Abstract
Cellular metabolism, apoptosis, fertilization, and proliferation of granulosa cells belong to a battery of processes where microRNAs can be detected and associated with infertility. The aim of the present review is to focus on mammalian oocyte maturation events and the association between oocyte growth and miRNA expression. PubMed/Medline, Google Scholar and Scopus databases were searched, and 33 studies were included. Regarding the correlation among miRNA expression and the regulation of granulosa cells and cumulus cells, the most important miRNAs were let-7b, let-7c and miR-21. Additionally, the loss of Dicer, an enzyme involved in miRNA biogenesis, is probably a crucial factor in oogenesis, oocyte maturation and embryogenesis. Furthermore, miRNAs interfere with different cellular mechanisms like apoptosis, steroidogenesis, genome integrity, angiogenesis, antioxidative response and, consequently, oocyte maturation. Hence, it is of major importance to clarify the role and mechanism of each miRNA as understanding its action may develop new tools and establish new diagnostic and treatment approaches for infertility and ovarian disorders.
1. Introduction
Infertility is commonly caused by ovulatory dysfunction, male factor problems, and tubal disease. Additionally, unexplained infertility remains a setback for approximately 15% of couples [1]. Furthermore, fertility is age-related, especially for women, as maternal age plays a crucial role and is related to ovary aging [2]. Oocyte maturation, initiates with the first meiotic progression of female germ cells during embryonic development and sustains at the dictyate stage of meiotic prophase I. The imperative event of folliculogenesis begins with fetal development at 4 months of pregnancy [3]. The oocytes from meiotic prophase I, through metaphase II (MII), have high transcriptional activity. Consequently, the mRNAs and proteins that are produced support early embryogenesis [4,5]. In the phase of primordial follicles, oocytes are surrounded by squamous granulosa cells (GCs), which promote steroidogenesis [6]. When primary follicles differentiate to secondary follicles, GCs proliferate and form multiple layers [7]. GC interactions play an important role in oocyte growth, cumulus triggering, expansion and, finally, ovulation, which prepares the oocyte to be fertilized [8,9].
In recent years, many disorders have been linked to miRNA expression leading to the proposition that it may be used as a biomarker [10]. MiRNAs are small, single-stranded, and non-coding RNA molecules (19–22 nucleotides), which regulate a gene expression during post-transcriptional stage by base-pairing to mRNAs [11,12]. Approximately 1% to 5% of all genes encode miRNAs, regulating 30% of gene expression [12]. MiRNAs are produced by two RNase III proteins, Drosha and Dicer, that transcript primary into mature miRNA [13,14]. These molecules interact first with Drosha and DGCR8 in the nucleus and then are released as pre-miRNAs into the cytoplasm, cleaved by Dicer and shaping the miRNA duplex [15]. Figure 1 provides a brief illustration of the canonical pathway of miRNA biogenesis.
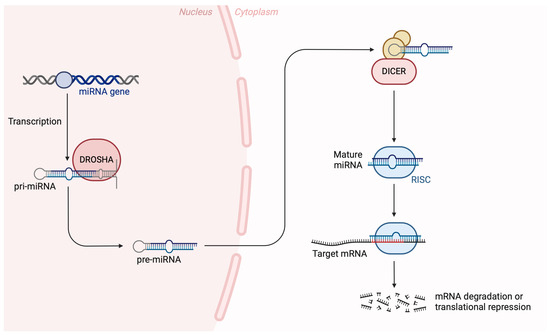
Figure 1.
The canonical pathway of miRNA biogenesis. Precursor miRNA molecules undergo nuclear and cytoplasmic processing events, carried out by the endoribonucleases DROSHA and DICER to produce mature miRNAs that are loaded onto the RISC (RNA-induced silencing complex) to exert their biological function. Created with BioRender.com on 25 April 2024.
As miRNAs are identified in developmental timing, fertilization, early embryonic development and granulosa cell proliferation, ovaries, oocytes, cumulus cells and preimplantation embryos may express many of them [16,17,18,19,20,21]. Single point mutations of miRNA sequences, or differential expression of miRNA transcripts, may be related to various cellular mechanisms [22]. Consequently, these molecules could be responsible for disorders like endometriosis, premature ovarian failure, and polycystic ovarian syndrome by initiation of different signaling pathways [23,24].
This article focuses on the events and development related to mammalian oocyte maturation and association of oocyte growth with miRNAs expression. We will discuss the fundamental related mechanisms in order to better understand female reproduction by providing information in detail.
2. Materials and Methods
A comprehensive literature search was conducted using the PubMed/Medline, Google Scholar, and Scopus databases from October 2023 to February 2024 with the aim to describe the relationship between miRNAs and oocyte maturation. The search terms used included “Oocyte maturation”, “miRNAs”, “micro RNAs”, “Infertility”, “Granulosa Cells”, “Female Reproduction” and “Assisted Reproduction”. Boolean operators (OR, AND) are administered, combined with those keywords either used as presented, separately and in combination. Two independent reviewers, E.N. and A.P., assessed abstracts of the retrieved results; the content of full-text publications that were eligible was further assessed. A third reviewer, D.M., was responsible for making the decision if a study was selected by only one reviewer.
A total of 1147 articles were collected via different databases. In total, 82 full text articles were assessed and 32 articles/studies were suitable for providing information in this literature review. The criteria for article eligibility consist of original articles, case reports, reviews, systematic reviews, and meta-analyses. No English language written articles were excluded. Figure 2 illustrates the study selection process.
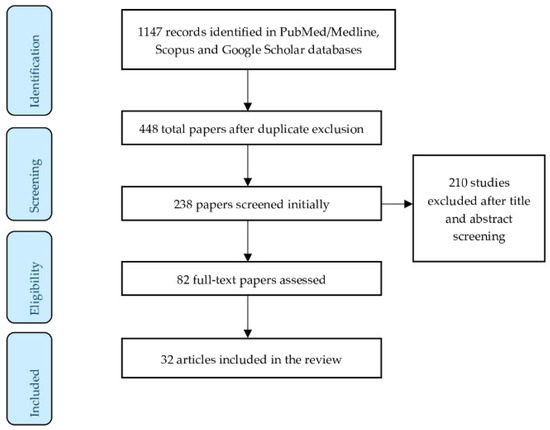
Figure 2.
Flow diagram of the study selection process.
3. Results
Cumulus–oocyte complex (COC) contains oocyte and cumulus cells (CCs), which supply molecular nutrients and help the development of oocytes [25]. GCs and CCs are controlled by various miRNAs, enhancing the conviction that miRNAs are key factors for reproduction [26,27]. Also, microRNA expression profiling shows that miRNAs may be detected in microvesicle and exosome preparations from follicular fluid (FF) [28]. Regarding the correlation among miRNAs expression and the regulation of GC and CC, many studies have been published. Sirotkin et al. showed that inhibiting miR-15a affects the proliferation of human GCs by decreasing the proliferating cell nuclear antigen levels (PCNA) [29]. Moreno et al. examined 30 women undergoing ICSI treatment and found the expression of 866 miRNAs in FFs and GCs. The results showed 13 differentially expressed miRNAs (2 up-regulated and 11 down-regulated from MII to GV oocytes). Also, the comparison between MII and MI revealed seven differentially expressed miRNAs in MII (three of them up-regulated and four down-regulated). Only one, hsa-miR-424, was detected in higher levels in women of advanced age [30]. Additionally, Santonocito et al. described the up-regulation of hsa-miR-483 and hsa-miR-339 in FF, which in the study of Moreno et al. have also been found highly expressed in FFs from MII [31]. Moreover, both studies detected the higher expression levels of has-mir-451 in FFs in the early phases of oocytes maturation [30,32]. Also, miRNAs such as hsa-miR-139-3p were shown to be down-regulated in the FF from GV oocytes compared with MII oocytes [33].
The miRNAs of human let-7 family were the subject in a series of studies. Assou et al. studied the expression of miRNA in human MII oocytes and in CCs and they revealed that the most abundant miRNAs in CCs were let-7b, let-7c and miR-21, while miR-184 and miR-10a were mainly detected in oocytes [34]. Andrei et al. revealed that miR-21-5p, let-7a-5p and let-7f-5p miRNAs were significantly over-expressed in GCs and CCs. Hence, the authors proposed that differentially expressed miRNAs are involved in the regulation of steroidogenesis, apoptosis and proliferation of GCs [35]. According to Jenabi et al., miR-21 was down-regulated in women with female factor infertility [36]. Therefore, miR-21 may have a major developmental role in oocytes via suppression of genes, promoting apoptosis in CCs [37,38,39]. When Bartolucci et al. examined microRNA-21 in human oocytes, the cultured CCs treated with an mir-21-5p inhibitor showed an increased apoptotic rate followed by a marked PTEN expression (a gene that is well-known for inhibiting the AKT-dependent survival pathway in cumulus cells) [40]. Figure 3 presents the most studied miRNAs expressed in cumulus cells (CCs), oocytes and follicular fluid (FF), which regulate and control oocyte meiotic maturation.
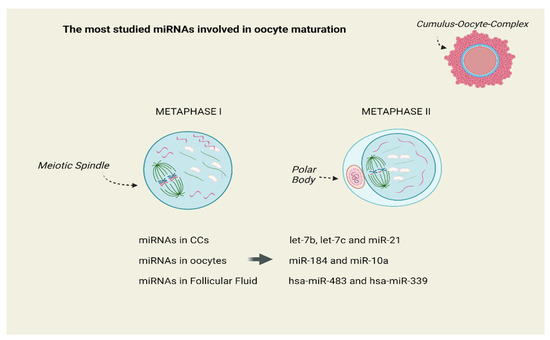
Figure 3.
The most studied miRNAs expressed in Cumulus Cells (CCs), Oocytes and Follicular Fluid (FF), which regulate and control oocyte meiotic maturation. Created with BioRender.com on 4 May 2024.
Dysfunctions in ovary tissue may cause various miRNAs alterations and affect oocytes. The miR-548 members seem to have a crucial role in maintaining oocyte function. Oltean et al. reported that hsa-miR-548au-3p was down-regulated and hsa-miR-548ae-5p, hsa-miR-548t-3p and hsa-miR-548au-5p were up-regulated [41]. Zhang et al. examined 68 patients by collecting ovarian FF and detected 47 miRNAs, which were involved in oocyte quality. Among two groups (high and poor oocyte quality), seven miRNAs were up-regulated in the poor oocyte quality group [42]. The down-regulation of hsa-miR-513c-5p in the poor oocyte quality group is expected to affect oocyte quality and is related according to other studies in breast cancer and hormonal dysregulation [43]. The up-regulation of miR-505-3p in the group of poor oocyte quality is possibly related to infertility. Its over-expression in mice was associated with delayed ovary maturation and lower fertility [44]. However, Barragán and colleagues, by examining a total of 36 in vivo matured MII oocytes collected from 30 healthy women recruited for oocyte donation, found that five miRNAs such as hsa-miR-220b, miR-4262 and miR-1260a were increased in the older group (age 32 ± 2 years and high antral follicular count approx. 29 ± 7 follicles) [45].
The miR-320 family plays a pivotal role in embryo development [46]. Machtinger et al., by collecting FF from 40 women that underwent IVF treatment, studied 754 exmiRNAs expression. The authors identified 207 extracellular microRNAs (exmiRNAs); miR-30d-5p, miR-320b, miR-10b-3p, miR-1291, and miR-720 were the most detected. Four ex-miRNAs (miR-202-5p, miR-206, miR-16-1-3p, and miR-1244) had a higher level of expression in fertilized oocytes [47]. Those findings are in alignment with the surveys of Sang et al. and Moreno et al., who also documented the up-regulated expression of miR-720 detected in FF from mature oocytes [30,48]. Additionally, Zhang et al. showed that hsa-miR-320e was up-regulated in the poor oocyte quality group, proposing that this up-regulation might affect the proliferation of ovarian GCs, resulting in oocyte degradation [42]. Santonocito et al. collected FF and plasma from 15 women undergoing IVF and identified miR-10b, miR-29a, miR-99a, miR-125b, miR-132, miR-202, miR-212, and miR-874 to be highly expressed in granulosa or in cumulus cells. They also supported that miR-29a, miR-99a, miR-100, miR-132, miR-212, miR-214, miR-218, miR-508-3p, and miR-654-3p may have a regulatory role through molecular signaling pathways (WNT, MAPK, ErbB, and TGFβ) [49].
Angiogenesis triggered by hypoxia happening in aging follicles remains important. According to this, miRNAs play an essential role in the cellular feedback to hypoxia. Al-Edani et al. studied CC samples of older women (>37 years) and compared them with CC samples of younger (<30 years) and median (31–34 years) age. Authors showed that mir-425, mir-744, mir-146b, and Let-7d were expressed in younger and median age women, while mir-202 and Let-7e were expressed in the older women. All the aforementioned miRNAs are targeting hypoxia- and angiogenesis-related genes [50,51].
Finally, an altered expression of miRNAs like miR-145, miR-92 and miR-21 has been reported, which target ACVR1b and SMAD7, and inhibit the apoptosis of GCs [52,53,54]. On the contrary, miR-205 and let-7g promote the apoptosis of GCs and inhibit estrogen synthesis via CREB1 and MAPK3K1 signaling pathways [55]. Lastly, a recent study in GCs from women with diminished ovarian reserve found increased expression of miR-484, correlated with ovarian function and triggering of granulosa cells [56]. Supplementary Table S1 illustrates the design and main outcomes of all the included studies in the review.
4. Discussion
Oocyte maturation is a multiplex process consisting of a battery of biological mechanisms; a plethora of molecules and signaling pathways regulate that process. The rapid technological development such as genetic modification of animal models, molecular biology, and biochemistry helps the researchers to gain a better understanding of oocyte GV arrest, meiosis I resumption and miRNAs expression. Because the oocyte maturation is not only self-depended, but it is based on mutual feedback and physical contact with follicular granulosa cells, it is interesting to focalize on different molecular pathways involving oocytes, granulosa cells, and oocyte microenvironment.
Studying the miRNAs in reproductive medicine represents a great challenge as it highlights important molecular pathways in female reproductive tissues and embryonic development. Consequently, better knowledge may provide new biomarkers and more personalized approaches for fertility issues. According to Eppig et al. oocyte maturation is a process in which the oocyte undergoes different transformations from the prophase I to metaphase II (MII) through meiotic division, chromatin remodeling and organelle reorganization [57]. Additionally, it acquires a transcriptomic and proteomic content essential for the early stages of embryonic development [58]. Consequently, understanding the mechanisms of protein expression and mRNA differentiation, should be the main field of research regarding female oogenesis. Specific miRNA profiling studies propose that changes in the expression pattern of miRNAs may be detected during the growth and development of mammalian oocytes. For example, miR-21, let-7family, miR-27a and miR-322 and DICER are essential in oocyte development and many research groups have established a possible interaction between their expression and oogenesis. miRNAs seem to regulate essential mechanisms like apoptosis, meiosis, hormone secretion and intracellular molecular pathways [33,34,36]. Figure 4 illustrates an overview of oocyte maturation failure and the affected signaling pathways.
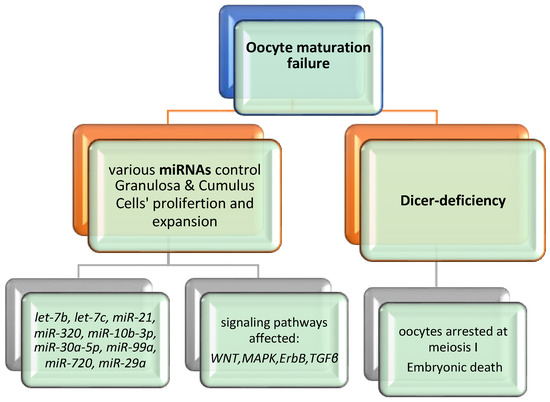
Figure 4.
Overview of oocyte maturation failure.
The loss of Dicer is a crucial factor in oogenesis, oocyte maturation and embryogenesis [59]. The vital role of Dicer can be documented in the case of Dicer deficiency, resulting in embryonic death [60,61]. Moreover, the involvement of Dicer in oogenesis was proposed through conditional knockout experiments, in which oocytes lacking Dicer arrest at meiosis I [62,63]. Liu et al. confirmed the above theory and proposed that Dicer and miRNA were essential for spindle organization, meiotic maturation and consequently control of the quality of oocytes [64]. As the most abundant miRNAs in CCs are revealed to be let-7b, let-7c and miR-21, members of let-7 family and mir-21 seem to have an essential role in female reproduction due to their involvement in the regulation of steroidogenesis, in apoptosis and in the proliferation of GCs [34,35,36,39,40]. Table 1 provides a summary list of the most studied miRNAs and their involvement in oocyte development and maturation.

Table 1.
Summary list of miRNAs and their involvement in oocyte development.
5. Conclusions
The present study is the first to our knowledge to provide an overview on miRNA expression related to oocyte maturation. Even though the current review highlights the number of miRNAs known to interfere with oocyte-related proteins, it would also be of great interest to focus on the polymorphisms detected on the corresponding genes and their effect on female reproduction.
Finally, it should be noted that even though the cause of miRNA-differentiated expression is unclear, epigenetic changes, genetic mutations and oxidative stress can be possible factors, causing their dysregulation. It is of major importance to clarify the role and mechanism of each miRNA as understanding its action may develop new tools or markers and establish pioneer approaches for diagnosis and treatment of infertile couples and ovarian dysfunction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diseases12060121/s1, Table S1: Included studies in the review.
Author Contributions
Conceptualization, E.N., T.V. and S.S.; methodology, A.P., D.M. and P.D.; validation, A.-A.V., V.S. and A.Z.; data curation, E.D., K.L. and C.S.; writing—original draft preparation, E.N. and A.P.; writing—review and editing, E.D., A.-A.V., A.Z., K.L. and P.P.; visualization, V.S.; supervision, C.S., P.P. and P.D.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bendarska-Czerwinska, A.; Zmarzly, N.; Morawiec, E.; Panfil, A.; Brys, K.; Czarniecka, J.; Ostenda, A.; Dziobek, K.; Sagan, D.; Boron, D.; et al. Endocrine disorders and fertility and pregnancy: An update. Front. Endocrinol. 2022, 13, 970439. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T.; Collins, J.; Egozcue, J.; Evers, L.H.; Gianaroli, L.; Leridon, H.; Sunde, A.; Templeton, A.; Van Steirteghem, A.; Cohen, J.; et al. Fertility and ageing. Hum. Reprod. Update 2005, 11, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Vallee, M.; Gravel, C.; Palin, M.F.; Reghenas, H.; Stothard, P.; Wishart, D.S.; Sirard, M.A. Identification of novel and known oocyte-specific genes using complementary DNA subtraction and microarray analysis in three different species. Biol. Reprod. 2005, 73, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Dalbies-Tran, R.; Mermillod, P. Use of heterologous complementary DNA array screening to analyze bovine oocyte transcriptome and its evolution during in vitro maturation. Biol. Reprod. 2003, 68, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Braw-Tal, R. The initiation of follicle growth: The oocyte or the somatic cells? Mol. Cell. Endocrinol. 2002, 187, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Sabouni, R.; Cayton Vaught, K.C.; Owen, C.M.; Albertini, D.F.; Segars, J.H. Biomechanics and mechanical signaling in the ovary: A systematic review. J. Assist. Reprod. Genet. 2018, 35, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.D.; Czekala, N.M.; Hsueh, A.J. Estrogen-producing ovarian granulosa cells: Use of the granulosa cell aromatase bioassay (GAB) to monitor FSH levels in body fluids. Adv. Exp. Med. Biol. 1987, 219, 275–298. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Gonadotropin stimulation of the expansion of cumulus oophori isolated from mice: General conditions for expansion in vitro. J. Exp. Zool. 1979, 208, 111–120. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The role of miRNAs in regulating gene expression networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: MicroRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Glasner, M.E.; Yekta, S.; Burge, C.B.; Bartel, D.P. Vertebrate microRNA genes. Science 2003, 299, 1540. [Google Scholar] [CrossRef] [PubMed]
- Hutvagner, G.; McLachlan, J.; Pasquinelli, A.E.; Balint, E.; Tuschl, T.; Zamore, P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Radmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Park, J.; Dang, T.L.; Choi, Y.G.; Kim, V.N. Microprocessor depends on hemin to recognize the apical loop of primary microRNA. Nucleic Acids Res. 2018, 46, 5726–5736. [Google Scholar] [CrossRef] [PubMed]
- Abd El Naby, W.S.; Hagos, T.H.; Hossain, M.M.; Salilew-Wondim, D.; Gad, A.Y.; Rings, F.; Cinar, M.U.; Tholen, E.; Looft, C.; Schellander, K.; et al. Expression analysis of regulatory microRNAs in bovine cumulus oocyte complex and preimplantation embryos. Zygote 2013, 21, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar] [PubMed]
- Dell’Aversana, C.; Cuomo, F.; Longobardi, S.; D‘Hooghe, T.; Caprio, F.; Franci, G.; Santonastaso, M.; Colacurci, N.; Barone, S.; Pisaturo, V.; et al. Age-related miRNome landscape of cumulus oophorus cells during controlled ovarian stimulation protocols in IVF cycles. Hum. Reprod. 2021, 36, 1310–1325. [Google Scholar] [CrossRef] [PubMed]
- Gonella-Diaza, A.M.; Lopes, E.; Ribeiro da Silva, K.; Perecin Nociti, R.; Mamede Andrade, G.; Atuesta-Bustos, J.E.; Coelho da Silveira, J.; Vieira Meirelles, F.; Binelli, M. Steroidal Regulation of Oviductal microRNAs Is Associated with microRNA-Processing in Beef Cows. Int. J. Mol. Sci. 2021, 22, 953. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Moya, J.M.; Vilella, F.; Simon, C. MicroRNA: Key gene expression regulators. Fertil. Steril. 2014, 101, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Soifer, H.S.; Rossi, J.J.; Saetrom, P. MicroRNAs in disease and potential therapeutic applications. Mol. Ther. 2007, 15, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Imbar, T.; Galliano, D.; Pellicer, A.; Laufer, N. Introduction: MicroRNAs in human reproduction: Small molecules with crucial regulatory roles. Fertil. Steril. 2014, 101, 1514–1515. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, W.J. Advance on Dicer gene and its role in female reproduction. Chin. J. Med. Genet. 2011, 28, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, H.; Sakurai, N.; Muto, N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In vitro maturation: Role of cumulus cells. Biol. Reprod. 2000, 63, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Kulus, M.; Stefanska, K.; Kranc, W.; Chermula, B.; Bryl, R.; Pienkowski, W.; Nawrocki, M.J.; Petitte, J.N.; Stelmach, B.; et al. Human Granulosa Cells-Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells 2021, 10, 1396. [Google Scholar] [CrossRef] [PubMed]
- Jagarlamudi, K.; Rajkovic, A. Oogenesis: Transcriptional regulators and mouse models. Mol. Cell Endocrinol. 2012, 356, 31–39. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Veeramachaneni, D.N.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: A possible new form of cell communication within the ovarian follicle. Biol. Reprod. 2012, 86, 71. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Laukova, M.; Ovcharenko, D.; Brenaut, P.; Mlyncek, M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J. Cell. Physiol. 2010, 223, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.; Nunez, M.J.; Quinonero, A.; Martinez, S.; de la Orden, M.; Simon, C.; Pellicer, A.; Diaz-Garcia, C.; Dominguez, F. Follicular fluid and mural granulosa cells microRNA profiles vary in in vitro fertilization patients depending on their age and oocyte maturation stage. Fertil. Steril. 2015, 104, 1037–1046. [Google Scholar] [CrossRef]
- Santonocito, M.; Vento, M.; Guglielmino, M.R.; Battaglia, R.; Wahlgren, J.; Ragusa, M.; Barbagallo, D.; Borzi, P.; Rizzari, S.; Maugeri, M.; et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: Bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014, 102, 1751–1761.e1. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Lu, Y.; Li, L. MiRNA-451 is a potential biomarker for estrogenicity in mouse uterus. Front. Environ. Sci. Eng. 2014, 8, 99–105. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Peng, S.; Wu, L.; Lin, H.Y.; Wang, S.; Wang, H. Differentially expressed plasma microRNAs in premature ovarian failure patients and the potential regulatory function of mir-23a in granulosa cell apoptosis. Reproduction 2012, 144, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Al-edani, T.; Haouzi, D.; Philippe, N.; Lecellier, C.H.; Piquemal, D.; Commes, T.; Ait-Ahmed, O.; Dechaud, H.; Hamamah, S. MicroRNAs: New candidates for the regulation of the human cumulus-oocyte complex. Hum. Reprod. 2013, 28, 3038–3049. [Google Scholar] [CrossRef] [PubMed]
- Andrei, D.; Nagy, R.A.; van Montfoort, A.; Tietge, U.; Terpstra, M.; Kok, K.; van den Berg, A.; Hoek, A.; Kluiver, J.; Donker, R. Differential miRNA Expression Profiles in Cumulus and Mural Granulosa Cells from Human Pre-ovulatory Follicles. Microrna 2019, 8, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Jenabi, M.; Khodarahmi, P.; Tafvizi, F.; Bostanabad, S.Z. Evaluation of the potential of miR-21 as a diagnostic marker for oocyte maturity and embryo quality in women undergoing ICSI. Sci. Rep. 2023, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.P.; Ferreira, M.C.F.; Silveira, C.O.; Campos, J.R.; Borges, I.T.; Baeta, P.G.; Silva, F.H.S.; Reis, F.M.; Del Puerto, H.L. Clinical correlation of apoptosis in human granulosa cells-A review. Cell Biol. Int. 2018, 42, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Carletti, M.Z.; Fiedler, S.D.; Christenson, L.K. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol. Reprod. 2010, 83, 286–295. [Google Scholar] [CrossRef]
- Han, X.; Xue, R.; Yuan, H.J.; Wang, T.Y.; Lin, J.; Zhang, J.; Liang, B.; Tan, J.H. MicroRNA-21 plays a pivotal role in the oocyte-secreted factor-induced suppression of cumulus cell apoptosis. Biol. Reprod. 2017, 96, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, A.F.; Uliasz, T.; Peluso, J.J. MicroRNA-21 as a regulator of human cumulus cell viability and its potential influence on the developmental potential of the oocyte. Biol. Reprod. 2020, 103, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Gaia-Oltean, A.I.; Braicu, C.; Gulei, D.; Ciortea, R.; Mihu, D.; Roman, H.; Irimie, A.; Berindan-Neagoe, I. Ovarian endometriosis, a precursor of ovarian cancer: Histological aspects, gene expression and microRNA alterations (Review). Exp. Ther. Med. 2021, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lv, J.; Tang, R.; Feng, Y.; Zhao, Y.; Fei, X.; Chian, R.; Xie, Q. Association of exosomal microRNAs in human ovarian follicular fluid with oocyte quality. Biochem. Biophys. Res. Commun. 2021, 534, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Muti, P.; Donzelli, S.; Sacconi, A.; Hossain, A.; Ganci, F.; Frixa, T.; Sieri, S.; Krogh, V.; Berrino, F.; Biagioni, F.; et al. MiRNA-513a-5p inhibits progesterone receptor expression and constitutes a risk factor for breast cancer: The hOrmone and Diet in the ETiology of breast cancer prospective study. Carcinogenesis 2018, 39, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tong, L.; Wang, M.; Chang, X.; Wang, S.; Li, K.; Xiao, J. miR-505-3p is a repressor of the puberty onset in female mice. J. Endocrinol. 2018, 240, 279–392. [Google Scholar] [CrossRef] [PubMed]
- Barragan, M.; Pons, J.; Ferrer-Vaquer, A.; Cornet-Bartolome, D.; Schweitzer, A.; Hubbard, J.; Auer, H.; Rodolosse, A.; Vassena, R. The transcriptome of human oocytes is related to age and ovarian reserve. Mol. Hum. Reprod. 2017, 23, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Sang, Q.; Zhu, Y.; Fu, W.; Liu, M.; Xu, Y.; Shi, H.; Xu, Y.; Qu, R.; Chai, R.; et al. MiRNA-320 in the human follicular fluid is associated with embryo quality in vivo and affects mouse embryonic development in vitro. Sci. Rep. 2015, 5, 8689. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Rodosthenous, R.S.; Adir, M.; Mansour, A.; Racowsky, C.; Baccarelli, A.A.; Hauser, R. Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: An exploratory study. J. Assist. Reprod. Genet. 2017, 34, 525–533. [Google Scholar] [CrossRef]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Hsieh, M.; Zamah, A.M.; Oh, J.S. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Al-Edani, T.; Assou, S.; Ferrieres, A.; Bringer Deutsch, S.; Gala, A.; Lecellier, C.H.; Ait-Ahmed, O.; Hamamah, S. Female aging alters expression of human cumulus cells genes that are essential for oocyte quality. Biomed. Res. Int. 2014, 2014, 964614. [Google Scholar] [CrossRef]
- Barragan, M.; Cornet-Bartolome, D.; Molina, N.; Vassena, R. The expression levels of NOS2, HMOX1, and VEGFC in cumulus cells are markers of oocyte maturation and fertilization rate. Mol. Reprod. Dev. 2023, 90, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhang, L.; Fang, T.; Zhang, Q.; Wu, S.; Jiang, Y.; Sun, H.; Hu, Y. MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett. 2012, 586, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, W.; Yao, Y.; Du, X.; Zhou, J.; Ma, B.; Liu, H.; Li, Q.; Pan, Z. MiR-92a inhibits porcine ovarian granulosa cell apoptosis by targeting Smad7 gene. FEBS Lett. 2014, 588, 4497–4503. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.B.; Tesfaye, D.; Rings, F.; Hossien, M.; Hoelker, M.; Held, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Salilew-Wondim, D. MicroRNA-130b is involved in bovine granulosa and cumulus cells function, oocyte maturation and blastocyst formation. J. Ovarian Res. 2017, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wu, W.; Zhou, X.; Liu, K.; Li, B.; Huang, X.; Zhang, Y.; Liu, H. Let-7g induces granulosa cell apoptosis by targeting MAP3K1 in the porcine ovary. Int. J. Biochem. Cell Biol. 2015, 68, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Mu, H.; Mei, Q.; Liu, Y.; Min, Z.; Zhang, L.; Su, P.; Xiang, W. Mir-484 contributes to diminished ovarian reserve by regulating granulosa cell function via YAP1-mediated mitochondrial function and apoptosis. Int. J. Biol. Sci. 2022, 18, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod. Fertil. Dev. 1996, 8, 485–489. [Google Scholar] [CrossRef]
- Li, L.; Zheng, P.; Dean, J. Maternal control of early mouse development. Development 2010, 137, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Zheng, M.; Hayashi, M.; Lee, J.D.; Yoshino, O.; Lin, S.; Han, J. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J. Clin. Investig. 2008, 118, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Kim, S.Y.; Carmell, M.A.; Murchison, E.P.; Alcorn, H.; Li, M.Z.; Mills, A.A.; Elledge, S.J.; Anderson, K.V.; Hannon, G.J. Dicer is essential for mouse development. Nat. Genet. 2003, 35, 215–217. [Google Scholar] [CrossRef]
- Yang, W.J.; Yang, D.D.; Na, S.; Sandusky, G.E.; Zhang, Q.; Zhao, G. Dicer is required for embryonic angiogenesis during mouse development. J. Biol. Chem. 2005, 280, 9330–9335. [Google Scholar] [CrossRef]
- Murchison, E.P.; Stein, P.; Xuan, Z.; Pan, H.; Zhang, M.Q.; Schultz, R.M.; Hannon, G.J. Critical roles for Dicer in the female germline. Genes Dev. 2007, 21, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Kaneda, M.; O‘Carroll, D.; Hajkova, P.; Barton, S.C.; Sun, Y.A.; Lee, C.; Tarakhovsky, A.; Lao, K.; Surani, M.A. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007, 21, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Tang, Y.; He, Z.; Rosenwaks, Z. Dicer is a key player in oocyte maturation. J. Assist. Reprod. Genet. 2010, 27, 571–580. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).