Pre-Stroke Antihypertensive Therapy Affects Stroke Severity and 3-Month Outcome of Ischemic MCA-Territory Stroke
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. dCA Assessment
2.3. Statistics
3. Results
3.1. Regression Analysis
Stroke Severity
3.2. Outcome
4. Discussion
5. Study Limitation
6. Future Perspective
7. Conclusions/Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Yanez, M.; Gomez-Choco, M.; Lopez-Cancio, E.; Amaro, S.; de Leciñana, M.A.; Arenillas, J.F.; Ayo-Martin, O.; Castellanos, M.; Freijo, M.M.; Garcia-Pastor, A.; et al. et ad hoc committee of the Spanish Society of Neurology’s Study Group for Cerebrovascular Diseases. Stroke prevention in patients with arterial hypertension: Recommendations of the Spanish Society of Neurology’s Stroke Study Group. Neurology (Engl. Ed.). 2021, 36, 462–471. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104, Erratum in Eur. Heart J. 2019, 40, 475. [Google Scholar] [CrossRef] [PubMed]
- Hamann, G.F.; Sander, D.; Röther, J.; Grau, A.; Deutsche Schlaganfall-Gesellschaft und Deutsche Gesellschaft für Neurologie. Sekundärprophylaxe ischämischer Schlaganfall und transitorische ischämische Attacke: Teil 1, S2kLeitlinie, 2022, in: Deutsche Gesellschaft für Neurologie (Hrsg.), Leitlinien für Diagnostik und Therapie in der Neurologie. Available online: www.dgn.org/leitlinien (accessed on 3 February 2024).
- Llwyd, O.; Fan, J.L.; Müller, M. Effect of drug interventions on cerebral hemodynamics in ischemic stroke patients. J. Cereb. Blood Flow Metab. 2022, 42, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Tziomalos, K.; Giampatzis, V.; Bouziana, S.D.; Spanou, M.; Papadopoulou, M.; Kazantzidou, P.; Kostaki, S.; Kouparanis, A.; Savopoulos, C.; Hatzitolios, A.I. Effects of different classes of antihypertensive agents on the outcome of acute ischemic stroke. J. Clin. Hypertens. 2015, 17, 275–280. [Google Scholar] [CrossRef]
- Renner, C.J.; Kasner, S.E.; Bath, P.M.; Bahouth, M.N. VISTA Acute Steering Committee. Stroke outcome related to initial volume status and diuretic use. J. Am. Heart Assoc. 2022, 11, e026903. [Google Scholar] [CrossRef] [PubMed]
- Eizenberg, Y.; Grossman, E.; Tanne, D.; Koton, S. Pre admission treatment with beta-blockers in hypertensive patients with acute stroke and 3-month outcome-data from a national stroke registry. J. Clin. Hypertens. 2018, 20, 568–572. [Google Scholar] [CrossRef]
- Selim, M.; Savitz, S.; Linfante, I.; Caplan, L.; Schlaug, G. Effect of pre-stroke use of ACE inhibitors on ischemic stroke severity. BMC Neurol. 2005, 5, 10. [Google Scholar] [CrossRef]
- Hwong, W.Y.; Bots, M.L.; Selvarajah, S.; Abdul Aziz, Z.; Sidek, N.N.; Spiering, W.; Kappelle, L.J.; Vaartjes, I. Use of antihypertensive drugs and ischemic stroke severity—Is there a role for angiotensin-II? PLoS ONE 2016, 11, e0166524. [Google Scholar] [CrossRef]
- Lyden, P.; Brott, T.; Tilley, B.; Welch, K.M.; Mascha, E.J.; Levine, S.; Haley, E.C.; Grotta, J.; Marler, J. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994, 25, 2220–2226. [Google Scholar] [CrossRef]
- Van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; Van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Lakatos, L.; Bolognese, M.; Müller, M.; Österreich, M.; von Hessling, A. Automated supra- and in-fratentorial brain infarct volume estimation on diffusion weighted imaging using the RAPID software. Front Neurol. 2022, 13, 907151. [Google Scholar] [CrossRef]
- Fazekas, F.; Barkhof, F.; Wahlund, L.O.; Pantoni, L.; Erkinjuntti, T.; Scheltens, P.; Schmidt, R. CT and MRI rating of white matter lesions. Cerebrovasc. Dis. 2002, 13 (Suppl. 2), 31–36. [Google Scholar] [CrossRef]
- Müller, M.; Österreich, M.; von Hessling, A.; Smith, R.S. Incomplete recovery of cerebral blood flow dynamics in sufficiently treated high blood pressure. J. Hypertens. 2019, 37, 372–379. [Google Scholar] [CrossRef]
- Panerai, R.B.; Brassard, P.; Burma, J.S.; Castro, P.; Claassen, J.A.; van Lieshout, J.J.; Liu, J.; Lucas, S.J.; Minhas, J.S.; Mitsis, G.D.; et al. Cerebrovascular Research Network (CARNet). Transfer function analysis of dynamic cerebral autoregulation: A CARNet white paper 2022 update. J. Cereb. Blood Flow Metab. 2023, 43, 3–25. [Google Scholar] [CrossRef]
- Koton, S.; Pike, J.R.; Johansen, M.; Knopman, D.S.; Lakshminarayan, K.; Mosley, T.; Patole, S.; Rosamond, W.D.; Schneider, A.L.; Sharrett, A.R.; et al. Association of ischemic stroke incidence, severity, and recurrence with dementia in the Atherosclerosis Risk in Communities Cohort Study. JAMA Neurol. 2022, 79, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Aries, M.J.; Elting, J.W.; De Keyser, J.; Kremer, B.P.; Vroomen, P.C. Cerebral autoregulation in stroke: A review of transcranial Doppler studies. Stroke 2010, 41, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.C.; Aries, M.; Minhas, J.S.; HPetersen, N.; Xiong, L.; Kainerstorfer, J.M.; Castro, P. Review of studies on dynamic cerebral autoregulation in the acute phase of stroke and the relationship with clinical outcome. J. Cereb. Blood Flow Metab. 2022, 42, 430–453. [Google Scholar] [CrossRef] [PubMed]
- Maïer, B.; Gory, B.; Lapergue, B.; Sibon, I.; Richard, S.; Kyheng, M.; Labreuche, J.; Desilles, J.P.; Blanc, R.; Piotin, M.; et al. BP TARGET Investigators. Effect of baseline antihypertensive treatments on stroke severity and outcomes in the BP TARGET trial. Stroke 2022, 53, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Milionis, H.; Faouzi, M.; Cordier, M.; D’Ambrogio-Remillard, S.; Eskandari, A.; Michel, P. Characteristics and early and long-term outcome in patients with acute ischemic stroke and low ejection fraction. Int. J. Cardiol. 2013, 168, 1082–1087. [Google Scholar] [CrossRef]
- Iwai, M.; Chen, R.; Ide, A.; Iwanami, J.; Tomochika, H.; Tomono, Y.; Mogi, M.; Horiuchi, M. The calcium-channel blocker, azelnidipine, enhances the inhibitory action of AT1 receptor blockade on ischemic brain damage. J. Hypertens. 2006, 24, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L. Cerebrovascular angiotensin AT1 receptor regulation in cerebral ischemia. Trends Cardiovasc. Med. 2008, 18, 98–103. [Google Scholar] [CrossRef]
- Krikov, M.; Thone-Reineke, C.; Müller, S.; Villringer, A.; Unger, T. Candesartan but not ramipril pretreatment improves outcome after stroke and stimulates neurotrophin BNDF/TrkB system in rats. J. Hypertens. 2008, 26, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Porritt, M.J.; Chen, M.; Rewell, S.S.; Dean, R.G.; Burrell, L.M.; Howells, D.W. ACE inhibition reduces infarction in normotensive but not hypertensive rats: Correlation with cortical ACE activity. J. Cereb. Blood Flow Metab. 2010, 30, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wakhloo, A.K.; Fisher, M. Advances in Acute Ischemic Stroke Therapy. Circ. Res. 2022, 130, 1230–1251. [Google Scholar] [CrossRef] [PubMed]
- Masthoff, M.; Krähling, H.; Akkurt, B.H.; Elsharkawy, M.; Köhler, M.; Ergawy, M.; Thomas, C.; Schwindt, W.; Minnerup, J.; Stracke, P. Evaluation of effectiveness and safety of the multizone NeVaTM stent retriever for mechanical thrombectomy in ischemic stroke. Neuroradiology 2023, 65, 1777–1785. [Google Scholar] [CrossRef]
- Yamal, J.M.; Martinez, J.; Osani, M.C.; Du, X.L.; Simpson, L.M.; Davis, B.R. Mortality and morbidity among individuals with hypertension receiving a diuretic, ACE inhibitor, or calcium channel blocker: A secondary analysis of a randomized clinical trial. JAMA Netw. Open 2023, 6, e2344998. [Google Scholar] [CrossRef]
- Nazarzadeh, M.; Canoy, D.; Bidel, Z.; Copland, E.; Rahimi, K.; Teo, K.; Davis, B.R.; Chalmers, J.; Pepine, C.J.; Woodward, M. Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment for prevention of major cardiovascular diseases in people with and without type 2 diabetes: An individual participant-level data meta-analysis. Lancet Diabetes Endocrinol. 2022, 10, 645–654. [Google Scholar] [CrossRef]
- Wright, J.M.; Musini, V.M.; Gill, R. First-line drugs for hypertension. Cochrane Database Syst. Rev. 2018, 4, CD001841. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, N.; Zhou, M.; Guo, J.; Zhu, C.; Zhou, J.; Ma, M.; He, L. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database Syst. Rev. 2022, 1, CD003654. [Google Scholar] [CrossRef]
- Sundström, J.; Lind, L.; Nowrouzi, S.; Hagström, E.; Held, C.; Lytsy, P.; Neal, B.; Marttala, K.; Östlund, O. Heterogeneity in blood pressure response to 4 antihypertensive drugs: A randomized clinical trial. JAMA 2023, 329, 1160–1169. [Google Scholar] [CrossRef]
| No High BP (n = 154) | High BP (n = 183) | |||||
|---|---|---|---|---|---|---|
| Beta-Blockers | Calcium Channel Blockers | Diuretics | RAAS Inhibition | |||
| ACE Inhibitors | AT-1 Receptor Inhibitors | |||||
| N | 154 | 76 | 60 | 77 | 59 | 79 |
| Sex (male/female) | 111/43 | 50/26 | 41/19 | 49/28 | 41/18 | 54/25 |
| Age (years) | 62.5 [IQR 51; 72] * | 76 [IQR 67; 82] | 75.5 [IQR 71; 83] | 76 [IQR 71.5; 82] | 72 [IQR 65; 77] | 74 [IQR 67; 80] |
| Diabetes mellitus | 23 ** | 27 | 19 | 26 | 21 | 24 |
| Dyslipidemia | 107 | 67 | 57 | 64 | 52 | 68 |
| Smoking | 42 | 15 | 13 | 14 | 19 | 12 |
| Large vessel disease | 27 | 13 | 16 | 17 | 7 | 17 |
| Ischemic heart disease | 11 | 38 | 17 | 32 | 20 | 29 |
| Atrial fibrillation | 18 | 27 | 13 | 21 | 11 | 15 |
| Left ventricular ejection fraction (%) | 60 [IQR 55; 64] | 60 [IQR 55; 64] | 60 [IQR 55; 64.5] | 60 [IQR 56; 65] | 60 [IQR 55; 65] | 60 [IQR 55; 63] |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 82 [IQR 61; 91] | 83 [IQR 67; 90] | 82 [IQR 69; 91] | 85 [IQR 76; 91] | 82 [IQR 71; 91] | 80 [IQR 73; 91] |
| Antihypertensive therapy with 0/1/2/3/>3 different drug classes | 0 | 0/17/29/22/8 | 0/9/23/20/8 | 0/6/36/27/8 | 0/19/29/7/4 | 0/18/34/23/4 |
| No High BP (n = 154) | High BP (n = 183) | |||||
|---|---|---|---|---|---|---|
| Beta-Blockers | Calcium Channel Blockers | Diuretics | RAAS Inhibition | |||
| ACE Inhibition | AT-1 Receptor Inhibitors | |||||
| N | 154 | 76 | 60 | 77 | 59 | 79 |
| Number of transient ischemic attack/stroke | 20/134 | 11/65 | 10/50 | 18/59 | 6/53 | 21/58 |
| National Institute of Health Score (NIHSS) at entry | 3 [IQR 1; 6.5] | 3 [IQR 1; 7.5] | 3.5 [IQR 1; 10] | 3 [IQR 2; 8] | 2 [IQR 1; 3.75] | 3 [IQR 1; 5] |
| Modified ranking score (mRs) at entry | 2 [IQR 1; 3.5] | 2 [IQR 1; 4] | 3 [IQR 2; 4] | 3 [IQR 2; 4] | 2 [IQR 1; 3] | 2 [IQR 1; 3] |
| CTP infarct core (mL) | 0 [IQR 0; 0], range 0–90 | 0 [IQR 0: 0], range 0–63 | 0 [IQR 0; 0], range 0–155 | 0 [IQR 0; 0], range 0–63 | 0 [IQR 0; 0] range 0–63 | 0 [IQR 0; 0] range 0–155 |
| CTP penumbra (mL) | 0 [IQR 0; 39.2], range 0–365 | 0 [IQR 0; 0], range 0–221 | 0 [IQR 0, 0], range 0–372 | 0 [IQR 0, 0], range 0–221 | 0 [IQR 0; 9] range 0–187 | 0 [IQR 0; 26.3] range 0–372 |
| Number of intravenous lysis (total n = 123) | 56 | 20 | 18 | 27 | 18 | 25 |
| Number of mechanical thrombectomy (total n = 65) | 30 | 21 | 10 | 18 | 9 | 14 |
| MRI infarct size (mL) | 0.97 [IQR 0.2; 6.97] | 0.70 [IQR 0.12; 8.65] | 0.77 [IQR 0.01; 7.00] | 0.51 [IQR 0.12; 7.90] | 0.30 [IQR 0.01; 2.94] | 0.56 [IQR 0.05; 5.40] |
| Lacunar/non-lacunar stroke | 22/132 | 20/56 | 10/50 | 9/68 | 20/39 | 15/64 |
| mRs 3 month | 0 [IQR 0; 2] | 1 [IQR 0; 3] | 1 [IQR 0; 3] | 1 [IQR 0; 3] | 1 [IQR 0; 3] | 1 [IQR 0; 2] |
| Variable | Reference Values from [14] in Mean ± SD | No High BP (n = 154) | High Blood Pressure (n = 183) | ||||
|---|---|---|---|---|---|---|---|
| Beta-Blockers | Calcium Channel Inhibitors | Diuretics | Renin–Angiotensin–Aldosterone System Inhibitors | ||||
| ACE Inhibitors | AT-1 Receptor Inhibitors | ||||||
| Mean velocity (cm/s) | 51 [IQR 43; 63] | 45 [IQR 39; 51.5] | 47.5 [IQR 42; 57] | 44.5 [IQR 37.5; 53] | 45 [IQR 38.5; 54] | 44 [IQR 38; 50] | |
| Pulsatility index | 0.86 [IQR 0.75; 0.97] * | 1.06 [IQR 0.87; 1.24] | 1.05 [IQR 0.93; 1.16] | 1.06 [IQR 0.90; 1.23] | 1.04 [IQR 0.89; 1.14] | 1.00 [IQR 0.85; 1.20] | |
| Coherence | |||||||
| −VLF | 0.47 ± 13 | 0.59 ± 0.15 0.61 [IQR 0.51; 0.73] | 0.58 ± 0.13 0.56 [IQR 0.48; 0.70] | 0.56 ± 0.11 0.57 [IQR 0.47; 0.70] | 0.56 ± 0.12 0.58 [IQR 0.48; 0.69] | 0.58 ± 0.14 0.64 [IQR 0.50; 0.70] | 0.59 ± 0.12 0.58 [IQR 0.50; 0.72] |
| -LF | 0.73 ± 0.15 | 0.67 ± 0.17 0.70 [IQR 0.59; 0.83] | 0.63 ± 0.16 0.65 [IQR 0.52; 0.75] | 0.60 ± 0.15 0.59 [IQR 0.50; 0.73] | 0.60 ± 0.15 0.63 [IQR 0.53; 0.75] | 0.64 ± 0.14 0.67 [IQR 0.53; 0.80] | 0.65 ± 0.15 0.68 [IQR 0.55; 0.78] |
| -HF | 0.65 ± 0.14 | 0.63 ± 0.14 0.68 [IQR 0.60; 0.77] | 65 ± 0.15 0.65 [IQR 0.55; 0.75] | 0.66 ± 0.16 0.61 [IQR 0.51; 0.69] | 0.63 ± 0.15 0.64 [IQR 0.54; 0.70] | 0.67 ± 0.12 0.67 [IQR 0.54; 0.73] | 0.66 ± 0.15 0.69 [IQR 0.58; 0.75] |
| Gain (cm/s/mmHg) | |||||||
| -VLF | 0.27 ± 0.29 | 0.31 ± 0.21 0.28 [IQR 0.14; 0.43] | 0.31 ± 0.20 0.23 [IQR 0.14; 0.44] | 0.25 ± 0.17 0.27 [IQR 0.15; 0.46] | 0.26 ± 0.19 0.25 [IQR 0.15; 0.44] | 0.30 ± 0.19 0.28 [IQR 0.17; 0.51] | 0.24 ± 0.18 0.25 [IQR 0.14; 0.35] |
| -LF | 0.69 ± 0.41 | 0.54 ± 0.32 0.41 [IQR 0.30; 0.62] | 0.47 ± 0.22 0.41 [IQR 0.29; 0.54] | 0.39 ± 0.21 0.40 [IQR 0.25; 0.53] | 0.46 ± 0.24 0.41 [IQR 0.31; 0.52] | 0.42 ± 0.24 0.40 [IQR 0.25; 0.53] | 0.40 ± 0.22 0.41 [IQR 0.34; 0.58] |
| -HF | 0.82 ± 0.51 | 0.56 ± 0.24 0.51 [IQR 0.40; 0.70] | 0.59 ± 0.30 0.53 [IQR 0.39; 0.67] | 0.52 ± 0.29 0.44 [IQR 0.36; 0.63] | 0.54 ± 0.33 0.52 [IQR 0.35; 0.64] | 0.55 ± 0.26 0.48 [IQR 0.39; 0.63] | 0.52 ± 0.26 0.48 [IQR 0.38; 0.62] |
| Phase (radian) | |||||||
| -VLF | 1.12 ± 0.35 | 0.81 ± 0.36 0.82 [IQR 0.59; 1.02] | 0.83 ± 0.39 0.79 [IQR 0.60; 0.96] | 0.82 ± 0.40 0.81 [IQR 0.64; 0.95] | 0.82 ± 0.35 0.69 [IQR 0.49; 1.04] | 0.80 ± 0.43 0.70 [IQR 0.53; 0.93] | 0.82 ± 0.35 0.81 [IQR 0.55; 1.07] |
| -LF | 0.74 ± 0.21 | 0.63 ± 0.31 0.57 [IQR 0.38; 0.80] | 0.64 ± 0.36 0.62 [IQR 0.46; 0.78] | 0.64 ± 0.30 0.58 [IQR 0.44; 0.83] | 0.62 ± 0.28 0.54 [IQR 0.43; 0.74] | 0.69 ± 0.35 0.58 [IQR 0.38; 0.79] | 0.65 ± 0.30 0.55 [IQR 0.43; 0.75] |
| -HF | 0. 37 ± 0.34 | 0.19 ±0.35 0.20 [IQR 0.03; 0.38] | 0.15 ± 0.38 0.20 [IQR 0.03; 0.38] | 0.14 ± 0.39 0.24 [IQR 0.10; 0.44] | 0.17 ± 0.37 0.18 [IQR 0.03; 0.35] | 0.11 ± 0.35 0.22 [IQR 0.03; 0.36] | 0.19 ± 0.34 0.18 [IQR 0.04; 0.35] |
| Variable | Reference Values from [14] in Mean ± SD | No High BP (n = 154) | High Blood Pressure (n = 183) | ||||
|---|---|---|---|---|---|---|---|
| Va | No High BP | Beta-Blockers | Calcium Channel Inhibitors | Diuretics | Renin–Angiotensin–Aldosterone System Inhibitors | ||
| ACE Inhibitors | AT-1 Receptor Inhibitors | ||||||
| Mean velocity (cm/s) | 52 [IQR 44; 65] | 45 [IQR 40; 52] | 48.5 [IQR 41; 59] | 45 [IQR 38; 54] | 46.5 [IQR 39.5; 55] | 45 [IQR 38.5; 51] | |
| Pulsatility index | 0.87 [IQR 0.78; 0.98] * | 1.07 [IQR 0.89; 1.26] | 1.05 [IQR 0.94; 1.17] | 1.05 [IQR 0.89; 1.22] | 1.04 [IQR 0.87; 1.13] | 1.01 [IQR 0.86; 1.20] | |
| Coherence | |||||||
| −VLF | 0.47 ± 13 | 56 ± 0.14 0.55 [IQR 0.49; 064] | 0.57 ± 12 0.59 [IQR 0.51; 0.72] | 0.56 ± 0.11 0.55 [IQR 0.47; 0.67] | 0.57 ± 0.13 0.60 [IQR 0.47; 0.71] | 0.57 ± 0.14 0.56 [IQR 0.48; 0.68] | 0.56 ± 0.11 0.60 [IQR 0.49; 0.70] |
| −LF | 0.73 ± 0.15 | 0.67 ± 0.15 0.65 [IQR 0.52; 0.78] | 0.64 ± 0.15 0.67 [IQR 0.53; 0.74] | 0.58 ± 0.14 0.62 [IQR 0.52; 0.75] | 0.61 ± 0.15 0.58 [IQR 0.50; 0.74] | 0.65 ± 0.13 0.61 [IQR 0.51; 0.74] | 0.61 ± 0.15 0.63 [IQR 0.52; 0.76] |
| −HF | 0.65 ± 0.14 | 0.65 ± 0.14 0.64 [IQR 0.51; 0.75] | 0.65 ± 0.14 0.66 [IQR 0.56; 0.76] | 0.65 ± 0.16 0.64 [IQR 0.56; 0.75] | 0.62 ± 0.16 0.61 [IQR 0.51; 0.73] | 0.67 ± 0.15 0.61 [IQR 0.53; 0.75] | 0.65 ± 0.15 0.64 [IQR 0.50; 0.76] |
| Gain (cm/s/mmHg) | |||||||
| −VLF | 0.27 ± 0.29 | 0.32 ± 0.24 0.26 [IQR 0.16; 0.43] | 0.31 ± 0.21 0.27 [IQR 0.14; 0.48] | 0.26 ± 0.19 0.26 [IQR 0.20; 0.48] | 0.27 ± 0.20 0.31 [IQR 0.13; 0.46] | 0.32 ± 0.21 0.28 [IQR 0.18; 0.44] | 0.23 ± 0.16 0.27 [IQR 0.15; 0.39] |
| −LF | 0.69 ± 0.41 | 0.48 ± 0.22 0.47 [IQR 0.30; 0.64] | 0.50 ± 0.23 0.42 [IQR 0.32; 0.54] | 0.41 ± 0.28 0.44 [IQR 0.31; 0.59] | 0.47 ± 0.27 0.43 [IQR 0.34; 0.56] | 0.41 ± 0.32 0.37 [IQR 0.30; 0.54] | 0.45 ± 0.28 0.44 [IQR 0.35; 0.64] |
| −HF | 0.82 ± 0.51 | 0.56 ± 0.22 0.55 [IQR 0.42; 0.82] | 0.63 ± 0.34 0.52 [IQR 0.38; 0.65] | 0.55 ± 0.26 0.52 [IQR 0.42; 0.67] | 0.54 ± 0.28 0.52 [IQR 0.36; 0.67] | 0.56 ± 0.23 0.55 [IQR 0.40; 0.63] | 0.55 ± 0.26 0.52 [IQR 0.40; 0.70] |
| Phase (radian) | |||||||
| −VLF | 1.12 ± 0.35 | 0.88 ± 0.43 0.88 [IQR 0.58; 1.20] | 0.84 ± 0.39 0.75 [IQR 0.53; 0.99] | 0.88 ± 0.38 0.79 [IQR 0.60; 0.99] | 0.84 ± 0.39 0.75 [IQR 0.37; 1.05] | 0.77 ± 0.36 0.74 [IQR 0.51; 1.00] | 0.88 ± 0.35 0.84 [IQR 0.41; 1.25] |
| −LF | 0.74 ± 0.21 | 0.60 ± 0.25 0.65 [IQR 0.44; 0.87] | 0.67 ± 0.38 0.60 [IQR 0.45; 0.80] | 0.72 ± 0.32 0.60 [IQR 0.48; 0.79] | 0.63 ± 0.27 0.56 [IQR 0.39; 0.72] | 0.71 ± 0.32 0.64 [IQR 0.46; 0.95] | 0.69 ± 0.32 0.54 [IQR 0.38; 0.73] |
| −HF | 0. 37 ± 0.34 | 0.19 ± 0.42 0.23 [IQR 0.05; 0.36] | 0.22 ± 0.38 0.20 [IQR 0.06; 0.35] | 0.16 ± 0.39 0.18 [IQR 0.12; 0.43] | 0.20 ± 0.34 0.24 [IQR 0.05; 0.43] | 0.14 ± 0.43 0.24 [IQR 0.05; 0.45] | 0.21 ± 0.27 0.19 [IQR 0.06; 0.37] |
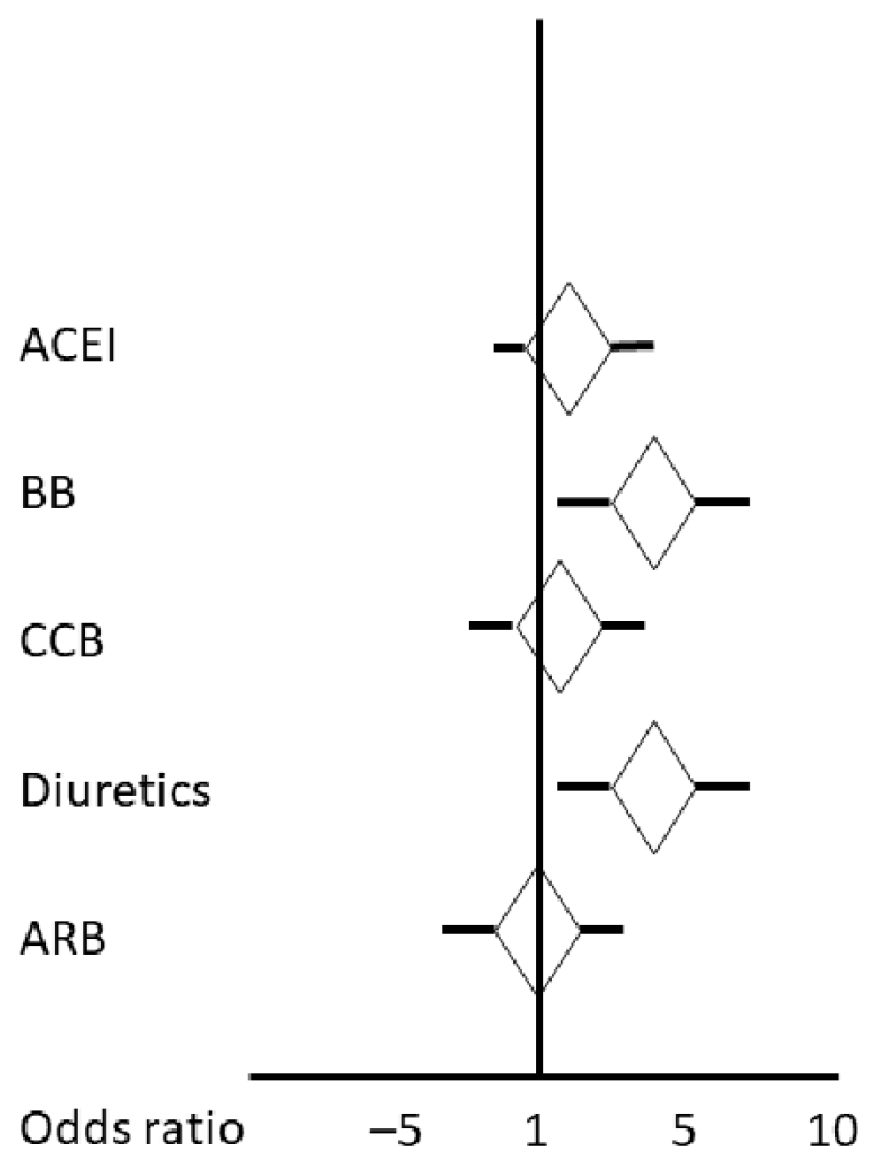
| Variable | Adjusted R2 | Beta | 95% CI of Beta | F-Statistic | Univariate Regression p = |
|---|---|---|---|---|---|
| ACEIs | 0.00 | 0.47 | −1.22 2.17 | 0.29 | 0.58 |
| BBs | 0.03 | 2.45 | 0.92 3.97 | 10.03 | 0.001 |
| CCBs | 0.00 | 0.23 | −1.45 1.92 | 0.07 | 0.78 |
| Diuretics | 0.02 | 2.18 | 0.66 370 | 8.00 | 0.005 |
| ARBs | 0.00 | 0.00 | −1.52 152 | 0.00 | 0.99 |
| Penumbra on CTP (mL) | 0.55 | 0.078 | 0.07 0.08 | 359.00 | 0.0000 |
| Infarct core on CTP (mL) | 0.33 | 0.20 | 0.17 0.24 | 145 | 0.0000 |
| Age | 0.02 | 0.05 | −0.01 0.1 | 6.45 | 0.01 |
| Systolic BP on admission | 0.01 | −0.03 | −0.0 0.05, | 5.23 | 0.02 |
| Diabetes mellitus | 0.009 | 1.38 | −0.12 2.89 | 3.25 | 0.07 |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 0.01 | −0.03 | −0.002 −0.07 | 4.44 | 0.03 |
| Large vessel disease | 0.01 | 1.68 | 0.10 3.35 | 3.93 | 0.04 |
| Coronary artery disease | 0.009 | 1.44 | −0.11 3.00 | 3.32 | 0.06 |
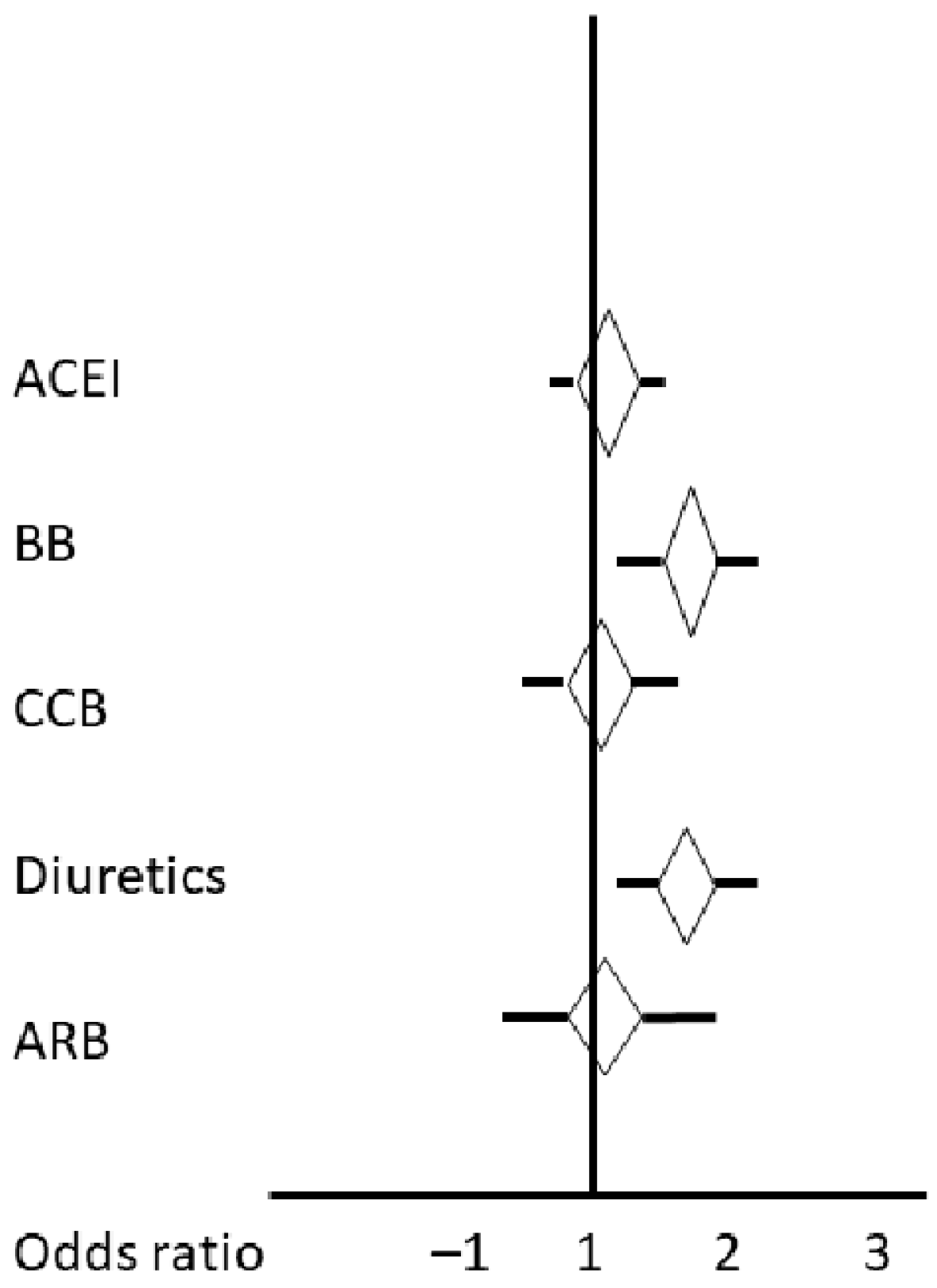
| Variable | Adjusted R2 | Beta | 95% CI of Beta | F-Statistic | Univariate Regression p = |
|---|---|---|---|---|---|
| ACEIs | 0.007 | 0.36 | −010 0.83 | 2.34 | 0.12 |
| BBs | 0.034 | 0.72 | 0.30 1.13 | 11.81 | 0.0007 |
| CCBs | 0.00 | 0.07 | 0.39 0.53 | 0.07 | 0.75 |
| Diuretics | 0.04 | 0.75 | 0.34 1.16 | 13.02 | 0.0004 |
| ARBs | 0.003 | 0.21 | −0.20 0.62 | 0.98 | 0.32 |
| Age | 0.05 | 0.022 | 0.01 0.03 | 19.29 | 0.0000 |
| CTP penumbra | 0.199 | 0.013 | 0.009 0.016 | 70.82 | 0.0000 |
| CTP infarct core | 0.11 | 0.032 | 0.02 0.04 | 36.20 | 0.0000 |
| MRI infarct volume | 0.23 | 0.027 | 0.02 0.03 | 95.08 | 0.0000 |
| SysBP on admission | 0.001 | −0.002 | −0.048 0.009 | 0.36 | 0.54 |
| IV lysis | 0.04 | 0.70 | 0.34 1.06 | 14.77 | 0.0001 |
| Mechanical thrombectomy | 0.14 | 0.99 | 0.73 1.25 | 56.05 | 0.0000 |
| High blood pressure | 0.005 | 0.23 | −0.11 0.58 | 1.80 | 0.18 |
| Diabetes mellitus | 0.003 | 0.211 | −0.20 0.62 | 0.98 | 0.32 |
| eGFR | 0.03 | −0.01 | −0.02 −0.06 | 10.44 | 0.001 |
| LVEF | 0.015 | −0.01 | −0.03 −0.002 | 4.85 | 0.02 |
| Atrial fibrillation | 0.016 | 0.54 | 0.08 1.00 | 5.49 | 0.01 |
| NIHSS on admission | 0.40 | 0.17 | 0.14 0.19 | 219.00 | 0.0000 |
| Large vessel disease | 0.001 | 0.263 | −0.19 0.71 | 0.49 | 0.48 |
| AHPhaseVLF | 0.003 | −0.37 | −0.82 0.08 | 2.60 | 0.10 |
| AHPhaseLF | 0.002 | −0.23 | −0.77 0.31 | 0.70 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakatos, L.-B.; Bolognese, M.; Österreich, M.; Weichsel, L.; Müller, M. Pre-Stroke Antihypertensive Therapy Affects Stroke Severity and 3-Month Outcome of Ischemic MCA-Territory Stroke. Diseases 2024, 12, 53. https://doi.org/10.3390/diseases12030053
Lakatos L-B, Bolognese M, Österreich M, Weichsel L, Müller M. Pre-Stroke Antihypertensive Therapy Affects Stroke Severity and 3-Month Outcome of Ischemic MCA-Territory Stroke. Diseases. 2024; 12(3):53. https://doi.org/10.3390/diseases12030053
Chicago/Turabian StyleLakatos, Lehel-Barna, Manuel Bolognese, Mareike Österreich, Laura Weichsel, and Martin Müller. 2024. "Pre-Stroke Antihypertensive Therapy Affects Stroke Severity and 3-Month Outcome of Ischemic MCA-Territory Stroke" Diseases 12, no. 3: 53. https://doi.org/10.3390/diseases12030053
APA StyleLakatos, L.-B., Bolognese, M., Österreich, M., Weichsel, L., & Müller, M. (2024). Pre-Stroke Antihypertensive Therapy Affects Stroke Severity and 3-Month Outcome of Ischemic MCA-Territory Stroke. Diseases, 12(3), 53. https://doi.org/10.3390/diseases12030053






