scholar] [CrossRef] [PubMed]
- de Velasco, G.; Bex, A.; Albiges, L.; Powles, T.; Rini, B.I.; Motzer, R.J.; Heng, D.Y.C.; Escudier, B. Sequencing and Combination of Systemic Therapy in Metastatic Renal Cell Carcinoma. Eur. Urol. Oncol. 2019, 2, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Powles, T.; Donskov, F.; Plimack, E.R.; Barthélémy, P.; Hammers, H.J.; et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J. Immunother. Cancer 2020, 8, e000891. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Motzer, R.J.; Rini, B.I.; Haanen, J.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Gravis-Mescam, G.; Uemura, M.; Lee, J.L.; et al. Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Bhindi, B.; Abel, E.J.; Albiges, L.; Bensalah, K.; Boorjian, S.A.; Daneshmand, S.; Karam, J.A.; Mason, R.J.; Powles, T.; Bex, A. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. Eur. Urol. 2019, 75, 111–128. [Google Scholar] [CrossRef]
- Flanigan, R.C.; Salmon, S.E.; Blumenstein, B.A.; Bearman, S.I.; Roy, V.; McGrath, P.C.; Caton, J.R., Jr.; Munshi, N.; Crawford, E.D. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 2001, 345, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Nelson, R.A.; Vogelzang, N. Disease-Specific Survival in De Novo Metastatic Renal Cell Carcinoma in the Cytokine and Targeted Therapy Era. PLoS ONE 2013, 8, e63341. [Google Scholar] [CrossRef] [PubMed]
- Bakouny, Z.; El Zarif, T.; Dudani, S.; Wells, J.C.; Gan, C.L.; Donskov, F.; Shapiro, J.; Davis, I.D.; Parnis, F.; Ravi, P.; et al. Upfront Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors or Targeted Therapy: An Observational Study from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. 2023, 83, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, C.; Slots, C.; Vasdev, N.; Rottey, S.; Fonteyne, V.; Andras, I.; Albersen, M.; De Meerleer, G.; Bex, A.; Decaestecker, K. Current role of cytoreductive nephrectomy in metastatic renal cell carcinoma. Turk. J. Urol. 2021, 47, S79–S84. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, L.; Manfredi, C.; Cirillo, L.; Fusco, G.M.; Passaro, F.; Abate, M.; La Rocca, R.; Mastrangelo, F.; Spirito, L.; Pandolfo, S.D.; et al. Cytoreductive Nephrectomy and Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives. Medicina 2023, 59, 767. [Google Scholar] [CrossRef] [PubMed]
- Mickisch, G.H.; Garin, A.; van Poppel, H.; de Prijck, L.; Sylvester, R.; European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet 2001, 358, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.B.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Mulders, P.; Jewett, M.; Wagstaff, J.; van Thienen, J.V.; Blank, C.U.; van Velthoven, R.; Del Pilar Laguna, M.; Wood, L.; van Melick, H.H.E. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients with Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019, 5, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Chevreau, C.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; Guy, L.; et al. Sunitinib Alone or After Nephrectomy for Patients with Metastatic Renal Cell Carcinoma: Is There Still a Role for Cytoreductive Nephrectomy? Eur. Urol. 2021, 80, 417–424. [Google Scholar] [CrossRef]
- Ghatalia, P.; Handorf, E.A.; Geynisman, D.M.; Deng, M.; Zibelman, M.R.; Abbosh, P.; Anari, F.; Greenberg, R.E.; Viterbo, R.; Chen, D.; et al. The Role of Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma: A Real-World Multi-Institutional Analysis. J. Urol. 2022, 208, 71–79. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, K.; Kameyama, K.; Taniguchi, T.; Kawada, K.; Ishida, T.; Takagi, K.; Nagai, S.; Enomoto, T.; Tomioka, M.; Kawase, M.; et al. Effectiveness and Safety of Molecular-Targeted Therapy after Nivolumab Plus Ipilimumab for Advanced or Metastatic Renal Cell Carcinoma: A Multicenter, Retrospective Cohort Study. Cancers 2022, 14, 4579. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, K.; Yamada, T.; Kameyama, K.; Taniguchi, T.; Kawada, K.; Ishida, T.; Nagai, S.; Enomoto, T.; Ueda, S.; Takagi, K.; et al. The Efficacy and Safety of Immune Checkpoint Inhibitor and Tyrosine Kinase Inhibitor Combination Therapy for Advanced or Metastatic Renal Cell Carcinoma: A Multicenter Retrospective Real-World Cohort Study. Cancers 2023, 15, 947. [Google Scholar] [CrossRef] [PubMed]
- Bhindi, B.; Graham, J.; Wells, J.C.; Bakouny, Z.; Donskov, F.; Fraccon, A.; Pasini, F.; Lee, J.L.; Basappa, N.S.; Hansen, A.; et al. Deferred Cytoreductive Nephrectomy in Patients with Newly Diagnosed Metastatic Renal Cell Carcinoma. Eur. Urol. 2020, 78, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, M.; Nguyen, D.D.; Modonutti, D.; Haeuser, L.; Lipsitz, S.; Mossanen, M.; Kibel, A.S.; Lughezzani, G.; Trinh, Q.D.; Cole, A.P. Impact of high-intensity local treatment on overall survival in stage IV upper tract urothelial carcinoma. Urol. Oncol. 2021, 39, 436.e1–436.e10. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Sun, M.; Meyer, C.P.; Nguyen, P.L.; Pal, S.K.; Chang, S.L.; de Velasco, G.; Trinh, Q.D.; Choueiri, T.K. Survival Analyses of Patients with Metastatic Renal Cancer Treated with Targeted Therapy with or without Cytoreductive Nephrectomy: A National Cancer Data Base Study. J. Clin. Oncol. 2016, 34, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Wells, J.C.; Donskov, F.; Lee, J.L.; Fraccon, A.; Pasini, F.; Porta, C.; Bowman, I.A.; Bjarnason, G.A.; Ernst, D.S.; et al. Cytoreductive Nephrectomy in Metastatic Papillary Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. Oncol. 2019, 2, 643–648. [Google Scholar] [CrossRef]
- Fujikawa, K.; Matsui, Y.; Oka, H.; Fukuzawa, S.; Takeuchi, H. Serum C-reactive protein level and the impact of cytoreductive surgery in patients with metastatic renal cell carcinoma. J. Urol. 1999, 162, 1934–1937. [Google Scholar] [CrossRef]
- Lahn, M.; Fisch, P.; Köhler, G.; Kunzmann, R.; Hentrich, I.; Jesuiter, H.; Behringer, D.; Muschal, B.; Veelken, H.; Kulmburg, P.; et al. Pro-inflammatory and T cell inhibitory cytokines are secreted at high levels in tumor cell cultures of human renal cell carcinoma. Eur. Urol. 1999, 35, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Bonomi, L.; Lillaz, B.; Paglino, C.; Rovati, B.; Imarisio, I.; Morbini, P.; Villa, C.; Danova, M.; Mensi, M.; et al. Renal cell carcinoma-induced immunosuppression: An immunophenotypic study of lymphocyte subpopulations and circulating dendritic cells. Anticancer Res. 2007, 27, 165–173. [Google Scholar] [PubMed]
- Lasorsa, F.; Rutigliano, M.; Milella, M.; Ferro, M.; Pandolfo, S.D.; Crocetto, F.; Autorino, R.; Battaglia, M.; Ditonno, P.; Lucarelli, G. Cancer Stem Cells in Renal Cell Carcinoma: Origins and Biomarkers. Int. J. Mol. Sci. 2023, 24, 13179. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Hutchinson, R.C.; Ghandour, R.A.; Freifeld, Y.; Fang, D.; Sagalowsky, A.I.; Lotan, Y.; Bagrodia, A.; Margulis, V.; Hammers, H.J.; et al. Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: An analysis of the National Cancer Database. Urol. Oncol. 2020, 38, 604.e9–604.e17. [Google Scholar] [CrossRef] [PubMed]
- Tully, K.H.; Berg, S.; Paciotti, M.; Janisch, F.; Reese, S.W.; Noldus, J.; Shariat, S.F.; Choueiri, T.; Müller, G.; McGregor, B.; et al. The Natural History of Renal-Cell Carcinoma with Sarcomatoid Differentiation, a Stage-by-Stage Analysis. Clin. Genitourin. Cancer 2023, 21, 63–68. [Google Scholar] [CrossRef]
- Powles, T.; Blank, C.; Chowdhury, S.; Henblas, S.; Peters, J.; Shamash, J.; Sarwar, N.; Boleti, E.; Sahdev, A.; O’Brien, T.; et al. The outcome of patients treated with sunitinib prior to planned nephrectomy in metastatic clear cell renal cancer. Eur. Urol. 2011, 60, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Sarwar, N.; Stockdale, A.; Sarker, S.J.; Boleti, E.; Protheroe, A.; Jones, R.; Chowdhury, S.; Peters, J.; Oades, G.; et al. Safety and Efficacy of Pazopanib Therapy Prior to Planned Nephrectomy in Metastatic Clear Cell Renal Cancer. JAMA Oncol. 2016, 2, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Esdaille, A.R.; Abel, E.J. Evolution of risk stratification systems is critical for improving patient selection for cytoreductive nephrectomy. Cancer 2021, 127, 3920–3923. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, R.; Wimalasingham, A.; Szabados, B.; Stewart, G.D.; Welsh, S.J.; Kuusk, T.; Blank, C.; Haanen, J.; Klatte, T.; Staehler, M.; et al. Deferred Cytoreductive Nephrectomy Following Presurgical Vascular Endothelial Growth Factor Receptor-targeted Therapy in Patients with Primary Metastatic Clear Cell Renal Cell Carcinoma: A Pooled Analysis of Prospective Trial Data. Eur. Urol. Oncol. 2020, 3, 168–173. [Google Scholar] [CrossRef]
- Bhindi, B.; Habermann, E.B.; Mason, R.J.; Costello, B.A.; Pagliaro, L.C.; Thompson, R.H.; Leibovich, B.C. Comparative survival following initial cytoreductive nephrectomy versus initial targeted therapy for metastatic renal cell carcinoma. J. Urol. 2018, 200, 528–534. [Google Scholar] [CrossRef]
- Tran, J.; Ornstein, M.C. Clinical Review on the Management of Metastatic Renal Cell Carcinoma. JCO Oncol. Pract. 2020, 18, 187–196. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kawasaki, Y.; Sato, S.; Katayama, H.; Satake, Y.; Shimada, S.; Sato, T.; Kawamorita, N.; Yamashita, S.; Mitsuzuka, K.; et al. Deliberation on Deferred Cytoreductive Nephrectomy and Postoperative Treatment for Advanced Renal Cell Carcinoma: A Case Report. Case Rep. Oncol. 2022, 15, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Buja, A.; De Luca, G.; Gatti, M.; Cozzolino, C.; Rugge, M.; Zorzi, M.; Gardi, M.; Sepulcri, M.; Bimbatti, D.; Baldo, V.; et al. Renal cell carcinoma: The population, real world, and cost-of-illness. BMC Urol. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Lichtbroun, B.J.; Srivastava, A.; Doppalapudi, S.K.; Chua, K.; Singer, E.A. New Paradigms for Cytoreductive Nephrectomy. Cancers 2022, 14, 2660. [Google Scholar] [CrossRef]
- Morshid, A.; Duran, E.S.; Choi, W.J.; Duran, C. A Concise Review of the Multimodality Imaging Features of Renal Cell Carcinoma. Cureus 2021, 13, e13231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).