Automated Atrial Fibrillation Diagnosis by Echocardiography without ECG: Accuracy and Applications of a New Deep Learning Approach
Abstract
1. Introduction
2. Materials and Methods
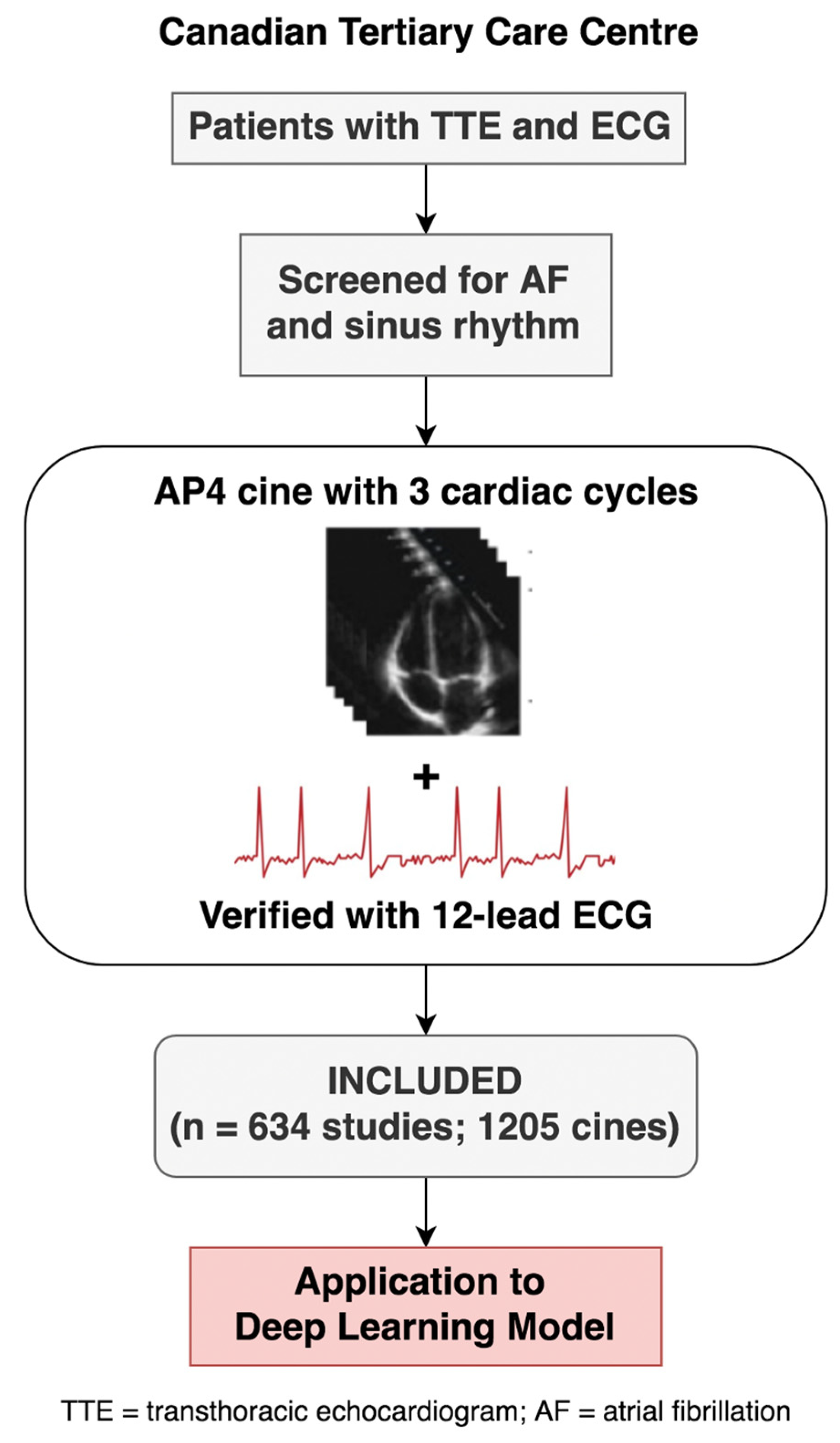
2.1. Study Overview
2.2. Echocardiography and ECG Inclusion Criteria
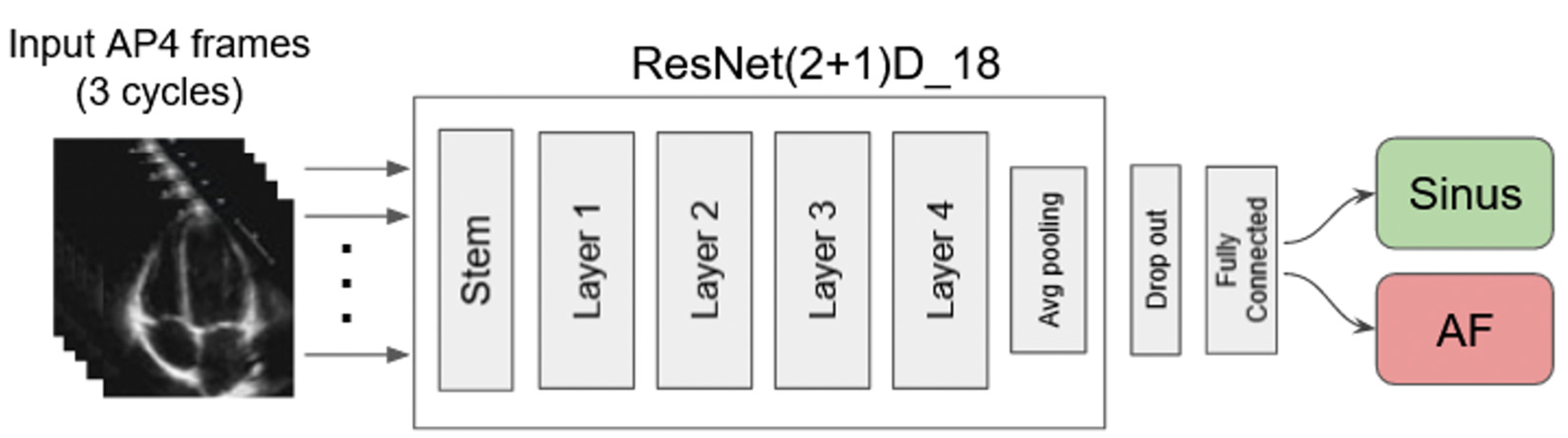
2.3. AI model Framework, Training Process, and Evaluation
2.4. Statistical Analysis
2.5. Qualitative Analysis
3. Results
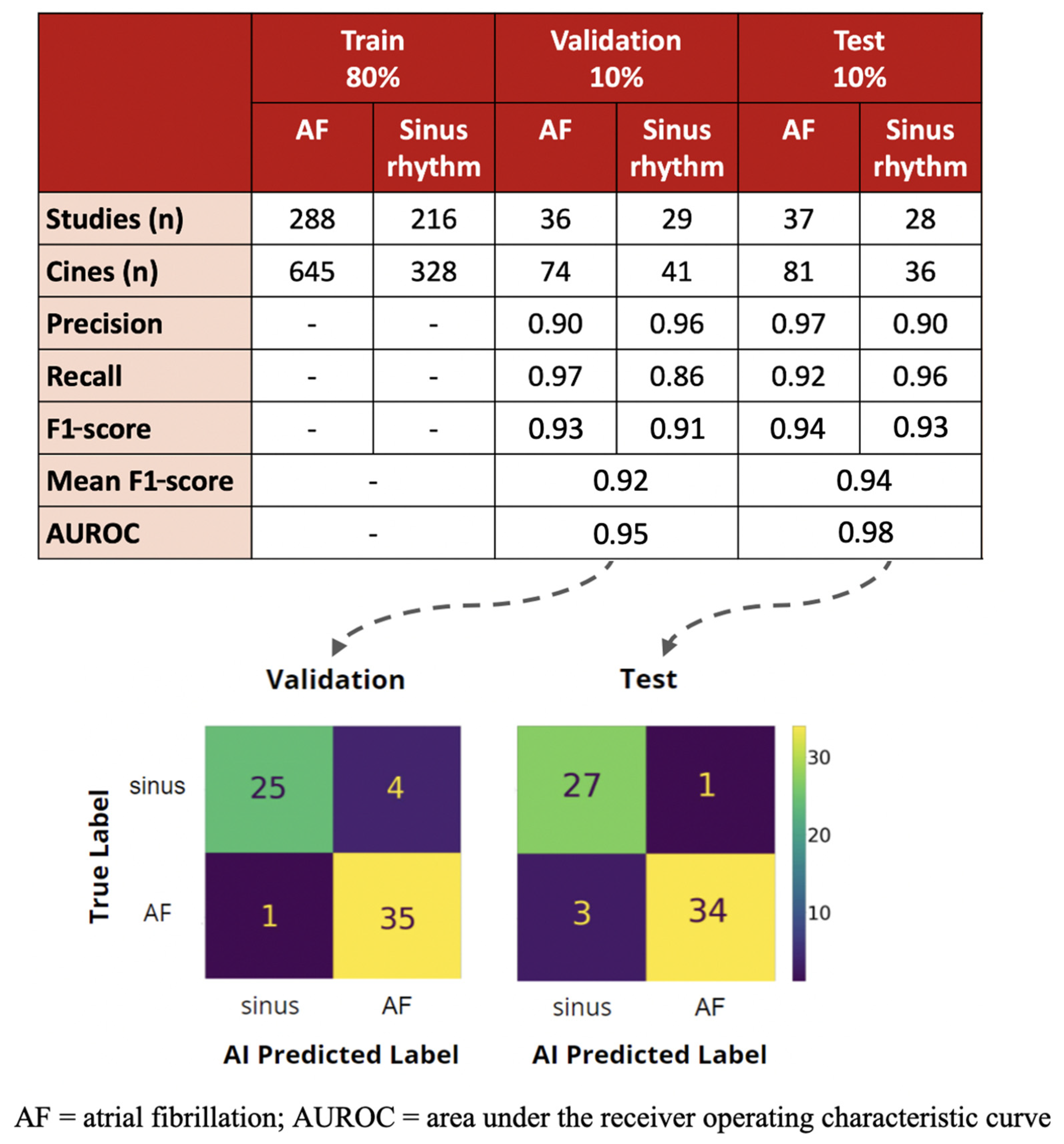
3.1. AI Training and Validation
3.2. Test Performance and Comparison to Echocardiography Cardiologist
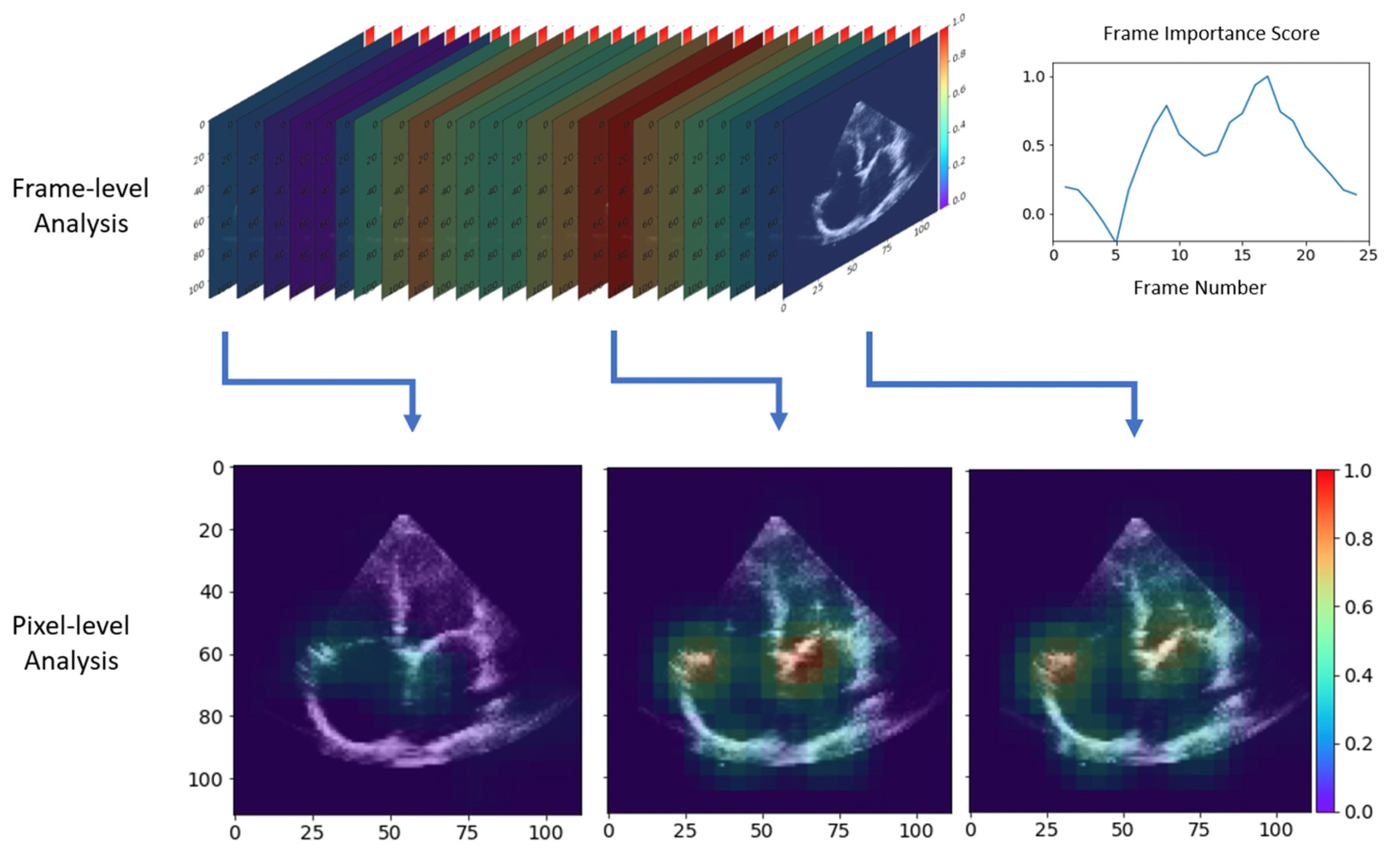
3.3. Qualitative Results with Occlusion-Based Importance Estimation
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mou, L.; Norby, F.L.; Chen, L.Y.; O’Neal, W.T.; Lewis, T.T.; Loehr, L.R.; Alonso, A. Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC study (Atherosclerosis Risk in Communities). Circ. Arrhythmia Electrophysiol. 2018, 11, e006350. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Aguilar, M.; Atzema, C.; Bell, A.; Cairns, J.A.; Cheung, C.C.; Cox, J.L.; Dorian, P.; Gladstone, D.J.; Healey, J.S.; et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can. J. Cardiol. 2020, 36, 1847–1948. [Google Scholar] [CrossRef] [PubMed]
- Mou, L.; Norby, F.L.; Chen, L.Y.; O’Neal, W.T.; Lewis, T.T.; Loehr, L.R.; Alonso, A. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Watkins, C.L. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Kim, T.-S.; Youn, H.-J. Role of echocardiography in atrial fibrillation. J. Cardiovasc. Ultrasound 2011, 19, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Billick, K.; Horton, K.; Jankowski, M.; Knoll, P.; Marshall, J.E.; Paloma, A.; Palma, R.; Adams, D.B. Artificial Intelligence and Echocardiography: A Primer for Cardiac Sonographers. J. Am. Soc. Echocardiogr. 2020, 33, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, F.T.; Luong, C.; Ginsberg, T.; Rohling, R.; Gin, K.; Abolmaesumi, P.; Tsang, T. Echo-SyncNet: Self-Supervised Cardiac View Synchronization in Echocardiography. IEEE Trans. Med. Imaging 2021, 40, 2092–2104. [Google Scholar] [CrossRef] [PubMed]
- Nabi, W.; Bansal, A.; Xu, B. Applications of artificial intelligence and machine learning approaches in echocardiography. Echocardiography 2021, 38, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, F.T.; Ginsberg, T.; Luong, C.; Vaseli, H.; Rohling, R.; Gin, K.; Tsang, T. Echo-Rhythm Net: Semi-Supervised Learning For Automatic Detection of Atrial Fibrillation in Echocardiography. In Proceedings of the 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI), Nice, France, 13–16 April 2021; pp. 110–113. [Google Scholar]
- Tran, D.; Wang, H.; Torresani, L.; Ray, J.; LeCun, Y.; Paluri, M. A Closer Look at Spatiotemporal Convolutions for Action Recognition. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition; IEEE: Salt Lake City, UT, USA, 2018; pp. 6450–6459. [Google Scholar]
- Gan, G.C.H.; Ferkh, A.; Boyd, A.; Thomas, L. Left atrial function: Evaluation by strain analysis. Cardiovasc. Diagn. Ther. 2018, 8, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.A.; Shah, R.V.; Murthy, V.L.; Praestgaard, A.; Shah, S.J.; Ventetuolo, C.E.; Kawut, S.M. Right Ventricular Structure and Function Are Associated With Incident Atrial Fibrillation: MESA-RV Study (Multi-Ethnic Study of Atherosclerosis-Right Ventricle). Circ. Arrhythmia Electrophysiol. 2017, 10, e004738. [Google Scholar] [CrossRef] [PubMed]
- Xie, E.; Yu, R.; Ambale-Venkatesh, B.; Bakhshi, H.; Heckbert, S.R.; Soliman, E.Z.; Bluemke, D.A.; Kawut, S.M.; Wu, C.O.; Nazarian, S.; et al. Association of right atrial structure with incident atrial fibrillation: A longitudinal cohort cardiovascular magnetic resonance study from the Multi-Ethnic Study of Atherosclerosis (MESA). J. Cardiovasc. Magn. Reson. 2020, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.T.; Kimura, B.J.; Korcarz, C.E.; Pellikka, P.A.; Rahko, P.S.; Siegel, R.J. Focused cardiac ultrasound: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Hamatani, Y.; Ogawa, H.; Takabayashi, K.; Yamashita, Y.; Takagi, D.; Esato, M.; Chun, Y.-H.; Tsuji, H.; Wada, H.; Hasegawa, K.; et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci. Rep. 2016, 6, 31042. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Bartz, T.M.; Elkind, M.S.; Okin, P.M.; Thacker, E.L.; Patton, K.K.; Stein, P.K.; Defilippi, C.R.; Gottesman, R.F.; Heckbert, S.R.; et al. Atrial Cardiopathy and the Risk of Ischemic Stroke in the CHS (Cardiovascular Health Study). Stroke 2018, 49, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Di Cara, M.; Nicolosi, G.L.; Eusebio, A.; Bordonali, M.; Santalucia, P.; Lombardo, M. Rapid Risk Stratification of Acute Ischemic Stroke Patients in the Emergency Department: The Incremental Prognostic Role of Left Atrial Reservoir Strain. J. Stroke Cerebrovasc. Dis. 2021, 30, 106100. [Google Scholar] [CrossRef] [PubMed]
- Luong, C.; Liao, Z.; Abdi, A.; Girgis, H.; Rohling, R.; Gin, K.; Jue, J.; Yeung, D.; Szefer, E.; Thompson, D.; et al. Automated estimation of echocardiogram image quality in hospitalized patients. Int. J. Cardiovasc. Imaging 2021, 37, 229–239. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Vaseli, H.; Mahdavi, M.; Taheri Dezaki, F.; Luong, C.; Yeung, D.; Gin, K.; Tsang, M.; Nair, P.; Jue, J.; et al. Automated Atrial Fibrillation Diagnosis by Echocardiography without ECG: Accuracy and Applications of a New Deep Learning Approach. Diseases 2024, 12, 35. https://doi.org/10.3390/diseases12020035
Lu N, Vaseli H, Mahdavi M, Taheri Dezaki F, Luong C, Yeung D, Gin K, Tsang M, Nair P, Jue J, et al. Automated Atrial Fibrillation Diagnosis by Echocardiography without ECG: Accuracy and Applications of a New Deep Learning Approach. Diseases. 2024; 12(2):35. https://doi.org/10.3390/diseases12020035
Chicago/Turabian StyleLu, Nelson, Hooman Vaseli, Mobina Mahdavi, Fatemah Taheri Dezaki, Christina Luong, Darwin Yeung, Ken Gin, Michael Tsang, Parvathy Nair, John Jue, and et al. 2024. "Automated Atrial Fibrillation Diagnosis by Echocardiography without ECG: Accuracy and Applications of a New Deep Learning Approach" Diseases 12, no. 2: 35. https://doi.org/10.3390/diseases12020035
APA StyleLu, N., Vaseli, H., Mahdavi, M., Taheri Dezaki, F., Luong, C., Yeung, D., Gin, K., Tsang, M., Nair, P., Jue, J., Barnes, M., Behnami, D., Abolmaesumi, P., & Tsang, T. S. M. (2024). Automated Atrial Fibrillation Diagnosis by Echocardiography without ECG: Accuracy and Applications of a New Deep Learning Approach. Diseases, 12(2), 35. https://doi.org/10.3390/diseases12020035





