Mortality and COVID Infection: Predictors of Mortality 10 Months after Discharge
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. In-Hospital Mortality
3.2. Long-Term Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Memish, Z.A.; Zumla, A.I.; Al-Hakeem, R.F.; Al-Rabeeah, A.A.; Stephens, G.M. Family Cluster of Middle East Respiratory Syndrome Coronavirus Infections. N. Engl. J. Med. 2013, 368, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating Excess Mortality Due to the COVID-19 Pandemic: A Systematic Analysis of COVID-19-Related Mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef] [PubMed]
- Clinical Management of COVID-19: Living Guideline; World Health Organization: Geneva, Switzerland, 2023.
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyashita, K.; Hozumi, H.; Furuhashi, K.; Nakatani, E.; Inoue, Y.; Yasui, H.; Karayama, M.; Suzuki, Y.; Fujisawa, T.; Enomoto, N.; et al. Changes in the Characteristics and Outcomes of COVID-19 Patients from the Early Pandemic to the Delta Variant Epidemic: A Nationwide Population-Based Study. Emerg. Microbes Infect. 2022, 12, 2155250. [Google Scholar] [CrossRef]
- Pijls, B.G.; Jolani, S.; Atherley, A.; Derckx, R.T.; Dijkstra, J.I.R.; Franssen, G.H.L.; Hendriks, S.; Richters, A.; Venemans-Jellema, A.; Zalpuri, S.; et al. Demographic Risk Factors for COVID-19 Infection, Severity, ICU Admission and Death: A Meta-Analysis of 59 Studies. BMJ Open 2021, 11, e044640. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, D.; Margină, D.; Tsarouhas, K.; Tekos, F.; Stan, M.; Nikitovic, D.; Kouretas, D.; Spandidos, D.A.; Tsatsakis, A. Obesity—A Risk Factor for Increased COVID-19 Prevalence, Severity and Lethality (Review). Mol. Med. Rep. 2020, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Grassi, G.; Mancia, G. COVID-19 and Arterial Hypertension: Hypothesis or Evidence? J. Clin. Hypertens 2020, 22, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk Factors for Mortality in Patients with Coronavirus Disease 2019 (COVID-19) Infection: A Systematic Review and Meta-Analysis of Observational Studies. Aging Male 2020, 23, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, Pathophysiology, Prognosis and Practical Considerations. Diabetes Metab. Syndr. 2020, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Arayici, M.E.; Kipcak, N.; Kayacik, U.; Kelbat, C.; Keskin, D.; Kilicarslan, M.E.; Kilinc, A.V.; Kirgoz, S.; Kirilmaz, A.; Kizilkaya, M.A.; et al. Effects of SARS-CoV-2 Infections in Patients with Cancer on Mortality, ICU Admission and Incidence: A Systematic Review with Meta-Analysis Involving 709,908 Participants and 31,732 Cancer Patients. J. Cancer Res. Clin. Oncol. 2022, 149, 2915–2928. [Google Scholar] [CrossRef]
- Kashyap, V.K.; Dhasmana, A.; Massey, A.; Kotnala, S.; Zafar, N.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Smoking and COVID-19: Adding Fuel to the Flame. Int. J. Mol. Sci. 2020, 21, 6581. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, U.; Casotto, V.; Barbiellini Amidei, C.; Vianello, A.; Guarnieri, G. COPD-Related Mortality before and after Mass COVID-19 Vaccination in Northern Italy. Vaccines 2023, 11, 1392. [Google Scholar] [CrossRef] [PubMed]
- Jothimani, D.; Venugopal, R.; Abedin, M.F.; Kaliamoorthy, I.; Rela, M. COVID-19 and the Liver. J. Hepatol. 2020, 73, 1231–1240. [Google Scholar] [CrossRef]
- Farouk, S.S.; Fiaccadori, E.; Cravedi, P.; Campbell, K.N. COVID-19 and the Kidney: What We Think We Know so Far and What We Don’t. J. Nephrol. 2020, 33, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Locke, E.; Green, P.; Berry, K.; O’Hare, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Dominitz, J.A.; Fan, V.S. Risk Factors for Hospitalization, Mechanical Ventilation, or Death among 10131 US Veterans with SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022310. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, L.; Ye, J.; Gu, Z.; Wang, S.; Xia, J.; Xie, Y.; Li, Q.; Xu, R.; Lin, N. Predictors of mortality in patients with coronavirus disease 2019: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karasneh, R.A.; Khassawneh, B.Y.; Al-Azzam, S.; Al-Mistarehi, A.H.; Lattyak, W.J.; Aldiab, M.; Kabbaha, S.; Hasan, S.S.; Conway, B.R.; Aldeyab, M.A. Risk Factors Associated with Mortality in COVID-19 Hospitalized Patients: Data from the Middle East. Int. J. Clin. Pract. 2022, 2022, 9617319. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, J.; Ma, F.Z.; Li, J.; Xue, S.; Xu, Z.G. The Common Risk Factors for Progression and Mortality in COVID-19 Patients: A Meta-Analysis. Arch. Virol. 2021, 166, 2071–2087. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Marzio, M.A.L.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic Factors for Severity and Mortality in Patients Infected with COVID-19: A Systematic Review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef]
- Zhang, J.J.; Cao, Y.Y.; Tan, G.; Dong, X.; Wang, B.C.; Lin, J.; Yan, Y.Q.; Liu, G.H.; Akdis, M.; Akdis, C.A.; et al. Clinical, Radiological, and Laboratory Characteristics and Risk Factors for Severity and Mortality of 289 Hospitalized COVID-19 Patients. Allergy 2021, 76, 533–550. [Google Scholar] [CrossRef]
- Chojnicki, M.; Neumann-Podczaska, A.; Seostianin, M.; Tomczak, Z.; Tariq, H.; Chudek, J.; Tobis, S.; Mozer-Lisewska, I.; Suwalska, A.; Tykarski, A.; et al. Long-Term Survival of Older Patients Hospitalized for COVID-19. Do Clinical Characteristics upon Admission Matter? Int. J. Environ. Res. Public Health 2021, 18, 10671. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Seelye, S.; Berkowitz, T.S.; Pura, J.; Bohnert, A.S.B.; Bowling, C.B.; Boyko, E.J.; Hynes, D.M.; Ioannou, G.N.; Maciejewski, M.L.; et al. Late Mortality After COVID-19 Infection Among US Veterans vs Risk-Matched Comparators: A 2-Year Cohort Analysis. JAMA Intern. Med. 2023, 183, 1111–1119. [Google Scholar] [CrossRef]
- Aghajani, M.H.; Sistanizad, M.; Toloui, A.; Neishaboori, A.M.; Pourhoseingholi, A.; Asadpoordezaki, Z.; Miri, R.; Yousefifard, M. Six-Month Follow-up of COVID-19 Patients: Mortality and Related Factors. Arch. Iran. Med. 2022, 25, 557–563. [Google Scholar] [CrossRef]
- Anaya, J.M.; Rojas, M.; Salinas, M.L.; Rodríguez, Y.; Roa, G.; Lozano, M.; Rodríguez-Jiménez, M.; Montoya, N.; Zapata, E.; Monsalve, D.M.; et al. Post-COVID Syndrome. A Case Series and Comprehensive Review. Autoimmun. Rev. 2021, 20, 102947. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carretero, R.; Vazquez-Gomez, O.; Gil-Prieto, R.; Gil-de-Miguel, A. Hospitalization Burden and Epidemiology of the COVID-19 Pandemic in Spain (2020–2021). BMC Infect. Dis. 2023, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.M.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.M.; Vargas-Núñez, J.A.; et al. Clinical Characteristics of Patients Hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry. Rev. Clin. Esp. 2020, 220, 480–494. [Google Scholar] [CrossRef]
- Chilimuri, S.; Sun, H.; Alemam, A.; Mantri, N.; Shehi, E.; Tejada, J.; Yugay, A.; Nayudu, S.K. Predictors of Mortality in Adults Admitted with COVID-19: Retrospective Cohort Study from New York City. West. J. Emerg. Med. 2020, 21, 779–784. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors Associated with Hospital Admission and Critical Illness among 5279 People with Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Baptista, A.; Vieira, A.M.; Capela, E.; Julião, P.; Macedo, A. COVID-19 Fatality Rates in Hospitalized Patients: A New Systematic Review and Meta-Analysis. J. Infect. Public Health 2023, 16, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.R.; Caro-Codón, J.; Rosillo, S.O.; Iniesta, A.M.; Castrejón-Castrejón, S.; Marco-Clement, I.; Martín-Polo, L.; Merino-Argos, C.; Rodríguez-Sotelo, L.; García-Veas, J.M.; et al. Heart Failure in COVID-19 Patients: Prevalence, Incidence and Prognostic Implications. Eur. J. Heart Fail. 2020, 22, 2205–2215. [Google Scholar] [CrossRef]
- Nasrullah, A.; Gangu, K.; Cannon, H.R.; Khan, U.A.; Shumway, N.B.; Bobba, A.; Sagheer, S.; Chourasia, P.; Shuja, H.; Avula, S.R.; et al. COVID-19 and Heart Failure with Preserved and Reduced Ejection Fraction Clinical Outcomes among Hospitalized Patients in the United States. Viruses 2023, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Li, X.M.; Liu, F.; Tian, T.; Luo, J.; Yang, Y. Acute Kidney Injury Is Associated with Severe Infection and Fatality in Patients with COVID-19: A Systematic Review and Meta-Analysis of 40 Studies and 24,527 Patients. Pharmacol. Res. 2020, 161, 105107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney Disease Is Associated with In-Hospital Death of Patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk Factors of Critical & Mortal COVID-19 Cases: A Systematic Literature Review and Meta-Analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef] [PubMed]
- Novelli, L.; Raimondi, F.; Carioli, G.; Carobbio, A.; Pappacena, S.; Biza, R.; Trapasso, R.; Anelli, M.; Amoroso, M.; Allegri, C.; et al. One-Year Mortality in COVID-19 Is Associated with Patients’ Comorbidities Rather than Pneumonia Severity. Respir. Med. Res. 2023, 83, 100976. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.M.; Aponte-Becerra, L.; Torres, C.; Rozas, J.; Francois, P.; Salcedo, J.; Barreto, A.; Rodriguez, J.; Suarez, M.; Fornoni, A.; et al. One-Year All-Cause Mortality in Hospitalized Patients with COVID-19 and End-Stage Renal Disease Undergoing Hemodialysis. Clin. Nephrol. 2023, 99, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Uusküla, A.; Jürgenson, T.; Pisarev, H.; Kolde, R.; Meister, T.; Tisler, A.; Suija, K.; Kalda, R.; Piirsoo, M.; Fischer, K. Long-Term Mortality Following SARS-CoV-2 Infection: A National Cohort Study from Estonia. Lancet Reg. Health. Eur. 2022, 18, 100394. [Google Scholar] [CrossRef]
- Ramzi, Z.S. Hospital Readmissions and Post-Discharge All-Cause Mortality in COVID-19 Recovered Patients; A Systematic Review and Meta-Analysis. Am. J. Emerg. Med. 2022, 51, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Renda, G.; Ricci, F.; Spinoni, E.G.; Grisafi, L.; D’ardes, D.; Mennuni, M.; Tana, C.; Rognoni, A.; Bellan, M.; Sainaghi, P.P.; et al. Predictors of Mortality and Cardiovascular Outcome at 6 Months after Hospitalization for COVID-19. J. Clin. Med. 2022, 11, 729. [Google Scholar] [CrossRef]
- Günster, C.; Busse, R.; Spoden, M.; Rombey, T.; Schillinger, G.; Hoffmann, W.; Weber-Carstens, S.; Schuppert, A.; Karagiannidis, C. 6-Month Mortality and Readmissions of Hospitalized COVID-19 Patients: A Nationwide Cohort Study of 8,679 Patients in Germany. PLoS ONE 2021, 16, e0255427. [Google Scholar] [CrossRef]
- Docherty, A.B.; Farrell, J.; Thorpe, M.; Egan, C.; Dunn, S.; Norman, L.; Shaw, C.A.; Law, A.; Leeming, G.; Norris, L.; et al. Patient Emergency Health-Care Use before Hospital Admission for COVID-19 and Long-Term Outcomes in Scotland: A National Cohort Study. Lancet Digit. Health 2023, 5, e446–e457. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhu, X.; Zhu, Z.; Yang, Y.; Tian, Z.; Wang, D.; Chen, S.; Gao, X.; Xu, Y.; Zhang, B.; et al. Non-Ischemic, Non-Hypoxic Myocardial Injury, and Long-Term Mortality in Patients with Coronavirus Disease 2019: A Retrospective Cohort Study. Cardiol. Discov. 2022, 2, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Betti, M.; Giacchero, F.; Grasso, C.; Franceschetti, G.; Carotenuto, M.; Odone, A.; Pacileo, G.; Ferrante, D.; Maconi, A. Long-Term Survival among Patients Hospitalized for COVID-19 during the First Three Epidemic Waves: An Observational Study in a Northern Italy Hospital. Int. J. Environ. Res. Public Health 2022, 19, 15298. [Google Scholar] [CrossRef] [PubMed]
- Sabanoglu, C.; Inanc, I.H.; Polat, E.; Peker, S.A. Long-Term Predictive Value of Cardiac Biomarkers in Patients with COVID-19 Infection. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6396–6403. [Google Scholar] [CrossRef] [PubMed]
- Lucijanić, M.; Živković, N.P.; Zelenika, M.; Barišić-Jaman, M.; Jurin, I.; Matijaca, A.; Zagorec, N.; Lagančić, M.; Osmani, B.; Bušić, I.; et al. Survival after Hospital Discharge in Patients Hospitalized for Acute Coronavirus Disease 2019: Data on 2586 Patients from a Tertiary Center Registry. Croat. Med. J. 2022, 63, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Putri, C.; Situmeang, R.F.V.; Kurniawan, A. Dementia Is a Predictor for Mortality Outcome from Coronavirus Disease 2019 (COVID-19) Infection. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 393–395. [Google Scholar] [CrossRef]
- Eligulashvili, A.; Gordon, M.; Lee, J.S.; Lee, J.; Mehrotra-Varma, S.; Mehrotra-Varma, J.; Hsu, K.; Hilliard, I.; Lee, K.; Li, A.; et al. Long-term outcomes of hospitalized patients with SARS-CoV-2/COVID-19 with and without neurological involvement: 3-year follow-up assessment. PLoS Med. 2024, 21, e1004263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walle-Hansen, M.M.; Ranhoff, A.H.; Mellingsæter, M.; Wang-Hansen, M.S.; Myrstad, M. Health-related quality of life, functional decline, and long-term mortality in older patients following hospitalisation due to COVID-19. BMC Geriatr. 2021, 21, 199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussien, H.; Nastasa, A.; Apetrii, M.; Nistor, I.; Petrovic, M.; Covic, A. Different aspects of frailty and COVID-19: Points to consider in the current pandemic and future ones. BMC Geriatr. 2021, 21, 389. [Google Scholar] [CrossRef]

| In-Hospital Mortality | Long-Term Mortality | ||||||
|---|---|---|---|---|---|---|---|
| All (762) | Survivors (654) | Dead (108) | p-Value | Survivors (567) | Dead (195) | p-Value | |
| Age (mean ± SD) >71; n (%) | 68.4 ± 17.9 376 (49.3) | 79.7 ± 13.0 290 (44.3) | 66.5 ± 17.8 86 (79.6) | <0.001 | 64.6 ± 17.5 220 (38.8) | 79.4 ± 13.9 156 (80.0) | <0.001 |
| Sex (M/F); n (%) | 403/359 (52.9/47.1) | 349/305 (53.4/46.6) | 50/50 (54/54) | NS | 52.4/47.6 (297/270) | 54.4/45.6 (106/89) | NS |
| Tobacco use (746) | NS | NS | |||||
| 71 (9.3) | 67 (10.2) | 4 (3.7) | 57 (10.0) | 14 (7.2) | ||
| 182 (23.9) | 152 (23.2) | 30 (27.8) | 132 (23.3) | 50 (25.6) | ||
| 493 (64.7) | 419 (64.1) | 74 (68.5) | 366 (64.6) | 127 (65.1) | ||
| Alcohol consumption (746) * | NS | NS | |||||
| 79 (10.4) | 72 (11.0) | 7 (6.5) | 60 (10.6) | 19 (9.7) | ||
| 32 (4.2) | 27 (4.1) | 5 (4.6) | 23 (4.1) | 9 (4.6) | ||
| 635 (83.3) | 539 (82.4) | 96 (88.9) | 472 (83.2) | 163 (83.6 | ||
| Metabolic syndrome (746) | 384 (51.5) | 312 (48.9) | 72 (66.7) | <0.001 | (265) 47.7 | (119) 62.3 | <0.001 |
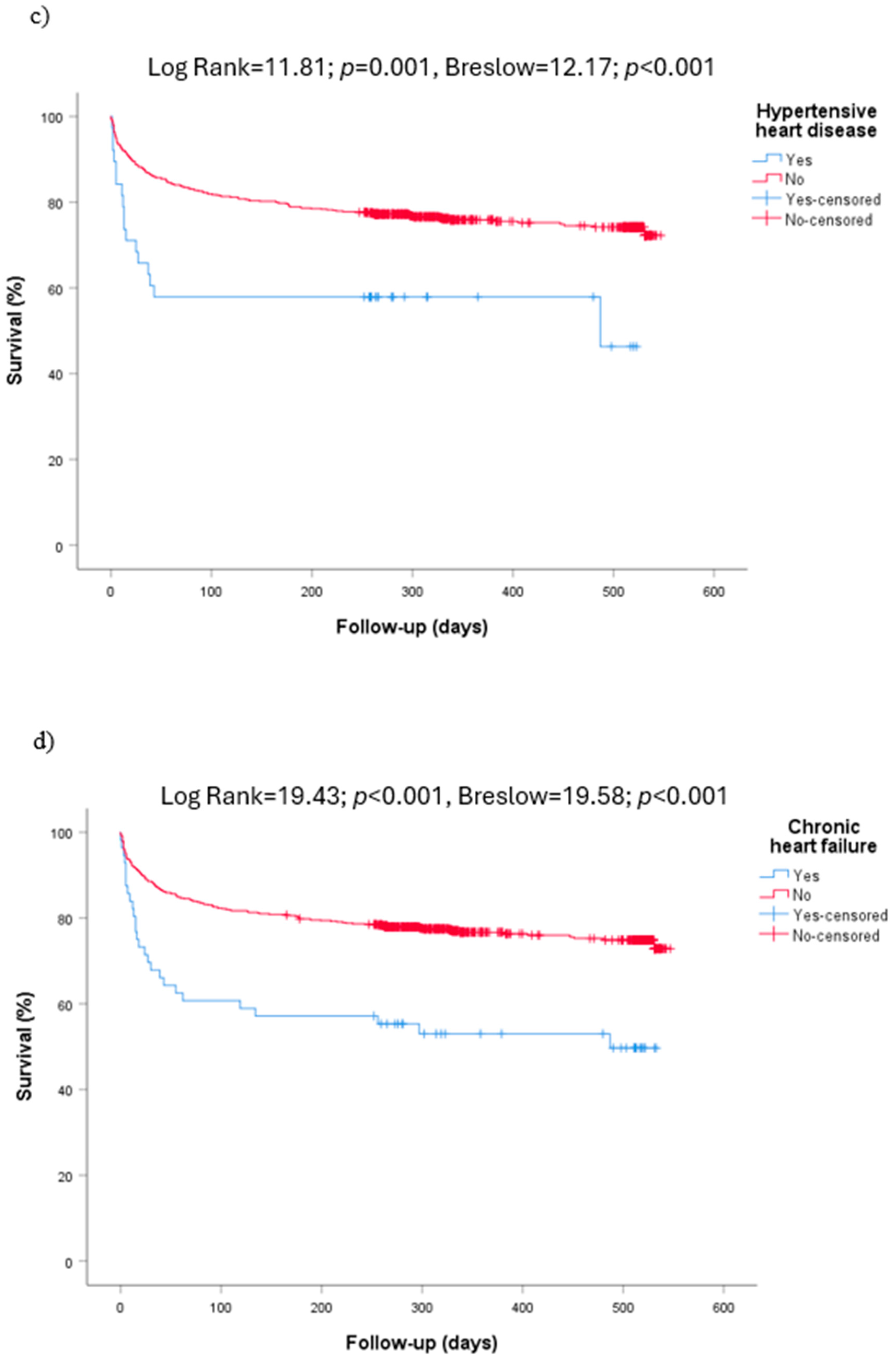
| Hypertension (746) | 428 (57.4) | 352 (55.2) | 76 (70.4) | NS | 295 (53.2) | 133 (69.6) | <0.001 |
| Dyslipidemia (746) | 365 (48.9) | 304 (47.6) | 61 (56.5) | NS | 258 (46.5) | 107 (56.0) | 0.026 |
| Diabetes mellitus (746) | 246 (33.0) | 203 (31.8) | 43 (39.8) | NS | 176 (31.7) | 70 (36.6) | NS |
| Obesity (746) | 191 (25.6) | 159 (24.9) | 32 (29.6) | NS | 139 (25.0) | 52 (27.2) | NS |
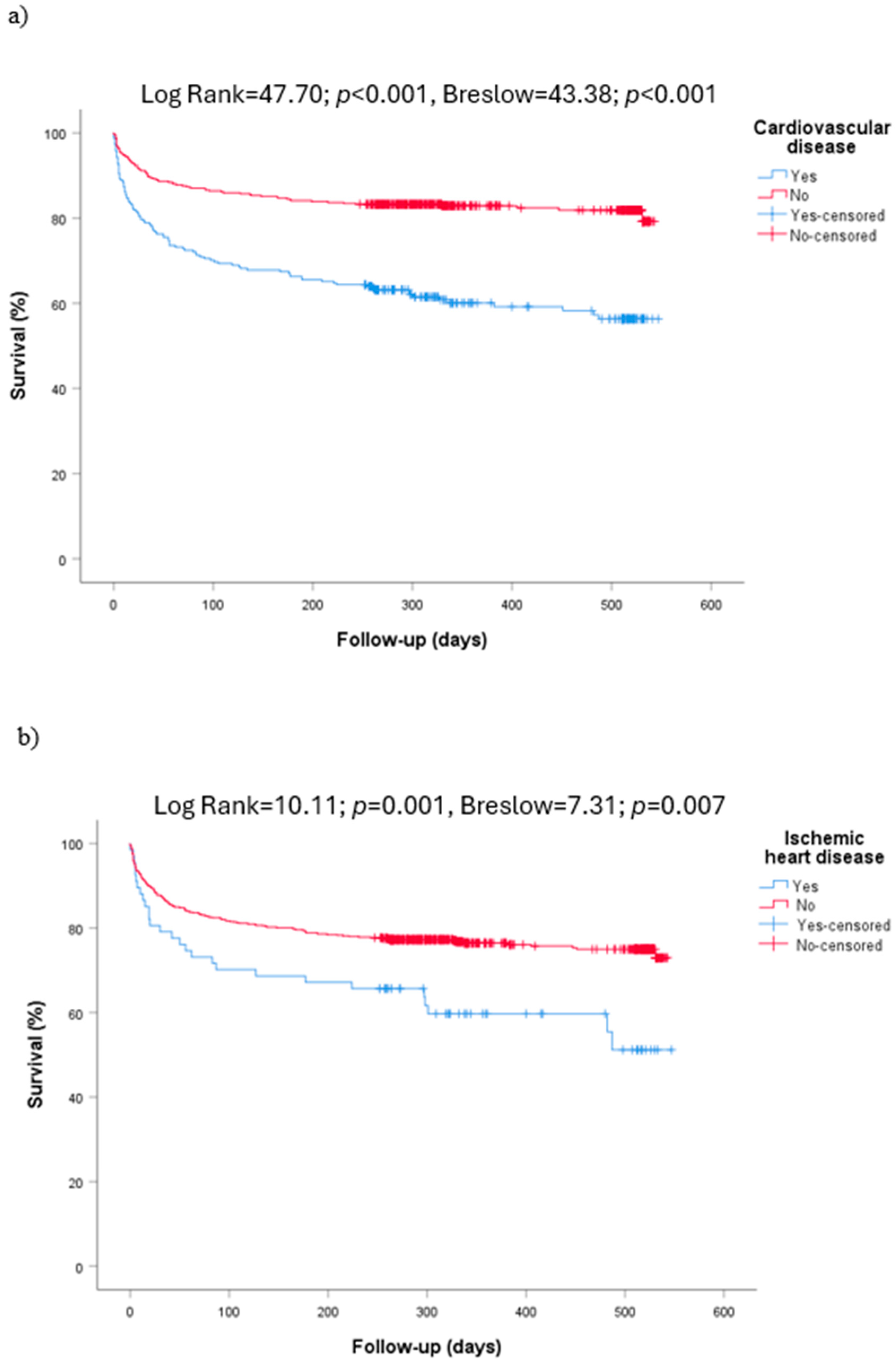
| Cardiovascular diseases (746) | 261 (35.0) | 204 (32.0) | 57 (52.8) | NS | 155 (27.9) | 106 (55.5) | <0.001 |
| 66 (9.0) | 51 (8.0) | 16 (14.8) | NS | 39 (7.0) | 28 (14.7) | 0.001 |
| 56 (7.5) | 38 (6.0) | 18 (16.7) | <0.001 | 29 (5.2) | 27 (14.1) | <0.001 |
| 38 (5.1) | 25 (3.9) | 13 (12.0) | <0.001 | 21 (3.8) | 17 (8.9) | 0.001 |
| 81 (10.9) | 63 (9.9) | 18 (16.7) | NS | 43 (7.8) | 38 (19.9) | <0.001 |
| Stroke (746) | 42 (5.6) | 33 (5.2) | 9 (8.3) | NS | 24 (4.3) | 18 (9.4) | 0.004 |
| COPD (746) | 59 (7.9) | 48 (7.5) | 11 (10.2) | NS | 34 (6.1) | 25 (13.1) | 0.002 |
| Asthma (746) | 55 (7.4) | 47 (7.4) | 8 (7.4) | NS | 44 (7.9) | 11 (5.8) | NS |
| Dementia (746) | 163 (21.8) | 122 (19.1) | 41 (38.0) | <0.001 | 79 (14.2) | 84 (44.0) | <0.001 |
| Parkinson’s disease (751) | 19 (2.5) | 15 (2.3) | 4 (3.7) | NS | 11 (2.0) | 8 (4.2) | 0.036 |
| Neoplasm (745) | 94 (12.6) | 67 (10.5) | 27 (25.0) | <0.001 | 52 (9.4) | 42 (22.0) | <0.001 |
| Chronic kidney disease | 50 (6.6) | 32 (4.9) | 18 (14.2) | <0.001 | 26 (4.6) | 24 (12.3) | <0.001 |
| Institutionalized (731) | 187 (25.6) | 151 (24.2) | 36 (33.6) | 0.004 | 106 (19.7) | 81 (42.2) | <0.001 |
| In-Hospital Mortality | Long-Term Mortality | ||||||
|---|---|---|---|---|---|---|---|
| All (762) | Survivors (654) | Dead (108) | p-Value | Survivors (567) | Dead (195) | p-Value | |
| Oxygen saturation <94% (724); n (%) | 399 (55.1) | 313 (50.6) | 86 (81.9) | <0.001 | 277 (51.5) | 122 (65.6) | <0.001 |
| Oxygen support (683) | NS | NS | |||||
| Conventional | 555 (81.3) | 479 (83.2) | 76 (71.0) | 398 (80.6) | 157 (83.1) | ||
| CPAP/NIVM | 69 (10.1) | 54 (9.4) | 15 (14.0) | 54 (10.9) | 15 (7.9) | ||
| Invasive MV | 59 (8.6) | 43 (7.5) | 16 (15.0) | 42 (8.5) | 17 (9.0) | ||
| Critical Care | 116 (15.2) | 95 (14.5) | 21 (19.4) | 0.040 | 94 (16.6) | 22 (11.3) | NS |
| Symptomatic | 664 (87.1) | 561 (85.8) | 103 (95.4) | <0.001 | 494 (87.1) | 170 (7.2) | NS |
| Severity of illness | <0.001 | <0.001 | |||||
| 98 (12.9) | 93 (14.2) | 5 (4.6) | 73 (12.9) | 25 (12.8) | ||
| 85 (11.0) | 82 (12.5) | 3 (2.8) | 62 (10.9) | 23 (11.8) | ||
| 355 (46.6) | 317 (48.5) | 38 (35.2) | 280 (49.3) | 75 (38.5) | ||
| 115 (15.1) | 74 (11.2) | 41 (38.0) | 67 (11.8) | 48 (24.6) | ||
| 110 (14.4) | 89 (13.6) | 21 (19.4) | 86 (15.1) | 24 (12.3) | ||
| Fever | 423 (55.5) | 360 (55.0) | 63 (58.3) | NS | 331 (58.4) | 92 (47.2) | 0.015 |
| Cough | 364 (47.8) | 324 (49.5) | 40 (37.0) | NS | 301 (53.1) | 63 (32.3) | <0.001 |
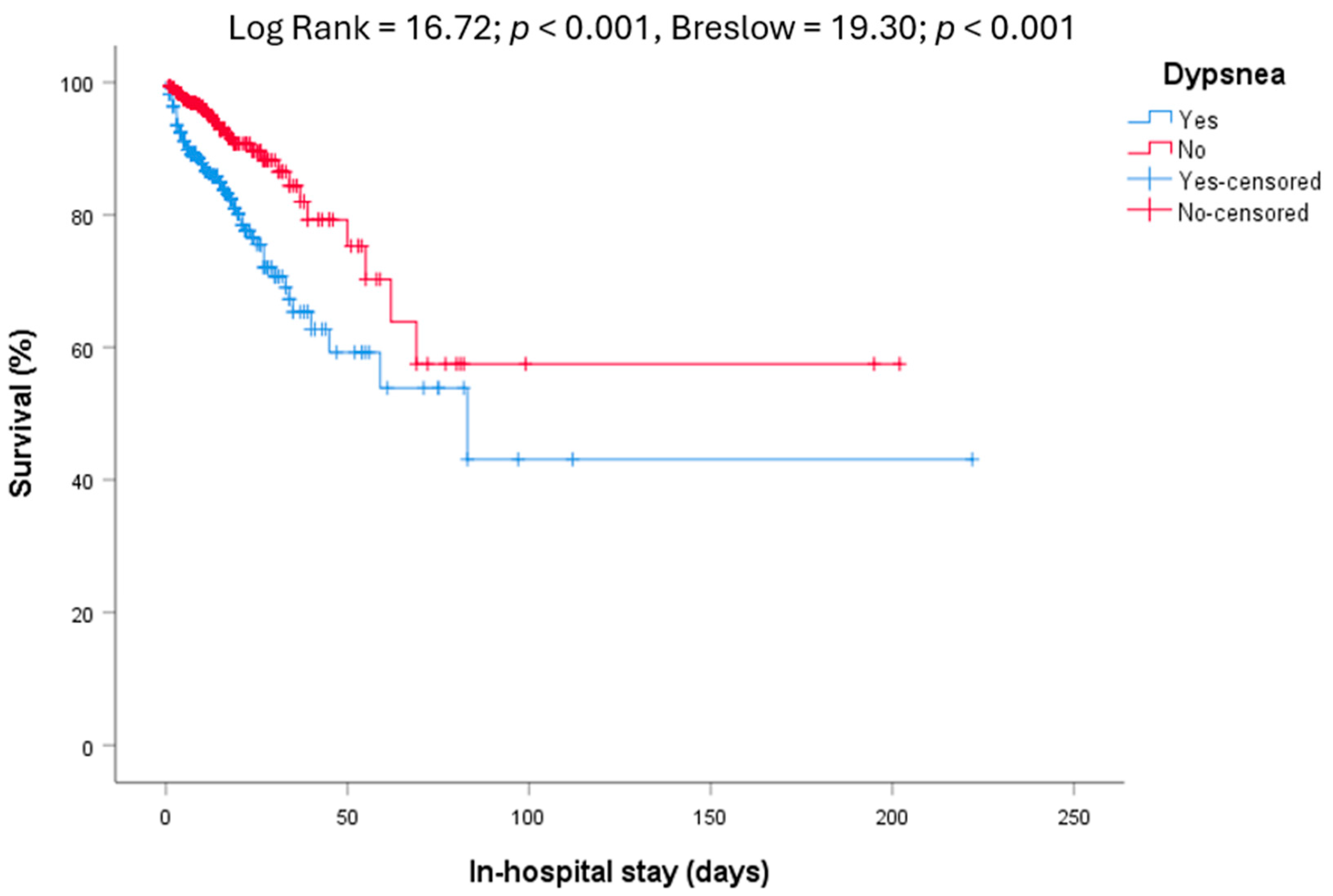
| Dyspnea | 390 (51.2) | 315 (48.2) | 75 (69.4) | <0.001 | 273 (48.1) | 117 (60.0) | 0.003 |
| Asthenia | 262 (34.4) | 236 (36.1) | 26 (24.1) | NS | 213 (37.6) | 49 (25.1) | 0.003 |
| Diarrhea | 132 (17.3) | 121 (18.5) | 11 (10.2) | NS | 113 (19.9) | 19 (9.7) | 0.001 |
| Leukocytes >6330/mm3 (733) | 367 (50.1) | 333 (53.1) | 34 (32.1) | <0.001 | 300 (55.1) | 67 (35.4) | <0.001 |
| Lymphocytes <1000/mm3 (733) | 368 (50.2) | 292 (46.6) | 76 (71.7) | <0.001 | 258 (47.4) | 110 (58.2) | 0.004 |
| Creatinine >0.87 mg/dL (735) | 368 (50.1) | 285 (45.5) | 83 (76.9) | <0.001 | 241 (44.3) | 127 (66.5) | 0.001 |
| Urea >37 mg/dL (735) | 368 (50.1) | 276 (44.0) | 92 (85.2) | <0.001 | 212 (39.0) | 156 (81.7) | 0.001 |
| C-reactive protein >48 mg/dL (731) | 366 (50.1) | 289 (46.3) | 77 (72.0) | <0.001 | 251 (46.4) | 115 (60.5) | 0.001 |
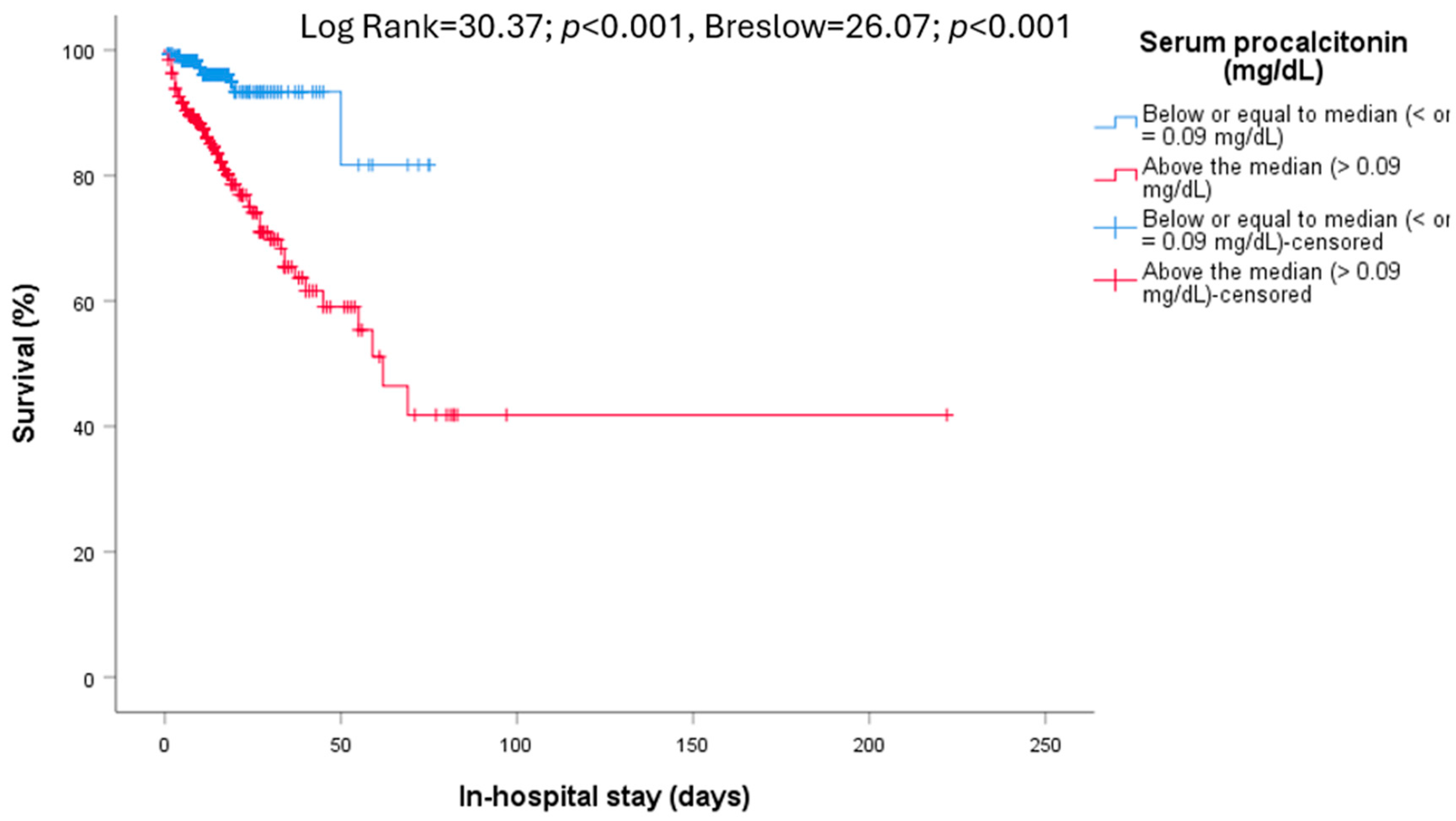
| Procalcitonin >0.09 mg/dL (631) | 326 (51.7) | 253 (46.3) | 73 (85.9) | <0.001 | 217 (46.0) | 109 (68.6) | <0.001 |
| LDH >270 UI/L (637) | 319 (50.1) | 252 (46.0) | 67 (75.3) | <0.001 | 229 (47.8) | 90 (57.0) | 0.011 |
| Ferritin >402 mg/dL (687) | 344 (50.1) | 280 (47.1) | 64 (69.6) | 0.002 | 248 (48.2) | 96 (55.8) | NS |
| D-dimer >855 mg/dL (675) | 338 (50.1) | 270 (46.3) | 68 (73.9) | 0.002 | 218 (43.2) | 120 (70.6) | <0.001 |
| Troponin >15.2 pg/mL (183) | 92 (50.3) | 63 (42.0) | 29 (87.9) | <0.001 | 49 (36.3) | 43 (89.6) | <0.001 |
| Natriuretic peptide >503.5 pg/mL (308) | 154 (50.0) | 99 (40.7) | 55 (84.6) | <0.001 | 74 (35.4) | 80 (80.8) | <0.001 |
| IL-6 >26.6 pg/mL (162) | 81 (50.0) | 60 (43.8) | 21 (84.0) | 0.045 | 58 (44.6) | 23 (71.9) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Delgado, V.; García-Rosado, D.; Pérez-Hernández, O.; Martín-Ponce, E.; de La Paz-Estrello, A.M.; García-Marichal, C.; Pérez-Fernández, S.; Rodríguez-Morón, V.; Alemán-Valls, R.; González-Reimers, E.; et al. Mortality and COVID Infection: Predictors of Mortality 10 Months after Discharge. Diseases 2024, 12, 123. https://doi.org/10.3390/diseases12060123
Vera-Delgado V, García-Rosado D, Pérez-Hernández O, Martín-Ponce E, de La Paz-Estrello AM, García-Marichal C, Pérez-Fernández S, Rodríguez-Morón V, Alemán-Valls R, González-Reimers E, et al. Mortality and COVID Infection: Predictors of Mortality 10 Months after Discharge. Diseases. 2024; 12(6):123. https://doi.org/10.3390/diseases12060123
Chicago/Turabian StyleVera-Delgado, Víctor, Dácil García-Rosado, Onán Pérez-Hernández, Esther Martín-Ponce, Alejandro Mario de La Paz-Estrello, Cristina García-Marichal, Sergio Pérez-Fernández, Valle Rodríguez-Morón, Remedios Alemán-Valls, Emilio González-Reimers, and et al. 2024. "Mortality and COVID Infection: Predictors of Mortality 10 Months after Discharge" Diseases 12, no. 6: 123. https://doi.org/10.3390/diseases12060123
APA StyleVera-Delgado, V., García-Rosado, D., Pérez-Hernández, O., Martín-Ponce, E., de La Paz-Estrello, A. M., García-Marichal, C., Pérez-Fernández, S., Rodríguez-Morón, V., Alemán-Valls, R., González-Reimers, E., & Martín-González, C. (2024). Mortality and COVID Infection: Predictors of Mortality 10 Months after Discharge. Diseases, 12(6), 123. https://doi.org/10.3390/diseases12060123







