Abstract
Introduction: Beta-lactamases are frequently prescribed for Gram-negative bloodstream infections (BSIs). However, chromosomally encoded AmpC-producing Enterobacterales (AE) could overproduce beta-lactamases when exposed to third-generation cephalosporins (3GCs), with a risk of clinical failure. There are few available in vivo data on the subject. Our goal was to assess the potential role of AE as a predictive factor for clinical failure in patients with BSIs. Materials and Methods: We retrospectively analyzed patients admitted to Cannes hospital between 2021 and 2022 for BSIs due to Enterobacterales. Patient demographics, comorbidities, and main clinical and laboratory parameters during hospitalization were collected. The risk factors for clinical instability after 48 h or death, as well as for ineffective initial empirical therapy, were assessed using univariate and multivariate analyses. Results: From January 2021 to December 2022, 101 subjects were included (mean age 79 years, 60% men, 97% with comorbidities, 17% with healthcare-associated infection, 13% with septic shock, 82% with qPitt severity score < 2, 58% with urinary tract infection, and 18% with AE). Septic shock [adjusted odds ratio (ORadj) = 5.30, 95% confidence interval (CI): 1.47–22.19, p = 0.014] and ineffective initial empirical therapy [ORadj 5.54, 95% CI: 1.95–17.01, p = 0.002] were independent predictive factors for clinical instability or death. Extended-spectrum beta-lactamases [ORadj 9.40, 95% CI: 1.70–62.14, p = 0.012], AE group [ORadj 5.89, 95% CI: 1.70–21.40, p = 0.006], and clinical instability or death [ORadj 4.71, 95% CI: 1.44–17.08, p = 0.012] were independently associated with ineffective empirical therapy. Conclusions: Infection with AE was associated with treatment failure. Empirical therapy may result in failure if restricted to 3GC.
1. Introduction
The burden of Gram-negative bacteria resistant to third-generation cephalosporins has been highlighted by the World Health Organization [1] and can be potentially life-threatening, especially in the case of bloodstream infections (BSIs), defined by the presence of viable microorganisms in the bloodstream eliciting an inflammatory response characterized by an alteration in clinical, laboratory and hemodynamic parameters [2]. The resistance of Enterobacterales mainly results from the plasmid-mediated acquisition of beta-lactamase enzymes or the deregulation of natural genetically encoded enzymes [3].
Chromosomally encoded AmpC-producing beta-lactamases (AE) include Enterobacterales species such as Enterobacter spp., Serratia marcescens, Citrobacter freundii, Providencia spp., and Morganella morganii (ESCPM), which have a natural resistance to aminopenicillins and first-generation cephalosporins [3,4]. When exposed to third-generation cephalosporins (3GCs) such as ceftriaxone and cefotaxime, these can select AmpC-overproducing strains, resulting in 3GC inactivation and treatment failure [5,6,7].
ESCPM microorganisms are implicated in a broad range of infections involving urinary and gastrointestinal tracts, lungs, skin, and soft tissues. Although several in vitro studies describe the risk of selecting AmpC-overproducing ESCPM strains when these are exposed to 3GCs, few in vivo data are available regarding the outcome of BSIs in the case of empirical treatment with 3GCs alone [8,9].
The aim of this study was to analyze the possible role of ESCPM organisms as a risk factor for clinical failure in patients with BSIs.
2. Materials and Methods
2.1. Study Design and Participants
We conducted an observational, retrospective cohort study of patients admitted to any clinical department in Cannes General Hospital in 2021 and 2022 for BSIs due to Enterobacterales. Data from each patient group were extracted from the hospital’s electronic database. Patient demographics, underlying comorbidities, duration of symptoms, clinical signs upon admission and during hospitalization, laboratory findings during hospital stay, and clinical outcomes were collected from their medical records. In order to assess sepsis severity, the qPitt score was calculated for each patient at the time of the first positive blood culture. Briefly, it consists of five binary parameters, one point being assigned to each of the following: temperature < 36°, systolic blood pressure ≤ 90 mmHg or vasopressor administration, respiratory rate ≥ 25/min or need of mechanical ventilation, altered mental status, and cardiac arrest. Sepsis is generally considered severe for a score of 2 points or above [10].
According to the Enterobacterales species responsible for the BSI, patients were divided into those infected and those not infected with ESCPM organisms, as defined elsewhere [4]. As the main goal of the study was to evaluate the potential correlation between the ESCPM group and outcome, patients were excluded in the case of polymicrobial BSI. Subjects were also excluded if the clinical outcome was not available, for example, in the case of admission to the emergency department and transfer to another hospital with no available clinical file.
The study was submitted to the Health Data Hub (https://www.health-data-hub.fr/depot, accessed on 27 November 2023), and all patients received written information regarding the study and gave their consent to participate.
2.2. Definitions and Statistical Analysis
After describing the study population’s main characteristics, we first searched whether the initial empirical antibiotic therapy was associated with a favorable outcome. Patients were therefore divided into those presenting with clinical instability after 48 h of BSI diagnosis or dying in-hospital, and those clinically stable at 48 h.
Clinical instability after 48 h of BSI diagnosis was defined by either clinical (i.e., persistence of fever or other clinical signs of infection) or microbiological (i.e., persistence of the same bacterial species on blood cultures) criteria. The empirical antibiotic treatment was defined as effective if the Enterobacterales species identified on blood cultures was susceptible to at least one of the antimicrobial agents prescribed according to bacterial susceptibility testing, realized using the disk diffusion method (i2a® disks, Montpellier, France) and interpreted in accordance with the European Committee on Antimicrobial Sensitivity Testing (EUCAST) guidelines. In the case of ESCPM microorganisms, 3GCs were considered ineffective.
Comparisons were performed using χ-square or Fisher’s exact tests and Student’s t-tests or the Wilcoxon–Mann–Whitney test. Independent risk factors associated with clinical outcomes were identified by logistic regression.
In case ofa significant association between ineffective therapy and a worse outcome, predictive factors for ineffective initial therapy were measured via univariate and multivariate analyses using the tests described above.
Kaplan–Meier curves were performed if parameters were significantly associated with mortality. A Cox regression analysis was used to obtain the hazard ratio. Analyses were performed using the R-4.3.0 software.
3. Results
3.1. Population Characteristics
From January 2021 to December 2022, 184 cases of BSIs due to Enterobacterales were collected. After excluding polymicrobial infections and cases with unavailable clinical data, 101 subjects were included in the study (mean age 79 years, 60% men, 97% with comorbid conditions including 30% with active cancer, 17% with healthcare-associated infection, 94% admitted in a medical department at the time of BSI, Table 1). Among the other comorbid conditions, the most frequent were chronic cardiopathies, diabetes, and neurocognitive disorders.

Table 1.
Patient characteristics.
The main suspected portals of entry for BSIs were urinary and gastrointestinal infections. Septic shock was diagnosed in 13% of subjects, while the majority of individuals had a low qPitt severity score at the time of their first positive blood culture (Table 1).
Among the isolated microorganisms, ESCPM microorganisms were identified in 18% of BSIs (nine Enterobacter sp., five Serratia sp., two Morganella sp., one Providencia sp., and one Pantoea sp.). The majority of BSIs were due to Escherichia coli (41%) and Klebsiella sp. (30%), while extended-spectrum beta-lactamase (ESBL)-producing organisms were identified in 8% of the cases.
For 59% of the patients, the empirical initial antibiotic therapy consisted of a single drug, in most cases a 3GC. Among patients receiving combination therapy, the main prescriptions consisted of 3GCs and metronidazole, followed by 3GCs and aminoglycosides.
3.2. Risk Factors for a Worse Clinical Outcome
Univariate analysis showed that clinical instability after 48 h from BSI diagnosis or in-hospital death was significantly associated with a shorter duration of antibiotic therapy, septic shock, ESCPM group, ineffective initial empirical therapy, and treatment with a single antibiotic agent (Table 2). As Enterobacter sp. are considered among the most AmpC-overproducing strains [11], we compared Enterobacter sp. with the other ESCPM group species, but we did not find any difference in clinical outcomes.

Table 2.
(a) Risk factors for clinical instability after 48 h of therapy or in-hospital death—univariate analysis; (b) independent risk factors for clinical instability after 48 h of therapy or in-hospital death.
In the multivariate analysis, the independent risk factors for clinical instability or death were septic shock [adjusted odds ratio (ORadj) = 5.30, 95% confidence interval (CI): 1.47–22.19, p = 0.014] and ineffective initial empirical therapy [ORadj 5.54, 95% CI: 1.95–17.01, p = 0.002] (Table 2).
3.3. Risk Factors for Ineffective Empirical Therapy
Univariate analysis revealed that ineffective initial empirical antibiotic therapy was associated with infection due to ESBL, the ESCPM group, clinical instability after 48 h or death, and the administration of a single antibiotic agent (Table 3).

Table 3.
(a) Risk factors for initial ineffective empirical antibiotic therapy—univariate analysis *; (b) independent factors associated with ineffective empirical antibiotic therapy.
Multivariate analysis revealed that ESBL [ORadj 9.4, 95% CI: 1.7–62.14, p = 0.012], the ESCPM group [ORadj 5.89, 95% CI: 1.7–21.40, p = 0.006], and clinical instability after 48 h or death [ORadj 4.71, 95% CI: 1.44–17.08, p = 0.012] were independently associated with ineffective empirical therapy (Table 3).

Table 4.
(a) Characteristics of patients with ESCPM BSIs and (b) characteristics of patients with ESBL BSIs.
3.4. Analysis of Deaths
Among the 101 patients included, 24 in-hospital deaths occurred. For 20 subjects, according to our in-depth file review, causes of death were very likely due to BSIs, while for the other 4 cases, the fatal outcome was probably linked to other causes, such as COVID-19 infection or cancer.
Kaplan–Meier curves showed a significant difference in survival according to the efficacy of initial antibiotic therapy, considering either crude mortality or BSI-related deaths (Figure 1).
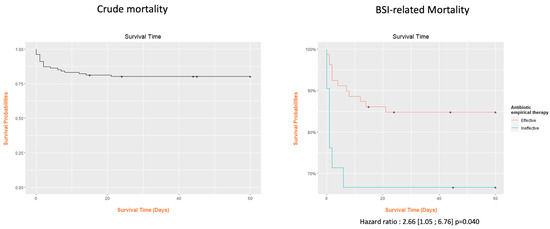
Figure 1.
Kaplan–Meier analysis of overall mortality and bloodstream infection-related mortality according to the initial effective therapy.
4. Discussion
In a retrospective cohort of patients admitted for BSIs due to Enterobacterales, we found high rates of unfavorable outcomes. Infection due to ESCPM organisms was an independent risk factor for ineffective initial antibiotic therapy.
The patients included in this study were representative of the rising rates of elderly and severely comorbid individuals presenting at emergency departments in most countries. Indeed, the British Geriatric Society reported that patients over 65 years constituted the majority of hospital admissions, bed days, and emergency readmissions, with high risks of mortality [12,13]. The increasing number of elderly populations worldwide [14,15] who are more likely to have complex presentations, multiple comorbidities, and multi-medication exposure represents a major challenge for clinical care.
Although the majority of BSIs were not severe at the time of diagnosis, we found high rates of unfavorable outcomes. These results are in line with previous studies showing that older patients have increased mortality rates compared to their younger counterparts [16]. Indeed, elderly patients are generally more prone to infection, as a consequence of aging, comorbidities, immune system dysfunction, and the use of invasive devices [17]. Moreover, classical manifestations of systemic inflammatory response to BSIs may be minimally present in older subjects. Indeed, the febrile response can be more frequently blunted than in younger subjects and may be replaced by other less evocative signs, such as weakness, confusion, falls, or loss of appetite [17]. Moreover, the prognosis for Gram-negative BSI is particularly poor due to virulence factors and antibiotic resistance [18,19,20]. However, the prevalence of ESBL was relatively low in our study, in line with current epidemiological data in France, which show significant differences among Enterobacterales but low levels of ESBL in E. coli species, the most frequently encountered organism in this study [21,22,23]. Our results are quite similar to those recently presented by Maillard et al., who showed higher rates of treatment failure to 3GC than cefepime in a retrospective analysis of patients with BSIs and pneumonia caused by AE. However, the authors did not find any difference in terms of mortality, probably because half of the patients included did not have sepsis [8].
If confirmed by larger and prospective studies, empirical antibiotic therapy for BSIs should take into account the ESCPM group and the possibility that single therapy with 3GCs could be associated with risks of failure. Therefore, we suggest that in cases of in-patients with a suspected BSI due to Enterobacterales or to positive blood cultures for Gram-negative bacteria, pending identification and susceptibility testing, cefepime should replace 3GCs as empirical treatment if a single compound is chosen, or alternatively, a double Gram-negative coverage with the addition of aminoglycosides could reduce risks of failure. Indeed, cefepime is a poor inducer of AmpC Beta-lactamases, and although comparisons with 3GCs of the effect on intestinal microbiota have not been specifically studied, McKinnell et al. showed lower rates of vancomycin-resistant Enterococci after cefepime than ceftriaxone use [24]. Moreover, cefepime use for BSIs due to AE is supported by recent data presented by Hermann et al., who showed its usefulness for treating BSIs if ESBL production can be excluded [25].
However, whether combination antimicrobial therapy is superior to monotherapy remains a controversial issue, and the main guidelines recommend limiting the use of combination therapies to critically ill patients or those with a high risk of multidrug-resistant pathogens [26,27]. Moreover, in a meta-analysis of monotherapy vs. beta-lactam-aminoglycoside combination strategy for sepsis, Paul et al. found no difference in efficacy but more frequent side effects in the case of combination therapy [28].
This study has many limitations, including its retrospective nature and the relatively small number of subjects included. Moreover, although the qPitt score previously showed good performance for predicting mortality [10], and patient files were thoroughly reviewed, we cannot exclude the risk of misinterpreting sepsis severity criteria. Furthermore, the study design did not allow microbiological confirmation that exposure to 3CGs increases the risk of developing resistant ESCMP strains, as published by Choi et al. [29].
5. Conclusions
In conclusion, in a population of elderly patients hospitalized for BSIs, we found that infection with ESCPM microorganisms was a predictive risk factor for treatment failure. Empirical therapy, regardless of initial clinical severity, should take into account the risk of failure in the case of 3GC monotherapy.
Author Contributions
M.V. conceptualized the study, collected data, and wrote the manuscript. R.F. and C.P. performed statistical analysis. L.L. and S.M. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to French laws. The study was submitted to the Health Data Hub (https://www.health-data-hub.fr/depot, accessed on 27 November 2023).
Informed Consent Statement
All patients received written information regarding the study and gave their consent to participate.
Data Availability Statement
Data respecting patient’s anonymity are available if necessary.
Acknowledgments
We wish to thank all the patients who participated in this study and their families. Our special thanks are also due to Nathalie Doux and Aurélie Leguillermic for organizing the study and to Brigitte Dunais for reviewing this paper. Finally, but no less important, we wish to thank all the nurses of the departments where patients had been admitted, without whose help this work could not have been achieved.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Antimicrobial Resistance and the United Nations Sustainable Development Cooperation Framework: Guidance for United Nations Country Teams. 2021. Available online: https://www.who.int/publications/i/item/9789240036024 (accessed on 25 November 2023).
- Viscoli, C. Bloodstream Infections: The peak of the iceberg. Virulence 2016, 7, 248–251. [Google Scholar] [CrossRef]
- Mizrahi, A.; Delerue, T.; Morel, H.; Le Monnier, A.; Carbonnelle, E.; Pilmis, B.; Zahar, J. Infections caused by naturally AmpC-producing Enterobacterales: Can we use third-generation cephalosporins? A narrative review. Int. J. Antimicrob. Agents 2019, 55, 105834. [Google Scholar] [CrossRef]
- Harris, P.; Ferguson, J. Antibiotic therapy for inducible AmpC β-lactamase-producing Gram-negative bacilli: What are the alternatives to carbapenems, quinolones and aminoglycosides? Int. J. Antimicrob. Agents 2012, 40, 297–305. [Google Scholar] [CrossRef]
- de Lastours, V.; Goulenok, T.; Guérin, F.; Jacquier, H.; Eyma, C.; Chau, F.; Cattoir, V.; Fantin, B. Ceftriaxone promotes the emergence of AmpC-overproducing Enterobacteriaceae in gut microbiota from hospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the treatment of AmpC β Lactamase producing Enterobacterales, Carbapenem resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Wolff, M.; Fagon, J.Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs. 15 days of antibiotic therapy for ventilator associated pneumonia in adults: A randomized trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef]
- Maillard, A.; Delory, T.; Bernier, J.; Villa, A.; Chaibi, K.; Escaut, L.; Contejean, A.; Bercot, B.; Robert, J.; El Alaoui, F.; et al. Effectiveness of third-generation cephalosporins or piperacillin compared with cefepime or carbapenems for severe infections caused by wild-type AmpC β-lactamase-producing Enterobacterales: A multi-centre retrospective propensity-weighted study. Int. J. Antimicrob. Agents 2023, 62, 106809. [Google Scholar] [CrossRef]
- Mounier, R.; Le Guen, R.; Woerther, P.-L.; Nacher, M.; Bonnefon, C.; Mongardon, N.; Langeron, O.; Levesque, E.; Couffin, S.; Houcke, S.; et al. Clinical outcome of wild-type AmpC-producing Enterobacterales infection in critically ill patients treated with β-lactams: A prospective multicenter study. Ann. Intensiv. Care 2022, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Battle, S.E.; Augustine, M.R.; Watson, C.M.; Bookstaver, P.B.; Kohn, J.; Owens, W.B.; Baddour, L.M.; Al-Hasan, M.N. Derivation of a quick Pitt bacteremia score to predict mortality in patients with Gram-negative bloodstream infection. Infection 2019, 47, 571–578. [Google Scholar] [CrossRef]
- Kang, C.I.; Kim, S.H.; Park, W.B.; Lee, K.D.; Kim, H.B.; Oh, M.D.; Kim, E.C.; Choe, K.W. Bloodstream infections caused by Enterobacter species: Predictors of 30-day mortality rate and impact of broad-spectrum cephalosporin resistance on outcome. Clin. Infect. Dis. 2004, 39, 812–818. [Google Scholar] [CrossRef]
- Kennelly, S.; McCabe, J.J. Acute care of older patients in the emergency department: Strategies to improve patient outcomes. Open Access Emerg. Med. 2015, 7, 45–54. [Google Scholar] [CrossRef][Green Version]
- Round, A.; Crabb, T.; Buckingham, K.; Mejzner, R.; Pearce, V.; Ayres, R.; Weeks, C.; Hamilton, W. Six month outcomes after emergency admission of elderly patients to a community or a district general hospital. Fam. Pr. 2004, 21, 173–179. [Google Scholar] [CrossRef][Green Version]
- Hwang, U.; Morrison, R.S. The geriatric emergency department. J. Am. Geriatr. Soc. 2007, 55, 1873–1876. [Google Scholar] [CrossRef]
- Rau, R.; Muszyńska, M.M.; Vaupel, J.W. Europe, the Oldest-Old Continent. In The Demography of Europe; Neyer, G., Andersson, G., Kulu, H., Bernardi, L., Bühler, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 119–137. [Google Scholar]
- Juneja, D.; Nasa, P.; Singh, O. Severe sepsis and septic shock in the elderly: An overview. World J. Crit. Care Med. 2012, 1, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Martín, S.; Pérez, A.; Aldecoa, C. Sepsis and Immunosenescence in the Elderly Patient: A Review. Front. Med. 2017, 4, 20. [Google Scholar] [CrossRef]
- Livorsi, D.J.; Stenehjem, E.; Stephens, D.S. Virulence factors of gram-negative bacteria in sepsis with a focus on Neisseria meningitides. Contrib. Microbiol. 2011, 17, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Baltas, I.; Stockdale, T.; Tausan, M.; Kashif, A.; Anwar, J.; Anvar, J.; Koutoumanou, E.; Sidebottom, D.; Garcia-Arias, V.; Wright, M.; et al. Long-term outcome and risk factors for late mortality in Gram-negative bacteraemia: A retrospective cohort study. J. Glob. Antimicrob. Resist. 2021, 25, 187–192. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Lemenand, O.; Caillon, J.; Coeffic, T.; Colomb-Cotinat, M.; Thibaut, S.; Birgand, G. National Primary Care Surveillance of Resistance to 3rd-Generation Cephalosporins and Fluoroquinolones in Urinary Isolates of Escherichia coli: 2015–2019 Trends in France. Article—Bulletin Epidémiologique Hebdomadaire. Available online: www.santepubliquefrance.fr (accessed on 7 December 2023).
- Lemenand, O.; Coeffic, T.; Thibaut, S.; Cotinat, M.C.; Caillon, J.; Birgand, G. Decreasing proportion of extended-spectrum beta-lactamase among E. coli infections during the COVID-19 pandemic in France. J. Infect. 2021, 83, 664–670. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Surveillance in Europe 2022–2020 Data. 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data (accessed on 1 December 2023).
- McKinnell, J.A.; Kunz, D.F.; Chamot, E.; Patel, M.; Shirley, R.M.; Moser, S.A.; Baddley, J.W.; Pappas, P.G.; Miller, L.G. Association between vancomycin-resistant Enterococci bacteremia and ceftriaxone usage. Infect. Control. Hosp. Epidemiol. 2012, 33, 718–724. [Google Scholar] [CrossRef]
- Herrmann, J.; Burgener-Gasser, A.-V.; Goldenberger, D.; Roth, J.; Weisser, M.; Tamma, P.D.; Tschudin-Sutter, S. Cefepime versus carbapenems for treatment of AmpC beta-lactamase-producing Enterobacterales bloodstream infections. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 43, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Lador, A.; Grozinsky-Glasberg, S.; Leibovici, L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst. Rev. 2014, 2014, CD003344. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, J.E.; Park, S.J.; Choi, S.-H.; Lee, S.-O.; Jeong, J.-Y.; Kim, M.-N.; Woo, J.H.; Kim, Y.S. Emergence of Antibiotic Resistance during Therapy for Infections Caused by Enterobacteriaceae Producing AmpC β-Lactamase: Implications for Antibiotic Use. Antimicrob. Agents Chemother. 2008, 52, 995–1000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).