Targeting Solute Carrier Transporters (SLCs) as a Therapeutic Target in Different Cancers
Abstract
1. Introduction
2. SLC Transporters in Cancer
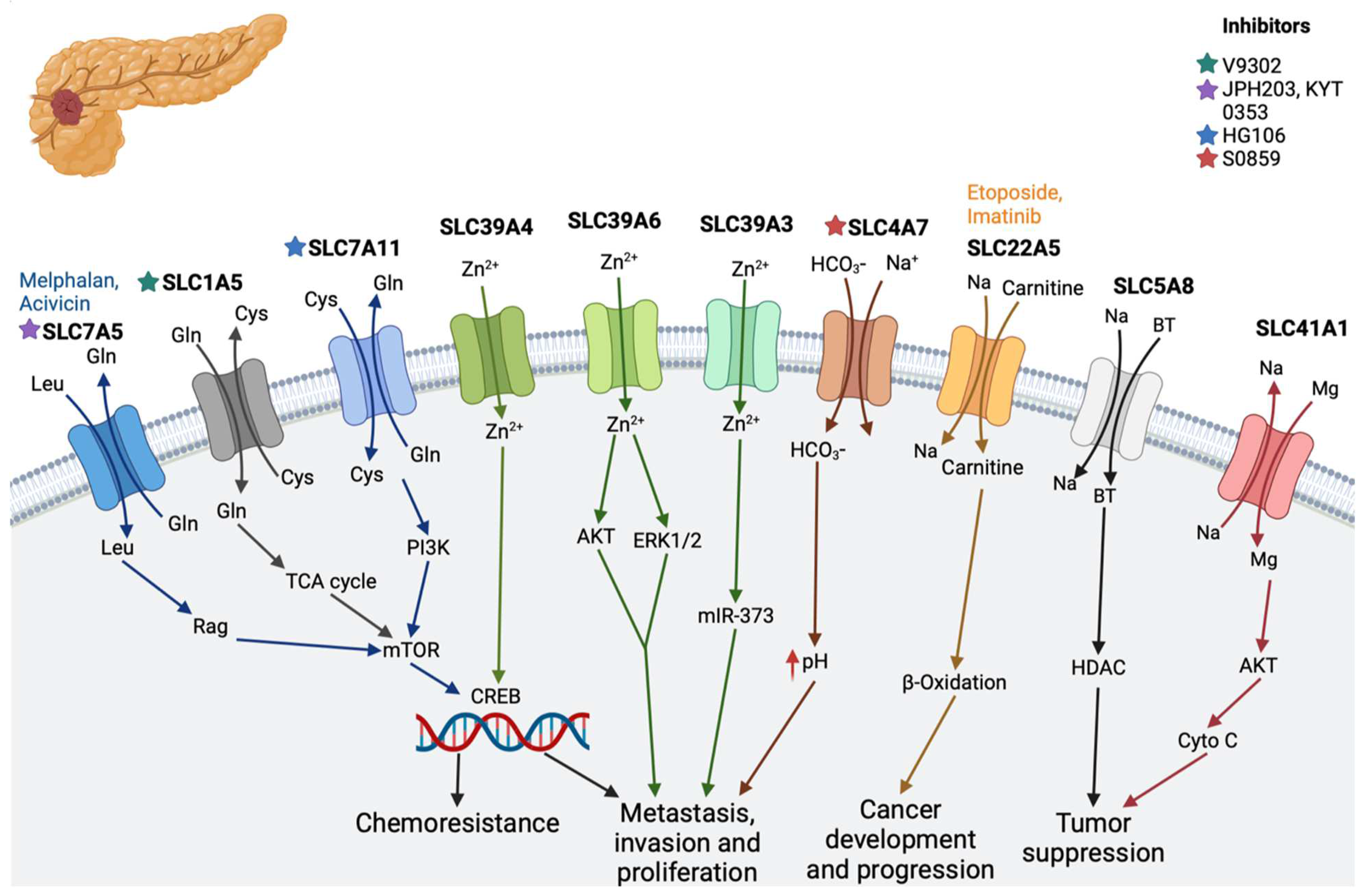
2.1. Pancreatic Cancer
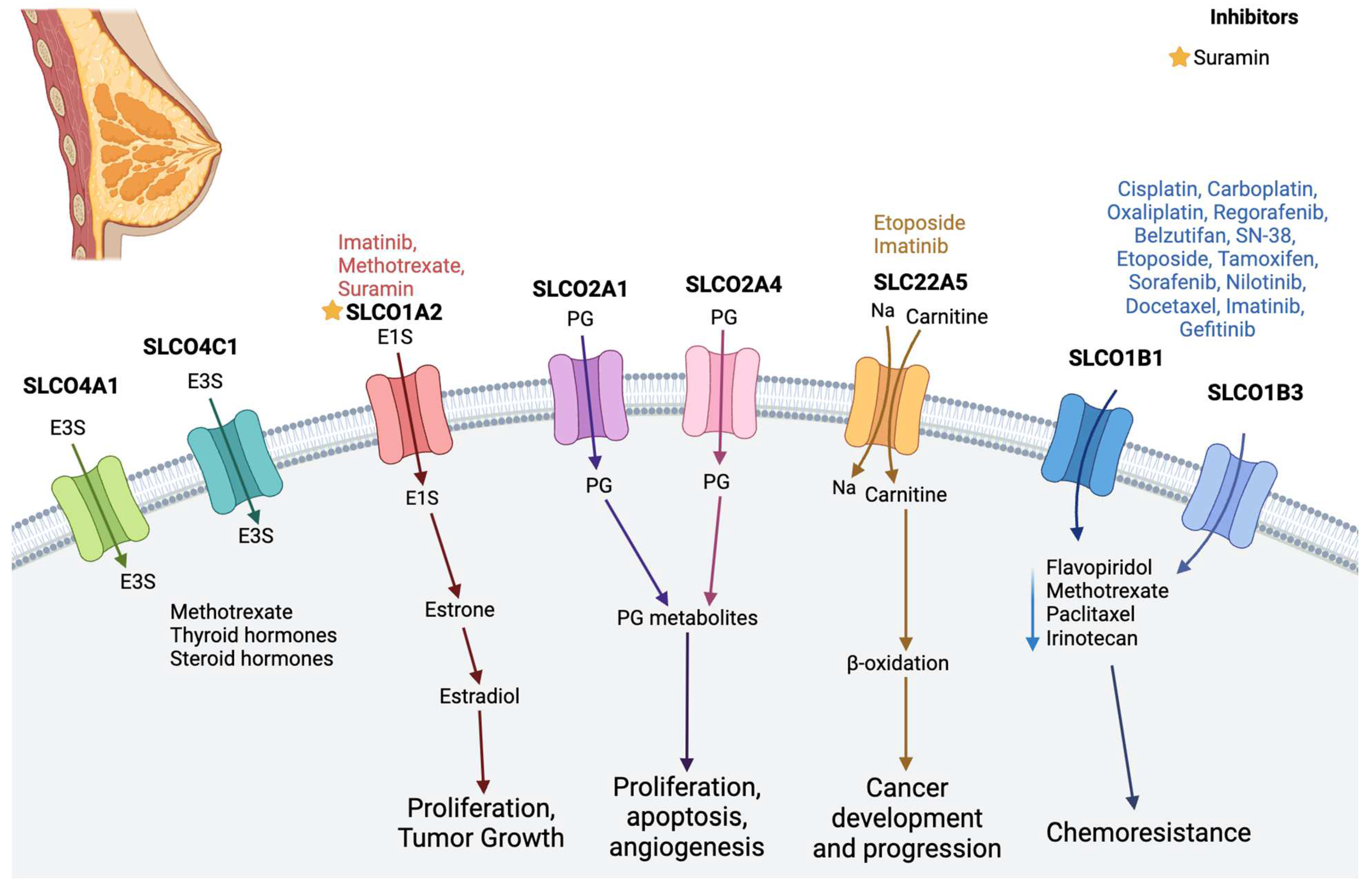
2.2. Breast Cancer
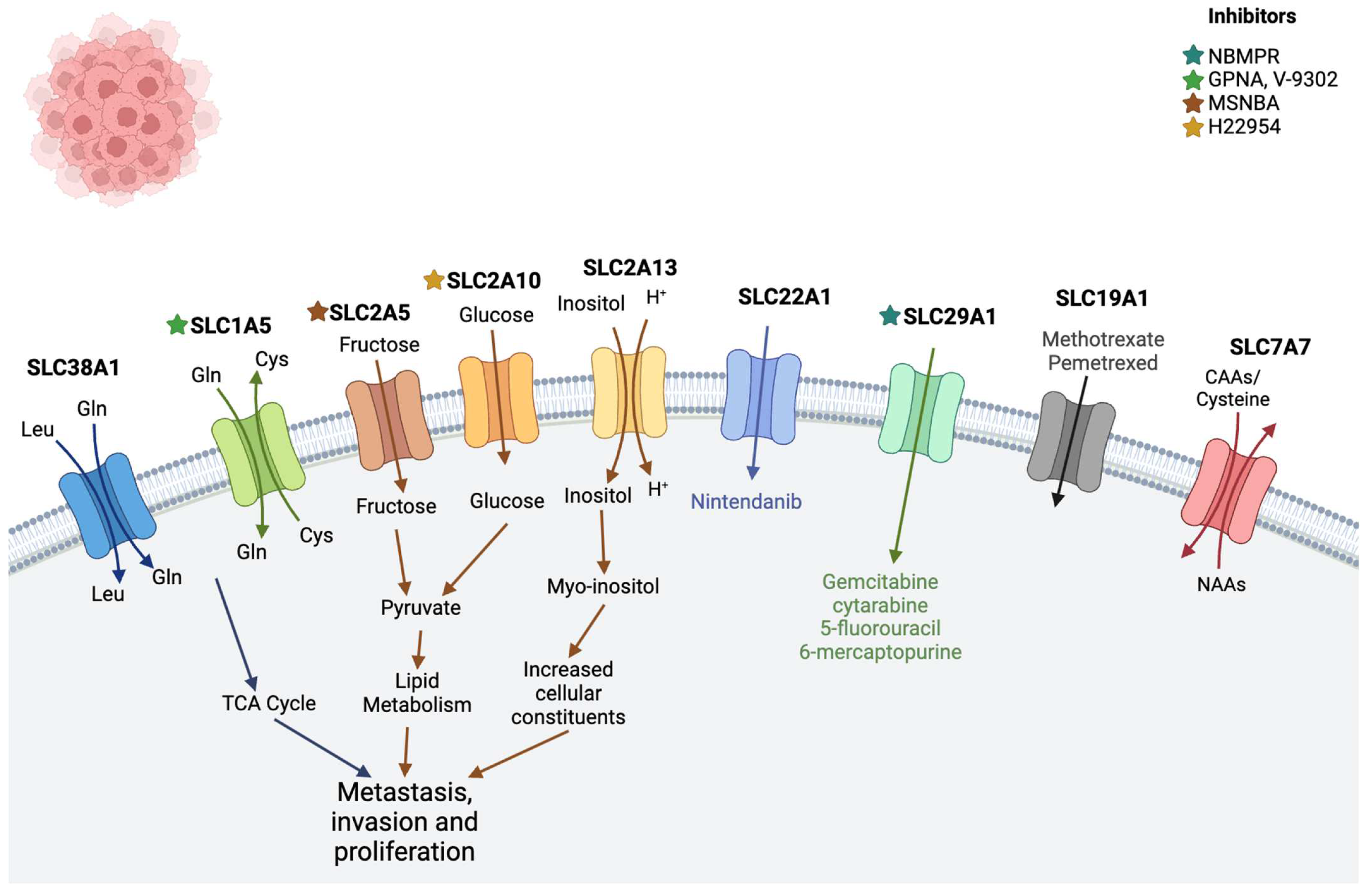
2.3. Leukemia
| Cancer | Solute Carrier Transporters | Function | Inhibitors/Substrates |
|---|---|---|---|
| Pancreatic cancer | SLC1A5 (alanine–serine–cysteine transporter 2) | Transports neutral amino acid with co-transportation in a sodium ion-dependent manner | V9302 [55] |
| SLC4A7 (sodium and bicarbonate ion co-transporter) | Plays a critical role in regulating the intracellular pH of pancreatic cells | S0859 [23] | |
| SLC7A5 (L-type amino acid transporter 1; LAT1) | Regulates the influx of L-leucine and the efflux of L-glutamine | JPH203 [56], KYT 0353 [57,58], melphalan, acivicin [11] | |
| SLC7A11 cystine/glutamate antiporter) | Raises glutathione biosynthesis through the uptake of cystine and the release of glutamate [22] | HG106 [11,59] | |
| SLC30 | Involved in the efflux of zinc | - | |
| SLC39A3, SLC39A4, and SLC39A6 | Involved in the absorption of zinc and increased cell proliferation | - | |
| SLC5A8 (Na2+-coupled monocarboxylate transporter 1 | Co-transports monocarboxylates such as lactate, butyrate, pyruvate, acetate, propionate, nicotinate, and β-hydroxybutyrate [29,30,31] | - | |
| SLC41A1 | Suppress tumor growth [33,34,35] | - | |
| SLC22A5 (OCTN2) | Zwitterions (L-carnitine), organic cations | Etoposide, imatinib [11] | |
| Breast Cancer | SLCO1A2 (OATP1A2) | Involved in transporting prostaglandin and steroid hormones/conjugates; estradiol-17β-glucuronide, estrone-3-sulfate (E-3-S), dehydroepiandrosterone sulfate (DHEA-S), and anticancer drugs; and imatinib, methotrexate, paclitaxel, doxorubicin, and docetaxel [39] | Imatinib, methotrexate [11] |
| SLCO1B1 (OATP1B1) and SLCO1B3 (OATP1B3) | Related to drug uptake | Cisplatin, carboplatin, oxaliplatin, regorafenib, belzutifan, SN-38, etoposide, tamoxifen, nilotinib, docetaxel, imatinib, gefitinib [11], sorafenib [60] | |
| SLCO2A1 (OATP2A1) and SLCO2A4 | Involved in prostaglandin transport | Suramin [61] | |
| SLCO4A1 and SLCO4C1 | Promote the elimination of external medications and internal substances such as methotrexate, thyroid hormones, and E-3-S [48,49] | - | |
| SLC22A5 (OCTN2) | Transports zwitterions (L-carnitine), organic cations | Etoposide, imatinib [11] | |
| Leukemia | SLC38A1 | Transports glutamine and plays a crucial role in maintaining the homeostasis of the human body [62] | - |
| SLC2 family (SLC2A5, SLC2A10, and SLC2A13) | Encodes glucose transporter (GLUT) proteins [63] | MSNBA and H22954 [64] | |
| SLC19A1 | Transports reduced folate and found to be associated with methotrexate drug | Methotrexate, pemetrexed [11] | |
| SLC29A1 (ENT1) | Cellular uptake of anticancer nucleoside agents as well as physiologic nucleosides | Gemcitabine, cytarabine, 5-fluorouracil, 6-mercaptopurine [11], NBMPR [65] | |
| SLC22A1 (OCT1) | Transport of organic cations, i.e., nutrients, neurotransmitters, metabolites, or drugs [66] | Nintendanib [11] | |
| SLC1A5 | Uptake of neutral amino acids, including glutamine (Gln), cysteine (Cys), serine (Ser), threonine (Thr), valine (Val), and alanine (Ala) [67,68] | V9302 [55] GPNA [69] | |
| SLC7A7 | Transports cationic amino acids such as arginine and lysine out of the cell | - | |
| Colon cancer | SLC19A3 (thiamine transporter 2; THTR2) | Manipulates the DNA methylation and histone deacetylation status of the nucleic acid of these cells | - |
| SLC3A2 | Putrescine and arginine importer | - | |
| SLCO4A1 | Transport of various compounds, including sugars, bile salts, organic acids, metal ions, amine compounds, and estrogen, and high expression has been reported in many cancer types, including colon cancer [70,71] | - | |
| SLC22A3 (OCT3) | Organic cation transporter 3 | Oxaliplatin [11] | |
| SLC35A2 and SLC29A1 | Nucleoside transporter | - | |
| SLC1A5 | Glutamine transporter | Cetuximab [72] | |
| SLC10A2 | Associated with the progression of colorectal cancer, especially in kids [73] | - | |
| SLC7A2 | Inducible transporter of the semi-essential amino acid L-arginine (L-Arg) | - | |
| SLC37A1 | Involved in the sugar–phosphate exchange | - | |
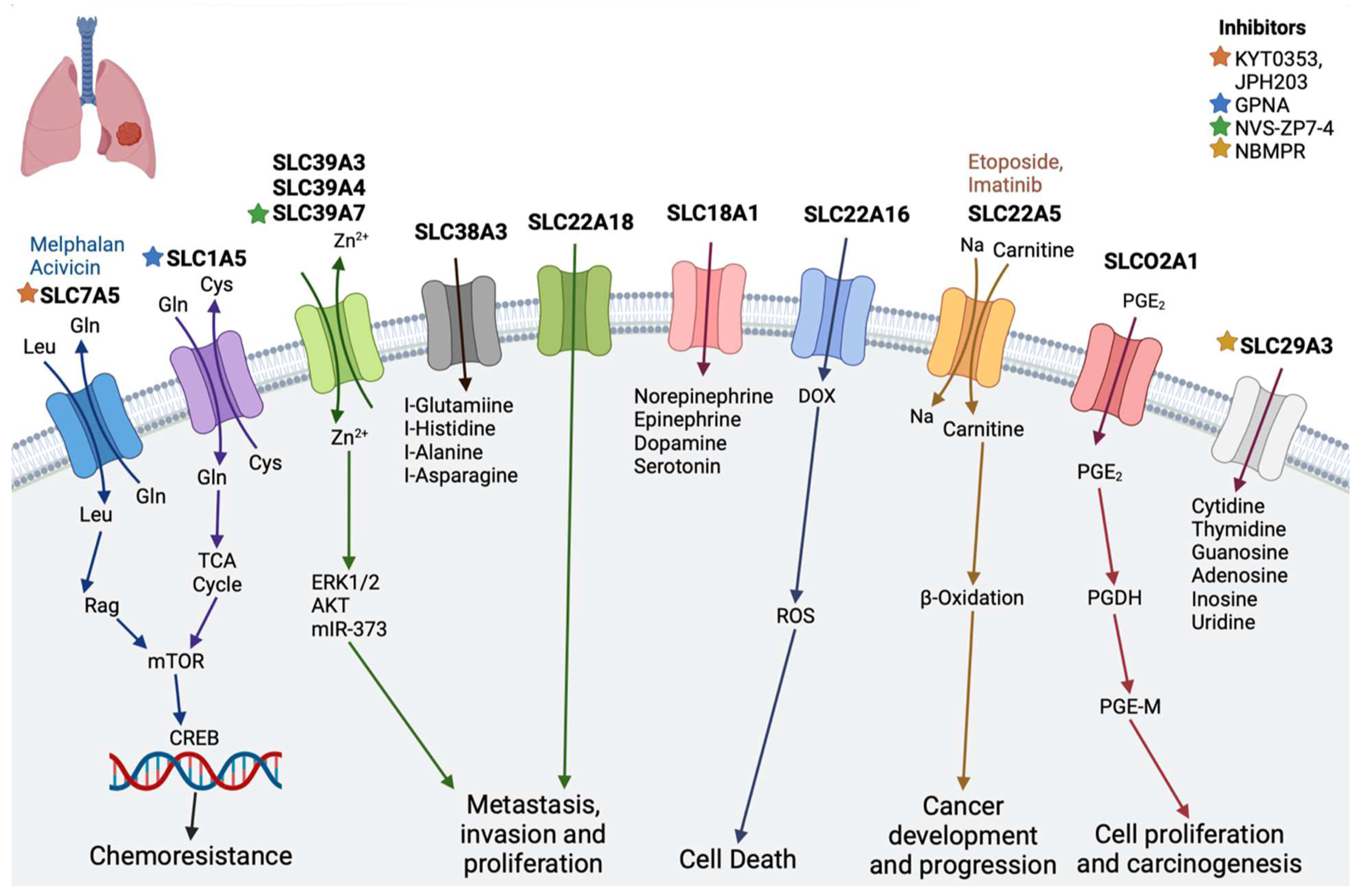
| Lung cancer | SLC18A1 | Transports monoamines, such as norepinephrine, epinephrine, dopamine, and serotonin, and has been associated with neuropsychiatric disorders [74] | - |
| SLC39A3, SLC39A4, and SLC39A7 | Zn2+ influx transporter, essential role in cell survival of lung adenocarcinoma | NVS-ZP7-4 [69] | |
| SLC1A5 | Transports glutamine and regulates the cell growth and oxidative stress in non-small cell lung cancer [75] | Gamma-l-glutamyl-p-nitroanilide (GPNA) [76] | |
| SLC29A3 | Nucleoside transporter | NBMPR [65] | |
| SLC7A5 | Plays important role in non-small cell lung cancer [77] | JPH203 [56], KYT 0353 [58], melphalan, acivicin [11] | |
| SLCO2A1 (OATP2A1) | Organic anion transporter, reported to function in the progression of lung cancer | Suramin [61] | |
| SLC38A3 | An amino acid transporter, specifically of L-glutamine, L -histidine, L -alanine, and L -asparagine | - | |
| SLC22A16 (OCT6) | Mediates platinum influx into cancer cells | - | |
| SLC22A18 | Regulates cellular metabolism, cellular growth, and drug sensitivity [78] | - | |
| SLC22A5 (OCTN2) | Zwitterions (L-carnitine), organic cations | Etoposide, imatinib [11] | |
| Liver cancer | SLC39A6 | Significantly upregulated in liver cancer tissues compared with normal liver tissues [79] | - |
| SLC26A6 | Nonselective anion exchanger that transports anions such as oxalate, formate, and sulfate [80] | DIDS [81] | |
| SLC2A1 | Glucose transporter and increased in HCC | GRg3, 1:1 CC-DG mixture [64] | |
| SLC2A2 | Glucose transporter and decreased in HCC [82,83] | Streptozotocin [11] | |
| SLC13A5 | A sodium-coupled citrate transporter, plays a key role in importing citrate from the circulation into liver cells [84,85] | BI01383298 [86] | |
| SLCO2B1 (OATP2B1) | An organic anion transporter; decreased expression in liver cancer | Etoposide, erlotinib [11] | |
| SLCO2A1 (OATP2A1) | Increased level of mRNA observed in hepatic tumors | Suramin [61] |
2.4. Colon Cancer
2.5. Lung Cancer
2.6. Liver Cancer
3. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stein, W.D.; Litman, T. Channels, Carriers, and Pumps: An Introduction to Membrane Transport; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Gautier, A.; Hinner, M.J. Site-Specific Protein Labeling; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- The International Transporter Consortium. Membrane Transporters in Drug Development. Nat. Rev. Drug Discov. 2010, 9, 215–236.
- Hediger, M.A.; Clémençon, B.; Burrier, R.E.; Bruford, E.A. The ABCs of Membrane Transporters in Health and Disease (SLC Series): Introduction. Mol. Asp. Med. 2013, 34, 95–107. [Google Scholar] [CrossRef]
- Rudnick, G.; Krämer, R.; Blakely, R.D.; Murphy, D.L.; Verrey, F. The SLC6 Transporters: Perspectives on Structure, Functions, Regulation, and Models for Transporter Dysfunction. Pflüg. Arch.-Eur. J. Physiol. 2014, 466, 25–42. [Google Scholar] [CrossRef]
- Bai, X.; Moraes, T.F.; Reithmeier, R.A. Structural Biology of Solute Carrier (SLC) Membrane Transport Proteins. Mol. Membr. Biol. 2017, 34, 1–32. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: A Worldwide Hub of Protein Knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Shi, Y. Common Folds and Transport Mechanisms of Secondary Active Transporters. Annu. Rev. Biophys. 2013, 42, 51–72. [Google Scholar] [CrossRef]
- Garibsingh, R.-A.A.; Schlessinger, A. Advances and Challenges in Rational Drug Design for SLCs. Trends Pharmacol. Sci. 2019, 40, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.; Boudker, O. Shared Molecular Mechanisms of Membrane Transporters. Annu. Rev. Biochem. 2016, 85, 543–572. [Google Scholar] [CrossRef] [PubMed]
- Puris, E.; Fricker, G.; Gynther, M. The Role of Solute Carrier Transporters in Efficient Anticancer Drug Delivery and Therapy. Pharmaceutics 2023, 15, 364. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, B.; Meng, S.; Xu, Y.; Guo, J.; Chen, L.J.; Xiao, J.; Zhang, W.; Tan, Z.R.; Tang, J.; et al. Increased Expression of SLC46A3 to Oppose the Progression of Hepatocellular Carcinoma and Its Effect on Sorafenib Therapy. Biomed. Pharmacother. 2019, 114, 108864. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Nyquist, M.D.; Prasad, B.; Mostaghel, E.A. Harnessing Solute Carrier Transporters for Precision Oncology. Molecules 2017, 22, 539. [Google Scholar] [CrossRef]
- Gyimesi, G.; Hediger, M.A. Transporter-Mediated Drug Delivery. Molecules 2023, 28, 1151. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Meredith, D. The SLC16 Gene Family-from Monocarboxylate Transporters (MCTs) to Aromatic Amino Acid Transporters and Beyond. Pflug. Arch. 2004, 447, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Bonglack, E.N.; Messinger, J.E.; Cable, J.M.; Ch’ng, J.; Parnell, K.M.; Reinoso-Vizcaíno, N.M.; Barry, A.P.; Russell, V.S.; Dave, S.S.; Christofk, H.R.; et al. Monocarboxylate Transporter Antagonism Reveals Metabolic Vulnerabilities of Viral-Driven Lymphomas. Proc. Natl. Acad. Sci. USA 2021, 118, e2022495118. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wu, Y.; Li, C.; Qu, Z.; Lou, G.; Guo, X.; Ji, J.; Li, N.; Guo, M.; Zhang, M.; et al. Comprehensive Analysis of the SLC16A Gene Family in Pancreatic Cancer Via Integrated Bioinformatics. Sci. Rep. 2020, 10, 7315. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Saksena, S.; Alrefai, W.A.; Sarwar, Z.; Goldstein, J.L.; Carroll, R.E.; Ramaswamy, K.; Dudeja, P.K. Expression and Membrane Localization of MCT Isoforms Along the Length of the Human Intestine. Am. J. Physiol. Cell Physiol. 2005, 289, C846–C852. [Google Scholar] [CrossRef]
- Lin, W.R.; Chiang, J.M.; Lim, S.N.; Su, M.Y.; Chen, T.H.; Huang, S.W.; Chen, C.W.; Wu, R.C.; Tsai, C.L.; Lin, Y.H.; et al. Dynamic Bioenergetic Alterations in Colorectal Adenomatous Polyps and Adenocarcinomas. EBioMedicine 2019, 44, 334–345. [Google Scholar] [CrossRef]
- Hong, C.S.; Graham, N.A.; Gu, W.; Espindola Camacho, C.; Mah, V.; Maresh, E.L.; Alavi, M.; Bagryanova, L.; Krotee, P.A.L.; Gardner, B.K.; et al. MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4. Cell Rep. 2016, 14, 1590–1601. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.; Robay, D.; Hindupur, S.K.; Pohlmann, J.; Colombi, M.; El-Shemerly, M.Y.; Maira, S.M.; Moroni, C.; Lane, H.A.; Hall, M.N. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin Due to NAD+ Depletion in Cancer Cells. Cell Rep. 2018, 25, 3047–3058.e4. [Google Scholar] [CrossRef]
- Ramirez, C.; Hauser, A.D.; Vucic, E.A.; Bar-Sagi, D. Plasma Membrane V-ATPase Controls Oncogenic RAS-Induced Macropinocytosis. Nature 2019, 576, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Arakawa, K.; Shimizu, K.; Oriuchi, N.; Nagamori, S.; Kanai, Y.; Oyama, T.; Takeyoshi, I. Relationship between CD147 and Expression of Amino Acid Transporters (LAT1 and ASCT2) in Patients with Pancreatic Cancer. Am. J. Transl. Res. 2015, 7, 356–363. [Google Scholar]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid Transporter SLC7A11/xCT at the Crossroads of Regulating Redox Homeostasis and Nutrient Dependency of Cancer. Cancer Commun. 2018, 38, 12. [Google Scholar] [CrossRef]
- Zhu, J.-H.; De Mello, R.A.; Yan, Q.-L.; Wang, J.-W.; Chen, Y.; Ye, Q.-H.; Wang, Z.-J.; Tang, H.-J.; Huang, T. MiR-139-5p/SLC7A11 Inhibits the Proliferation, Invasion and Metastasis of Pancreatic Carcinoma Via PI3K/Akt Signaling Pathway. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165747. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.-M.; Cousins, R.J. Aberrant Expression of Zinc Transporter ZIP4 (SLC39A4) Significantly Contributes to Human Pancreatic Cancer Pathogenesis and Progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef]
- Unno, J.; Satoh, K.; Hirota, M.; Kanno, A.; Hamada, S.; Ito, H.; Masamune, A.; Tsukamoto, N.; Motoi, F.; Egawa, S. LIV-1 Enhances the Aggressive Phenotype through the Induction of Epithelial to Mesenchymal Transition in Human Pancreatic Carcinoma Cells. Int. J. Oncol. 2009, 35, 813–821. [Google Scholar] [PubMed]
- Gopal, E.; Miyauchi, S.; Martin, P.M.; Ananth, S.; Roon, P.; Smith, S.B.; Ganapathy, V. Transport of Nicotinate and Structurally Related Compounds by Human SMCT1 (SLC5A8) and Its Relevance to Drug Transport in the Mammalian Intestinal Tract. Pharm. Res. 2007, 24, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, M.; Gopal, E.; Martin, P.M.; Ananth, S.; Smith, S.B.; Prasad, P.D.; Sterneck, E.; Ganapathy, V. SLC5A8 Triggers Tumor Cell Apoptosis through Pyruvate-Dependent Inhibition of Histone Deacetylases. Cancer Res. 2006, 66, 11560–11564. [Google Scholar] [CrossRef]
- Martin, P.M.; Gopal, E.; Ananth, S.; Zhuang, L.; Itagaki, S.; Prasad, B.M.; Smith, S.B.; Prasad, P.D.; Ganapathy, V. Identity of SMCT1 (SLC5A8) as a Neuron-Specific Na+-Coupled Transporter for Active Uptake of L-Lactate and Ketone Bodies in the Brain. J. Neurochem. 2006, 98, 279–288. [Google Scholar] [CrossRef]
- Coothankandaswamy, V.; Elangovan, S.; Singh, N.; Prasad, P.D.; Thangaraju, M.; Ganapathy, V. The Plasma Membrane Transporter SLC5A8 Suppresses Tumour Progression through Depletion of Survivin without Involving Its Transport Function. Biochem. J. 2013, 450, 169–178. [Google Scholar] [CrossRef]
- Helm, J.; Coppola, D.; Ganapathy, V.; Lloyd, M.; Centeno, B.A.; Chen, D.-T.; Malafa, M.P.; Park, J.Y. SLC5A8 Nuclear Translocation and Loss of Expression Are Associated with Poor Outcome in Pancreatic Ductal Adenocarcinoma. Pancreas 2012, 41, 904–909. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Yang, S.; Thangaraju, M.; Ganapathy, V. SLC Transporters as a Novel Class of Tumour Suppressors: Identity, Function and Molecular Mechanisms. Biochem. J. 2016, 473, 1113–1124. [Google Scholar] [CrossRef]
- Costello, L.C.; Levy, B.A.; Desouki, M.M.; Zou, J.; Bagasra, O.; Johnson, L.A.; Hanna, N.; Franklin, R.B. Decreased Zinc and Downregulation of ZIP3 Zinc Uptake Transporter in the Development of Pancreatic Adenocarcinoma. Cancer Biol. Ther. 2011, 12, 297–303. [Google Scholar] [CrossRef]
- Xie, J.; Cheng, C.-s.; Zhu, X.Y.; Shen, Y.H.; Song, L.B.; Chen, H.; Chen, Z.; Liu, L.M.; Meng, Z.Q. Magnesium Transporter Protein Solute Carrier Family 41 Member 1 Suppresses Human Pancreatic Ductal Adenocarcinoma through Magnesium-Dependent Akt/mTOR Inhibition and Bax-Associated Mitochondrial Apoptosis. Aging 2019, 11, 2681–2698. [Google Scholar] [CrossRef]
- Takagishi, T.; Hara, T.; Fukada, T. Recent Advances in the Role of SLC39A/ZIP Zinc Transporters In Vivo. Int. J. Mol. Sci. 2017, 18, 2708. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Prev. Biomark. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Hee Choi, Y.; Yu, A.-M. ABC Transporters in Multidrug Resistance and Pharmacokinetics, and Strategies for Drug Development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Miki, Y.; Suzuki, T.; Kitada, K.; Yabuki, N.; Shibuya, R.; Moriya, T.; Ishida, T.; Ohuchi, N.; Blumberg, B.; Sasano, H. Expression of the Steroid and Xenobiotic Receptor and Its Possible Target Gene, Organic Anion Transporting Polypeptide-A, in Human Breast Carcinoma. Cancer Res. 2006, 66, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, A.; Roth, M.; Hagenbuch, B. The Expression and Function of Organic Anion Transporting Polypeptides in Normal Tissues and in Cancer. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Nakanishi, T.; Yanagihara, C.; Nishimoto, T.; Wakayama, T.; Mizokami, A.; Namiki, M.; Kawai, K.; Tamai, I. Enhanced Expression of Organic Anion Transporting Polypeptides (OATPs) in Androgen Receptor-Positive Prostate Cancer Cells: Possible Role of OATP1A2 in Adaptive Cell Growth under Androgen-Depleted Conditions. Biochem. Pharmacol. 2012, 84, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Onogawa, T.; Suzuki, T.; Ishida, T.; Rikiyama, T.; Katayose, Y.; Ohuchi, N.; Sasano, H.; Abe, T.; Unno, M. Human Liver-Specific Organic Anion Transporter-2 Is a Potent Prognostic Factor for Human Breast Carcinoma. Cancer Sci. 2007, 98, 1570–1576. [Google Scholar] [CrossRef]
- Kindla, J.; Rau, T.T.; Jung, R.; Fasching, P.A.; Strick, R.; Stoehr, R.; Hartmann, A.; Fromm, M.F.; König, J. Expression and Localization of the Uptake Transporters OATP2B1, OATP3A1 and OATP5A1 in Non-Malignant and Malignant Breast Tissue. Cancer Biol. Ther. 2011, 11, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Wlcek, K.; Taferner, B.; Hering, S.; Stieger, B.; Tong, D.; Zeillinger, R.; Thalhammer, T.; Jäger, W. Expression of Organic Anion-Transporting Polypeptides 1B1 and 1B3 in Ovarian Cancer Cells: Relevance for Paclitaxel Transport. Biomed. Pharmacother. 2011, 65, 417–426. [Google Scholar] [CrossRef]
- Bleasby, K.; Castle, J.; Roberts, C.; Cheng, C.; Bailey, W.; Sina, J.; Kulkarni, A.; Hafey, M.; Evers, R.; Johnson, J. Expression Profiles of 50 Xenobiotic Transporter Genes in Humans and Pre-Clinical Species: A Resource for Investigations into Drug Disposition. Xenobiotica 2006, 36, 963–988. [Google Scholar] [CrossRef] [PubMed]
- Kochel, T.J.; Goloubeva, O.G.; Fulton, A.M. Upregulation of Cyclooxygenase-2/Prostaglandin E2 (COX-2/PGE2) Pathway Member Multiple Drug Resistance-Associated Protein 4 (MRP4) and Downregulation of Prostaglandin Transporter (PGT) and 15-Prostaglandin Dehydrogenase (15-PGDH) in Triple-Negative Breast Cancer. Breast Cancer 2016, 10, 61–70. [Google Scholar] [PubMed]
- Nozawa, T.; Suzuki, M.; Takahashi, K.; Yabuuchi, H.; Maeda, T.; Tsuji, A.; Tamai, I. Involvement of Estrone-3-Sulfate Transporters in Proliferation of Hormone-Dependent Breast Cancer Cells. J. Pharmacol. Exp. Ther. 2004, 311, 1032–1037. [Google Scholar] [CrossRef]
- Wlcek, K.; Svoboda, M.; Riha, J.; Zakaria, S.; Olszewski, U.; Dvorak, Z.; Sellner, F.; Ellinger, I.; Jäger, W.; Thalhammer, T. The Analysis of Organic Anion Transporting Polypeptide (OATP) mRNA and Protein Patterns in Primary and Metastatic Liver Cancer. Cancer Biol. Ther. 2011, 11, 801–811. [Google Scholar] [CrossRef]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The Organic Anion and Cation Transporters of the SLCO and SLC22A Gene Superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef]
- Banerjee, N.; Allen, C.; Bendayan, R. Differential Role of Organic Anion-Transporting Polypeptides in Estrone-3-Sulphate Uptake by Breast Epithelial Cells and Breast Cancer Cells. J. Pharmacol. Exp. Ther. 2012, 342, 510–519. [Google Scholar] [CrossRef]
- Kuo, K.-L.; Zhu, H.; McNamara, P.J.; Leggas, M. Localization and Functional Characterization of the Rat Oatp4c1 Transporter in an In Vitro Cell System and Rat Tissues. PLoS ONE 2012, 7, e39641. [Google Scholar] [CrossRef]
- Okabe, M.; Szakács, G.; Reimers, M.A.; Suzuki, T.; Hall, M.D.; Abe, T.; Weinstein, J.N.; Gottesman, M.M. Profiling SLCO and SLC22 Genes in the NCI-60 Cancer Cell Lines to Identify Drug Uptake Transporters. Mol. Cancer Ther. 2008, 7, 3081–3091. [Google Scholar] [CrossRef]
- Albers, A.; Bröer, A.; Wagner, C.A.; Setiawan, I.; Lang, P.A.; Kranz, E.U.; Lang, F.; Bröer, S. Na+ Transport by the Neural Glutamine Transporter ATA1. Pflüg. Arch. 2001, 443, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, I.; Yoshino, H.; Fukumoto, W.; Tamai, M.; Okamura, S.; Osako, Y.; Sakaguchi, T.; Inoguchi, S.; Matsushita, R.; Yamada, Y.; et al. Targeting of the Glutamine Transporter SLC1A5 Induces Cellular Senescence in Clear Cell Renal Cell Carcinoma. Biochem. Biophys. Res. Commun. 2022, 611, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y. Amino Acid Transporter LAT1 (SLC7A5) as a Molecular Target for Cancer Diagnosis and Therapeutics. Pharmacol. Ther. 2022, 230, 107964. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Anonick, M.V.; Jaiswal, S.; Mashayekh, S.; Brown, A.; Wodzanowski, K.A.; Okuda, K.; Silverman, N.; Grimes, C.L. Synthesis and Validation of Click-Modified NOD1/2 Agonists. Innate Immun. 2023, 29, 186–200. [Google Scholar] [CrossRef]
- Oda, K.; Hosoda, N.; Endo, H.; Saito, K.; Tsujihara, K.; Yamamura, M.; Sakata, T.; Anzai, N.; Wempe, M.F.; Kanai, Y.; et al. L-Type Amino Acid Transporter 1 Inhibitors Inhibit Tumor Cell Growth. Cancer Sci. 2010, 101, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, K.; Lv, J.; Feng, J.; Chen, J.; Wu, H.; Cheng, F.; Jiang, W.; Wang, J.; Pei, H.; et al. Suppression of the SLC7A11/Glutathione Axis Causes Synthetic Lethality in KRAS-Mutant Lung Adenocarcinoma. J. Clin. Investig. 2020, 130, 1752–1766. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Sánchez-Martín, A.; Rodríguez-Macías, G.; Sánchez-Abarca, L.I.; Lozano, E.; Herraez, E.; Odero, M.D.; Díez-Martín, J.L.; Marin, J.J.G.; Briz, O. Role of Drug Transporters in the Sensitivity of Acute Myeloid Leukemia to Sorafenib. Oncotarget 2018, 9, 28474–28485. [Google Scholar] [CrossRef]
- Kamo, S.; Nakanishi, T.; Aotani, R.; Nakamura, Y.; Gose, T.; Tamai, I. Impact of FDA-Approved Drugs on the Prostaglandin Transporter OATP2A1/SLCO2A1. J. Pharm. Sci. 2017, 106, 2483–2490. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ganapathy, V. Glutamine Transporters in Mammalian Cells and Their Functions in Physiology and Cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2531–2539. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) Family of Membrane Transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Ismail, A.; Tanasova, M. Importance of GLUT Transporters in Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 8698. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, O.; Papini, F.; Mantini, G.; Gregori, A.; Parrino, B.; Liu, D.S.K.; Cascioferro, S.; Carbone, D.; Peters, G.J.; Frampton, A.E.; et al. “Open Sesame?”: Biomarker Status of the Human Equilibrative Nucleoside Transporter-1 and Molecular Mechanisms Influencing its Expression and Activity in the Uptake and Cytotoxicity of Gemcitabine in Pancreatic Cancer. Cancers 2020, 12, 3206. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, D.; Gorboulev, V.; Gambaryan, S.; Veyhl, M.; Koepsell, H. Drug Excretion Mediated by a New Prototype of Polyspecific Transporter. Nature 1994, 372, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Kekuda, R.; Prasad, P.D.; Fei, Y.-J.; Torres-Zamorano, V.; Sinha, S.; Yang-Feng, T.L.; Leibach, F.H.; Ganapathy, V. Cloning of the Sodium-Dependent, Broad-Scope, Neutral Amino Acid Transporter Bo from a Human Placental Choriocarcinoma Cell Line. J. Biol. Chem. 1996, 271, 18657–18661. [Google Scholar] [CrossRef]
- Fuchs, B.C.; Bode, B.P. Amino Acid Transporters ASCT2 and LAT1 in Cancer: Partners in Crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef]
- Nolin, E.; Gans, S.; Llamas, L.; Bandyopadhyay, S.; Brittain, S.M.; Bernasconi-Elias, P.; Carter, K.P.; Loureiro, J.J.; Thomas, J.R.; Schirle, M.; et al. Discovery of a ZIP7 Inhibitor from a Notch Pathway Screen. Nat. Chem. Biol. 2019, 15, 179–188. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Meier, P.J. Organic Anion Transporting Polypeptides of the OATP/SLC21 Family: Phylogenetic Classification as OATP/SLCO Superfamily, New Nomenclature and Molecular/Functional Properties. Pflüg. Arch. 2004, 447, 653–665. [Google Scholar] [CrossRef]
- Ancona, N.; Maglietta, R.; Piepoli, A.; D’Addabbo, A.; Cotugno, R.; Savino, M.; Liuni, S.; Carella, M.; Pesole, G.; Perri, F. On the Statistical Assessment of Classifiers Using DNA Microarray Data. BMC Bioinform. 2006, 7, 387. [Google Scholar] [CrossRef]
- Ma, H.; Wu, Z.; Peng, J.; Li, Y.; Huang, H.; Liao, Y.; Zhou, M.; Sun, L.; Huang, N.; Shi, M.; et al. Inhibition of SLC1A5 Sensitizes Colorectal Cancer to Cetuximab. Int. J. Cancer 2018, 142, 2578–2588. [Google Scholar] [CrossRef]
- Da Silva, T.C.; Polli, J.E.; Swaan, P.W. The Solute Carrier Family 10 (SLC10): Beyond Bile Acid Transport. Mol. Asp. Med. 2013, 34, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Kayabaşı, Y.; Güneş, B.; Erbaş, O. Serotonin Receptors and Depression. J. Exp. Basic Med. Sci. 2021, 2, 240–246. [Google Scholar]
- Hassanein, M.; Hoeksema, M.D.; Shiota, M.; Qian, J.; Harris, B.K.; Chen, H.; Clark, J.E.; Alborn, W.E.; Eisenberg, R.; Massion, P.P. SLC1A5 Mediates Glutamine Transport Required for Lung Cancer Cell Growth and Survival. Clin. Cancer Res. 2013, 19, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Dominici, S.; Piaggi, S.; Belcastro, E.; Chiu, M.; Taurino, G.; Pacini, S.; Bussolati, O.; Pompella, A. γ-Glutamyltransferase enzyme activity of cancer cells modulates L-γ-glutamyl-p-nitroanilide (GPNA) cytotoxicity. Sci. Rep. 2019, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Tamai, I. Solute Carrier Transporters as Targets for Drug Delivery and Pharmacological Intervention for Chemotherapy. J. Pharm. Sci. 2011, 100, 3731–3750. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Zhou, Z.; Karri, S.; Li, Z.; Zhao, J. In Vitro and In Vivo Radiosensitization of Human Glioma U251 Cells Induced by Upregulated Expression of SLC22A18. Cancer Gene Ther. 2014, 21, 103–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hassanein, M.; Qian, J.; Hoeksema, M.D.; Wang, J.; Jacobovitz, M.; Ji, X.; Harris, F.T.; Harris, B.K.; Boyd, K.L.; Chen, H. Targeting SLC1a5-Mediated Glutamine Dependence in Non-Small Cell Lung Cancer. Int. J. Cancer 2015, 137, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Alper, S.L.; Sharma, A.K. The SLC26 Gene Family of Anion Transporters and Channels. Mol. Asp. Med. 2013, 34, 494–515. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yang, J.; Fok, K.L.; Ye, Y.H.; Jin, L.; Chen, Z.Y.; Zhang, X.M.; Huang, H.F.; Chan, H.C. Involvement of Cl(-)/HCO3(-) Exchanger SLC26A3 and SLC26A6 in Preimplantation Embryo Cleavage. Sci. Rep. 2016, 6, 28402. [Google Scholar] [CrossRef]
- Amann, T.; Maegdefrau, U.; Hartmann, A.; Agaimy, A.; Marienhagen, J.; Weiss, T.S.; Stoeltzing, O.; Warnecke, C.; Schölmerich, J.; Oefner, P.J.; et al. GLUT1 Expression Is Increased in Hepatocellular Carcinoma and Promotes Tumorigenesis. Am. J. Pathol. 2009, 174, 1544–1552. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeong, D.C.; Pak, K.; Han, M.E.; Kim, J.Y.; Liangwen, L.; Kim, H.J.; Kim, T.W.; Kim, T.H.; Hyun, D.W.; et al. SLC2A2 (GLUT2) as a Novel Prognostic Factor for Hepatocellular Carcinoma. Oncotarget 2017, 8, 68381–68392. [Google Scholar] [CrossRef]
- Inoue, K.; Fei, Y.J.; Zhuang, L.; Gopal, E.; Miyauchi, S.; Ganapathy, V. Functional Features and Genomic Organization of Mouse NaCT, a Sodium-Coupled Transporter for Tricarboxylic Acid Cycle Intermediates. Biochem. J. 2004, 378, 949–957. [Google Scholar] [CrossRef]
- Pajor, A.M. Sodium-Coupled Dicarboxylate and Citrate Transporters from the SLC13 Family. Pflug. Arch. 2014, 466, 119–130. [Google Scholar] [CrossRef]
- opnMe.com Boehringer Ingelheim (2024): OpnMe—Boehringer Ingelheim Open Innovation Portal. Available online: https://www.opnme.com/molecules/slc13a5-bi01383298 (accessed on 11 March 2024).
- Li, Y.; Shao, H.; Da, Z.; Pan, J.; Fu, B. High Expression of SLC38A1 Predicts Poor Prognosis in Patients with De Novo Acute Myeloid Leukemia. J. Cell. Physiol. 2019, 234, 20322–20328. [Google Scholar] [CrossRef]
- Lai, B.; Lai, Y.; Zhang, Y.; Zhou, M.; Sheng, L.; OuYang, G. The Solute Carrier Family 2 Genes Are Potential Prognostic Biomarkers in Acute Myeloid Leukemia. Technol. Cancer Res. Treat. 2020, 19, 1533033819894308. [Google Scholar] [CrossRef]
- Wang, S.-M.; Sun, L.-L.; Zeng, W.-X.; Wu, W.-S.; Zhang, G.-L. Effects of a microRNA Binding Site Polymorphism in SLC19A1 on Methotrexate Concentrations in Chinese Children with Acute Lymphoblastic Leukemia. Med. Oncol. 2014, 31, 62. [Google Scholar] [CrossRef]
- Ni, F.; Yu, W.-M.; Li, Z.; Graham, D.K.; Jin, L.; Kang, S.; Rossi, M.R.; Li, S.; Broxmeyer, H.E.; Qu, C.-K. Critical Role of ASCT2-Mediated Amino Acid Metabolism in Promoting Leukaemia Development and Progression. Nat. Metab. 2019, 1, 390–403. [Google Scholar] [CrossRef]
- Bröer, S.; Palacin, M. The Role of Amino Acid Transporters in Inherited and Acquired Diseases. Biochem. J. 2011, 436, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Bröer, A.; Wagner, C.A.; Lang, F.; Bröer, S. The Heterodimeric Amino Acid Transporter 4F2hc/y+ LAT2 Mediates Arginine Efflux in Exchange with Glutamine. Biochem. J. 2000, 349, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Grzes, K.M.; Swamy, M.; Hukelmann, J.L.; Emslie, E.; Sinclair, L.V.; Cantrell, D.A. Control of Amino Acid Transport Coordinates Metabolic Reprogramming in T-Cell Malignancy. Leukemia 2017, 31, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yang, X.; Wang, N.; Kang, M.; Wang, Y.; Rong, L.; Fang, Y.; Xue, Y. Function of SLC7A7 in T-Cell Acute Lymphoblastic Leukemia. Cell. Physiol. Biochem. 2018, 48, 731–740. [Google Scholar] [CrossRef]
- Ikehata, M.; Ueda, K.; Iwakawa, S. Different Involvement of DNA Methylation and Histone Deacetylation in the Expression of Solute-Carrier Transporters in 4 Colon Cancer Cell Lines. Biol. Pharm. Bull. 2012, 35, 301–307. [Google Scholar] [CrossRef]
- Jonker, J.W.; Schinkel, A.H. Pharmacological and Physiological Functions of the Polyspecific Organic Cation Transporters: OCT1, 2, and 3 (SLC22A1-3). J. Pharmacol. Exp. Ther. 2004, 308, 2–9. [Google Scholar] [CrossRef]
- Zhang, S.; Lovejoy, K.S.; Shima, J.E.; Lagpacan, L.L.; Shu, Y.; Lapuk, A.; Chen, Y.; Komori, T.; Gray, J.W.; Chen, X. Organic Cation Transporters Are Determinants of Oxaliplatin Cytotoxicity. Cancer Res. 2006, 66, 8847–8857. [Google Scholar] [CrossRef]
- Hamada, A.; Sissung, T.; Price, D.K.; Danesi, R.; Chau, C.H.; Sharifi, N.; Venzon, D.; Maeda, K.; Nagao, K.; Sparreboom, A. Effect of SLCO1B3 Haplotype on Testosterone Transport and Clinical Outcome in Caucasian Patients with Androgen-Independent Prostatic Cancer. Clin. Cancer Res. 2008, 14, 3312–3318. [Google Scholar] [CrossRef]
- Lee, W.; Belkhiri, A.; Lockhart, A.C.; Merchant, N.; Glaeser, H.; Harris, E.I.; Washington, M.K.; Brunt, E.M.; Zaika, A.; Kim, R.B. Overexpression of OATP1B3 Confers Apoptotic Resistance in Colon Cancer. Cancer Res. 2008, 68, 10315–10323. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Stringer, D.E.; Blohm-Mangone, K.A.; Gerner, E.W. Polyamine Transport is Mediated by Both Endocytic and Solute Carrier Transport Mechanisms in the Gastrointestinal Tract. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G517–G522. [Google Scholar] [CrossRef] [PubMed]
- Ban, M.J.; Ji, S.H.; Lee, C.-K.; Bae, S.B.; Kim, H.J.; Ahn, T.S.; Lee, M.S.; Baek, M.-J.; Jeong, D. Solute Carrier Organic Anion Transporter Family Member 4A1 (SLCO4A1) as a Prognosis Marker of Colorectal Cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1437–1447. [Google Scholar] [CrossRef]
- Phua, L.C.; Mal, M.; Koh, P.K.; Cheah, P.Y.; Chan, E.C.Y.; Ho, H.K. Investigating the Role of Nucleoside Transporters in the Resistance of Colorectal Cancer to 5-Fluorouracil Therapy. Cancer Chemother. Pharmacol. 2013, 71, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, K.; Goto, Y.; Sekikawa, K.; Takenoshita, S.; Ishida, N.; Kawakita, M.; Kannagi, R. Increased Expression of UDP-Galactose Transporter Messenger RNA in Human Colon Cancer Tissues and Its Implication in Synthesis of Thomsen-Friedenreich Antigen and Sialyl Lewis A/X Determinants. Cancer Res. 2001, 61, 4620–4627. [Google Scholar]
- Coburn, L.A.; Singh, K.; Asim, M.; Barry, D.P.; Allaman, M.M.; Al-Greene, N.T.; Hardbower, D.M.; Polosukhina, D.; Williams, C.S.; Delgado, A.G. Loss of Solute Carrier Family 7 Member 2 Exacerbates Inflammation-Associated Colon Tumorigenesis. Oncogene 2019, 38, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, D.; Saito, M.; Saito, K.; Watanabe, Y.; Matsumoto, Y.; Kanke, Y.; Onozawa, H.; Hayase, S.; Sakamoto, W.; Ishigame, T. Upregulated Solute Carrier Family 37 Member 1 in Colorectal Cancer is Associated with Poor Patient Outcome and Metastasis. Oncol. Lett. 2018, 15, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Banerjee, N.; Chatterjee, S. Solute Carrier Proteins and c-Myc: A Strong Connection in Cancer Progression. Drug Discov. Today 2020, 25, 891–900. [Google Scholar] [CrossRef]
- Neal, R.D.; Sun, F.; Emery, J.D.; Callister, M.E. Lung Cancer. BMJ 2019, 365, l1725. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. Expression of the Vesicular Monoamine Transporter Gene Solute Carrier Family 18 Member 1 (SLC18A1) in Lung Cancer. Cancer Genom. Proteom. 2018, 15, 387–393. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian Zinc Transporters: Nutritional and Physiologic Regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, Y.; Qi, H.; Liang, L.; Wu, H.; Yuan, J.; Hu, Q. Evaluation of the Prognostic Values of Solute Carrier (SLC) Family 39 Genes for Patients with Lung Adenocarcinoma. Aging 2021, 13, 5312–5331. [Google Scholar] [CrossRef]
- Nair, S.; Strohecker, A.M.; Persaud, A.K.; Bissa, B.; Muruganandan, S.; McElroy, C.; Pathak, R.; Williams, M.; Raj, R.; Kaddoumi, A. Adult Stem Cell Deficits Drive Slc29a3 Disorders in Mice. Nat. Commun. 2019, 10, 2943. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Ren, S.; Li, X.; Zhou, F.; Li, W.; Gao, G.; He, Y.; Zhou, C. Genomic Polymorphisms of SLC29A3 Associated with Overall Survival in Advanced Non-Small-Cell Lung Cancer Treated with Gemcitabine. Med. Oncol. 2014, 31, 865. [Google Scholar] [CrossRef]
- Lemstrová, R.; Souček, P.; Melichar, B.; Mohelnikova-Duchonova, B. Role of Solute Carrier Transporters in Pancreatic Cancer: A Review. Pharmacogenomics 2014, 15, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liang, X.; Dai, J.; Guan, X. Prostaglandin Transporter, SLCO2A1, Mediates the Invasion and Apoptosis of Lung Cancer Cells Via PI3K/AKT/mTOR Pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 9175–9181. [Google Scholar] [PubMed]
- Nakata, R.; Nakamura, Y.; Hosomi, S.; Okuda, H.; Nishida, Y.; Sugita, N.; Itani, S.; Nadatani, Y.; Otani, K.; Tanaka, F. Slco 2 a1 Deficiency Exacerbates Experimental Colitis Via Inflammasome Activation in Macrophages: A Possible Mechanism of Chronic Enteropathy Associated with SLCO2A1 gene. Sci. Rep. 2020, 10, 4883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, L.; Cui, M.; Wang, Y.; Xu, Y.; Li, M.; Mi, J. Amino Acid Transporter SLC38A3 Promotes Metastasis of Non-Small Cell Lung Cancer Cells by Activating PDK1. Cancer Lett. 2017, 393, 8–15. [Google Scholar] [CrossRef]
- Oguri, T.; Kunii, E.; Fukuda, S.; Sone, K.; Uemura, T.; Takakuwa, O.; Kanemitsu, Y.; Ohkubo, H.; Takemura, M.; Maeno, K. Organic Cation Transporter 6 Directly Confers Resistance to Anticancer Platinum Drugs. Biomed. Rep. 2016, 5, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Kunii, E.; Oguri, T.; Kasai, D.; Ozasa, H.; Uemura, T.; Takakuwa, O.; Ohkubo, H.; Takemura, M.; Maeno, K.; Niimi, A. Organic Cation Transporter OCT6 Mediates Cisplatin Uptake and Resistance to Cisplatin in Lung Cancer. Cancer Chemother. Pharmacol. 2015, 75, 985–991. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xu, C.; Zhao, Z.; Qin, X.; Xu, H.; Zhang, H. Low Expression of SLC22A18 Predicts Poor Survival Outcome in Patients with Breast Cancer after Surgery. Cancer Epidemiol. 2011, 35, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef]
- Idilman, R.; De Maria, N.; Colantoni, A.; Van Thiel, D.H. Pathogenesis of Hepatitis B and C-Induced Hepatocellular Carcinoma. J. Viral Hepat. 1998, 5, 285–299. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Duan, Z.; Yu, X.; White, D.; El-Serag, H.B. Trends in the Burden of Nonalcoholic Fatty Liver Disease in a United States Cohort of Veterans. Clin. Gastroenterol. Hepatol. 2016, 14, 301–308.e2. [Google Scholar] [CrossRef]
- Morgan, T.R.; Mandayam, S.; Jamal, M.M. Alcohol and Hepatocellular Carcinoma. Gastroenterology 2004, 127, S87–S96. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, X. Role of SLC39A6 in the Development and Progression of Liver Cancer. Oncol. Lett. 2022, 23, 77. [Google Scholar] [CrossRef]
- Cao, J.; Wang, P.; Chen, J.; He, X. Systemic Characterization of the SLC Family Genes Reveals SLC26A6 as a Novel Oncogene in Hepatocellular Carcinoma. Transl. Cancer Res. 2021, 10, 2882–2894. [Google Scholar] [CrossRef]
- Peng, Q.; Hao, L.Y.; Guo, Y.L.; Zhang, Z.Q.; Ji, J.M.; Xue, Y.; Liu, Y.W.; Lu, J.L.; Li, C.G.; Shi, X.L. Solute Carrier Family 2 Members 1 and 2 as Prognostic Biomarkers in Hepatocellular Carcinoma Associated with Immune Infiltration. World J. Clin. Cases 2022, 10, 3989–4019. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Liu, M.; Zou, Z.; Wang, F.; Hu, H.; Sun, B.; Zhang, S. Risk of Developing Hepatocellular Carcinoma following Depressive Disorder Based on the Expression Level of Oatp2a1 and Oatp2b1. Biomed. Res. Int. 2019, 2019, 3617129. [Google Scholar] [CrossRef]
- Wu, X.; Gong, C.; Weinstock, J.; Cheng, J.; Hu, S.; Venners, S.A.; Hsu, Y.H.; Wu, S.; Zha, X.; Jiang, S.; et al. Associations of the SLCO1B1 Polymorphisms With Hepatic Function, Baseline Lipid Levels, and Lipid-lowering Response to Simvastatin in Patients With Hyperlipidemia. Clin. Appl. Thromb. Hemost. 2018, 24, 240s–247s. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Lusi, C.F.; Mashayekh, S.; Nagar, A.; Subbarao, M.; Kane, G.I.; Wodzanowski, K.A.; Brown, A.R.; Okuda, K.; Monahan, A.; et al. Methotrexate Suppresses Psoriatic Skin Inflammation by Inhibiting Muropeptide Transporter SLC46A2 Activity. Immunity 2023, 56, 998–1012.e8. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC transporters as Therapeutic Targets: Emerging Opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharadwaj, R.; Jaiswal, S.; Velarde de la Cruz, E.E.; Thakare, R.P. Targeting Solute Carrier Transporters (SLCs) as a Therapeutic Target in Different Cancers. Diseases 2024, 12, 63. https://doi.org/10.3390/diseases12030063
Bharadwaj R, Jaiswal S, Velarde de la Cruz EE, Thakare RP. Targeting Solute Carrier Transporters (SLCs) as a Therapeutic Target in Different Cancers. Diseases. 2024; 12(3):63. https://doi.org/10.3390/diseases12030063
Chicago/Turabian StyleBharadwaj, Ravi, Swati Jaiswal, Erandi E. Velarde de la Cruz, and Ritesh P. Thakare. 2024. "Targeting Solute Carrier Transporters (SLCs) as a Therapeutic Target in Different Cancers" Diseases 12, no. 3: 63. https://doi.org/10.3390/diseases12030063
APA StyleBharadwaj, R., Jaiswal, S., Velarde de la Cruz, E. E., & Thakare, R. P. (2024). Targeting Solute Carrier Transporters (SLCs) as a Therapeutic Target in Different Cancers. Diseases, 12(3), 63. https://doi.org/10.3390/diseases12030063







