Global Prevalence of Zika and Chikungunya Coinfection: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Guideline and Protocol
2.2. Literature Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction and Quality Assessment
2.5. Data Synthesis and Statistical Analysis
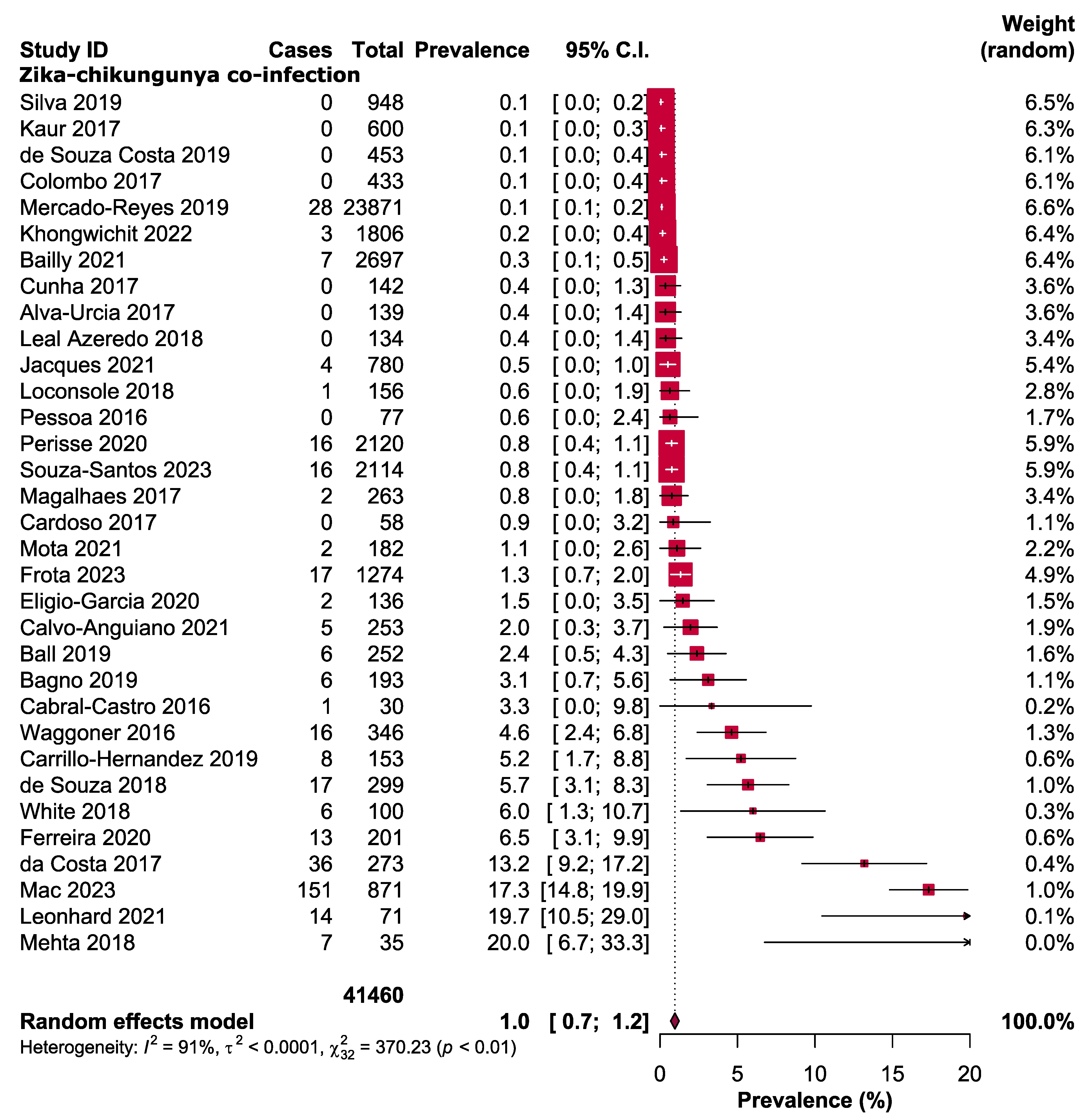
3. Results
3.1. Selection of the Relevant Studies
3.2. Major Features of the Included Studies
3.3. Major Outcomes
3.4. Publication Bias and Quality Assessment
3.5. Outlier and Sensitivity Analysis
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Staples, J.E.; Breiman, R.F.; Powers, A.M. Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef]
- Zeller, H.; Van Bortel, W.; Sudre, B. Chikungunya: Its history in Africa and Asia and its spread to new regions in 2013–2014. J. Infect. Dis. 2016, 214, S436–S440. [Google Scholar] [CrossRef]
- Monath, T.P. The Arboviruses: Epidemiology and Ecology; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A. Emerging and re-emerging viruses: A global challenge illustrated by Chikungunya virus outbreaks. World J. Virol. 2012, 1, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Puntasecca, C.J.; King, C.H.; LaBeaud, A.D. Measuring the global burden of chikungunya and Zika viruses: A systematic review. PLoS Negl. Trop. Dis. 2021, 15, e0009055. [Google Scholar] [CrossRef] [PubMed]
- Khatib, A.N. Climate Change and Travel: Harmonizing to Abate Impact. Curr. Infect. Dis. Rep. 2023, 25, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Scheday, S.; Boenecke, J.; Gogoi, A.; Maharaj, A.; Korovou, S. Climate Change, Health and Mosquito-Borne Diseases: Trends and Implications to the Pacific Region. Int. J. Environ. Res. Public. Health 2019, 16, 5114. [Google Scholar] [CrossRef]
- Asaga Mac, P.; Airiohuodion, P.E.; Yako, A.B.; Makpo, J.K.; Kroeger, A. The Seroprevalence and Hidden Burden of Chikungunya Endemicity and Malaria Mono- and Coinfection in Nigeria. Int. J. Environ. Res. Public. Health 2022, 19, 8896. [Google Scholar] [CrossRef]
- Carrillo-Hernandez, M.Y.; Ruiz-Saenz, J.; Villamizar, L.J.; Gomez-Rangel, S.Y.; Martinez-Gutierrez, M. Co-circulation and simultaneous co-infection of dengue, chikungunya, and zika viruses in patients with febrile syndrome at the Colombian-Venezuelan border. BMC Infect. Dis. 2018, 18, 61. [Google Scholar] [CrossRef]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef]
- Hasan, S.S.; Sevvana, M.; Kuhn, R.J.; Rossmann, M.G. Structural biology of Zika virus and other flaviviruses. Nat. Struct. Mol. Biol. 2018, 25, 13–20. [Google Scholar] [CrossRef]
- Leier, H.C.; Messer, W.B.; Tafesse, F.G. Lipids and pathogenic flaviviruses: An intimate union. PLoS Pathog. 2018, 14, e1006952. [Google Scholar] [CrossRef]
- Wikan, N.; Smith, D.R. Zika virus: History of a newly emerging arbovirus. Lancet Infect. Dis. 2016, 16, e119–e126. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Tumban, E. Zika Virus on a Spreading Spree: What we now know that was unknown in the 1950’s. Virol. J. 2016, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef]
- Gregory, C.J.; Oduyebo, T.; Brault, A.C.; Brooks, J.T.; Chung, K.W.; Hills, S.; Kuehnert, M.J.; Mead, P.; Meaney-Delman, D.; Rabe, I.; et al. Modes of Transmission of Zika Virus. J. Infect. Dis. 2017, 216, S875–S883. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, Y.; Rojas, M.; Ramirez-Santana, C.; Acosta-Ampudia, Y.; Monsalve, D.M.; Anaya, J.M. Autonomic symptoms following Zika virus infection. Clin. Auton. Res. 2018, 28, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, U.; Pandey, N.; Rastogi, M.; Singh, S.K. Gist of Zika Virus pathogenesis. Virology 2021, 560, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lessler, J.; Chaisson, L.H.; Kucirka, L.M.; Bi, Q.; Grantz, K.; Salje, H.; Carcelen, A.C.; Ott, C.T.; Sheffield, J.S.; Ferguson, N.M.; et al. Assessing the global threat from Zika virus. Science 2016, 353, aaf8160. [Google Scholar] [CrossRef]
- Anaya, J.M.; Rodriguez, Y.; Monsalve, D.M.; Vega, D.; Ojeda, E.; Gonzalez-Bravo, D.; Rodriguez-Jimenez, M.; Pinto-Diaz, C.A.; Chaparro, P.; Gunturiz, M.L.; et al. A comprehensive analysis and immunobiology of autoimmune neurological syndromes during the Zika virus outbreak in Cucuta, Colombia. J. Autoimmun. 2017, 77, 123–138. [Google Scholar] [CrossRef]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martinez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N.; Group, W.H.O.Z.C.W. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barre Syndrome: Systematic Review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Deng, C.L.; Li, J.Q.; Li, N.; Zhang, Q.Y.; Ye, H.Q.; Yuan, Z.M.; Zhang, B. Infectious Chikungunya Virus (CHIKV) with a Complete Capsid Deletion: A New Approach for a CHIKV Vaccine. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Chikungunya Worldwide Overview. ECDC 2023. Available online: https://www.ecdc.europa.eu/en/chikungunya-monthly (accessed on 13 October 2023).
- Vega-Rua, A.; Zouache, K.; Girod, R.; Failloux, A.B.; Lourenco-de-Oliveira, R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J. Virol. 2014, 88, 6294–6306. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Carbonnier, M.; Pasquet, M.; Bouhmani, B.; Ghazouani, J.; Noormahomed, T.; Beullier, G.; Attali, T.; Samperiz, S.; Fourmaintraux, A.; et al. Mother-to-child transmission of Chikungunya virus infection. Pediatr. Infect. Dis. J. 2007, 26, 811–815. [Google Scholar] [CrossRef]
- Couderc, T.; Lecuit, M. Chikungunya virus pathogenesis: From bedside to bench. Antivir. Res. 2015, 121, 120–131. [Google Scholar] [CrossRef]
- Lobkowicz, L.; Miranda-Filho, D.B.; Montarroyos, U.R.; Martelli, C.M.T.; Barreto de Araujo, T.V.; De Souza, W.V.; Bezerra, L.C.A.; Dhalia, R.; Marques, E.T.A.; Clemente, N.S.; et al. Co-circulation of Chikungunya Virus during the 2015–2017 Zika Virus Outbreak in Pernambuco, Brazil: An Analysis of the Microcephaly Epidemic Research Group Pregnancy Cohort. Am. J. Trop Med. Hyg. 2022, 106, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Irekeola, A.A.; Engku Nur Syafirah, E.A.R.; Islam, M.A.; Shueb, R.H. Global prevalence of dengue and chikungunya coinfection: A systematic review and meta-analysis of 43,341 participants. Acta Trop. 2022, 231, 106408. [Google Scholar] [CrossRef]
- Jacques, I.; Katz, L.; Sena, M.A.; Guimaraes, A.B.G.; Silva, Y.L.; Albuquerque, G.D.M.; Pereira, R.O.; de Albuquerque, C.; Silva, M.A.L.; Oliveira, P.A.S.; et al. High Incidence of Zika or Chikungunya Infection among Pregnant Women Hospitalized Due to Obstetrical Complications in Northeastern Brazil-Implications for Laboratory Screening in Arbovirus Endemic Area. Viruses 2021, 13, 744. [Google Scholar] [CrossRef]
- Mac, P.A.; Airiohuodion, P.E.; Zubair, S.; Tadele, M.; Aighobahi, J.O.; Anyaike, C.; Kroeger, A.; Panning, M. Antibody seropositivity and endemicity of chikungunya and Zika viruses in Nigeria. Anim. Dis. 2023, 3, 7. [Google Scholar] [CrossRef]
- Khongwichit, S.; Chuchaona, W.; Vongpunsawad, S.; Poovorawan, Y. Molecular surveillance of arboviruses circulation and co-infection during a large chikungunya virus outbreak in Thailand, October 2018 to February 2020. Sci. Rep. 2022, 12, 22323. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Metallo, A.; De Robertis, A.L.; Morea, A.; Quarto, M.; Chironna, M. Seroprevalence of Dengue Virus, West Nile Virus, Chikungunya Virus, and Zika Virus in International Travelers Attending a Travel and Migration Center in 2015–2017, Southern Italy. Vector Borne Zoonotic Dis. 2018, 18, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Bailly, S.; Rousset, D.; Fritzell, C.; Hoze, N.; Ben Achour, S.; Berthelot, L.; Enfissi, A.; Vanhomwegen, J.; Salje, H.; Fernandes-Pellerin, S.; et al. Spatial Distribution and Burden of Emerging Arboviruses in French Guiana. Viruses 2021, 13, 1299. [Google Scholar] [CrossRef]
- Pessoa, R.; Patriota, J.V.; Lourdes de Souza, M.; Felix, A.C.; Mamede, N.; Sanabani, S.S. Investigation into an Outbreak of Dengue-like Illness in Pernambuco, Brazil, Revealed a Cocirculation of Zika, Chikungunya, and Dengue Virus Type 1. Medicine 2016, 95, e3201. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Jaffar-Bandjee, M.C.; Hoarau, J.J.; Krejbich Trotot, P.; Denizot, M.; Lee-Pat-Yuen, G.; Sahoo, R.; Guiraud, P.; Ramful, D.; Robin, S.; et al. Chikungunya fever: CNS infection and pathologies of a re-emerging arbovirus. Prog. Neurobiol. 2010, 91, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J.; Villamil-Gomez, W.E.; Franco-Paredes, C. The arboviral burden of disease caused by co-circulation and co-infection of dengue, chikungunya and Zika in the Americas. Travel. Med. Infect. Dis. 2016, 14, 177–179. [Google Scholar] [CrossRef]
- Kam, Y.W.; Pok, K.Y.; Eng, K.E.; Tan, L.K.; Kaur, S.; Lee, W.W.; Leo, Y.S.; Ng, L.C.; Ng, L.F. Sero-prevalence and cross-reactivity of chikungunya virus specific anti-E2EP3 antibodies in arbovirus-infected patients. PLoS Negl. Trop. Dis. 2015, 9, e3445. [Google Scholar] [CrossRef]
- Zanotto, P.M.A.; Leite, L.C.C. The Challenges Imposed by Dengue, Zika, and Chikungunya to Brazil. Front. Immunol. 2018, 9, 1964. [Google Scholar] [CrossRef]
- Nkengasong, J.N.; Nsubuga, P.; Nwanyanwu, O.; Gershy-Damet, G.M.; Roscigno, G.; Bulterys, M.; Schoub, B.; DeCock, K.M.; Birx, D. Laboratory systems and services are critical in global health: Time to end the neglect? Am. J. Clin. Pathol. 2010, 134, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zika Virus. WHO 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/zika-virus (accessed on 25 December 2023).
- Chikungunya Virus. CDC 2023. Available online: https://www.cdc.gov/chikungunya/hc/treatment-prevention.html (accessed on 25 December 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.; Khalil, H.; Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis; JBI: Florence County, SC, USA, 2020; pp. 406–451. [Google Scholar] [CrossRef]
- Ahmed, S.; Chowdhury, M.I.H.; Sultana, S.; Alam, S.S.; Marzan, M.; Islam, M.A. Prevalence of Antibiotic-Resistant Shigella spp. in Bangladesh: A Systematic Review and Meta-Analysis of 44,519 Samples. Antibiotics 2023, 12, 817. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane, 2022. Available online: www.training.cochrane.org/handbook (accessed on 10 October 2023).
- Huedo-Medina, T.B.; Sanchez-Meca, J.; Marin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Souza-Santos, R.; Sobral, A.; Perisse, A.R.S. High-risk spatial clusters for Zika, dengue, and chikungunya in Rio de Janeiro, Brazil. Rev. Saude Publica 2023, 57, 32. [Google Scholar] [CrossRef]
- Frota, C.C.; Correia, F.G.S.; Alves Vasconcelos, L.R.; de Sousa, P.R.C.; Ferreira, M.; Saraiva, S.P.; Mota Ferreira, R.; Romcy, K.A.M.; Pinheiro, R.F.; de Oliveira, R.T.G.; et al. Positivity of dengue, chikungunya, and Zika infections in women in Northeast Brazil post-Zika epidemic. Pathog. Glob. Health 2023, 117, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Anguiano, G.; Lugo-Trampe, J.J.; Ponce-Garcia, G.; Lugo-Trampe, A.; Martinez-Garza, L.E.; Ibarra-Ramirez, M.; Campos-Acevedo, L.D.; Caballero-Sosa, S.; Juache-Villagrana, A.E.; Fernandez-Salas, I.; et al. Molecular Characterization of Associated Pathogens in Febrile Patients during Inter-Epidemic Periods of Urban Arboviral Diseases in Tapachula Southern Mexico. Pathogens 2021, 10, 1450. [Google Scholar] [CrossRef]
- Mota, M.L.; Dos Santos Souza Marinho, R.; Duro, R.L.S.; Hunter, J.; de Menezes, I.R.A.; de Lima Silva, J.M.F.; Pereira, G.L.T.; Sabino, E.C.; Grumach, A.; Diaz, R.S.; et al. Serological and molecular epidemiology of the Dengue, Zika and Chikungunya viruses in a risk area in Brazil. BMC Infect. Dis. 2021, 21, 704. [Google Scholar] [CrossRef] [PubMed]
- Leonhard, S.E.; Halstead, S.; Lant, S.B.; Militao de Albuquerque, M.F.P.; de Brito, C.A.A.; de Albuquerque, L.B.B.; Ellul, M.A.; de Oliveira Franca, R.F.; Gourlay, D.; Griffiths, M.J.; et al. Guillain-Barre syndrome during the Zika virus outbreak in Northeast Brazil: An observational cohort study. J. Neurol. Sci. 2021, 420, 117272. [Google Scholar] [CrossRef] [PubMed]
- Eligio-Garcia, L.; Crisostomo-Vazquez, M.D.P.; Caballero-Garcia, M.L.; Soria-Guerrero, M.; Mendez-Galvan, J.F.; Lopez-Cancino, S.A.; Jimenez-Cardoso, E. Co-infection of Dengue, Zika and Chikungunya in a group of pregnant women from Tuxtla Gutierrez, Chiapas: Preliminary data. 2019. PLoS Negl. Trop. Dis. 2020, 14, e0008880. [Google Scholar] [CrossRef]
- Brito Ferreira, M.L.; Militao de Albuquerque, M.F.P.; de Brito, C.A.A.; de Oliveira Franca, R.F.; Porto Moreira, A.J.; de Morais Machado, M.I.; da Paz Melo, R.; Medialdea-Carrera, R.; Dornelas Mesquita, S.; Lopes Santos, M.; et al. Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: A prospective observational study. Lancet Neurol. 2020, 19, 826–839. [Google Scholar] [CrossRef]
- Perisse, A.R.S.; Souza-Santos, R.; Duarte, R.; Santos, F.; de Andrade, C.R.; Rodrigues, N.C.P.; Schramm, J.M.A.; da Silva, E.D.; Jacobson, L.; Lemos, M.C.F.; et al. Zika, dengue and chikungunya population prevalence in Rio de Janeiro city, Brazil, and the importance of seroprevalence studies to estimate the real number of infected individuals. PLoS ONE 2020, 15, e0243239. [Google Scholar] [CrossRef] [PubMed]
- Bagno, F.F.; Figueiredo, M.M.; Villarreal, J.; Pereira, G.C.; Godoi, L.C.; da Fonseca, F.G. Undetected Chikungunya virus co-infections in a Brazilian region presenting hyper-endemic circulation of Dengue and Zika. J. Clin. Virol. 2019, 113, 27–30. [Google Scholar] [CrossRef]
- Ball, J.D.; Elbadry, M.A.; Telisma, T.; White, S.K.; Chavannes, S.; Anilis, M.G.; Prosperi, M.; Cummings, D.A.T.; Lednicky, J.A.; Morris, J.G.; et al. Clinical and Epidemiologic Patterns of Chikungunya Virus Infection and Coincident Arboviral Disease in a School Cohort in Haiti, 2014–2015. Clin. Infect Dis. 2019, 68, 919–926. [Google Scholar] [CrossRef]
- de Souza Costa, M.C.; Siqueira Maia, L.M.; Costa de Souza, V.; Gonzaga, A.M.; Correa de Azevedo, V.; Ramos Martins, L.; Chavez Pavoni, J.H.; Gomes Naveca, F.; Dezengrini Slhessarenko, R. Arbovirus investigation in patients from Mato Grosso during Zika and Chikungunya virus introdution in Brazil, 2015–2016. Acta Trop. 2019, 190, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Tauro, L.B.; Kikuti, M.; Anjos, R.O.; Santos, V.C.; Gonçalves, T.S.; Paploski, I.A.; Moreira, P.S.; Nascimento, L.C.; Campos, G.S. Concomitant transmission of dengue, chikungunya, and Zika viruses in Brazil: Clinical and epidemiological findings from surveillance for acute febrile illness. Clin. Infect. Dis. 2019, 69, 1353–1359. [Google Scholar] [CrossRef]
- Mercado-Reyes, M.; Acosta-Reyes, J.; Navarro-Lechuga, E.; Corchuelo, S.; Rico, A.; Parra, E.; Tolosa, N.; Pardo, L.; Gonzalez, M.; Martin-Rodriguez-Hernandez, J.; et al. Dengue, chikungunya and zika virus coinfection: Results of the national surveillance during the zika epidemic in Colombia. Epidemiol. Infect. 2019, 147, e77. [Google Scholar] [CrossRef]
- de Souza, T.M.A.; Ribeiro, E.D.; Correa, V.C.E.; Damasco, P.V.; Santos, C.C.; de Bruycker-Nogueira, F.; Chouin-Carneiro, T.; Faria, N.; Nunes, P.C.G.; Heringer, M.; et al. Following in the Footsteps of the Chikungunya Virus in Brazil: The First Autochthonous Cases in Amapa in 2014 and Its Emergence in Rio de Janeiro during 2016. Viruses 2018, 10, 623. [Google Scholar] [CrossRef]
- Azeredo, E.L.; Dos Santos, F.B.; Barbosa, L.S.; Souza, T.M.A.; Badolato-Correa, J.; Sanchez-Arcila, J.C.; Nunes, P.C.G.; de-Oliveira-Pinto, L.M.; de Filippis, A.M.; Dal Fabbro, M.; et al. Clinical and Laboratory Profile of Zika and Dengue Infected Patients: Lessons Learned from the Co-circulation of Dengue, Zika and Chikungunya in Brazil. PLoS Curr. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Soares, C.N.; Medialdea-Carrera, R.; Ellul, M.; da Silva, M.T.T.; Rosala-Hallas, A.; Jardim, M.R.; Burnside, G.; Pamplona, L.; Bhojak, M.; et al. The spectrum of neurological disease associated with Zika and chikungunya viruses in adults in Rio de Janeiro, Brazil: A case series. PLoS Negl. Trop. Dis. 2018, 12, e0006212. [Google Scholar] [CrossRef]
- White, S.K.; Mavian, C.; Elbadry, M.A.; Beau De Rochars, V.M.; Paisie, T.; Telisma, T.; Salemi, M.; Lednicky, J.A.; Morris, J.G., Jr. Detection and phylogenetic characterization of arbovirus dual-infections among persons during a chikungunya fever outbreak, Haiti 2014. PLoS Negl. Trop Dis. 2018, 12, e0006505. [Google Scholar] [CrossRef]
- Alva-Urcia, C.; Aguilar-Luis, M.A.; Palomares-Reyes, C.; Silva-Caso, W.; Suarez-Ognio, L.; Weilg, P.; Manrique, C.; Vasquez-Achaya, F.; Del Valle, L.J.; Del Valle-Mendoza, J. Emerging and reemerging arboviruses: A new threat in Eastern Peru. PLoS ONE 2017, 12, e0187897. [Google Scholar] [CrossRef]
- Cardoso, C.W.; Kikuti, M.; Prates, A.P.; Paploski, I.A.; Tauro, L.B.; Silva, M.M.; Santana, P.; Rego, M.F.; Reis, M.G.; Kitron, U.; et al. Unrecognized Emergence of Chikungunya Virus during a Zika Virus Outbreak in Salvador, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0005334. [Google Scholar] [CrossRef]
- Colombo, T.E.; Estofolete, C.F.; Reis, A.F.N.; da Silva, N.S.; Aguiar, M.L.; Cabrera, E.M.S.; Dos Santos, I.N.P.; Costa, F.R.; Cruz, L.; Rombola, P.L.; et al. Clinical, laboratory and virological data from suspected ZIKV patients in an endemic arbovirus area. J. Clin. Virol. 2017, 96, 20–25. [Google Scholar] [CrossRef]
- Cunha, M.D.P.; Santos, C.A.D.; Neto, D.F.L.; Schanoski, A.S.; Pour, S.Z.; Passos, S.D.; Souza, M.S.F.; Costa, D.D.; Zanotto, P.M.A. Outbreak of chikungunya virus in a vulnerable population of Sergipe, Brazil-A molecular and serological survey. J. Clin. Virol. 2017, 97, 44–49. [Google Scholar] [CrossRef]
- Charlys da Costa, A.; Theze, J.; Komninakis, S.C.V.; Sanz-Duro, R.L.; Felinto, M.R.L.; Moura, L.C.C.; Barroso, I.M.O.; Santos, L.E.C.; Nunes, M.A.L.; Moura, A.A.; et al. Spread of Chikungunya Virus East/Central/South African Genotype in Northeast Brazil. Emerg. Infect. Dis. 2017, 23, 1742–1744. [Google Scholar] [CrossRef]
- Kaur, N.; Jain, J.; Kumar, A.; Narang, M.; Zakaria, M.K.; Marcello, A.; Kumar, D.; Gaind, R.; Sunil, S. Chikungunya outbreak in Delhi, India, 2016: Report on coinfection status and comorbid conditions in patients. New Microbes New Infect. 2017, 20, 39–42. [Google Scholar] [CrossRef]
- Magalhaes, T.; Braga, C.; Cordeiro, M.T.; Oliveira, A.L.S.; Castanha, P.M.S.; Maciel, A.P.R.; Amancio, N.M.L.; Gouveia, P.N.; Peixoto-da-Silva, V.J., Jr.; Peixoto, T.F.L.; et al. Zika virus displacement by a chikungunya outbreak in Recife, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0006055. [Google Scholar] [CrossRef]
- Cabral-Castro, M.J.; Cavalcanti, M.G.; Peralta, R.H.S.; Peralta, J.M. Molecular and serological techniques to detect co-circulation of DENV, ZIKV and CHIKV in suspected dengue-like syndrome patients. J. Clin. Virol. 2016, 82, 108–111. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Gresh, L.; Vargas, M.J.; Ballesteros, G.; Tellez, Y.; Soda, K.J.; Sahoo, M.K.; Nunez, A.; Balmaseda, A.; Harris, E.; et al. Viremia and Clinical Presentation in Nicaraguan Patients Infected with Zika Virus, Chikungunya Virus, and Dengue Virus. Clin. Infect. Dis. 2016, 63, 1584–1590. [Google Scholar] [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.; Teissier, A.; Huart, M.; Merceron, S.; Vanhomwegen, J.; Roche, C.; Vial, A.L.; Teururai, S.; Sicard, S.; Paulous, S.; et al. Zika Virus Seroprevalence, French Polynesia, 2014–2015. Emerg. Infect. Dis. 2017, 23, 669–672. [Google Scholar] [CrossRef]
- Buathong, R.; Hermann, L.; Thaisomboonsuk, B.; Rutvisuttinunt, W.; Klungthong, C.; Chinnawirotpisan, P.; Manasatienkij, W.; Nisalak, A.; Fernandez, S.; Yoon, I.K.; et al. Detection of Zika Virus Infection in Thailand, 2012–2014. Am. J. Trop. Med. Hyg. 2015, 93, 380–383. [Google Scholar] [CrossRef]
- Aubry, M.; Teissier, A.; Roche, C.; Richard, V.; Yan, A.S.; Zisou, K.; Rouault, E.; Maria, V.; Lastere, S.; Cao-Lormeau, V.M.; et al. Chikungunya outbreak, French Polynesia, 2014. Emerg. Infect. Dis. 2015, 21, 724–726. [Google Scholar] [CrossRef]
- Sadarangani, S.P.; Hsu, L.Y. The 2016 Outbreak of Zika in Singapore. Ann. Acad Med. 2016, 45, 381–382. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Liu-Helmersson, J.; Stenlund, H.; Wilder-Smith, A.; Rocklov, J. Vectorial capacity of Aedes aegypti: Effects of temperature and implications for global dengue epidemic potential. PLoS ONE 2014, 9, e89783. [Google Scholar] [CrossRef]
- Nhan, T.X.; Musso, D. The burden of chikungunya in the Pacific. Clin. Microbiol. Infect. 2015, 21, e47–e48. [Google Scholar] [CrossRef]
- Pastula, D.M.; Hancock, W.T.; Bel, M.; Biggs, H.; Marfel, M.; Lanciotti, R.; Laven, J.; Chen, T.H.; Staples, J.E.; Fischer, M.; et al. Chikungunya virus disease outbreak in Yap State, Federated States of Micronesia. PLoS Negl. Trop. Dis. 2017, 11, e0005410. [Google Scholar] [CrossRef]
- Venturi, G.; Di Luca, M.; Fortuna, C.; Remoli, M.E.; Riccardo, F.; Severini, F.; Toma, L.; Del Manso, M.; Benedetti, E.; Caporali, M.G.; et al. Detection of a chikungunya outbreak in Central Italy, August to September 2017. Eurosurveillance 2017, 22, 17–00646. [Google Scholar] [CrossRef]
- Kabir, I.; Dhimal, M.; Muller, R.; Banik, S.; Haque, U. The 2017 Dhaka chikungunya outbreak. Lancet Infect. Dis. 2017, 17, 1118. [Google Scholar] [CrossRef]
- Gregianini, T.S.; Ranieri, T.; Favreto, C.; Nunes, Z.M.A.; Tumioto Giannini, G.L.; Sanberg, N.D.; da Rosa, M.T.M.; da Veiga, A.B.G. Emerging arboviruses in Rio Grande do Sul, Brazil: Chikungunya and Zika outbreaks, 2014–2016. Rev. Med. Virol. 2017, 27, e1943. [Google Scholar] [CrossRef]
- Henry, M.; Francis, L.; Asin, V.; Polson-Edwards, K.; Olowokure, B. Chikungunya virus outbreak in Sint Maarten, 2013–2014. Rev. Panam. Salud Publica 2017, 41, e61. [Google Scholar] [CrossRef]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2018, 13, e0210122. [Google Scholar] [CrossRef] [PubMed]
- Charrel, R.N.; Leparc-Goffart, I.; Gallian, P.; de Lamballerie, X. Globalization of Chikungunya: 10 years to invade the world. Clin. Microbiol. Infect. 2014, 20, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(st) Century. Trop. Med. Health 2011, 39, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Boga, J.A.; Alvarez-Arguelles, M.E.; Rojo-Alba, S.; Rodriguez, M.; de Ona, M.; Melon, S. Simultaneous detection of Dengue virus, Chikungunya virus, Zika virus, Yellow fever virus and West Nile virus. J. Virol. Methods 2019, 268, 53–55. [Google Scholar] [CrossRef]
- Aguiar, B.S.; Lorenz, C.; Virginio, F.; Suesdek, L.; Chiaravalloti-Neto, F. Potential risks of Zika and chikungunya outbreaks in Brazil: A modeling study. Int. J. Infect. Dis. 2018, 70, 20–29. [Google Scholar] [CrossRef]
- Lowe, R.; Barcellos, C.; Brasil, P.; Cruz, O.G.; Honorio, N.A.; Kuper, H.; Carvalho, M.S. The Zika Virus Epidemic in Brazil: From Discovery to Future Implications. Int. J. Environ. Res. Public. Health 2018, 15, 96. [Google Scholar] [CrossRef]
- Nunes, M.R.; Faria, N.R.; de Vasconcelos, J.M.; Golding, N.; Kraemer, M.U.; de Oliveira, L.F.; Azevedo Rdo, S.; da Silva, D.E.; da Silva, E.V.; da Silva, S.P.; et al. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med. 2015, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, I.G.; Marandino, R.; Mendonca, A.P.; Nogueira, R.M.; Vasconcelos, P.F.; Guerra, L.R.; Brandao, B.C.; Mendonca, A.P.; Aguiar, G.R.; Bacco, P.A. Chikungunya virus infection: Report of the first case diagnosed in Rio de Janeiro, Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Power, G.M.; Vaughan, A.M.; Qiao, L.; Sanchez Clemente, N.; Pescarini, J.M.; Paixao, E.S.; Lobkowicz, L.; Raja, A.I.; Portela Souza, A.; Barreto, M.L.; et al. Socioeconomic risk markers of arthropod-borne virus (arbovirus) infections: A systematic literature review and meta-analysis. BMJ Glob. Health 2022, 7, e007735. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.; Lee, P. Publication bias in meta-analysis: Its causes and consequences. J. Clin. Epidemiol. 2000, 53, 207–216. [Google Scholar] [CrossRef] [PubMed]



| No. | Study ID (References) | Study Period | Country | Type of Participants | Number of Participants (Female) | Age of the Participant (Mean ± SD, Range) (Years) | Detection Technique | Adult/ Paediatric |
|---|---|---|---|---|---|---|---|---|
| 1 | Souza-Santos 2023 [50] | 07/2018 to 10/2018 | Brazil | Random selection based on socioeconomic status | 2114 (NR) | 10–14 | Rapid test kit | Paediatric |
| 2 | Mac 2023 [32] | 12/2020 to 11/2021 | Nigeria | All outpatients, pregnant women, and people living with HIV | 871 (619) | 36.6 (0–80+) | Immunoblot assay | Both adult and paediatric |
| 3 | Frota 2023 [51] | 02/2018 to 12/2018 | Brazil | Women with suspected arbovirus infection | 1289 (all female) | 15–39 | RT-PCR | Both adult and paediatric |
| 4 | Khongwichit 2022 [33] | 10/2018 to 02/2020 | Thailand | Chikungunya suspected patients | 1806 (NR) | ≤10–>50 | RT-PCR | Both adult and paediatric |
| 5 | Bailly 2021 [35] | 06/2017 to 10/2017 | French Guiana | Patients with suspected arbovirus infection | 2697 (NR) | 34.1 (25–75) | Microsphere immunoassay | Adult |
| 6 | Calvo-Anguiano 2021 [52] | 04/2015 to 06/2015 and 02/2016 to 03/2016 | Mexico | Patients with suspected arbovirus infection | 253 (169) | 0–>50 | RT-qPCR and nested-PCR | Both adult and paediatric |
| 7 | Jacques 2021 [31] | 10/2018 to 05/2019 | Brazil | Pregnant women with obstetric complications | 780 (all female) | 26.5 ± 3.6 | RT-qPCR | Adult |
| 8 | Mota 2021 [53] | 2016 | Brazil | Patients with compatible symptoms of arbovirus infection | 182 (131) | 40.06 ± 19.86 | RT-qPCR | Adult |
| 9 | Leonhard 2021 [54] | 12/2014 to 02/2017 | Brazil | Patients with a suspected preceding arbovirus infection and an acute neurological disease | 71 (36) | 46 (32–56) | RT-PCR and ELISA | Adult |
| 10 | Eligio-Garcia 2020 [55] | 02/2019 to 08/2019 | Mexico | Asymptomatic pregnant women | 136 (all female) | 14–43 | RT-PCR and ELISA | Adult |
| 11 | Ferreira 2020 [56] | 12/2014 to 12/2016 | Brazil | Suspected arbovirus-associated neurological disease | 201 (106) | 48 (34–60) | RT-PCR and PRNT | Adult |
| 12 | Perisse 2020 [57] | 07/2018 to 10/2018 | Brazil | Suspected patients with both symptomatic and asymptomatic arboviral infections | 2120 (1624) | 43.7 ± 21.4 | Rapid test kit | Adult |
| 13 | Bagno 2019 [58] | NR | Brazil | Pregnant woman and their respective new-borns with symptoms of arboviral infection | 193 (NR) | NR | RT-PCR and ELISA | Both adult and paediatric |
| 14 | Ball 2019 [59] | 05/2014 To 02/2015 | Haiti | Acute febrile illness | 252 (120) | 7.8 ± 4.5 | RT-PCR | paediatric |
| 15 | de Souza Costa 2019 [60] | 2015 to 2016 | Brazil | Acute febrile illness | 453 (266) | NR | Rapid colorimetric tests and RT-PCR | Both adult and paediatric |
| 16 | Silva 2019 [61] | 09/2014 to 07/2016 | Brazil | Acute febrile illness | 948 (NR) | NR | RT-PCR and ELISA | Adult |
| 17 | Mercado-Reyes 2019 [62] | 10/2015 to 12/2016 | Colombia | Patients suspected of arbovirus infection | 23,871 (NR) | NR | RT-PCR | Both adult and paediatric |
| 18 | Carrillo-Hernandez 2019 [11] | 08/2015 to 04/2016 | Colombia | Patients with febrile syndrome | 157 (103) | 26.81 ± 14.54 | Conventional PCR and RT-PCR | Both adult and Paediatric |
| 19 | de Souza 2018 [63] | 2014 to 2015 | Brazil | Patients suspected of arbovirus infection | 299 (NR) | NR | RT-PCR and ELISA | Both adult and paediatric |
| 20 | Leal Azeredo 2018 [64] | 02/2016 to 03/2016 | Brazil | Patients suspected of arbovirus infection | 134 (NR) | NR | RT-PCR and ELISA | Both adult and paediatric |
| 21 | Loconsole 2018 [34] | 03/2015 to 06/2017 | Italy | Vector-borne disease suspected international travellers | 156 (77) | 33 (median) | ELISA | Adult |
| 22 | Mehta 2018 [65] | 11/2015 to 06/2016 | Brazil | Patients with new neurological conditions associated with suspected ZIKV infection | 35 (NR) | NR | RT-PCR | Adult |
| 23 | White 2018 [66] | 05/2014 to 07/2014 | Haiti | Acute febrile illness | 100 (NR) | NR | RT-PCR | paediatric |
| 24 | Alva-Urcia 2017 [67] | 01/2016 to 03/2016 | Peru | Acute febrile illness | 139 (63) | NR | RT-PCR | Both adult and paediatric |
| 25 | Cardoso 2017 [68] | 07/2015 to 04/2016 | Brazil | Patients suspected of arbovirus infection | 58 (NR) | NR | RT-PCR and ELISA | NR |
| 26 | Colombo 2017 [69] | 01/2016 to 11/2016 | Brazil | Patients with suspected zika virus | 433 (287) | 36.7 ± 16.8 | RT-PCR | Both adult and paediatric |
| 27 | Cunha 2017 [70] | 02/2016 | Brazil | Symptoms of arboviral infections | 142 (NR) | NR | RT-PCR and ELISA | Both adult and paediatric |
| 28 | da Costa 2017 [71] | 03/2016 to 05/2016 | Brazil | Symptoms compatible with dengue, chikungunya zika virus infection | 273 (175) | 37 ± NR | Molecular diagnostics and virus discovery methods | Both adult and paediatric |
| 29 | Kaur 2017 [72] | 08/2016 to 12/2016 | India | Suspected chikungunya virus | 600 (NR) | 35 ± NR | RT-PCR | Both adult and paediatric |
| 30 | Magalhaes 2017 [73] | 05/2015 to 05/2016 | Brazil | Acute febrile patients with arboviral symptoms | 263 (NR) | 29 (median) | RT-PCR and ELISA | Both adult and paediatric |
| 31 | Cabral-Castro 2016 [74] | 04/2015 to 01/2016 | Brazil | Patients with suspected dengue fever | 30 (NR) | NR | RT-PCR | NR |
| 32 | Pessoa 2016 [36] | 05/2015 | Brazil | Suspected dengue patients | 77 (52) | NR | RT-PCR and ELISA | Both adult and paediatric |
| 33 | Waggoner 2016 [75] | 09/2015 to 04/2016 | Nicaragua | Suspected arboviral illness | 346 (NR) | NR | RT-PCR | NR |
| Subgroups | Prevalence of Zika- Chikungunya Coinfection [95% CIs] (%) | Number of Studies Analysed | Total Number of Subjects | Heterogeneity | |
|---|---|---|---|---|---|
| I2 | p-Value | ||||
| ZIKV-CHIKV coinfection from different countries | |||||
| Brazil | 1.0 [0.6–1.4] | 19 | 10,003 | 87% | <0.01 |
| Colombia | 2.4 [0.0–7.3] | 2 | 24,024 | 88% | <0.01 |
| Haiti | 3.5 [0.2–6.8] | 2 | 352 | 50% | 0.16 |
| Mexico | 1.8 [0.5–3.1] | 2 | 389 | 0% | 0.71 |
| ZIKV-CHIKV coinfection from different regions | |||||
| South America | 0.6 [0.4–0.9] | 24 | 36,940 | 85% | <0.01 |
| North America | 2.8 [1.5–4.1] | 5 | 1087 | 43% | 0.13 |
| Asia | 0.1 [0.0–0.3] | 2 | 2406 | 0% | 0.59 |
| ZIKV-CHIKV coinfection in adult and paediatric | |||||
| Adult | 0.7 [0.2–1.1] | 10 | 7326 | 84% | <0.01 |
| Paediatric | 2.1 [0.0–4.2] | 3 | 2466 | 73% | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; Sultana, S.; Kundu, S.; Alam, S.S.; Hossan, T.; Islam, M.A. Global Prevalence of Zika and Chikungunya Coinfection: A Systematic Review and Meta-Analysis. Diseases 2024, 12, 31. https://doi.org/10.3390/diseases12020031
Ahmed S, Sultana S, Kundu S, Alam SS, Hossan T, Islam MA. Global Prevalence of Zika and Chikungunya Coinfection: A Systematic Review and Meta-Analysis. Diseases. 2024; 12(2):31. https://doi.org/10.3390/diseases12020031
Chicago/Turabian StyleAhmed, Saleh, Shabiha Sultana, Shoumik Kundu, Sayeda Sadia Alam, Tareq Hossan, and Md Asiful Islam. 2024. "Global Prevalence of Zika and Chikungunya Coinfection: A Systematic Review and Meta-Analysis" Diseases 12, no. 2: 31. https://doi.org/10.3390/diseases12020031
APA StyleAhmed, S., Sultana, S., Kundu, S., Alam, S. S., Hossan, T., & Islam, M. A. (2024). Global Prevalence of Zika and Chikungunya Coinfection: A Systematic Review and Meta-Analysis. Diseases, 12(2), 31. https://doi.org/10.3390/diseases12020031








