Dengue Fever Epidemics and the Prospect of Vaccines: A Systematic Review and Meta-Analysis Using Clinical Trials in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Study Screening Process
2.5. Quality Assessment
Risk of Bias and Quality of Evidence
2.6. Statistical Analysis
2.7. Data Extraction
3. Results
3.1. Study Subjects, Study Design, Locations, and Major Findings
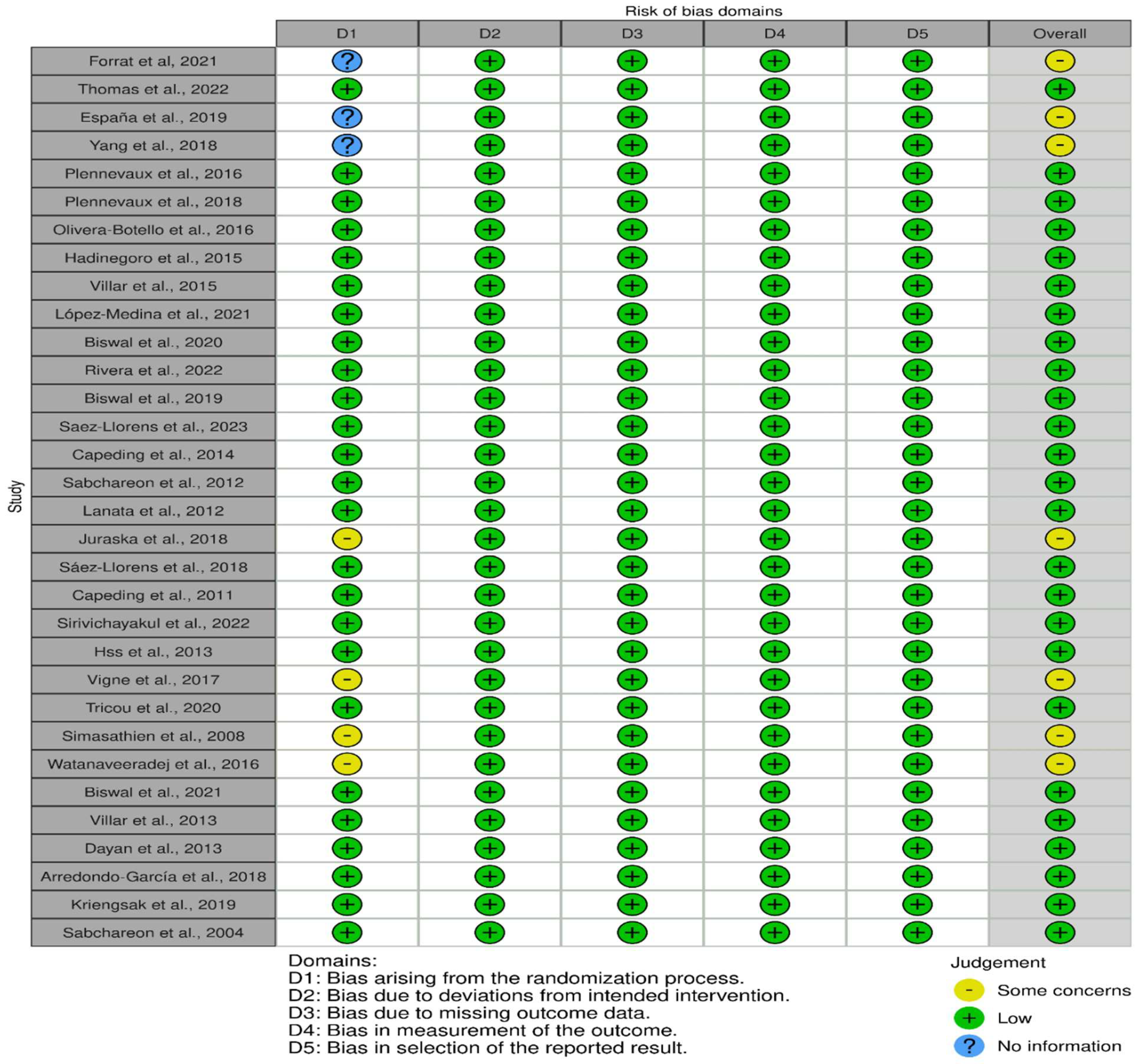
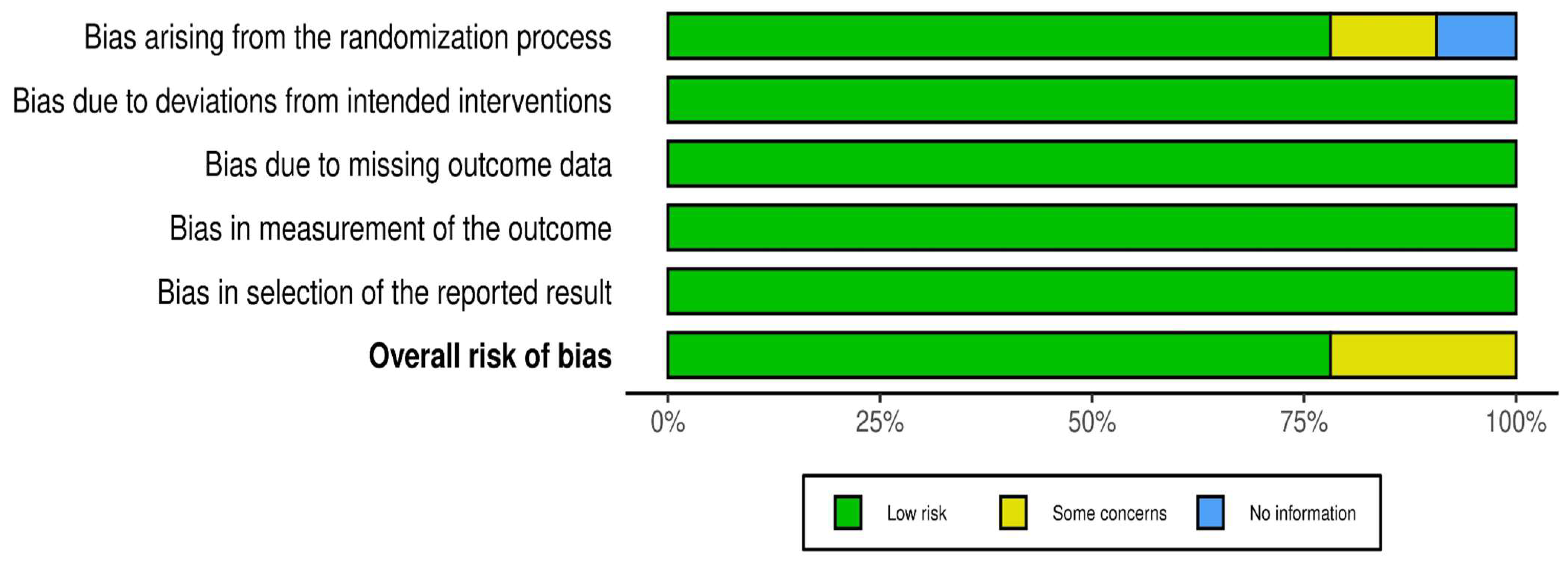
3.2. Outcome of the Risk Assessment
3.3. Domains of Risk of Bias
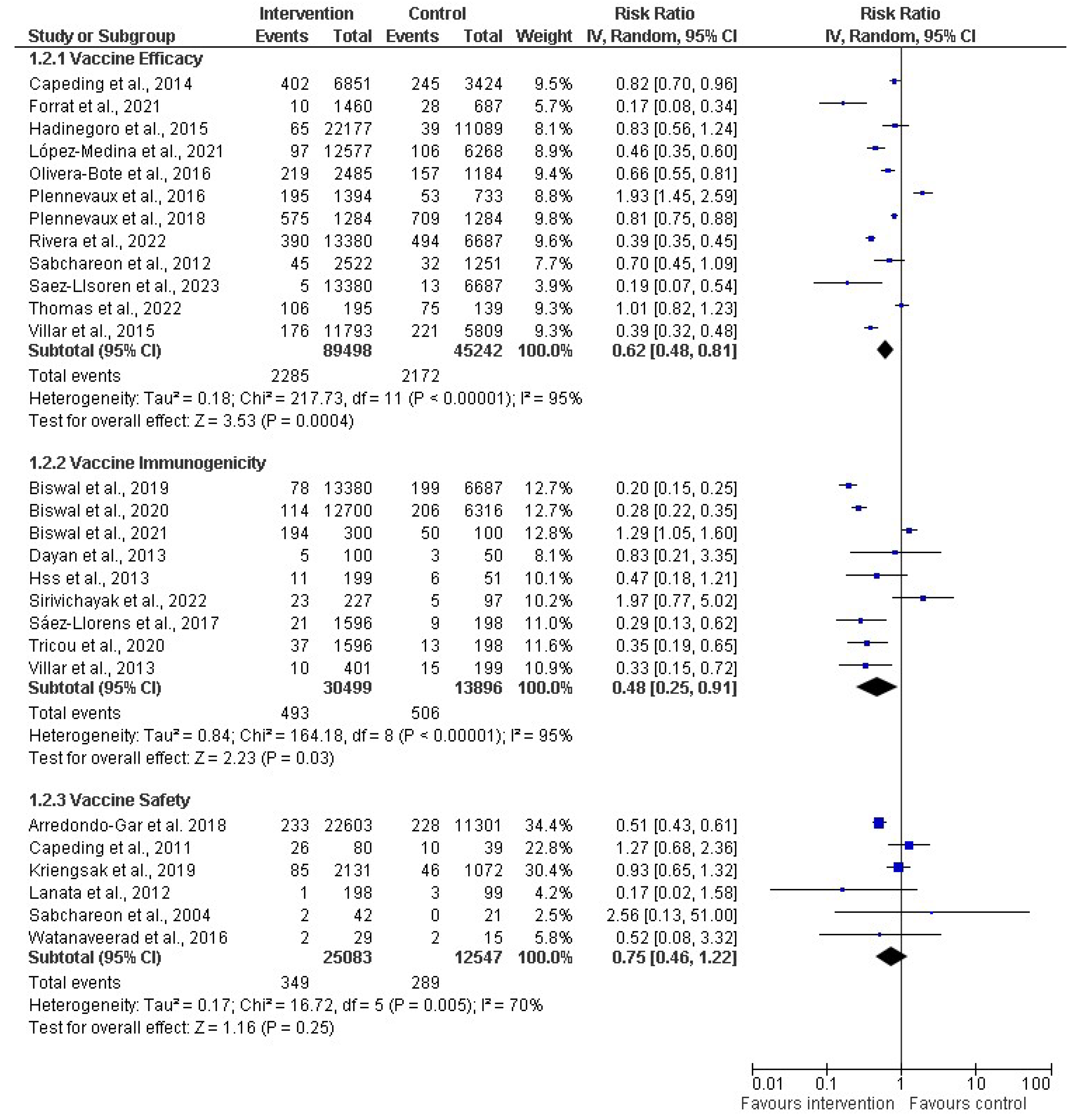
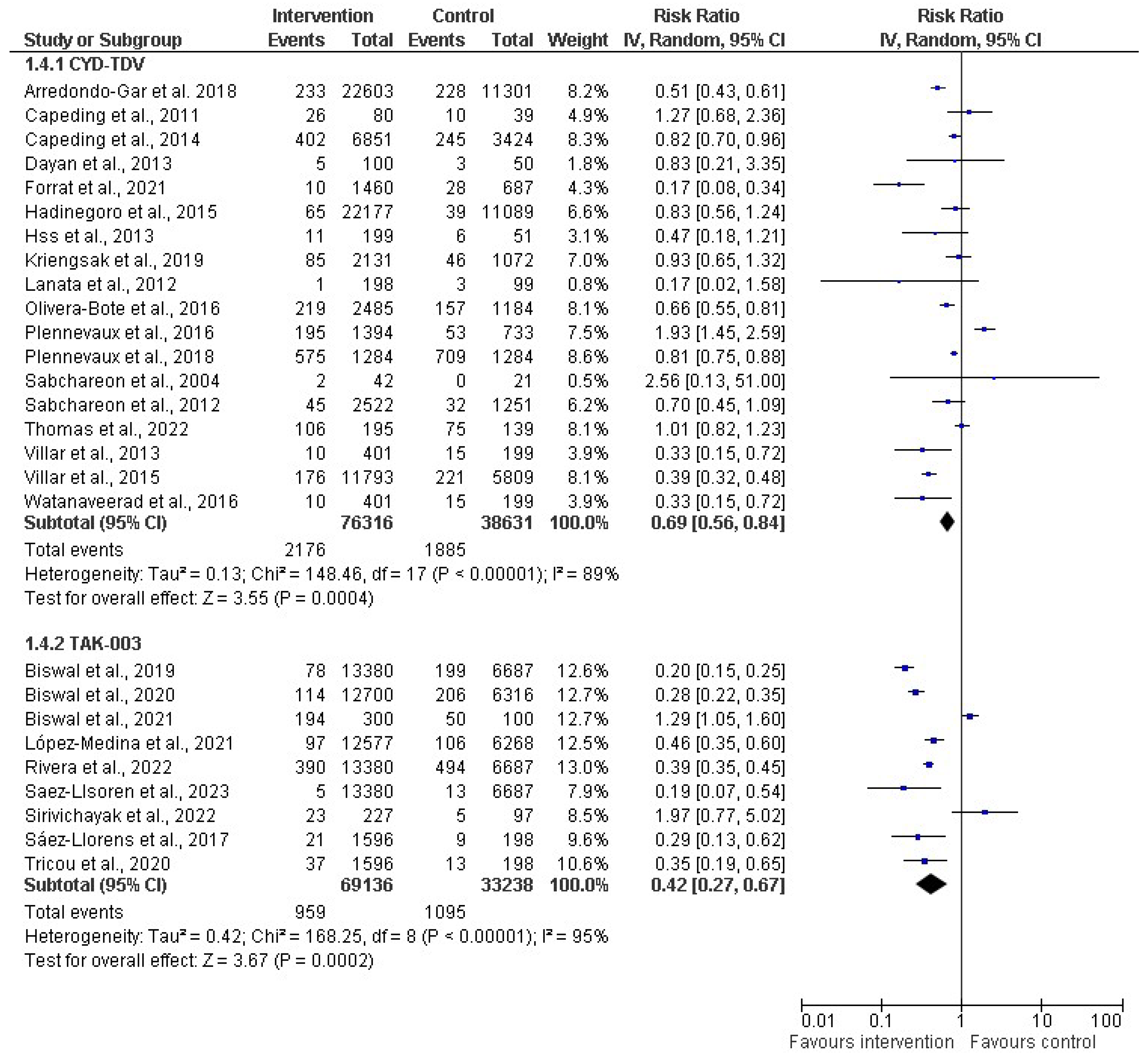
3.4. Vaccine Efficacy, Safety, and Immunonogenicity
3.5. Effectiveness and Safety of Dengue Vaccines: Findings of Systematic Review
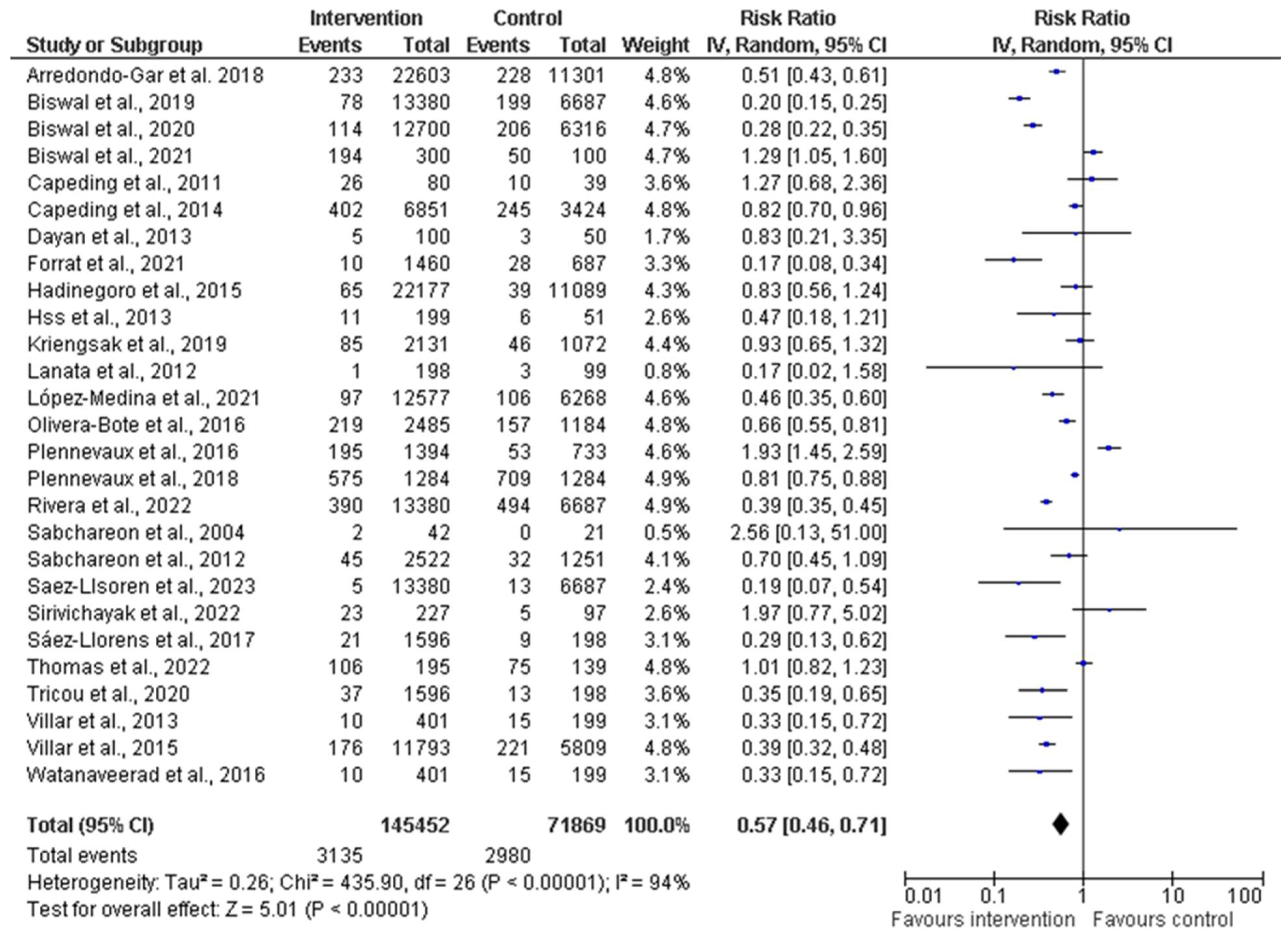
3.6. Efficacy, Immunogenicity, and Safety of Dengue Vaccine: Findings of Meta-Analysis
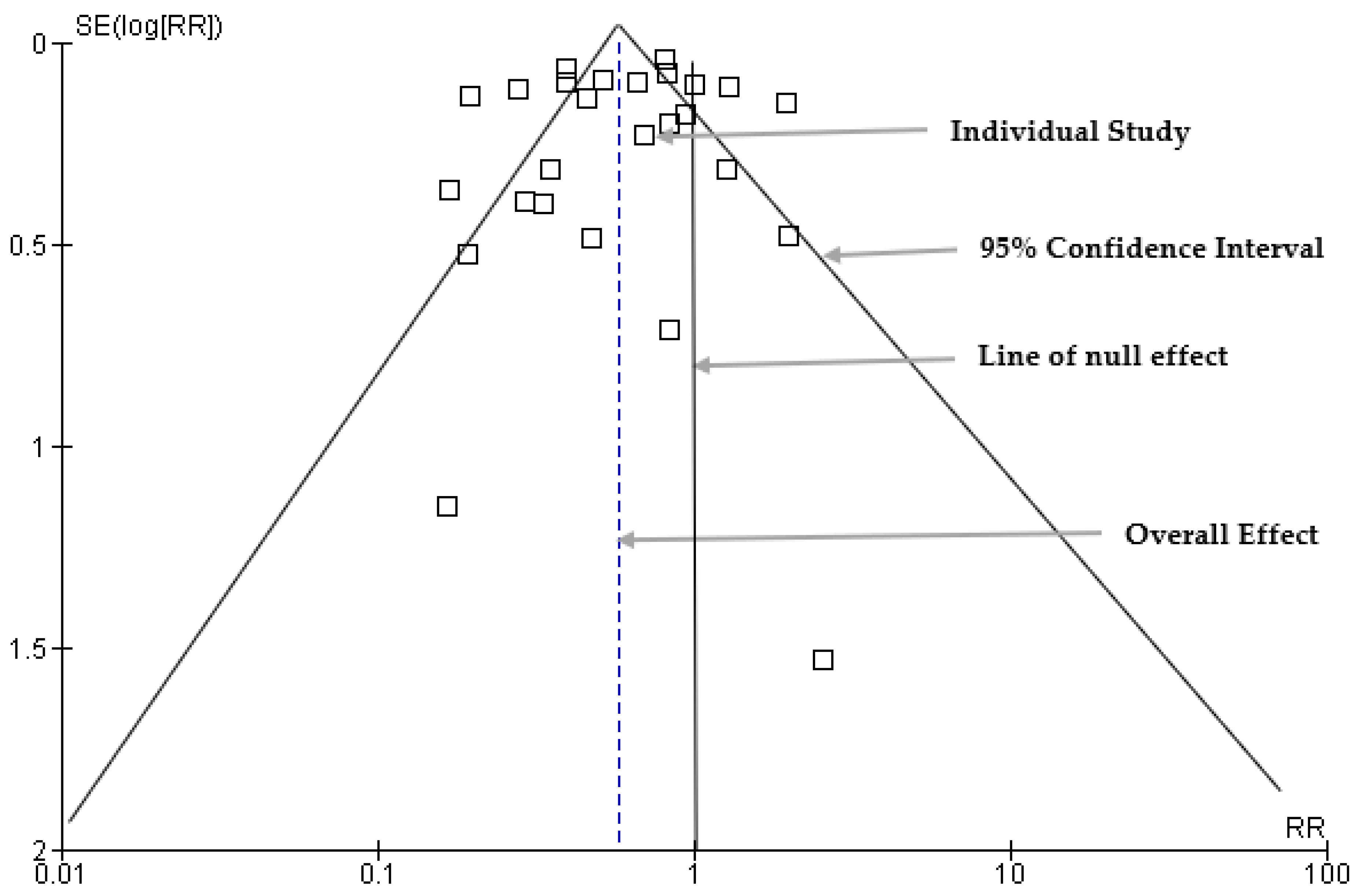
3.7. Funnel Plot: Publication Bias Results
3.8. Egger’s Regression Analysis Results for Publication Bias
4. Discussion
4.1. Strength of the Study
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Author (Year) | Study Design | Sample Size | Age Group | Country | Major Findings |
|---|---|---|---|---|---|
| Forrat et al., 2021 [30] | RCT | 29,229 | 2–16 years | Asia and Latin America | CYD-TDV demonstrated robust protection against hospitalized and severe VCD over the entire 6-year follow-up. |
| Thomas et al., 2022 [31] | RCT | 334 | 4–11 years | Thailand | Specific HLA alleles were significantly associated with dengue NAb titers. |
| España et al., 2019 [32] | RCT | 51,253 | 2–16 years | Peru | Lower detectability of primary DENV infections among seronegative individuals in the vaccinated group. |
| Yang et al., 2018 [33] | RCT | 31,125 | 5–11 years | USA | CYD-TDV vaccine was highly efficacious for all dengue serotypes among children. |
| Plennevaux et al., 2016 [34] | RCT | 2266 | 4–11 years | Thailand | A significant number of false positives occurred during routine clinical practice and surveillance following the introduction of the dengue vaccine. |
| Sridhar et al., 2018 [35] | Case–cohort study | 3578 | 2–16 years | Asia-Pacific region, Latin America, and Thailand | CYD-TDV protected against severe VCD and hospitalization. |
| Plennevaux et al., 2018 [36] | RCT | 31,000 | 2–16 years | Asia and Latin America | Baseline dengue serostatus (as defined by the PRNT50) had an impact on the IgM and IgG levels observed in VCD. |
| Moodie et al., 2018 [37] | Case–cohort study | 31,144 | 2–16 years | Asia and Latin America | High antibody titers are associated with high VE for all serotypes. |
| Olivera-Botello et al., 2016 [38] | RCT | 31,126 | 2–16 years | Asia and Latin America (Colombia, Brazil, Mexico, Puerto Rico, and Honduras) | Vaccine efficacy was marginally higher in subjects aged 9–16 years. |
| Hadinegoro et al., 2015 [39] | RCT | 33,266 | 2–16 years | Asia–Pacific countries, and Latin American countries | The vaccine was efficacious. |
| Villar et al., 2015 [40] | RCT | 20,869 | 9–16 years | Colombia, Brazil, Mexico, Puerto Rico, and Honduras | The CYD-TDV dengue vaccine was efficacious against VCD and severe VCD. |
| Dayan et al., 2015 [41] | Case–cohort study | 436 | 3–9 years | Asia-Pacific and Latin America | CYD-TDV provided long-term efficacy against symptomatic VCD in seropositive participants. |
| Dayan et al., 2020 [42] | Case–cohort study | 31,126 | 9–14 years | Philippines | A single dose of CYD-TDV protected children from severe dengue. |
| López-Medina et al., 2021 [43] | RCT | 20,099 | 4–16 years | Latin America (Brazil, Colombia, Dominican Republic, Panama and Nicaragua), Sri Lanka, Thailand, Philippines | TAK-003 demonstrated continued benefit independent of baseline serostatus in reducing dengue. |
| Biswal et al., 2020 [44] | RCT | 20,099 | 4–16 years | Asia and Latin America | TAK-003 was well tolerated and efficacious against symptomatic dengue. |
| Rivera et al., 2022 [45] | RCT | 20,099 | 4–16 years | Latin America and Asia (Philippines, Sri Lanka) | TAK-003 was safe and efficacious against symptomatic dengue over 3 years. |
| Biswal et al., 2019 [46] | RCT | 20,071 | 4–16 years | Brazil, Colombia, Dominican Republic, Nicaragua, Panama, Philippines, Sri Lanka, and Thailand | TAK-003 was efficacious against virologically confirmed dengue fever among healthy children, irrespective of previous dengue exposure. |
| Saez-Llorens et al., 2023 [47] | RCT | 13,380 | 4–16 years | Latin America (Columbia) and Asia (Philippines, Sri Lanka, Thailand) | TAK-003 vaccination resulted in a reduced risk of episodes of symptomatic dengue. |
| Reynales et al., 2020 [48] | Case–cohort study | 9740 | 9–16 years | Colombia | CYD-TDV protected against severe VCD and hospitalization. |
| Ylade et al., 2021 [49] | Case–cohort study | 490 | 9–14 years | Philippines | A single dose of CYD-TDV conferred protection against dengue. |
| Capeding et al., 2014 [50] | RCT | 10,275 | 2–14 years | Asia-Pacific countries (Indonesia, Malaysia, Philippines, Thailand, and Vietnam | The dengue vaccine is efficacious when given as three injections at months 0, 6, and 12 to children. |
| Sabchareon et al., 2012 [51] | RCT | 4002 | 4–11 years | Thailand | The vaccine is efficacious but differed by serotype. |
| Juraska et al., 2018 [52] | RCT | 563 | 2–16 years | Brazil and Thailand | Greater estimated vaccine efficacy of CYD-TDV against serotypes. |
| Sáez-Llorens et al., 2018 [53] | RCT | 1800 | 2–17 years | Dominican Republic, Panama, and the Philippines | Takeda vaccine was well tolerated and immunogenic against all four dengue serotypes, irrespective of baseline dengue serostatus. |
| Capeding et al., 2011 [54] | RCT | 126 | 2–17 years | Philippines | Supports the safety and tolerability of the vaccine in a flavivirus-endemic population. |
| Sirivichayakul et al., 2022 [55] | RCT | 212 | 1–11 years | Puerto Rico, Columbia, Singapore, and Thailand | Persistence of neutralizing antibody titers against TAK-003 over 3 years. |
| Hss et al., 2013 [56] | RCT | 250 | 2–11 years | Malaysia | A balanced humoral immune response against all four DENV serotypes for CYD-TDV administered in three doses. |
| Vigne et al., 2017 [57] | RCT | 5780 | 9–17 years | Asia Pacific (including Australia), Latin America, and the USA | CYD-TDV elicits neutralizing antibody responses against all dengue serotypes. |
| Tricou et al., 2020 [58] | RCT | 1800 | 2–17 years | Dominican Republic, Panama, and the Philippines | TAK-003 elicited antibody responses against all four serotypes, which persisted to 48 months postvaccination. |
| Simasathien et al., 2008 [59] | RCT | 89 | 6–7 years | Thailand | The vaccine was well -tolerated. |
| Watanaveeradej et al., 2016 [60] | RCT | 56 | 2–8 years | Thailand | The live-attenuated DENV candidate vaccine did not elicit a durable primary humoral immune response. |
| Biswal et al., 2021 [61] | RCT | 400 | 12–17 years | Mexico | TAK-003 was immunogenic against all four serotypes and was well tolerated. |
| Villar et al., 2013 [62] | RCT | 600 | 2–16 years | Colombia, Honduras, Mexico, and Puerto Rico | CYD-TDV had a favorable safety profile and elicited antibody responses against all 4 dengue virus serotypes. |
| Dayan et al., 2013 [63] | RCT | 150 | 9–16 years | Brazil | CYD-TDV vaccination elicited a neutralizing antibody response against all 4 serotypes and was well tolerated. |
| Arredondo-García et al., 2018 [64] | RCT | 23,429 | 2–16 years | 5 Asian-Pacific countries, 5 Latin American countries and Thailand | A higher protective effect in the 6–8 year olds than in the 2–5-year-old children. |
| Kriengsak et al., 2019 [65] | RCT | 3997 | 4–11 years | Thailand | The risk of hospitalization decreased with CYD-TDV. |
| Sabchareon et al., 2004 [66] | RCT | 1587 | 5–12 years | Thailand | No serious adverse event except mild-to-moderate fever, rash, headache, and myalgia occurring within 12 days after dose 1 and generally lasting 3 days or less. |
| Lanata et al., 2012 [67] | RCT | 300 | 2–11 years | Peru | No adverse events after vaccination. |
Appendix B
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Dengue Vaccines (CYD_TDV and Takeda) c | Control | Relative (95% CI) | Absolute (95% CI) | ||
| Efficacy | ||||||||||||
| 12 | Randomized trials | not serious a | not serious | not serious | not serious b | none | 2285/89,498 (2.6%) | 2172/45,242 (4.8%) | RR 0.62 (0.48 to 0.81) | 18 fewer per 1000 (from 25 fewer to 9 fewer) | High | CRITICAL |
| Immunogenicity | ||||||||||||
| 9 | Randomized trials | not serious | not serious | not serious | not serious | none | 493/30,499 (1.6%) | 506/13,896 (3.6%) | RR 0.48 (0.25 to 0.91) | 19 fewer per 1000 (from 27 fewer to 3 fewer) | High | CRITICAL |
| Safety | ||||||||||||
| 6 | Randomized trials | not serious | not serious | not serious | not serious | none | 349/25,083 (1.4%) | 289/12,547 (2.3%) | RR 0.75 (0.46 to 1.22) | 6 fewer per 1000 (from 12 fewer to 5 more) | High | CRITICAL |
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Ferreira-de-Lima, V.H.; Lima-Camara, T.N. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: A systematic review. Parasites Vectors 2018, 11, 77. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Dengue and Severe Dengue. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 18 December 2023).
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of the fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef]
- Wahala, W.M.; de Silva, A.M. The human antibody response to dengue virus infection. Viruses 2011, 3, 2374–2395. [Google Scholar] [CrossRef]
- Centers for Disease Control & Prevention (CDC). Dengue: Clinical Presentation. 2023. Available online: https://www.cdc.gov/dengue/healthcare-providers/clinical-presentation.html#:~:text=Severe%20dengue%20is%20defined%20by,impaired%20consciou-sness%2C%20or%20heart%20impairment (accessed on 18 September 2023).
- Houtman, J.; Shultz, L.; Glassman, R.; Gilmour, J.; Rivera, J.M.; Scarpino, S. The Increasing Burden of Dengue Fever in a Changing Climate; The Rockefeller Foundation: New York, NY, USA, 2023; Available online: https://www.rockefellerfoundation.org/blog/the-increasing-burden-of-dengue-fever-in-a-changing-climate/#:~:text=In%20the%20last%20fifty%20years,are%20at%20risk%20of%20infection (accessed on 1 October 2023).
- Centers for Disease Control & Prevention (CDC). Potential Range of Aedes aegypti and Aedes albopictus in the United States in 2017. 2023. Available online: https://www.cdc.gov/mosquitoes/mosquito-control/professionals/range.html#:~:text=These%20mosquitoes%20live%20in%20tropical,than%20other%20types%20of%20mosquitoes (accessed on 20 October 2023).
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Rocklöv, J.; Tozan, Y. Climate change and the rising infectiousness of dengue. Emerg. Top. Life Sci. 2019, 3, 133–142. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Disease Outbreak News; Dengue in Bangladesh. 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON481 (accessed on 14 September 2023).
- PAHO. Epidemiological Update: Dengue. PAHO/WHO, Washington 2019. Available online: https://www.paho.org/sites/default/files/2019-10/2019-sept-13-phe-dengue-epi-update.pdf (accessed on 18 December 2023).
- WHO. Dengue—The Region of the Americas. 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON475#:~:text=Dengue%20is%20the%20arbovirus%20that,were%20recorded%20in%20South%20America (accessed on 18 December 2023).
- World Health Organization (WHO). Vaccines and Immunization: Dengue. 2018. Available online: https://www.who.int/news-room/questions-and-answers/item/dengue-vaccines (accessed on 28 November 2023).
- Liu, Y.; Liu, J.; Cheng, G. Vaccines, and immunization strategies for dengue prevention. Emerg. Microbes Infect. 2016, 5, e77. [Google Scholar] [CrossRef]
- Collins, M.H.; Metz, S.W. Progress and works in progress: Update on flavivirus vaccine development. Clin. Ther. 2017, 39, 1519–1536. [Google Scholar] [CrossRef]
- Pang, T.; Mak, T.K.; Gubler, D.J. Prevention and control of dengue—The light at the end of the tunnel. Lancet Infect. Dis. 2017, 17, e79–e87. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent developments in recombinant protein-based dengue vaccines. Front. Immunol. 2018, 9, 1919. [Google Scholar] [CrossRef]
- Guy, B.; Barrere, B.; Malinowski, C.; Saville, M.; Teyssou, R.; Lang, J. From research to phase III: Preclinical, industrial, and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 2011, 29, 7229–7241. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Rodríguez-Barraquer, I.; Dorigatti, I.; Mier-Y-Teran-Romero, L.; Laydon, D.J.; Cummings, D.A. Benefits, and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science 2016, 353, 1033–1036. [Google Scholar] [CrossRef]
- Aguiar, M.; Halstead, S.B.; Stollenwerk, N. Consider stopping dengvaxia administration without immunological screening. Expert Rev. Vaccines 2017, 16, 301–302. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017, 35, 6355–6358. [Google Scholar] [CrossRef]
- Aguiar, M.; Stollenwerk, N. Dengvaxia: Age as surrogate for serostatus. Lancet Infect. Dis. 2018, 18, 245. [Google Scholar] [CrossRef]
- Osorio, J.E.; Partidos, C.D.; Wallace, D.; Stinchcomb, D.T. Development of a recombinant, chimeric tetravalent dengue vaccine candidate. Vaccine 2015, 33, 7112–7120. [Google Scholar] [CrossRef]
- Durbin, A.P.; Kirkpatrick, B.D.; Pierce, K.K.; Carmolli, M.P.; Tibery, C.M.; Grier, P.L.; Hynes, N.; Opert, K.; Jarvis, A.P.; Sabundayo, B.P.; et al. A 12-month-interval dosing study in adults indicates that a single dose of the national institute of allergy and infectious diseases tetravalent dengue vaccine induces a robust neutralizing antibody response. J. Infect. Dis. 2016, 214, 832–835. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Cochrane: London, UK, 2023. [Google Scholar]
- Deeks, J.J.; Higgins, J.; Altman, D.G. Chapter 9: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2008; pp. 243–296. [Google Scholar] [CrossRef]
- Thomas, J.; Harden, A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med. Res. Methodol. 2008, 8, 45. [Google Scholar] [CrossRef]
- Forrat, R.; Dayan, G.H.; DiazGranados, C.A.; Bonaparte, M.; Laot, T.; Capeding, M.R.; Sanchez, L.; Coronel, D.L.; Reynales, H.; Chansinghakul, D.; et al. Analysis of hospitalized and severe dengue cases over the 6 years of follow-up of the tetravalent dengue vaccine (CYD-TDV) efficacy trials in Asia and Latin America. Clin. Infect. Dis. 2021, 73, 1003–1012. [Google Scholar] [CrossRef]
- Thomas, R.; Chansinghakul, D.; Limkittikul, K.; Gilbert, P.B.; Hattasingh, W.; Moodie, Z.; Shangguan, S.; Frago, C.; Dulyachai, W.; Li, S.S.; et al. Associations of human leukocyte antigen with neutralizing antibody titers in a tetravalent dengue vaccine phase 2 efficacy trial in Thailand. Hum. Immunol. 2022, 83, 53–60. [Google Scholar] [CrossRef]
- España, G.; Hogea, C.; Guignard, A.; ten Bosch, Q.A.; Morrison, A.C.; Smith, D.L.; Scott, T.W.; Schmidt, A.; Perkins, T.A. Biased efficacy estimates in phase-III dengue vaccine trials due to heterogeneous exposure and differential detectability of primary infections across trial arms. PLoS ONE 2019, 14, e0210041. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, Y.; Halloran, M.E.; Longini, I.M., Jr. Dependency of vaccine efficacy on preexposure and age: A closer look at a tetravalent dengue vaccine. Clin. Infect. Dis. 2018, 66, 178–184. [Google Scholar] [CrossRef]
- Plennevaux, E.; Sabchareon, A.; Limkittikul, K.; Chanthavanich, P.; Sirivichayakul, C.; Moureau, A. Detection of dengue cases by serological testing in a dengue vaccine efficacy trial: Utility for efficacy evaluation and impact of future vaccine introduction. Vaccine 2016, 34, 2707–2712. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef]
- Plennevaux, E.; Moureau, A.; Arredondo-García, J.L.; Villar, L.; Pitisuttithum, P.; Tran, N.H.; Bonaparte, M.; Chansinghakul, D.; Coronel, D.L.; L’azou, M.; et al. Impact of dengue vaccination on serological diagnosis: Insights from phase III dengue vaccine efficacy trials. Clin. Infect. Dis. 2018, 66, 1164–1172. [Google Scholar] [CrossRef]
- Moodie, Z.; Juraska, M.; Huang, Y.; Zhuang, Y.; Fong, Y.; Carpp, L.N.; Self, S.G.; Chambonneau, L.; Small, R.; Jackson, N.; et al. Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J. Infect. Dis. 2018, 217, 742–753. [Google Scholar] [CrossRef]
- Olivera-Botello, G.; Coudeville, L.; Fanouillere, K.; Guy, B.; Chambonneau, L.; Noriega, F.; Jackson, N.; CYD-TDV Vaccine Trial Group. Tetravalent dengue vaccine reduces symptomatic and asymptomatic dengue virus infections in healthy children and adolescents Aged 2–16 years in Asia and Latin America. J. Infect. Dis. 2016, 214, 994–1000. [Google Scholar] [CrossRef]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Ismail, H.H.M.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- Dayan, G.; Arredondo, J.L.; Carrasquilla, G.; Deseda, C.C.; Dietze, R.; Luz, K.; Costa, M.S.; Cunha, R.V.; Rey, L.C.; Morales, J.; et al. Prospective cohort study with active surveillance for fever in four dengue-endemic countries in Latin America. Am. J. Trop. Med. Hyg. 2015, 93, 18–23. [Google Scholar] [CrossRef][Green Version]
- Dayan, G.H.; Langevin, E.; Gilbert, P.B.; Wu, Y.; Moodie, Z.; Forrat, R.; Price, B.; Frago, C.; Bouckenooghe, A.; Cortes, M.; et al. Assessment of the long-term efficacy of a dengue vaccine against symptomatic, virologically-confirmed dengue disease by baseline dengue serostatus. Vaccine 2020, 38, 3531–3536. [Google Scholar] [CrossRef]
- López-Medina, E.; Biswal, S.; Saez-Llorens, X.; Borja-Tabora, C.; Bravo, L.; Sirivichayakul, C.; Vargas, L.M.; Alera, M.T.; Velásquez, H.; Reynales, H.; et al. Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination. J. Infect. Dis. 2022, 225, 1521–1532. [Google Scholar] [CrossRef]
- Biswal, S.; Borja-Tabora, C.; Martinez Vargas, L.; Velásquez, H.; Theresa Alera, M.; Sierra, V.; Yu, D.; Moreira, E.D.; Fernando, A.D.; Gunasekera, D.; et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: A randomized, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1423–1433. [Google Scholar] [CrossRef]
- Rivera, L.; Biswal, S.; Sáez-Llorens, X.; Reynales, H.; López-Medina, E.; Borja-Tabora, C.; Bravo, L.; Sirivichayakul, C.; Kosalaraksa, P.; Vargas, L.M.; et al. Three-year Efficacy and Safety of Takeda’s Dengue Vaccine Candidate (TAK-003). Clin. Infect. Dis. 2022, 75, 107–117. [Google Scholar] [CrossRef]
- Biswal, S.; Reynales, H.; Saez-Llorens, X.; Lopez, P.; Borja-Tabora, C.; Kosalaraksa, P.; Sirivichayakul, C.; Watanaveeradej, V.; Rivera, L.; Espinoza, F.; et al. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N. Engl. J. Med. 2019, 381, 2009–2019. [Google Scholar] [CrossRef]
- Sáez-Llorens, X.; Biswal, S.; Borja-Tabora, C.; Fernando, L.; Liu, M.; Wallace, D.; Folschweiller, N.; Reynales, H.; LeFevre, I. Effect of the tetravalent dengue vaccine TAK-003 on sequential episodes of symptomatic dengue. Am. J. Trop. Med. Hyg. 2023, 108, 722–726. [Google Scholar] [CrossRef]
- Reynales, H.; Carrasquilla, G.; Zambrano, B.; Cortes, S.M.; Machabert, T.; Jing, J.; Pallardy, S.M.; Haney, O.; Faccini, M.N.; Quintero, J.; et al. Secondary analysis of the efficacy and safety trial data of the tetravalent dengue vaccine in children and adolescents in Colombia. Pediatr. Infect. Dis. J. 2020, 39, e30–e36. [Google Scholar] [CrossRef]
- Ylade, M.; Agrupis, K.A.; Daag, J.V.; Crisostomo, M.V.; Tabuco, M.O.; Sy, A.K.; Nealon, J.; Macina, D.; Sarol, J.; Deen, J.; et al. Effectiveness of a single-dose mass dengue vaccination in Cebu, Philippines: A case-control study. Vaccine 2021, 39, 5318–5325. [Google Scholar] [CrossRef]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.; Ismail, H.I.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomized, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- Sabchareon, A.; Wallace, D.; Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Suvannadabba, S.; Jiwariyavej, V.; Dulyachai, W.; Pengsaa, K.; Wartel, T.A.; et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomized, controlled phase 2b trial. Lancet 2012, 380, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Juraska, M.; Magaret, C.A.; Shao, J.; Carpp, L.N.; Fiore-Gartland, A.J.; Benkeser, D.; Girerd-Chambaz, Y.; Langevin, E.; Frago, C.; Guy, B.; et al. Viral genetic diversity and protective efficacy of a tetravalent dengue vaccine in two phase 3 trials. Proc. Natl. Acad. Sci. USA 2018, 115, E8378. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Llorens, X.; Tricou, V.; Yu, D.; Rivera, L.; Jimeno, J.; Villarreal, A.C.; Dato, E.; Mazara, S.; Vargas, M.; Brose, M.; et al. Immunogenicity and safety of one versus two doses of tetravalent dengue vaccine in healthy children aged 2–17 years in Asia and Latin America: 18-month interim data from a phase 2, randomized, placebo-controlled study. Lancet Infect. Dis. 2018, 18, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Capeding, R.Z.; Luna, I.A.; Bomasang, E.; Lupisan, S.; Lang, J.; Forrat, R.; Wartel, A.; Crevat, D. Live-attenuated, tetravalent dengue vaccine in children, adolescents, and adults in a dengue-endemic country: Randomized controlled phase I trial in the Philippines. Vaccine 2011, 29, 3863–3872. [Google Scholar] [CrossRef] [PubMed]
- Sirivichayakul, C.; Barranco-Santana, E.A.; Rivera, I.E.; Kilbury, J.; Raanan, M.; Borkowski, A.; Papadimitriou, A.; Wallace, D. Long-term safety and immunogenicity of a tetravalent dengue vaccine candidate in children and adults: A randomized, placebo-controlled, phase 2 study. J. Infect. Dis. 2022, 225, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Hss, A.S.; Koh, M.T.; Tan, K.K.; Chan, L.G.; Zhou, L.; Bouckenooghe, A.; Crevat, D.; Hutagalung, Y. Safety and immunogenicity of a tetravalent dengue vaccine in healthy children aged 2–11 years in Malaysia: A randomized, placebo-controlled, Phase III study. Vaccine 2013, 31, 5814–5821. [Google Scholar] [CrossRef] [PubMed]
- Vigne, C.; Dupuy, M.; Richetin, A.; Guy, B.; Jackson, N.; Bonaparte, M.; Hu, B.; Saville, M.; Chansinghakul, D.; Noriega, F.; et al. Integrated immunogenicity analysis of a tetravalent dengue vaccine up to 4 y after vaccination. Hum. Vaccines Immunother. 2017, 13, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Tricou, V.; Sáez-Llorens, X.; Yu, D.; Rivera, L.; Jimeno, J.; Villarreal, A.C.; Dato, E.; de Suman, O.S.; Montenegro, N.; DeAntonio, R.; et al. Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2–17 years: A randomized, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1434–1443. [Google Scholar] [CrossRef]
- Simasathien, S.; Thomas, S.J.; Watanaveeradej, V.; Nisalak, A.; Barberousse, C.; Innis, B.; Sun, W.; Putnak, J.R.; Eckels, K.H.; Hutagalung, Y.; et al. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus naive children. Am. J. Trop. Med. Hyg. 2008, 78, 426–433. [Google Scholar] [CrossRef]
- Watanaveeradej, V.; Simasathien, S.; Mammen, M.P.; Nisalak, A.; Tournay, E.; Kerdpanich, P.; Samakoses, R.; Putnak, R.J.; Gibbons, R.V.; Yoon, I.-K.; et al. Long-term safety and immunogenicity of a tetravalent live-attenuated dengue vaccine and evaluation of a booster dose administered to healthy Thai children. Am. J. Trop. Med. Hyg. 2016, 94, 1348–1358. [Google Scholar] [CrossRef]
- Biswal, S.; Mendez Galvan, J.F.; Macias Parra, M.; Galan-Herrera, J.F.; Carrascal Rodriguez, M.B.; Rodriguez Bueno, E.P.; Brose, M.; Rauscher, M.; LeFevre, I.; Wallace, D.; et al. Immunogenicity, and safety of a tetravalent dengue vaccine in dengue-naïve adolescents in Mexico City. Rev. Panam. Salud Publica 2021, 45, e67. [Google Scholar] [CrossRef]
- Villar, L.Á.; Rivera-Medina, D.M.; Arredondo-García, J.L.; Boaz, M.; Starr-Spires, L.; Thakur, M.; Zambrano, B.; Miranda, M.C.; Rivas, E.; Dayan, G.H. Safety, and immunogenicity of a recombinant tetravalent dengue vaccine in 9–16-year-olds: A randomized, controlled, phase II trial in Latin America. Pediatr. Infect. Dis. J. 2013, 32, 1102–1109. [Google Scholar] [CrossRef]
- Dayan, G.H.; Thakur, M.; Boaz, M.; Johnson, C. Safety, and immunogenicity of three tetravalent dengue vaccine formulations in healthy adults in the USA. Vaccine 2013, 31, 5047–5054. [Google Scholar] [CrossRef]
- Arredondo-García, J.L.; Hadinegoro, S.R.; Reynales, H.; Chua, M.N.; Rivera Medina, D.M.; Chotpitayasunondh, T.; Tran, N.; Deseda, C.; Wirawan, D.; Supelano, M.C.; et al. Four-year safety follow-up of the tetravalent dengue vaccine efficacy randomized controlled trials in Asia and Latin America. Clin. Microbiol. Infect. 2018, 24, 755–763. [Google Scholar] [CrossRef]
- Kriengsak, L.; Chanthavanich, P.; Lee, K.S.; Lee, J.-S.; Chatchen, S.; Lim, S.-K.; Arunsodsai, W.; Yoon, I.-K.; Lim, J.K. Dengue virus seroprevalence study in Bangphae district, Ratchaburi, Thailand: A cohort study in 2012–2015. PLoS Negl. Trop. Dis. 2022, 16, e0010021. [Google Scholar] [CrossRef]
- Sabchareon, A.; Lang, J.; Chanthavanich, P.; Yoksan, S.; Forrat, R.; Attanath, P.; Sirivichayakul, C.; Pengsaa, K.; Pojjaroen-Anant, C.; Chambonneau, L.; et al. Safety and immunogenicity of a three-dose regimen of two tetravalent live-attenuated dengue vaccines in five- to twelve-year-old Thai children. Pediatr. Infect. Dis. J. 2004, 23, 99–109. [Google Scholar] [CrossRef]
- Lanata, C.F.; Andrade, T.; Gil, A.I.; Terrones, C.; Valladolid, O.; Zambrano, B.; Saville, M.; Crevat, D. Immunogenicity and safety of tetravalent dengue vaccine in 2–11-year-olds previously vaccinated against yellow fever: Randomized, controlled, phase II study in Piura, Peru. Vaccine 2012, 30, 5935–5941. [Google Scholar] [CrossRef]
- Saada, A.; Lieu, T.A.; Morain, S.R.; Zikmund-Fisher, B.J.; Wittenberg, E. Parents’ choices and rationales for alternative vaccination schedules: A qualitative study. Clin. Pediatr. 2015, 54, 236–243. [Google Scholar] [CrossRef]
- Harmsen, I.A.; Mollema, L.; Ruiter, R.A.; Paulussen, T.G.; de Melker, H.E.; Kok, G. Why parents refuse childhood vaccination: A qualitative study using online focus groups. BMC Public Health 2013, 13, 1183. [Google Scholar] [CrossRef]
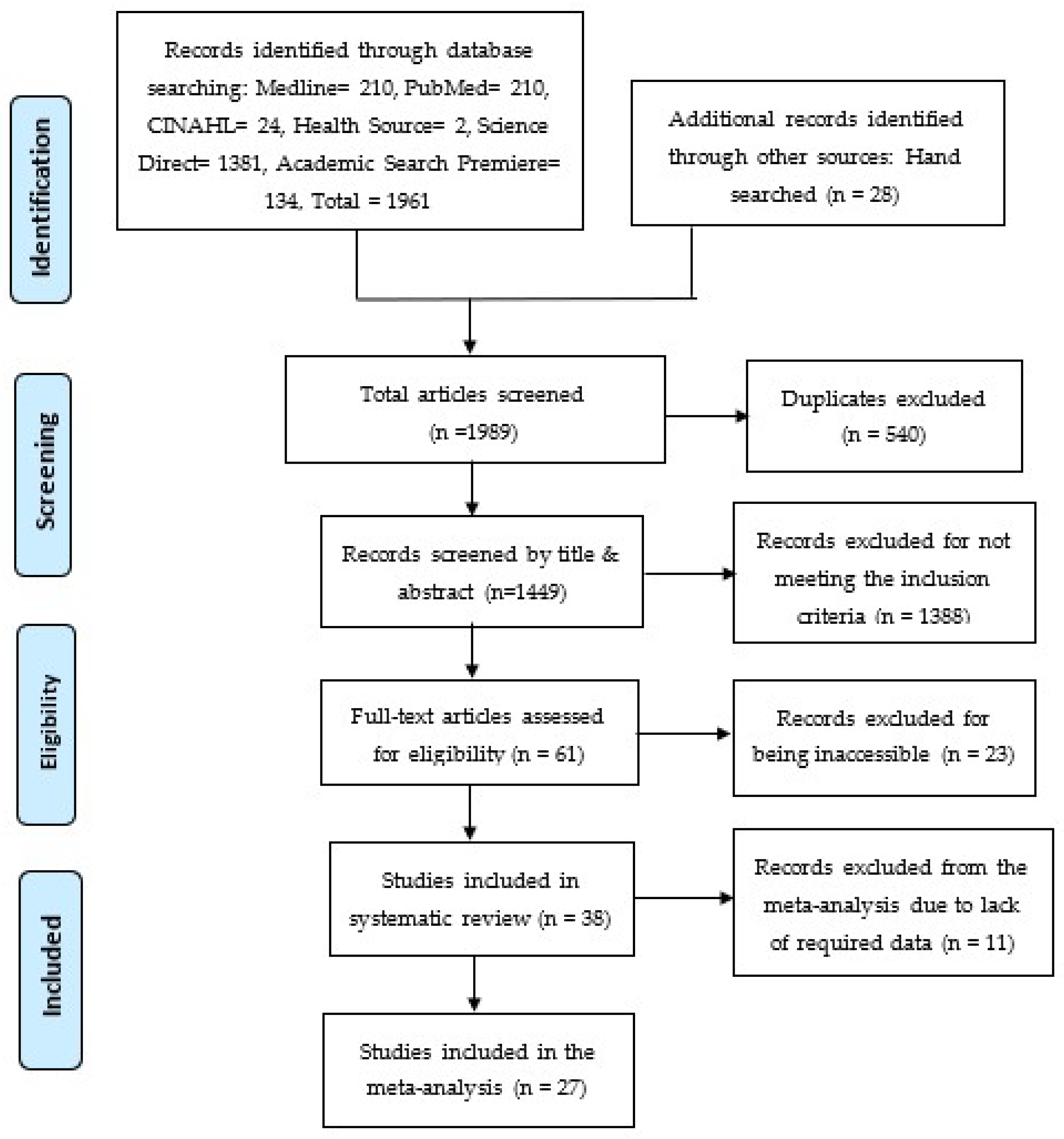
| Databases | Search Keywords | Number of Articles Found |
|---|---|---|
| PubMed (1) | “Dengue fever” OR “Dengue epidemics” OR “Dengue vaccine” OR “Dengue Vaccine prospects” | 129 |
| PubMed (2) | “Dengue fever” OR “Dengue epidemics” AND “Dengue vaccine” OR “Dengue vaccine development” OR “Dengue vaccine prospects” AND “Dengue vaccine efficacy” OR “Dengue vaccine safety” OR “Dengue serotypes” | 81 |
| CINAHL | “Dengue fever” OR “Dengue epidemics” AND “Dengue vaccine” OR “Dengue vaccine development” OR “Dengue vaccine prospects” AND “Dengue vaccine efficacy” OR “Dengue vaccine safety” OR “Dengue serotypes” OR “Clinical trials” OR “Epidemiological studies” | 24 |
| Medline | “Dengue fever” OR “Dengue epidemics” AND “Dengue vaccine” OR “Dengue vaccine development” OR “Dengue vaccine prospects” AND “Dengue vaccine efficacy” OR “Dengue vaccine safety” OR “Dengue serotypes” OR “Clinical trials” OR “Epidemiological studies | 210 |
| Health Source | “Dengue fever” OR “Dengue epidemics” AND “Dengue vaccine” OR “Dengue vaccine development” OR “Dengue vaccine prospects” AND “Dengue vaccine efficacy” OR “Dengue vaccine safety” OR “Dengue serotypes” | 2 |
| Science Direct | “Dengue fever” OR “Dengue epidemics” AND “Dengue vaccine” OR “Dengue vaccine development” AND “Dengue vaccine efficacy” OR “Dengue vaccine safety” OR “Dengue serotypes” OR “Clinical trials” OR “Epidemiological studies” | 1381 |
| Academic Search Premiere | “Dengue fever” OR “Dengue epidemics” AND “Dengue vaccine” OR “Dengue vaccine development” OR “Dengue vaccine prospects” AND “Dengue vaccine efficacy” OR “Dengue vaccine safety” OR “Dengue serotypes” OR “Clinical trials” OR “Epidemiological studies” | 134 |
| Intercept | −0.94387 |
|---|---|
| Standard error | 1.22659 |
| 95% CI lower limit (2-tailed) | −3.47009 |
| 95% CI upper limit (2-tailed) | 1.58235 |
| t-value | 0.76950 |
| df | 25 |
| p-value (1-tailed) | 0.22440 |
| p-value (2-tailed) | 0.44881 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoye, E.C.; Mitra, A.K.; Lomax, T.; Nunaley, C. Dengue Fever Epidemics and the Prospect of Vaccines: A Systematic Review and Meta-Analysis Using Clinical Trials in Children. Diseases 2024, 12, 32. https://doi.org/10.3390/diseases12020032
Okoye EC, Mitra AK, Lomax T, Nunaley C. Dengue Fever Epidemics and the Prospect of Vaccines: A Systematic Review and Meta-Analysis Using Clinical Trials in Children. Diseases. 2024; 12(2):32. https://doi.org/10.3390/diseases12020032
Chicago/Turabian StyleOkoye, Ebele C., Amal K. Mitra, Terica Lomax, and Cedric Nunaley. 2024. "Dengue Fever Epidemics and the Prospect of Vaccines: A Systematic Review and Meta-Analysis Using Clinical Trials in Children" Diseases 12, no. 2: 32. https://doi.org/10.3390/diseases12020032
APA StyleOkoye, E. C., Mitra, A. K., Lomax, T., & Nunaley, C. (2024). Dengue Fever Epidemics and the Prospect of Vaccines: A Systematic Review and Meta-Analysis Using Clinical Trials in Children. Diseases, 12(2), 32. https://doi.org/10.3390/diseases12020032







